Abstract
Clinical data underscores the fact that subsequent high mortality rates occur in patients who survive acute septic episodes. Herein, we described a clinically relevant model of experimental sepsis that we believe will allow further investigation of the manner in which the pulmonary innate immune response is modulated after sepsis. C57BL/6 mice were subjected to cecal ligation and puncture (CLP) model, whereby the cecum was partially ligated and punctured nine times with a 21-gauge needle. This procedure was associated with 100% mortality at 3 days after surgery. In contrast, when mice subjected to CLP were treated with antibiotic beginning at 8 hours after surgery, and every 12 hours thereafter until 3 days, ∼60% of the mice survived. Interestingly, CLP survivors quickly succumbed (100% mortality) to pulmonary infection when intratracheally challenged, at day 3 after CLP, with viable Aspergillus fumigatus conidia. No mortality was observed in conidia-challenged sham-operated mice. The defective innate immune response against A. fumigatus in CLP mice could not be explained by a failure of neutrophils to infiltrate the lungs. Instead, gene array analysis revealed that several components of the innate immune response, including the nuclear factor-κB signaling pathway, were down-regulated. Thus, we describe a system of sepsis-induced innate immune failure in the lungs of C57BL/6 mice.
More than 500,000 cases of sepsis occur annually in the United States. 1 Despite the availability of an increasing array of potent antibiotics and intensive medical care, this mortality remains significantly high. 1-3 The initiation and maintenance of sepsis and acute lung injury are dependent on a diverse collection of poorly understood cellular and molecular mechanisms, which are likely triggered by an initially overwhelmed innate immune response leading to a host-derived cytokine storm. The exaggerated systemic inflammatory response syndrome that occurs during sepsis is counterbalanced by a subsequent sustained expression of potent anti-inflammatory cytokines resulting in a prolonged and profound state of immunosuppression. 4 This impaired immune activity is likely caused by a compensatory anti-inflammatory response syndrome, which is characterized by substantially altered monocyte/macrophage effector cell function. Patients with sepsis are clearly immunosuppressed, as evidenced by their development of immune anergy, their frequent inability to eradicate the primary infection that produced the sepsis response, and their propensity to acquire secondary nosocomial infections. 5 Interestingly, clinical investigations have emphasized that the risk of dying after the septic episode continues to significantly rise throughout the first year after a septic episode. Further studies using clinical models have demonstrated that, after surviving a severe septic event, the risk of dying exceeded predictions for the next 5 years. A study by Perl et al 1 found that only 40% of severe sepsis patients in 4 years were alive and another investigation by Quartin and colleagues, 6 showed similar results, as only 20% of severe septic patient leaving the hospital were alive within 8 years. Thus, the paradigm that sepsis is only an acute disease with acute clinical consequences must be re-assessed in terms of the potential chronic medical problems caused by this syndrome.
Innate immunity provides a rapid response to invasive or infectious stimuli. This response is characterized by the activation tissue resident cells and recruited leukocytes via their ability to respond to a variety of proinflammatory cytokines, such as interleukin (IL)-1, tumor necrosis factor (TNF)-α, and specific chemokines. 7-10 The latter group of mediators may be of critical importance because the chemokines are known to be involved in both innate and acquired immunity, and appear to link these two immune responses. 11,12 The manner in which chemokines and chemokines receptors provide this immune linkage is a growing focus of research in the sepsis field.
The purpose of this study was to develop and provide an initial characterization of a clinically relevant and reproducible model of septic peritonitis, in which pulmonary innate immune responses to secondary infections could be studied. Our initial investigations demonstrated a marked increase in the survival and an improvement of the clinical characteristics of C57BL/6 mice after severe cecal ligation and puncture (CLP)-induced peritonitis via the implementation of intense fluid resuscitation and antibiotic treatment. However, it was further demonstrated that the survivors were susceptible to an intrapulmonary challenge with Aspergillus fumigatus, a ubiquitous fungus that normally only causes mortality in an immunocompromised hosts. 13,14 Mechanistically, the altered response in this clinically relevant model of septic peritonitis is associated with depressed pulmonary innate immune responses.
Materials and Methods
Severe Peritonitis Model Induced by CLP
Specific-pathogen-free, female, C57BL/6 mice were subjected to CLP surgery as previously described in detail. 15 Briefly, mice were anesthetized by the intraperitoneal injection of a mixture of ketamine (Ketamine HCL, 112.5 mg/kg; Abbott Laboratories, N. Chicago, IL) and xylazine (Anased, 7.5 mg/kg; Lloyd Laboratories, Shenandoah, IA). Under sterile surgical conditions, a 1- to 2-cm midline incision was made to the ventral surface of the abdomen, and the cecum was fully exposed through this incision. The cecum was ligated (without causing bowel obstruction) at its base with a 4-0 silk suture, and the ligated cecum was punctured at total of three or nine times with 21-gauge needle. Unless otherwise stated, the nine-puncture CLP surgery model was used in the experiments described below. Sham-operated mice underwent an identical laparotomy but did not undergo cecal ligation or puncture and served as controls. In both groups of mice, the abdominal incision was closed using a surgical staple, and 1 ml of sterile normal saline was administered subcutaneously for fluid resuscitation. In the first set of experiments, survival was monitored in groups of 15 mice subjected to a sham operation or CLP surgery for up to 7 days after these procedures. Because this model was associated with 100% mortality (see Results), we next examined whether survival could be improved with a course of antibiotic treatment. In the second set of experiments, groups of 8 to 16 mice subjected to sham operation or CLP surgery were treated with an antibiotic preparation containing Imipenem conjugated with Cilastatin (Primaxin I.V., 10 mg/kg, i.p.; Merck & Co., Inc, West Point, PA) beginning at 8, 12, or 18 hours after CLP surgery, and antibiotic was then readministered every 12 hours thereafter for up to 3 days later.
Neutrophil and Monocyte/Macrophage Migration into the Peritoneal Cavity after CLP Surgery
Neutrophil and monocyte/macrophage migration was evaluated at 4 and 18 hours after sham or CLP (three or nine punctures) surgery in mice that did not receive antibiotic and at days 1, 3, and 7 after CLP surgery in mice that received the antibiotic therapy. At each of these time points, a minimum of five mice was euthanized by anesthesia overdose, and the cells present in the peritoneal cavity were harvested. Total counts were determined using a hemocytometer and differential cell counts were determined from microscope slides onto which peritoneal cells were cytospun (Cytospin; Shandon Scientific, Runcorn, UK). The Diff-Quick method was used to stain each microscope slide.
A. fumigatus Culture Conditions and Intrapulmonary Infection
To examine alterations in the pulmonary innate immune response, we examined the impact of intratracheally introduced A. fumigatus conidia in mice previously subjected to CLP surgery and antibiotic therapy. A well-described strain of A. fumigatus (strain 13073; American Type Culture Collection, Rockville, MD) was grown on Sabouraud dextrose agar culture plates (Becton & Dickinson, Cockeysville, MD) at 37°C, and A. fumigatus conidia were harvested from these cultures plates between days 8 and 12 after the initiation of the cultures. Conidia were harvested from each culture plate by washing with 50 ml of 1× phosphate-buffered saline (PBS) containing 0.1% Tween-20 and scraping the conidia from the mycelium with plastic pipette. Conidia were passed through four layers of sterile gauze to remove hyphal material and debris, and counted using a hemocytometer. The suspension of conidia was then diluted to the desired concentration and kept on ice until introduced into mice. At day 3 after sham or CLP surgery and 3 days of antibiotic therapy (described above), each mouse was anesthetized by the intraperitoneal injection of ketamine and xylazine, and an incision of 0.5 cm in length was applied to separate the skin and muscle immediately above the trachea. Each mouse then received 1.0 × 107 or 5.0 × 107 A. fumigatus conidia suspended in 30 μl of PBS via the intratracheal route using a 1-ml syringe controlled by a Stepper (Tridak Division, Indicon, Inc., Brookfield, CT). The incision was closed with a surgical staple. Mouse survival and pulmonary response was monitored for up to 7 days after the intratracheal A. fumigatus conidia challenge.
Bronchoalveolar Lavage (BAL) after CLP Surgery
BAL was performed at 6 and 18 hours after CLP surgery in mice that did not receive antibiotic and at day 3 after CLP surgery in mice that received the antibiotic therapy. In another set of experiments, BAL was performed at 6 hours after saline or A. fumigatus conidia injections into mice that had been subjected to CLP surgery and antibiotic therapy. At each of these time points, groups of at least five mice were euthanized by anesthesia overdose, the trachea was exposed and a 1-ml syringe with 18-gauge needle was inserted into the trachea. BAL was performed by instilling 1 ml of normal saline. Differential cell counts were made from microscope slides onto which BAL samples were cytospun and stained by the Diff-Quick method.
Lung Histology: Hematoxylin and Eosin (H&E) Staining
Lungs were collected at 6 and 18 hours after sham or CLP surgery in groups of mice that did not receive antibiotic and at day 3 in both surgery groups that received antibiotic treatment. Whole lung samples for histological examination were excised, perfused with 10% formalin to improve resolution of anatomical relationships, and placed in fresh formalin for an additional 24 hours. Routine histological techniques were used to paraffin-embed this tissue, and 3-μm sections of whole lung were stained with H&E.
Cytokine and Chemokine Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
At designated time points (at 6 and 18 hours in the sham and CLP surgery groups not treated with antibiotic, and at 1, 3, and 7 days after sham or CLP surgery in the antibiotic-treated groups), mice were anesthetized with ketamine plus xylazine and euthanized by medular dislocation. Lung lobes were homogenized in 1 ml of lysis buffer, containing PBS, 0.5% Triton X-100 and protease inhibitor, (Roche Diagnostics, Mannheim, Germany) using a tissue homogenizer. Tissue homogenates were incubated on ice for 30 minutes, centrifuged at 2000 × g for 10 minutes, and supernatants were then collected. These supernatants were analyzed for murine TNF-α, interferon-γ, CCL2 (MCP-1), MIP-2, KC, IL-13, CCL22 (MDC), CCL6, IL-4, and IL-10 using a modified double-ligand ELISA assay as previously described. 16 Also, whole lung levels of all of the above cytokines and chemokines were analyzed at 6 hours after A. fumigatus conidia challenge in mice subjected to sham or CLP surgery. The results were normalized to the amount of protein (mg) present in cell-free preparations of each sample by Bradford assay, as described elsewhere. 17
Transmission Electron Microscopy
Lungs were collected 2 days after A. fumigatus conidia challenge in sham or CLP surgery groups that received antibiotic treatment. At this time point, groups of three mice were euthanized by anesthesia overdose, the trachea was exposed and a 1-ml syringe with 18-gauge needle was inserted into the trachea. Lungs were inflated with 4% glutaraldehyde buffered with 0.1 mol/L of cacodylate. The trachea was tied with 4-0 silk suture, and the lungs were en bloc excised and immersed into a small beaker of 4% buffered glutaraldehyde on ice for 10 minutes. The lung was minced into 1-mm3 pieces with a new razor blade. Samples were processed for routine transmission electron microscopy. Briefly, the procedure is as follows. These lung pieces were fixed overnight at 4°C in the 4% buffered glutaraldehyde. Samples were postfixed in 1% osmium tetroxide for 1 hour, dehydrated in graded alcohols and propylene oxide, infiltrating in increasing concentration of Epon (Electron Microscopy Sciences, Fort Washington, PA, USA) and propylene oxide, to finally embedding in pure Epon. Ultrathin sections (90 Å) were cut, stained with uranyl acetate and lead citrate, and observed on a Philips transmission electron microscope at 60KV.
Lung Histology: Gomori Methanamine Silver Staining of Fungal Material
Mice challenged with A. fumigatus conidia or saline were euthanized by anesthesia overdose 2 days after the intratracheal conidia challenge and the lungs were collected for histological processing as described above. Three-μm sections of whole lung were stained with Gomori methanamine silver to localize A. fumigatus conidia and hyphal elements (black staining).
SuperArray Analysis
SuperArray analysis of gene expression was performed according to the manufacturer’s directions (GEArray Q series KIT nonradioactive; SuperArray Inc., Bethesda, MD). Briefly, pooled mouse lungs were homogenized and the total RNA was isolated using the Trizol reagent (Invitrogen/Life Technologies, Carlsbad, CA). Once isolated, 2.5 μg of mRNA was used as the template for biotin-labeled cDNA probe synthesis. The labeled probes were then hybridized to the GEArray Q series membrane containing 96 genes related to the mouse nuclear factor (NF)-κB signaling pathway. After an overnight incubation in a 60°C incubator, the membranes were washed several times in hybridization tubes. Subsequently, membranes were blocked and subjected to chemiluminescent detection (alkaline phosphatase-conjugated streptavidin; 1:10,000 dilution) with the chemiluminescent substrate for alkaline phosphatase, phenylphosphate substituted 1,2 dioxetane (CDP-star). The membranes were exposed to X-ray film, and the developed X-ray film was scanned to create a raw image file, and this file was analyzed using an image analysis software program (Scanalyze by Michael Eisen). Each GEArray Q series membrane was spotted with a negative control of pUC18 DNA and housekeeping genes such as β-actin, GAPDH, cyclophilin, and ribosomal protein L13a. The relative abundance of a particular transcript was estimated by directly comparing its signal intensity to the signal derived from three or four combined housekeeping genes. The results were expressed as the ratio of the normalized spot intensity in the sham plus saline group versus sham plus conidia group, and in the between sham plus conidia group versus CLP plus conidia group. Fold changes in gene expression were calculated and three to four separate experiments were conducted. Only twofold changes were considered significant and are reported below.
Statistical Analysis
All data shown are means ± SE and are representative of two to four separate experiments. The means between different treatments were compared by analysis of variance. If significance was detected, individual differences were detected using the Bonferroni’s t-test for unpaired values. Statistical significance was set at P < 0.05. Survival rates were expressed as percentages, and a log rank test (chi-square test) was used to detect differences in mouse survival.
Results
Severe Sepsis Is Associated with the Failure of Neutrophil and Monocyte Recruitment into the Peritoneal Cavity and the Lung
As has been previously described, 18 C57BL/6 mice subjected to a severe peritonitis induced by nine punctures with a 21-gauge needle die quickly. As shown in Figure 1A ▶ , 80% mice were dead at day 2, and 100% were dead at day 3 (Figure 1A) ▶ . Similar to other published reports, 19,20 mice subjected to this severe type of CLP surgery procedure experienced a significant failure of neutrophil (at 4 and 18 hours after CLP), and mononuclear cell (at 18 hours after CLP) recruitment into the peritoneal cavity (Figure 1B) ▶ compared with mice subjected to CLP with three punctures, which showed a significant peritoneal migration of neutrophils 13.4 ± 2.08 × 106/cavity (Figure 1B) ▶ . The migration of neutrophils observed in the sham group was because of the abdominal incision, and this response was short-lived.
Figure 1.
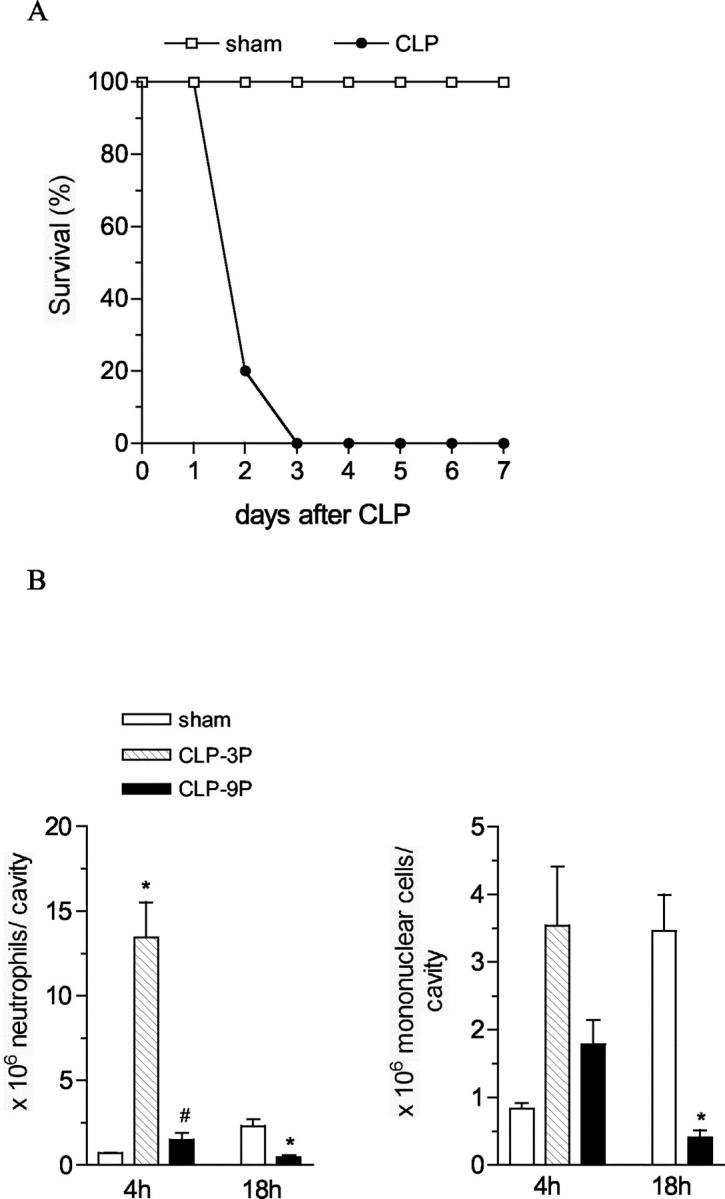
Survival curve and leukocyte migration to the peritoneal cavity in mice subjected to severe sepsis. A: Mice were subjected to CLP or sham operation and the survival of these mice was followed until day 7. The results are expressed as percentage of survival mice per day and each group has 10 mice. The data are representative of four experiments. P < 0.05 as compared with sham-operated mice. B: Mice subjected to CLP with nine punctures (CLP-9P) or with three punctures (CLP-3P) or sham operation were sacrificed 4 or 18 hours after surgery for neutrophil and mononuclear cell migration evaluation The results are expressed as mean ± SEM of cell per cavity, each group has five to eight mice. The data are representative of two experiments. *, P < 0.05 compared to sham-operated mice; #, P < 0.05 compared to CLP-3P group.
Antibiotic Therapy Markedly Improves Survival and Leukocyte Migration during Severe Sepsis
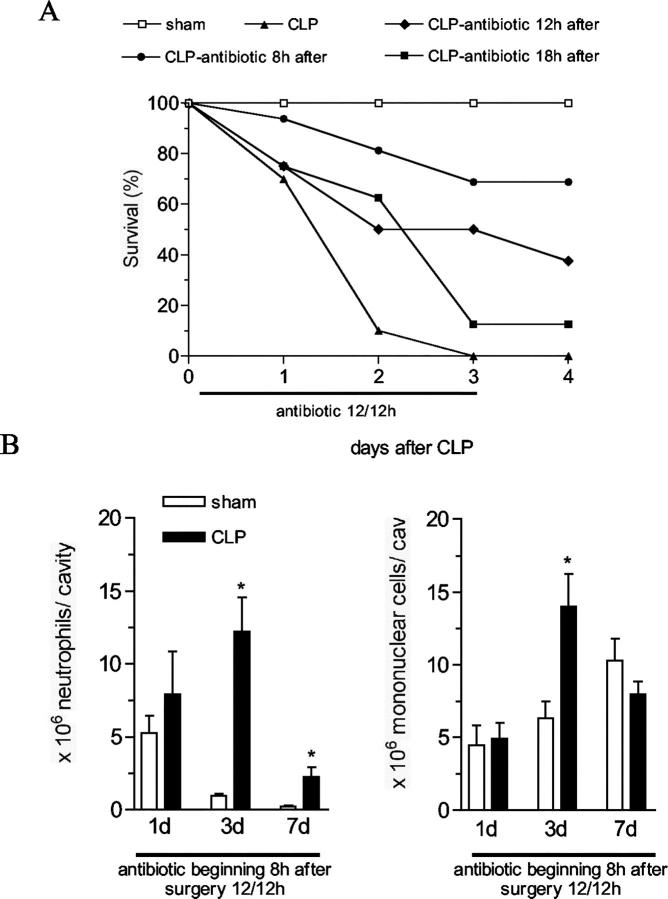
Given the rapid demise of experimental animals after severe sepsis it was apparent that other interventions were required to improve mouse survival. Therefore, we examined the impact of various antibiotic therapeutic regimens in this severe sepsis model. After nine puncture CLP surgery, groups of 8 to 16 mice were treated intraperitoneally with antibiotic beginning at 8, 12, or 18 hours after CLP surgery, and every 12 hours thereafter up to day 3 (Figure 2A) ▶ . Sham surgery groups did not receive antibiotic therapy in this experiment. The CLP group that received antibiotic at 8 hours after surgery exhibited the greatest improvement in survival, as ∼70% of these mice were alive at day 4. In contrast, CLP groups that receivedantibiotics commencing either at 12 or 18 hours after surgery showed more modest improvements in survival of 40% and 10%, respectively. In all subsequent experiments we used the antibiotic treatment protocol beginning at 8 hours after sham or CLP surgery. We next determined the extent of leukocyte migration in the CLP mice treated with antibiotic. The improvement in mouse survival appeared to be related, in part, to the restoration of neutrophil and mononuclear cell migration into the peritoneal cavity (Figure 2B) ▶ . In contrast, neutrophils were not found in the BAL after CLP in the absence (6 hours and 18 hours) or in the presence (3 days) of the antibiotic treatment (data not shown).
Figure 2.
Survival curve and leukocyte migration to the peritoneal cavity in mice subjected to CLP and treated with antibiotic. A: Mice received an intraperitoneal injection of antibiotic Imipenem (200 μg/0.2 ml/mouse) 8, 12, or 18 hours after CLP surgery, and then every 12 hours until day 3 after surgery. Mouse survival was followed until day 4, and the results were expressed as survival percentage, each group has 10 mice. The data are representative of three experiments. P < 0.05 between CLP antibiotic 8-hour group and CLP without antibiotic treatment group. B: Mice were subjected to sham or CLP surgery and received antibiotic 8 hours after, and then every 12 hours until day 3. Neutrophil and mononuclear cell recruitment to peritoneal cavity was evaluated at 1, 3, and 7 days after surgery. The results are expressed as mean ± SEM, each group has five to eight mice. The data are representative of three experiments. *, P < 0.05 compared to sham-operated group.
Histological Improvement in the Lungs of Mice Receiving Antibiotic Treatment
In the absence of antibiotic therapy, the experimental animals rapidly succumbed to sepsis suggesting that the pulmonary system was a target in this severe sepsis model. As shown in Figure 3B ▶ , whole lung samples collected at 6 hours after CLP surgery showed leukocyte infiltration and a major thickening of the alveolar wall, two features that were not observed in sham-operated mice, which appeared histologically normal (Figure 3A) ▶ . At 18 hours after CLP surgery, major edematous and inflammatory alterations to the lung architecture were apparent (Figure 3D) ▶ in contrast to lung samples from mice subjected to sham surgery (Figure 3C) ▶ , confirming that a septic insult in the peritoneal cavity has a deleterious effect on the lung. Surprisingly, the lung architecture in mice subjected to CLP surgery and antibiotic therapy (commencing at 8 hours after CLP) appeared normal at day 3 (Figure 3F) ▶ and histologically similar to the lung samples from sham-operated mice that also received antibiotic therapy (Figure 3E) ▶ .
Figure 3.
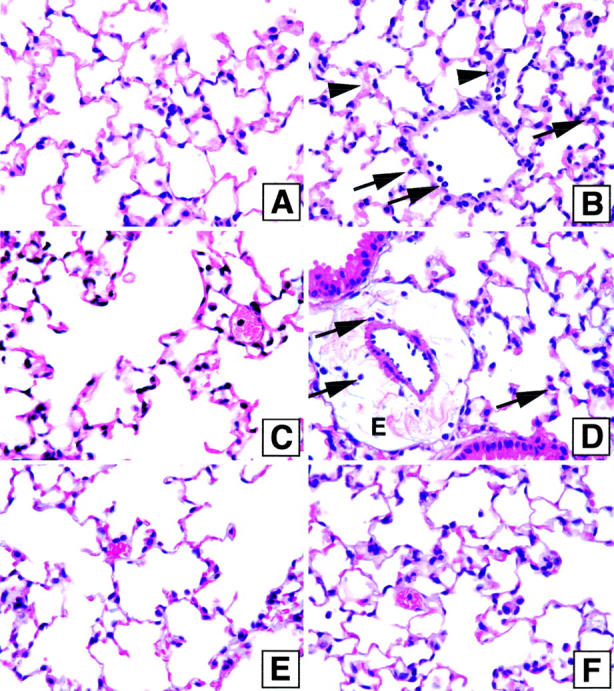
Lung histology in mice subjected to sham or CLP surgery with and without antibiotic treatment. Mice were sacrificed 6 (A and B) and 18 hours (C and D) after sham (A and C) and CLP (B and D) surgery without antibiotic treatment. Sham (E) and CLP (F) groups treated with antibiotic were sacrificed 3 days after surgery. The lungs were perfused with formalin 10% by trachea, harvested, cut, embedded in paraffin, and stained with H&E. The arrowheads indicate the alveolar wall, which is thickened, and the arrows indicate neutrophil infiltration (B and D), edema (D). The histology shown is representative of three separate experiments, with five mice in each group.
Chemokines and Cytokines Profiles in Whole Lung Samples from CLP Groups without and with Antibiotic Therapy
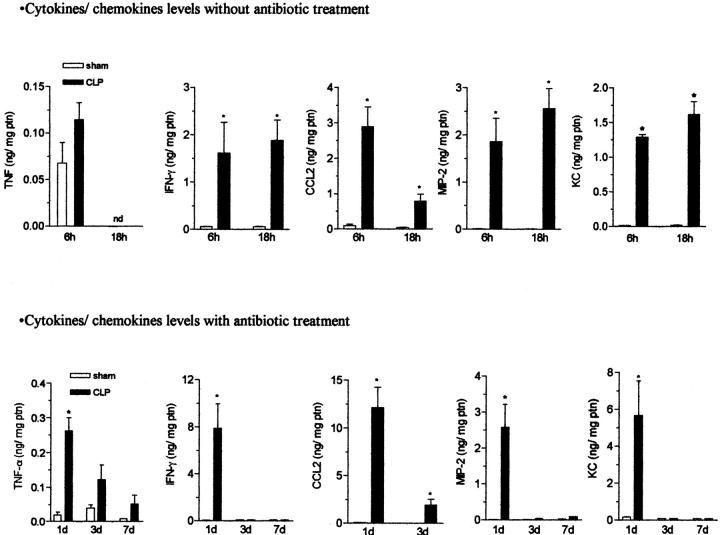
We next examined whole lung samples from CLP and sham surgery groups to determine whether the antibiotic therapy altered the cytokine and chemokine profile in the lung. Because untreated mice died very soon after CLP surgery, cytokine and chemokine levels were measured in whole lung samples at early time points (6 and 18 hours after surgery). In the absence of antibiotics, the whole lung levels of interferon-γ, CCL2 (MCP-1), MIP-2, and KC were significantly elevated in the CLP mice, as compared to the sham control mice (Figure 4 ▶ , top). The same cytokines from lung samples of antibiotic-treated CLP (commencing at 8 hours after CLP) and sham groups were analyzed at 1, 3, and 7 days. Significant elevations in the proinflammatory cytokines and chemokines were observed at 24 hours after CLP surgery compared with the sham group (Figure 4 ▶ , bottom). Interestingly, only CCL2 remained significantly elevated at later times after the CLP surgery in antibiotic-treated mice compared with the sham group.
Figure 4.
The effect of sham or CLP surgery on lung levels of TNF-α, interferon-γ, CCL22, MIP-2, and KC in mice with or without antibiotic therapy. Sham- and CLP-operated mice that did not receive antibiotic treatment were sacrificed 6 and 18 hours (top) after surgery, whereas sham- and CLP-operated mice that received antibiotic treatment (bottom) were sacrificed 1, 3, and 7 days after surgery. The left lung was collected and snap-frozen for ELISA assay. The results are expressed as mean ± SEM of ng of cytokine/chemokine per mg of protein, each group has five to six mice and the data are representative of two experiments. *, P < 0.05 compared to sham-operated group.
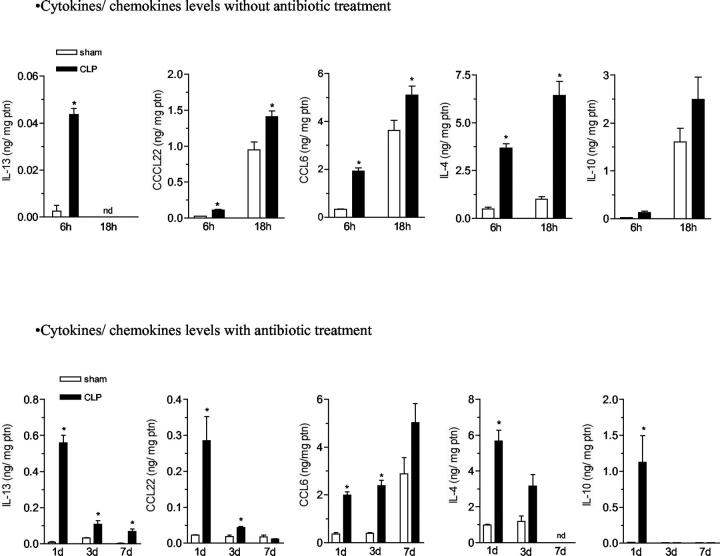
Examination of immunomodulatory or anti-inflammatory cytokines and chemokines also yielded interesting results. In the absence of antibiotic therapy, IL-4, IL-10, IL-13, CCL6, and CCL22 levels in whole lung samples were significantly increased at 6 and 18 hours after surgery in the CLP group compared with the sham group (Figure 5 ▶ , top). In the presence of antibiotic therapy, whole lung levels of all these immunomodulatory and anti-inflammatory cytokines and chemokines were also significantly elevated in the CLP group compared with the sham group. However, in contrast to the transient levels of inflammatory cytokine and chemokines (found in Figure 4 ▶ ), IL-13, CCL22, and CCL6 remained significantly elevated up to day 7 after CLP surgery relative to the appropriate sham group (Figure 5 ▶ , bottom). IL-4 was also elevated, but it was only evaluated up to day 3. Only IL-10 levels appeared to follow the pattern of the proinflammatory chemokines and cytokines. Thus, these data suggested that lung cytokine and chemokine levels were altered in mice that survived sepsis because of an intense antibiotic therapeutic regimen.
Figure 5.
The effect of sham and CLP surgery on lung levels of IL-13, CCL22, CCL6, IL-4, and IL-10 in mice with or without antibiotic therapy. Sham- and CLP-operated mice that did not receive antibiotic treatment were sacrificed 6 and 18 hours (top) after surgery, whereas sham- and CLP-operated mice that received antibiotic treatment (bottom) were sacrificed 1, 3, and 7 days after surgery. The left lung was collected and snap-frozen for ELISA assay. The results are expressed as mean ± SEM of ng of cytokine/chemokine per mg of protein, each group has five to six mice and the data are representative of two separate experiments. *, P < 0.05 compared to sham-operated group. Nd, not determined.
Survivors of CLP Surgery Are Susceptible to Pulmonary Infection with A. fumigatus
The inclusion of a 3-day antibiotic regimen clearly enhanced mouse survival after severe CLP surgery. With these survivors, we addressed whether the innate immune response in the lung was altered by the previous septic insult. We chose to examine the impact of A. fumigatus challenge in the CLP survivors for two reasons: clearance of this ubiquitous fungus requires the concerted actions of recruited neutrophils, monocytes, and the lung resident alveolar macrophages, 13,21,22 and immunocompromised hosts, including those that have experienced sepsis, are extremely susceptible to A. fumigatus infection. 23-26 In these studies, C57BL/6 mice were subjected to CLP or sham surgery and antibiotic treatment was initiated 8 hours later. At day 3, CLP and sham-operated mice received 1 or 5 × 107 A. fumigatus conidia or saline by intratracheal injection and mouse survival was followed for 7 days. No deaths were recorded in the sham groups challenged either with A. fumigatus conidia or saline (Figure 6A) ▶ . In contrast, although the CLP surgery groups tolerated the intratracheal saline challenge, ∼50% of these mice succumbed after a challenge with 1 × 107 A. fumigatus conidia by day 7, and all of the CLP survivors were dead by day 3 after the 5 × 107 A. fumigatus conidia challenge (Figure 6A) ▶ . Interestingly, the neutrophil migration into the airway at 6 hours after conidia challenge was significantly reduced in mice after CLP surgery (less than 25% total cells in the BAL were neutrophils) compared to the sham group (greater than 80% of the BAL cells were neutrophils) (Figure 6B) ▶ .
Figure 6.
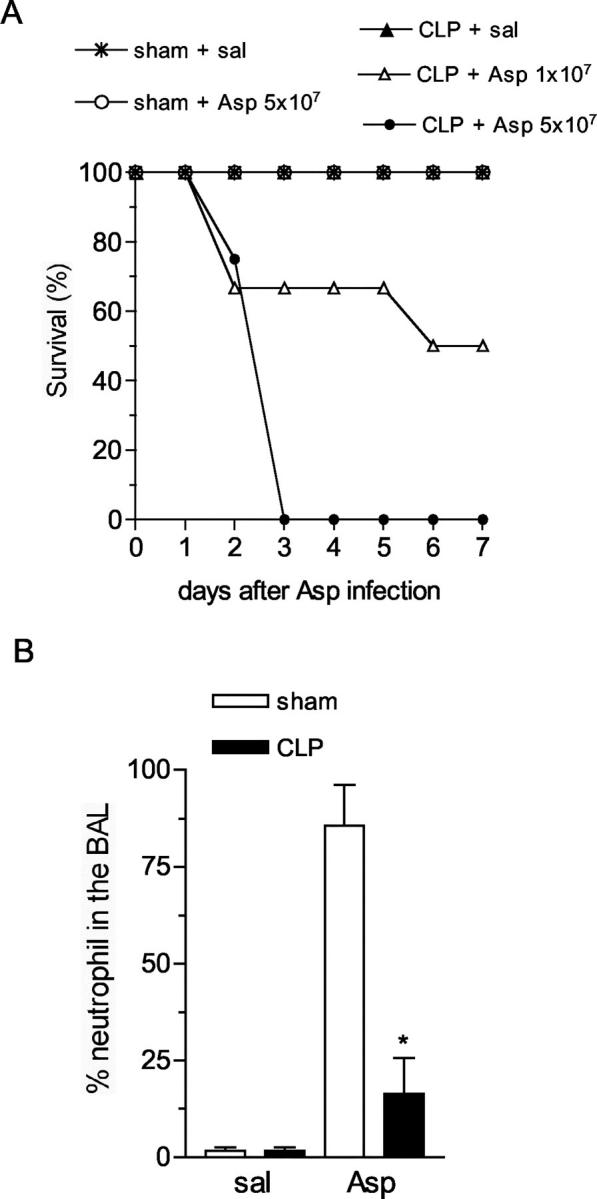
Survival curve and neutrophil migration into the BAL of sham or CLP-operated mice challenged with A. fumigatus. A: On the third day after sham or CLP surgery and antibiotic treatment, groups of mice either received an intratracheal injection of 30 μl of saline (control group) or 5 × 107 conidia of A. fumigatus (Asp) in 30 μl of saline. Only the CLP surgery group received 1 × 107 conidia. These mice were monitored for 7 days. Survival results are expressed as percentage of surviving mice per day, and each group started with 10 mice. The data are representative of three separate experiments. P < 0.05, CLP plus Asp 1 × 107 and CLP plus Asp 5 × 107 compared to CLP plus saline. B: On the third day after sham or CLP surgery and antibiotic treatment, both groups of mice were subjected to an intratracheal injection of 5 × 107 of A. fumigatus conidia. The animals were sacrificed 6 hours after saline or conidia challenge and BAL was performed to evaluate the cell population. The results are expressed as a percentage of neutrophils in the BAL, and each group has six to eight mice. *, P < 0.05 compared to sham group challenged with conidia. The data are representative of three separate experiments.
Uncontrolled Growth of A. fumigatus in the Lungs of Mice that Survive Severe CLP Surgery
We next examined putative explanations for the increased mortality associated with A. fumigatus conidia challenge in CLP survivors. Representative histological lung samples, collected from CLP survivors at day 2 after a 5 × 107 A. fumigatus conidia challenge, are shown in Figure 7 ▶ . A distinctly different histological picture emerged in comparing sham-operated (Figure 7 ▶ ; A, C, and E) with CLP mice (Figure 7 ▶ ; B, D, and F) at this time after the conidia challenge. First, it was apparent that the inflammatory response (ie, neutrophil and monocyte accumulation) elicited to the lung interstitium in the conidia-challenged CLP survivors (Figure 7B) ▶ was much more intense than that observed in the conidia-challenged sham surgery group (Figure 7A) ▶ . However, the inflammatory response elicited in the A. fumigatus-challenged CLP was not sufficient to contain the growth of this fungus. In sham-operated mice, debris and few intact conidia (stained black) were present in lung sections from this group (Figure 7C) ▶ . The majority of the conidia were dead (ie, ghosts) and no hyphal elements were present. This histological picture differed greatly from the CLP survivor group in which multiple intact conidia and hyphal elements were present (Figure 7D) ▶ despite the presence of neutrophils and monocytes. These data suggested that the innate immune response necessary for the clearance of A. fumigatus in the lungs of CLP survivors was clearly impaired. Interestingly, intact conidia (C) within macrophages and neutrophils, and also classic examples of apoptotic cells (A) were observed at day 2 after A. fumigatus challenged in CLP mice, by transmission electron microscopy (Figure 7F) ▶ . In contrast, in the sham surgery group, dead or dying conidia or ghosts (G) were observed inside macrophages (M) (Figure 7E) ▶ , and fewer apoptotic cells were seen compared with the CLP group.
Figure 7.
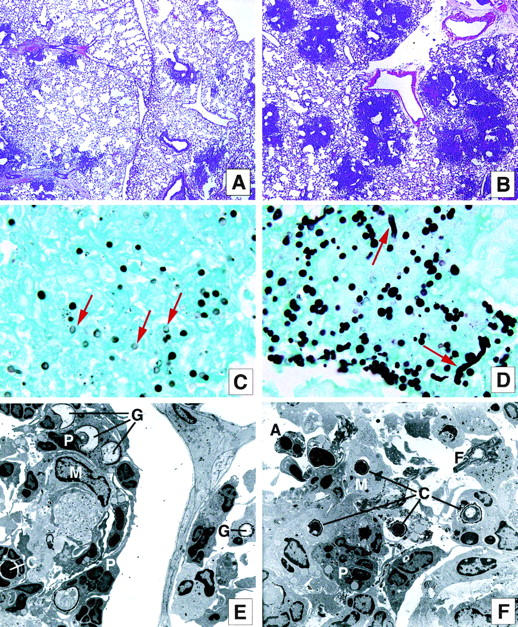
Leukocyte infiltration and the presence of A. fumigatus conidia and hyphae in the lung of mice subjected to sham or CLP surgery and challenged with fungus. A and B: Sham (A) and CLP (B) mice were injected intratracheally with 5 × 107 conidia of A. fumigatus, sacrificed 2 days after this challenge, and the lung tissue was harvested and processed to evaluate the inflammatory response by H&E stain. The histology shown is representative of three separate experiments, with five mice in each group. C and D: High-power magnification of the inflammatory sites of the lung in the day 2 after conidia challenge in sham (C) and CLP (D) mice. The slides were stained with Gomori methanamine silver to evaluate the presence of conidia and hyphae (black dots—arrowheads indicate ghost conidia in C and hyphae growth in D). The histology shown is representative of three separate experiments with five mice in each group. E and F: Electron micrographs of lungs obtained 2 days after conidia challenge in the sham (E) and CLP groups (F). G, Ghost conidia; C, intact conidia; M, macrophage; P, neutrophil; A, apoptotic cell; F, fibrin. The electron micrograph shown is representative of two separate experiments, with three mice in each group. Original magnifications: ×40 (A and B); × 1000 (C and D); ×2600 (E and F).
Altered Whole Lung CCL6 Levels in Postseptic Mice after A. fumigatus Conidia Challenge
To determine the effect of the Aspergillus conidia challenge on the generation of cytokines and chemokines in the lung, we measured whole lung levels of several cytokines and chemokines at 6 hours after A. fumigatus challenge in sham or CLP operated mice. We observed a statistically significant reduction in the whole lung CCL6 levels compared with the sham group (Figure 8) ▶ . Conversely, the whole lung levels of IL-10 in the CLP group mice challenged with conidia was similar to the sham group (Figure 8) ▶ . This latter observation was recapitulated with several other cytokines and chemokines that were measured in the same whole lung samples. Thus, the immunosuppression in the lungs of CLP mice challenged with A. fumigatus conidia did not appear to be because of excess IL-10 generation but possibly because of a deficit in a potent protective chemokine. 27
Figure 8.
The effect of sham and CLP surgery on whole lung levels of CCL6 and IL-10 in mice subjected to CLP and challenged with A. fumigatus conidia. Sham- and CLP-operated mice treated with antibiotic were sacrificed 6 hours after A. fumigatus conidia challenge and the left lung was collected and snap-frozen for ELISA assay for CCL6 and IL-10. The results are expressed as mean ± SEM of ng of cytokine/chemokine per mg of protein, each group has five to six mice and the data are representative of two separate experiments. *, P < 0.05 compared to sham-operated group.
Gene Array Analysis Revealed that Key Innate Immune Signal Transduction Pathways May Be Impaired in CLP Survivors Relative to Sham-Operated Mice after A. fumigatus Conidia Challenge
The discordance in the pulmonary innate immune response between the sham and CLP surgery groups was further highlighted by gene array analysis for several signal transduction pathways necessary for innate immunity (Table 1) ▶ . Genes encoding transcription factors, signal transduction kinase, NF-κB family members, adaptor proteins, cytokines, and cytokine receptors were all increased more than or equal to twofold in sham-operated mice treated with antibiotic and challenged with conidia compared with sham-operated mice that received intratracheal saline. Many of these same genes were decreased more than or equal to twofold in CLP survivors challenged with A. fumigatus conidia relative to gene expression in sham group that received intratracheal conidia. Interestingly, we did not observe a decrease in the expression of any gene in the sham-operated mice challenged with conidia (24.5 ± 7.5% of genes in this gene array analysis were up-regulated), in contrast we did not observe an increase in these same genes in the CLP group challenged with fungus. In fact, ∼33.3 ± 2.9% of the genes in this gene array were down-regulated. Together, these data suggest that the pulmonary innate immune response appears to be markedly impaired in the fungus challenge CLP survivors compared to fungus-challenged sham-operated control groups.
Table 1.
Gene Expression for Mouse NFkB Signaling at 6 Hours after A. fumigatus Conidia Challenge in Mice Subjected to Sham or CLP Surgery
| Groups | Genes | Sham + asp | CLP + asp | |||||
|---|---|---|---|---|---|---|---|---|
| up | down | |||||||
| 1° | 2° | 3° | 4° | 1° | 2° | 3° | ||
| CREBP1/ATF-2 | 2.2 | 3.2 | 2.5 | −3.9 | 0 | |||
| Elk-3 | 2.9 | 3.7 | −2.7 | 0 | 0 | |||
| Signal transduction kinase | IKK-γ | 3.1 | 12.0 | 3.8 | ||||
| JNK1 | 6.7 | 3.0 | 2.1 | |||||
| MKK4 (JNKK1) | 3.7 | 2.4 | 0 | −2.7 | ||||
| IkBα | 4.7 | 2.6 | 3.0 | 0 | 0 | |||
| Rel/NFkB/IkB family | NFkBIB | 4.0 | 3.2 | 3.0 | 3.0 | −2.2 | 0 | |
| NFkB1 | 5.4 | 2.1 | ||||||
| NFkB2 | 3.9 | 2.0 | −4.2 | −5.3 | ||||
| c-rel | 9.1 | 6.8 | 2.6 | 3.7 | 0 | 0 | ||
| Adaptor proteins | IRAK2 | 2.1 | 2.4 | |||||
| MyD88 | 2.1 | 2.4 | 2.0 | −6.4 | −2.9 | |||
| Cytokine/cytokine receptor | IL-1β | 2.9 | 10.3 | 11.5 | 6.4 | −2.6 | 0 | −3.6 |
| IL-1R1 | 3.3 | 2.6 | 0 | 0 | 0 | |||
| Others | L-selectin | 5.2 | 2.9 | 0 | −2.5 | |||
Mice were sacrificed 6 hours after A. fumigatus injection and the lung was harvested and immediately snap-frozen to subsequent mRNA purification and gene expression analysis by Gene Array. The results are expressed as fold increase or decrease relative to the control group, and values of each group were normalized with three or four housekeeping genes present on the membrane. The first column contains genes expressed in the sham + Asp challenged mice compared with sham + saline group; the second column contains genes expressed in the CLP + Asp compared to sham + Asp group. The O value means that the gene was not expressed when compared to sham + Asp group which showed expression of this gene. The experiment was repeated four times for sham + Asp compared to sham + saline and three times for CLP + Asp compared to sham + Asp. Each membrane was hybridized with mRNA purified from lung obtained from three to four mice per group.
Discussion
The mechanism underlying the immunosuppression followed by sepsis remains poorly characterized, despite the biological importance of the phenomenon as well as the considerable experimental effort that has been expended in trying to understand this response. With this background, we characterized a clinically relevant sepsis model, which allows for the study of mechanisms that contribute to the immunosuppression during sepsis. The early overwhelming systemic inflammatory response induced by CLP surgery significantly alters the subsequent host response against a secondary pulmonary infection. These mice become susceptible to nosocomial/opportunistic infection by A. fumigatus, although this fungus normally poses little health concern to immunocompetent mice. In our model, C57 BL/6 mice subjected to a severe sepsis exhibited a significant failure in neutrophil migration permitting the persistence of bacteria in the peritoneal cavity. Even though the lung is not the primary site of infection or insult during CLP, the lung was affected by the peritonitis, as evidenced by alterations in cytokines and chemokines, leukocyte infiltration, and thickness of alveolar wall at 6 hours after CLP surgery. This effect was temporary, as by day 3, the survivors exhibited no histological evidence of lung injury. Despite the fact that the mice that recovered from sepsis after 3 days of antibiotic treatment were clinically healthy, they were susceptible to a secondary infection caused by conidia of A. fumigatus. Given that, previous studies have shown that only immunocompromised mice develop invasive Aspergillosis, 13,23 we examined the effect of this fungus in the CLP survivors. In addition, the fact that the model was developed in C57 BL/6 mice is useful because of the availability of deficient or transgenic mice that have the genetic background in this strain. Our data support an interesting clinical phenomenon, in which patients recovered from a severe first septic insult are susceptible to opportunistic disease, such lung infection, and have a high mortality within 1 year. 1 In addition, the fact that the incidence of sepsis because of a fungal organisms has been increased 207% since 1979, 28 reinforces the relevance of our model.
There was a therapeutic window for antibiotic treatment in the CLP model, which confers protection up to 60 to 70% of the mice. When mice received antibiotic treatment beginning more than 8 hours after CLP surgery, no significant improvement in mouse survival was noted. The antibiotic therapy reversed the paralysis of neutrophil migration into the peritoneal cavity observed in the nonantibiotic-treated animals, as the numbers of neutrophils, and mononuclear cells, were significantly increased by the third day after antibiotic treatment. The protective effect of the antibiotic therapy was presumably related to its antimicrobial activity, but it is noteworthy that the antibiotic treatment increased mononuclear cell accumulation in the peritoneal cavity of sham-operated mice. This result is consistent with the ability of this antibiotic to significantly increase in vitro and in vivo adherence, spontaneous mobility, and chemotaxis and phagocytosis properties of macrophages. 29 Also, the antibiotic imipenem has been shown to have chemoattractant activity for neutrophils and increase cGMP levels in these cells. 30 Taking these previous findings into account, the combined bactericidal activity and proinflammatory effects may have accounted for the improved survival of CLP mice who received antibiotic therapy.
On the third day after CLP and antibiotic treatment, whole lung levels of several immunomodulatory cytokines (IL-13, IL-4) were significantly elevated in the lung, whereas proinflammatory cytokines such as interferon-γ and TNF-α were low or undetectable. The persistence of these modulatory cytokines may be contributing to the susceptibility of CLP mice to a secondary insult, preventing the host from mounting an appropriate immune response against the fungus infection. We have previously observed that IL-10 contributes to the immunosuppression associated with a mild sepsis induced by a not severe CLP surgery model. 31 However changes in IL-10 levels did not appear to explain the findings in the present study, because IL-10 levels were not detected in whole lung samples form CLP mice at day 3 after surgery and antibiotic treatment. In addition, the whole lung levels of IL-10 after A. fumigatus conidia challenge in CLP mice were not different from sham-operated mice, suggesting, once again, that this cytokine is not responsible for the susceptibility for a secondary fungus infection in this model.
In addition, it was apparent that chemokine levels (ie, MIP-2 and KC) were altered in the lung before and after the conidia challenge (data not shown). CCL6 was also elevated before A. fumigatus infection, but 6 hours after fungus challenge, the levels of CCL6 were significantly lower compared to the level observed in the sham plus fungus group. Previously, we have shown that CCL6 is protective during sepsis because of its ability to augment bacterial clearance and modulate the release of inflammatory mediators. 27 In addition, in a preliminary study we have observed that mice with augmented pulmonary CCL6 expression because of a pulmonary transgene are completely protected during CLP-induced sepsis and fungus challenge. Another chemokine that was elevated before and after conidia challenge in CLP mice was CCL2 (data not shown). In future experiments we will investigate whether CCL6 and CCL2 are mechanistically involved in the altered pulmonary innate response after CLP.
The lung histology at 2 days after A. fumigatus challenge in the CLP survivors revealed intense neutrophil and macrophage recruitment. However, these cells appeared to have an impaired ability to kill and digest A. fumigatus conidia and hyphae, as a marked increase in the numbers of conidia and hyphae were observed in the CLP group and not in the sham-operated mice challenged with the same amount of conidia. Using electron microscopy, it was observed that neutrophils and macrophages from both groups were able to phagocyte the conidia, however the conidia inside the cells of CLP group were intact (presumably alive), whereas a great number or ghost conidia (degraded or dying) were observed in the sham group. These results indicate that leukocyte phagocytosis activity may be normal, but the killing activity of these cells appears to be impaired in the CLP-operated mice. Another immune aspect that could explain the inability to clear the fungus relates to the observation that the CLP group had an increased number of apoptotic cells compared to sham group. It has been shown that A. fumigatus conidia release toxins that induce apoptosis of leukocytes. 32,33 The impact of A. fumigatus on the apoptotic process in the lung will be a subject of our future investigations.
Finally, to characterize the model and understand the immunosuppression that follows sepsis, we investigated the gene expression of the NF-κB signaling pathway in the lung of mice subjected to CLP and A. fumigatus challenge, as compared to sham controls also subjected to the fungus. Although sham-operated mice exhibited an up-regulation in signal transduction kinases, NF-κB, adaptors protein, and IL-1β and its receptor, the CLP group demonstrated a down-regulation of many of these same genes. These data provide evidence that the lung environment, after a severe septic response, is not appropriate for the elimination of pathogens, indicating that the early events induced by the CLP injury may result in a critical alteration that impairs the pulmonary innate response against an opportunistic secondary infection. Overall discussion of each gene affected by fungal challenge in the sham and CLP group is beyond the scope of this article. However, we are presently examining how various innate receptors and their corresponding signal transduction pathway lead to the skewing of the host immune response after sepsis.
Thus, in the present study, we have described a model of severe sepsis that is characterized by profound immunosuppression. A number of mediators and putative mechanism(s) that could drive this altered immune state are also described, and they include a variety of cytokines, chemokines, and signaling proteins. Our data demonstrated that the long-term susceptibility of CLP survivors in C57 BL/6 mice after a severe septic response may be because of a skewed Th2-type response, which results in an inappropriate immune response in the face of an A. fumigatus infection. We believe that this model will provide important insights into why long-term survivors of clinical sepsis are susceptible to a higher rate of morbidity and mortality.
Acknowledgments
We thank Robin Kunkel for her artistic assistance; and Holly Evanoff, Aaron Berlin, and Pamela Lincoln for their technical assistance.
Footnotes
Address reprint requests to Steven L. Kunkel, Department of Pathology, Box 0602, 1301 Catherine Rd., MS I, 5214, University of Michigan, Ann Arbor, MI 48109-0602. E-mail: slkunkel@umich.edu.
Supported in part by the National Institutes of Health (grants P50 HL60289 and HL31237 to S. L. K.), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (fellowship to C. F. B.).
References
- 1.Perl TM, Dvorak L, Hwang T, Wenzel RP: Long-term survival and function after suspected Gram-negative sepsis. JAMA 1995, 274:338-345 [PubMed] [Google Scholar]
- 2.Bone RC: Gram-negative sepsis. Background, clinical features, and intervention. Chest 1991, 100:802-808 [DOI] [PubMed] [Google Scholar]
- 3.Martin MA: Epidemiology and clinical impact of gram-negative sepsis. Infect Dis Clin North Am 1991, 5:739-752 [PubMed] [Google Scholar]
- 4.Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL: Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun 1996, 64:4733-4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE: Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol 2002, 168:2493-2500 [DOI] [PubMed] [Google Scholar]
- 6.Quartin AA, Schein RM, Kett DH, Peduzzi PN: Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA 1997, 277:1058-1063 [PubMed] [Google Scholar]
- 7.Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami K, Nagai Y, Takeuchi O, Akira S, Matsuguchi T: Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol 2002, 169:2026-2033 [DOI] [PubMed] [Google Scholar]
- 8.Tabeta K, Yamazaki K, Akashi S, Miyake K, Kumada H, Umemoto T, Yoshie H: Toll-like receptors confer responsiveness to lipopolysaccharide from Porphyromonas gingivalis in human gingival fibroblasts. Infect Immun 2000, 68:3731-3735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK: Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol 2000, 164:966-972 [DOI] [PubMed] [Google Scholar]
- 10.Takeda K, Kaisho T, Akira S: Toll-like receptors. Annu Rev Immunol 2003, 21:335-376 [DOI] [PubMed] [Google Scholar]
- 11.Zisman DA, Kunkel SL, Strieter RM, Tsai WC, Bucknell K, Wilkowski J, Standiford TJ: MCP-1 protects mice in lethal endotoxemia. J Clin Invest 1997, 99:2832-2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackay CR: Chemokines: immunology’s high impact factors. Nat Immunol 2001, 2:95-101 [DOI] [PubMed] [Google Scholar]
- 13.Kontoyiannis DP, Bodey GP: Invasive aspergillosis in 2002: an update. Eur J Clin Microbiol Infect Dis 2002, 21:161-172 [DOI] [PubMed] [Google Scholar]
- 14.Mehrad B, Strieter RM, Standiford TJ: Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J Immunol 1999, 162:1633-1640 [PubMed] [Google Scholar]
- 15.Baker CC, Chaudry IH, Gaines HO, Baue AE: Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery 1983, 94:331-335 [PubMed] [Google Scholar]
- 16.Evanoff H, Burdick M, Moore S, Kunkel S, Strieter R: A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1). Immunol Invest 1992, 21:39-45 [DOI] [PubMed] [Google Scholar]
- 17.Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72:248-254 [DOI] [PubMed] [Google Scholar]
- 18.Benjamim CF, Ferreira SH, Cunha FQ: Role of nitric oxide in the failure of neutrophil migration in sepsis. J Infect Dis 2000, 182:214-223 [DOI] [PubMed] [Google Scholar]
- 19.Benjamim CF, Silva JS, Fortes ZB, Oliveira MA, Ferreira SH, Cunha FQ: Inhibition of leukocyte rolling by nitric oxide during sepsis leads to reduced migration of active microbicidal neutrophils. Infect Immun 2002, 70:3602-3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matute-Bello G, Frevert CW, Kajikawa O, Skerrett SJ, Goodman RB, Park DR, Martin TR: Septic shock and acute lung injury in rabbits with peritonitis: failure of the neutrophil response to localized infection. Am J Respir Crit Care Med 2001, 163:234-243 [DOI] [PubMed] [Google Scholar]
- 21.Mehrad B, Moore TA, Standiford TJ: Macrophage inflammatory protein-1 alpha is a critical mediator of host defense against invasive pulmonary aspergillosis in neutropenic hosts. J Immunol 2000, 165:962-968 [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim-Granet O, Philippe B, Boleti H, Boisvieux-Ulrich E, Grenet D, Stern M, Latge JP: Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect Immun 2003, 71:891-903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis M: Invasive fungal infections: evolving challenges for diagnosis and therapeutics. Mol Immunol 2002, 38:947-957 [DOI] [PubMed] [Google Scholar]
- 24.Colquhoun IW, Gascoigne AD, Gould K, Corris PA, Dark JH: Native pulmonary sepsis after single-lung transplantation. Transplantation 1991, 52:931-933 [PubMed] [Google Scholar]
- 25.Rodgers GL, Mortensen J, Fisher MC, Lo A, Cresswell A, Long SS: Predictors of infectious complications after burn injuries in children. Pediatr Infect Dis J 2000, 19:990-995 [DOI] [PubMed] [Google Scholar]
- 26.Latge JP: The pathobiology of Aspergillus fumigatus. Trends Microbiol 2001, 9:382-389 [DOI] [PubMed] [Google Scholar]
- 27.Steinhauser ML, Hogaboam CM, Matsukawa A, Lukacs NW, Strieter RM, Kunkel SL: Chemokine C10 promotes disease resolution and survival in an experimental model of bacterial sepsis. Infect Immun 2000, 68:6108-6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin GS, Mannino DM, Eaton S, Moss M: The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003, 348:1546-1554 [DOI] [PubMed] [Google Scholar]
- 29.Nunez RM, Rodriguez AB, Barriga C, De la Fuente M: In vitro and in vivo effects of Imipenem on phagocytic activity of murine peritoneal macrophages. APMIS 1989, 97:879-886 [PubMed] [Google Scholar]
- 30.Rodriguez AB, Barriga C, De la Fuente M: Mechanisms of action involved in the chemoattractant activity of three beta-lactamic antibiotics upon human neutrophils. Biochem Pharmacol 1991, 41:931-936 [DOI] [PubMed] [Google Scholar]
- 31.Steinhauser ML, Hogaboam CM, Kunkel SL, Lukacs NW, Strieter RM, Standiford TJ: IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol 1999, 162:392-399 [PubMed] [Google Scholar]
- 32.Mendes-Giannini MJ, Taylor ML, Bouchara JB, Burger E, Calich VL, Escalante ED, Hanna SA, Lenzi HL, Machado MP, Miyaji M, Monteiro Da Silva JL, Mota EM, Restrepo A, Restrepo S, Tronchin G, Vincenzi LR, Xidieh CF, Zenteno E: Pathogenesis II: fungal responses to host responses: interaction of host cells with fungi. Med Mycol 2000, 38(Suppl 1):113-123 [PubMed] [Google Scholar]
- 33.Sutton P, Newcombe NR, Waring P, Mullbacher A: In vivo immunosuppressive activity of gliotoxin, a metabolite produced by human pathogenic fungi. Infect Immun 1994, 62:1192-1198 [DOI] [PMC free article] [PubMed] [Google Scholar]