Abstract
Pathological osteolyses are considered a consequence of a disturbance in the mechanisms that govern the bone remodeling, mainly the communication between osteoclasts and osteoblasts. Osteoprotegerin (OPG) and receptor activator of NF-κB ligand (RANKL) are newly discovered molecules that play a key role in these communications. RANKL is essential for osteoclast differentiation via its receptor RANK located on the osteoclast membrane. OPG is a soluble decoy receptor that inhibits osteoclast differentiation through its binding to RANKL. The aim of this study is the analysis of the RANKL/OPG balance by complementary methods (semiquantitative reverse transcription-polymerase chain reaction, immunohistochemistry, and enzyme-linked immunosorbent assay) in human osteolysis associated to various bone etiologies (n = 60), tumoral (primitive, secondary) or not, compared to healthy tissues (n = 16). Results demonstrated that RANKL/OPG ratio was significantly increased in patients suffering from severe osteolysis compared to the control group and that this imbalance is involved in bone resorption mechanisms. In this study, OPG expression appears to reflect a protective mechanism of the skeleton to compensate increased bone resorption by inhibiting osteoclast formation and bone resorbing activity. Moreover, as revealed by immunohistochemistry, RANKL and OPG were colocalized in all of the tissues analyzed. To define the veracity of RANKL/OPG index in assessing and managing patients with severe osteolysis, an extended population of patients suffering from severe osteolysis must be now monitored.
Bone is a specialized connective tissue formed by a mineralized matrix that confers its elastic and strength properties. Bone remodeling allows to adapt bone tissue to mechanical constraints and to maintain phosphocalcic homeostasis through coordinated phases of formation and resorption. Thus, bone remodeling involves synthesis of organic matrix by osteoblasts, and bone resorption by osteoclasts. This equilibrium is tightly regulated by physical parameters (ie, mechanical stimulations) and numerous polypeptides (hormones, cytokines). 1 Any disturbance between these effectors leads to the development of skeletal abnormalities, characterized by decreased (osteoporosis) or increased (osteopetrosis) bone mass. Increased osteoclast activity is observed in many osteopathic disorders, including postmenopausal osteoporosis, Paget’s disease, primary bone tumors, lytic bone metastases, multiple myeloma, or rheumatoid arthritis, leading to increased bone resorption and a loss of bone mass. 2-4 Concerning bone tumors, tumor cells release agents (hormones, eicosanoid, growth factors, cytokines) into the bone microenvironment, which act on osteoblastic stromal cells to enhance the production of osteoclast-activating factors. Most notable of these is the osteoprotegerin (OPG) ligand, also named receptor activator of nuclear factor κB ligand (RANKL), which is a member of the tumor necrosis factor (TNF) cytokine family.
OPG and RANKL have been recently identified as members of a ligand-receptor system that directly regulates osteoclast differentiation and bone resorption. 5-8 RANKL has been shown to both activate mature osteoclasts and mediate osteoclastogenesis in the presence of M-CSF. 9,10 Soluble- and membranous-form of RANKL is preferentially expressed by committed preosteoblastic cells, whereas their specific receptor RANK is expressed on hematopoietic osteoclast progenitors. 8,11 This interaction is necessary and, together with M-CSF, sufficient for osteoclast formation, since mice lacking RANKL are unable to produce osteoclasts and since exogenously provided soluble RANKL and M-CSF stimulate osteoclastogenesis in the absence of stromal/osteoblastic cells. 7,8 In this system, OPG produced by osteoblasts acts as a decoy receptor for RANKL, preventing it from binding to and activating RANK. It also inhibits the development of osteoclasts 6 and down-regulates the RANKL signaling through RANK. 5 The evidence of OPG as an inhibitor of osteoclastogenesis emerges from experiments with transgenic mice, where overexpression of OPG leads to severe osteopetrosis and reduced number of mature osteoclasts. 5 In contrast, OPG knockout mice are osteoporotic. 12 The biological effects of OPG on bone cells include the inhibition of terminal stages of osteoclast differentiation, suppression of mature osteoclast activation, and induction of apoptosis. 13,14 In fine, bone remodeling appears to be mainly controlled by the balance RANKL/OPG.
RANKL and OPG have been already detected in several tumor cells. Thus, soluble RANKL was produced by human prostate cancer cells when injected to SCID mice. 15 Similarly, Brown et al 16 reported that RANKL was heterogeneously expressed in 10 of 11 prostate carcinoma specimens and the proportion of tumor cells expressing RANKL was significantly increased in all bone metastases. Moreover, RANKL was expressed in squamous cell carcinoma cell lines derived from malignancy tissues associated with hypercalcemia 17 and was detected in more than 90% of metastatic tumor cells in lesions of breast, lung, and thyroid adenocarcinoma. 18 The studies of Thomas et al 19 and Chikatsu et al 20 thus revealed that RANKL mRNA has not been detected in melanoma and breast cancer cells, whereas Good et al 21 have demonstrated recently by immunohistochemical techniques that primary benign bone tumors, primary malignant tumors, and bone metastases were positive for RANKL. These data suggested that tumor cells could switch to become positive for RANKL in bone. To our knowledge, serum RANKL has never been investigated. In addition, few data are available on the production and expression of OPG in these pathologies. 22-25
The present work studied the expression and the production of RANKL and OPG by semiquantitative reverse transcription-polymerase chain reaction (RT-PCR), enzyme-linked immunosorbent assay (ELISA) and immunohistochemistry, in a large number of bone pathological situations where osteolysis occurred. The balance RANKL/OPG was systematically analyzed, compared to healthy controls and correlated to the severity of osteolysis.
Materials and Methods
Specimens
Seventy-one patients were included in the present study and all were treated at the University Hospital of Nantes (France) between September 2001 and December 2002 (Table 1) ▶ . Sixty patients, 31 women [59.9 ± 18.3 years (mean age ± SD), range 17 to 80] and 29 men [47 ± 20.8 years, range 15 to 86] with pathological osteolysis referred to the Department of Orthopedic Surgery (University Hospital of Nantes) were included in the various pathological groups. Eleven patients, 8 women [65.6 ± 21.8 years, range 23 to 83] and 3 men [44.6 ± 11 years, range 33 to 55] were included in the control group; healthy tissues (n = 16; muscle, bone, capsule) were harvested during resection of epiphysis for metaphyseal bone tumors or during operations for intramedullary nail removal. Pathological osteolysis cases included prosthesis aseptic loosening [total hip arthroplasty (THA); n = 16], giant cell tumors (GCT) (n = 6), osteitis (n = 2), primary benign bone tumors (PBBT) (desmoplastic fibroma, essential cyst, aneurismal bone cyst, chondromyxoid fibroma, fibrous dysplasia, eosinophile granuloma, osteo-cartilaginous exostose, chondroma; n = 13), tumors of soft tissues (liposarcoma, synovial sarcoma, villonodular synovitis; n = 4), hematological malignancies (myeloma; n = 2), primary malignant bone tumors (PMBT) (angiosarcoma, Ewing sarcoma, chondrosarcoma, osteosarcoma; n = 9) and bone metastases from other primary origins (lung, renal, breast; n = 8). Osteolysis degree was measured arbitrarily by analogical scale: the reference group, free of any osteolysis is noted “−”; when osteolysis and osteocondensation coexist within the same tissue, the sample is noted “0”; grade 1, 2, and 3 are attributed according to the classification of Campanacci et al (Table 1) ▶ . 26 This classification initially described for the GCT is subdivided in 3 grades: grade 1 or “quiet form” corresponds to the tumors whose osteolysis is multilocular without cortical destruction; grade 3 or “aggressive form” corresponds to the tumors associated with a rupture of the cortical bone and grade 2 or “active form” is between the two grades previously described. When osteolysis occurred without information, the sample was noted “+” (Table 1) ▶ . Part of the specimens obtained during surgery were frozen and kept at −80°C until RNA transcript analyses, while the other part was fixed immediately in 10% formaldehyde solution until immunohistochemical analysis.
Table 1.
Characteristics of Patients with Pathologic Osteolysis and Control Groups
| Group | Patient | Classification | Osteolysis | Sex |
|---|---|---|---|---|
| Control | 1 | Bone | − | F |
| 2 | Bone | − | M | |
| 3 | Bone | − | F | |
| 4 | Bone | − | F | |
| 5 | Bone | − | F | |
| 6 | Bone | − | M | |
| 7 | Bone | − | F | |
| 8 | Bone | − | M | |
| 9 | Bone | − | F | |
| 10 | Cartilage | − | F | |
| 11 | Cartilage | − | F | |
| 4a | Capsule | − | F | |
| 5a | Capsule | − | F | |
| 7a | Capsule | − | F | |
| 5b | Muscle | − | F | |
| 7b | Muscle | − | F | |
| THA | 12 | THA | + | F |
| 13 | THA | + | M | |
| 14 | THA | + | F | |
| 15 | THA | + | F | |
| 16 | THA | + | F | |
| 17 | THA | + | F | |
| 18 | THA | + | M | |
| 19 | THA | + | F | |
| 20 | THA | + | F | |
| 21 | THA | + | M | |
| 22 | THA | + | F | |
| 23 | THA | + | M | |
| 24 | THA | + | F | |
| 25 | THA | + | F | |
| 26 | THA | + | M | |
| 27 | THA | + | F | |
| GCTs | 28 | GCT | 3 | F |
| 29 | GCT | 3 | F | |
| 30 | GCT | 3 | M | |
| 31 | GCT | 2 | M | |
| 32 | GCT | 3 | M | |
| 33 | GCT | 3 | M | |
| Osteitis | 34 | Osteitis | + | M |
| 35 | Osteitis | + | M | |
| PBBTs | 36 | Desmoplastic fibroma | 1 | M |
| 37 | Desmoplastic fibroma | 1 | F | |
| 38 | Chondromyxoid fibroma | 2 | M | |
| 39 | Aneurismal bone cyst | 2 | F | |
| 40 | Essential cyst | 1 | M | |
| 41 | Fibrous dysplasia | 1 | M | |
| 42 | Eosinophile granuloma | 1 | M | |
| 43 | Osteo-cartilaginous exostose | 0 | F | |
| 44 | Osteo-cartilaginous exostose | 0 | M | |
| 45 | Osteo-cartilaginous exostose | 0 | M | |
| 46 | Chondroma | 0 | M | |
| 47 | Chondroma | 0 | M | |
| 48 | Chondroma | 0 | F | |
| Tumors of the soft tissues | 49 | Liposarcoma | − | M |
| 50 | Liposarcoma | − | F | |
| 51 | Synovial-sarcoma | 3 | F | |
| 52 | Villonodular synovitis | 2 | M | |
| Hematologic malignancies | 53 | Myeloma | 3 | F |
| 54 | Myeloma | 3 | M | |
| PMBTs | 55 | Chondrosarcoma | 0 | F |
| 56 | Angiosarcoma | 3 | F | |
| 57 | Ewing sarcoma | 0 | F | |
| 58 | Ewing sarcoma | 0 | M | |
| 59 | Osteosarcoma | 3 | F | |
| 60 | Osteosarcoma | 3 | F | |
| 61 | Osteosarcoma | + | F | |
| 62 | Osteosarcoma | 3 | M | |
| 63 | Osteosarcoma | + | F | |
| Bone metastases from other primary origins | 64 | Lung | 3 | M |
| 65 | Lung | 3 | M | |
| 66 | Kidney | 3 | M | |
| 67 | Kidney | 3 | M | |
| 68 | Breast | 3 | F | |
| 69 | Uterus | 3 | F | |
| 70 | Unknown | 3 | F | |
| 71 | Unknown | 3 | F |
Osteolysis was graded as described in Materials and Methods. Ages of the patients are given in years.
Preparation of Total RNA and Semiquantitative RT-PCR
Total RNA was extracted from frozen samples and RT-PCR performed as described elsewhere. 27 Briefly, dissected samples were ground in liquid nitrogen before dissolution in Trizol reagent (Invitrogen, Eragny, France) according to the manufacturer’s instructions. Total RNA was quantified by measuring OD260, and integrity was checked by 1% agarose/formaldehyde gel electrophoresis. RNA samples were then treated with DNase I (0.1 U/μl) before the reverse-transcription step, to exclude the possibility of interference with contaminating genomic DNA. cDNA was then amplified by PCR to generate products corresponding to mRNA encoding human RANKL, OPG, and 18S (corresponding oligonucleotides are listed Table 2 ▶ ) using Taq Polymerase (Promega, Charbonnières, France) under semiquantitative conditions. PCR products were then analyzed in 1% agarose gels, stained with ethidium bromide, and photographed. Band densities were measured using the ImageQuant computer software program. Relative expression of the RANKL and OPG genes were calculated as the ratio to 18S signal. After the number of PCR cycles was increased for each studied gene, a plot was done for each sample allowing the determination of the cycle values corresponding to the linear part of the amplification curve (Table 2) ▶ used to quantify the messages versus the 18S signal determined in the same way.
Table 2.
Oligonucleotide Primers Used for RT-PCR
| Designation | Sequences | Conditions (cycle number) | Message size |
|---|---|---|---|
| 18S | +: 5′ TCAAGAACGAAAGTCGGAGGTTCG 3′ | 1 minute 94°C | 475 bp |
| −: 5′ TTATTGCTCAATCTCGGGTGGCTG 3′ | 1 minute 62°C | ||
| 1 minute 72°C | |||
| (28 cycles) | |||
| OPG | +: 5′ GCTAACCTCACCTTCGAG 3′ | 30 seconds 94°C | 324 bp |
| 30 seconds 55°C | |||
| −: 5′ TGATTGGACCTGGTTACC 3′ | 30 seconds 72°C | ||
| (40 cycles) | |||
| RANKL | +: 5′ GCCAGTGGGAGATGTTAG 3′ | 30 seconds 94°C | 486 bp |
| 30 seconds 55°C | |||
| −: 5′ TTAGCTGCAAGTTTTCCC 3′ | 30 seconds 72°C | ||
| (40 cycles) |
Primers are represented in a 5′ to 3′ orientation, with that for the coding strand (+) and the non-coding strand (−). The product size generated by reverse transcription and PCR amplification of the authentic mRNA is indicated, together with semi-quantitative PCR conditions.
Immunochemistry
In all cases, tissue samples were harvested from pathological osteolysis bearing patients during incisional or excisional biopsies and immediately fixed in 10% formaldehyde solution. The samples were decalcified by electrolysis, and after embedding in paraffin augmented by pycolytis (a process to add 10% to 15% of “pycolyte” to the paraffin to improve the quality of the sections) (Dubbar Electronique France, Rueil-Malmaison, France), 5-μm-thick sections were mounted on glass slides. Deparaffinized sections were treated with 3% hydrogen peroxide (H2O2) for 5 minutes to block endogenous peroxydase. The sections were then incubated with primary polyclonal anti-RANKL or anti-OPG antibodies (R&D Systems, Abingdon, UK), diluted 1:20 and 1:6, respectively, for 2 hours at room temperature in a humidified atmosphere. After the sections were incubated with 1:500 anti-rabbit biotinylated immunoglobulin (Amersham, Little Chalfont, UK) for 1 hour and streptavidin-horseradish peroxidase (HRP) at 1:500 (Dako, Copenhagen, Denmark) for 45 minutes at room temperature, they were revealed with a staining kit (Sigma, St. Louis, MO). Preparations were counterstained with hematoxylin, dehydrated and mounted with Gel Mount (Biomedica, Foster City, CA). All antibody and streptavidin dilutions were prepared with phosphate-buffered saline (pH 7.4). The negative controls included 1) suppression of the primary antibody and 2) substitution of the primary polyclonal antibody with polyclonal anti-κ IgG fraction antiserum.
ELISA for RANKL and OPG
Sera were available from healthy volunteers (n = 14) and from part of patients with pathological osteolysis associated with aseptic loosening (n = 16), GCTs (n = 4), osteitis (n = 2), PBBTs (n = 12), tumors of the soft tissue (n = 3), hematological malignancies (n = 2), PMBTs (n = 9), and bone metastases from other primary origins (n = 8). All sera were obtained at the time of biopsies and were frozen at −80°C until assay. All assays were performed as blind study. OPG were assayed using a specific ELISA test (Duoset; R&D Systems) according the recommendations of the manufacturer. Briefly, wells were coated overnight with 100 μl anti-hOPG at 2 μg/ml in 1% PBS, then blocked by addition of 100 μl 1 mol/L sulfuric acid and incubated with samples or standard of recombinant human OPG for 2 hours. After 3 washes, the plates were incubated with biotinylated anti-hOPG mAb at 200 ng/ml for 2 hours and then with streptavidin-HRP conjugate (1:200) and tetramethylbenzidine (TMB) substrate solution for 20 to 30 minutes. The reaction was stopped with 1 mol/L sulfuric acid, and absorbance was determined at 450 nm, with correction at 540 nm in a microtiter reader (PerkinElmer, Shelton, CT). Similar methodology was used for RANKL assay using rabbit polyclonal anti-RANKL antibodies (PeproTech, London, UK), and recombinant human RANKL (R&D Systems) as standard.
Statistical Analysis
Statistical analysis were carried out using a nonparametric test for unpaired samples according to the method of Mann and Whitney (Statview 5000; Abacus Concepts Inc., Berkeley, CA). P value of less than 0.05 was considered significant.
Results
RANKL Expression and RANKL/OPG mRNA Ratio are Significantly Increased in Severe Osteolysis
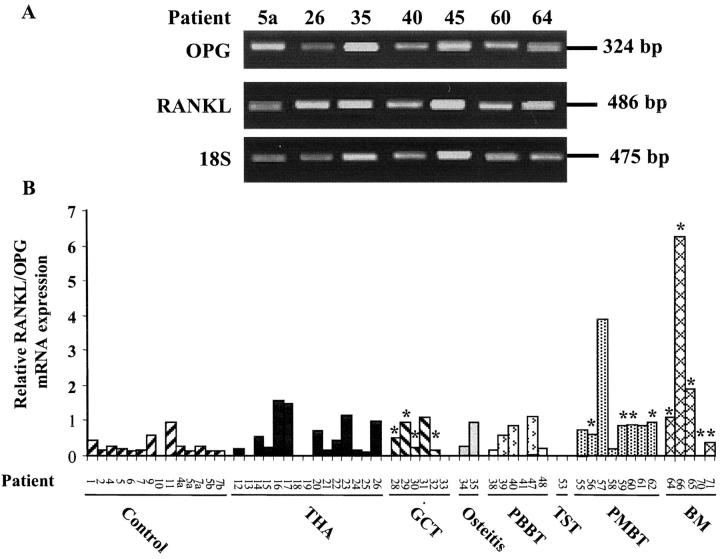
To determine the potential involvement of RANKL and OPG in pathological osteolysis, relative OPG and RANKL gene expressions were investigated in control and pathological samples. Results of representative samples from different osteolytic pathologies are presented in Figure 1A ▶ . Total mRNAs could be extracted from 75% of samples studied (controls, 14 of 16; pathological groups, 43 of 60), and OPG transcript was detectable in each case. Its expression was greatly variable both in control and in pathological groups (minimum to maximum, 0.13 to 8.16 and 0.11 to 3.27, respectively) and no significant difference was found between each group (Table 3) ▶ . RANKL transcript was detected in 89.6% of the above-described samples (Figure 1B) ▶ . Indeed, in 7 cases [1 cartilage control (patient 10), 3 THA (patients 13, 18, 19), 1 GCT (patient 33), 1 PMBT (patient 41) and 1 metastases (patient 70)] no RANKL mRNA was detected. No significant difference could be evidenced between each osteolytic pathology and the control group (Table 3) ▶ . As bone remodeling is controlled by the RANKL/OPG balance, RANKL/OPG mRNA ratio were calculated in each case. Thus, relative RANKL/OPG mRNA expression was significantly increased in all of the pathological samples compared to the control [0.94 ± 1.36 and 0.27 ± 0.23, respectively; P < 0.05]. More specifically, relative RANKL/OPG mRNA ratio was increased in PMBTs (1.08 ± 1.29, P < 0.01), in bone metastases (1.92 ± 2.50, P < 0.01), both compared to the control group. Similarly, RANKL/OPG mRNA expression was significantly increased in severe grade 3 osteolysis (1.03 ± 1.58, P < 0.05) (Figure 1B ▶ ; Table 3 ▶ ). These data demonstrate that the RANKL/OPG balance is disturbed in severe osteolysis in favor of RANKL.
Figure 1.
Gene expression of OPG and RANKL in samples from osteolytic pathologies. The relative mRNA levels were determined at 40 cycles, which corresponds to the linear part of their respective amplification curves. The relative mRNA expressions were normalized versus the 18S signal determined at 26 cycles. The size of OPG, RANKL, and 18S PCR products were 324, 486, and 475 bp, respectively. A: A representative illustration of OPG and RANKL expression from one sample of each pathological group studied (patient 26, 35, 40, 45, 60, 64) compared to healthy tissue (patient 5) (see Table 1 ▶ ). B: Relative RANKL/OPG mRNA expression of each biopsy analyzed. TST, tumors of soft tissues; BM, bone metastases. *, samples associated with increased severe osteolysis (grade 3 according to the classification of Campanacci et al). 26
Table 3.
RANKL and OPG Expression per Group of Pathologies Studied
| Group | Serum concentration (pg/ml) mean ± SD | mRNA expression (relative expression/18S) mean ± SD | ||||
|---|---|---|---|---|---|---|
| OPG | RANKL | RANKL/OPG | OPG | RANKL | RANKL/OPG | |
| Control | 236.5 ± 66.6 (n = 13) | 66.4 ± 86.9 (n = 13) | 0.32 ± 0.38 | 1.82 ± 2.00 (n = 14) | 0.45 ± 0.44 (n = 14) | 0.27 ± 0.23 |
| Total hip arthroplasty | 437.2 ± 306.9 (n = 16) | 198.1 ± 163 (n = 16) | 0.99 ± 1.50 | 1.47 ± 1.58 (n = 15) | 0.49 ± 0.4 (n = 15) | 0.51 ± 0.53 |
| Giant cell tumors | 207.2 ± 126.8 (n = 4) | 160 ± 138.5 (n = 4) | 1.13 ± 1.20 | 0.97 ± 0.49 (n = 6) | 0.54 ± 0.51 (n = 6) | 0.48 ± 0.44 |
| Osteitis | 285 ± 106.1 (n = 2) | 285 ± 91.9 (n = 2) | 1.13 ± 0.74 | 1.08 ± 1.11 (n = 2) | 0.72 ± 0.7 (n = 2) | 0.60 ± 0.47 |
| Primary benign bone tumors | 229.3 ± 152.2 (n = 12) | 263.3 ± 349.1 (n = 12) | 0.45 ± 0.44 | 2.16 ± 2.03 (n = 6) | 0.66 ± 0.43 (n = 6) | 0.48 ± 0.43 |
| Tumors of the soft tissues | 350 ± 264.6 (n = 3) | 396.6 ± 306.6 (n = 3) | 1.23 ± 1.50 | 0.26 (n = 1) | 0 (n = 1) | 0 |
| Hematologic malignancies | 505 ± 176.7 (n = 2) | 140 ± 198 (n = 2) | 0.22 ± 0.31 | ND | ND | NA |
| Primary malignant bone tumors | 299.1 ± 234.5 (n = 9) | 235.4 ± 272.3 (n = 9) | 1.05 ± 1.53* | 1.23 ± 1.02 (n = 8) | 0.75 ± 0.40 (n = 8) | 1.08 ± 1.29** |
| Bone metastasis from other primary origins | 319.6 ± 163.4 (n = 8) | 393.7 ± 267.2 (n = 8)* | 1.55 ± 1.54** | 0.66 ± 0.46 (n = 5) | 0.69 ± 0.54 (n = 5) | 1.92 ± 2.50** |
| Tumors associated with severe osteolysis | 318.8 ± 190.6 (n = 18) | 226.6 ± 243.5 (n = 18) | 1.04 ± 1.36* | 0.75 ± 0.50 (n = 9) | 0.57 ± 0.47 (n = 9) | 1.03 ± 1.58* |
*P < 0.05 and **P < 0.01 compared to the control group; ND, not determined; NA, non-applicable.
Severity of Osteolysis is Correlated with the Increase of Serum RANKL and RANKL/OPG Levels
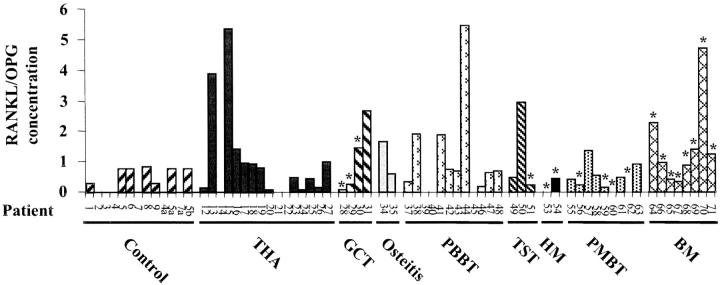
To determine the serum levels of RANKL and OPG of each patient suffering from osteolytic lesions included in the present study, both cytokines were measured on 69 sera using ELISA assays. Although OPG levels were not significantly modified between pathological groups and controls, serum RANKL was significantly increased in overall pathological groups (P < 0.05). Specifically, RANKL concentrations were increased in bone metastases (393.75 ± 267.2; P < 0.05) compared to the control (66.4 ± 86.9) (Table 3) ▶ . As for the transcript analysis, RANKL/OPG ratio were calculated for each pathology (Table 3 ▶ ; Figure 2 ▶ ). Thus, RANKL/OPG ratio was significantly increased in all of the pathological groups (1.02 ± 1.32) compared to the control group (0.32 ± 0.38; P < 0.05). Moreover, RANKL/OPG ratio was increased in PMBTs (1.05 ± 1.53; P < 0.05) and in bone metastases (1.55 ± 1.54; P < 0.01) compared to the control group (0.32 ± 0.38). Similarly, RANKL/OPG mRNA ratio was significantly increased in severe grade 3 osteolysis (1.04 ± 1.36; P < 0.05). These results confirm at the protein level that RANKL/OPG balance is also disturbed in severe osteolysis in favor of RANKL, strengthening the key role of both proteins in osteolysis.
Figure 2.
Serum RANKL and OPG levels of patients included in the present study and suffering from osteolytic lesions. Serum RANKL and OPG concentrations were determined using specific ELISA tests. Patients are referenced in Table 1 ▶ . TST, tumors of soft tissues; HM, hematological malignancies; BM, bone metastases. Samples associated with increased severe osteolysis (grade 3 according to the classification of Campanacci et al). 26
OPG and RANKL Proteins are Co-Localized Either in Osteoblasts and in Osteoclasts
As RANKL/OPG ratio are increased in severe osteolysis, immunolocalization studies of both proteins were conducted in pathological tissues associated with osteolysis (Figure 4 ▶ ; Table 4 ▶ ) and compared to healthy tissues (cartilage, bone, skeletal muscle) (Figure 3 ▶ ; Table 4 ▶ ). Immunolocalization data of both proteins in all tissues studied were in agreement with the analysis of the corresponding transcripts (Figure 1 ▶ ; Table 3 ▶ ). In articular hyaline cartilage, weak positive OPG and RANKL immunostaining were observed in the uncalcified matrix of hyaline cartilage with no apparent staining of the chondrocytes (Figure 3, A to C) ▶ . However, extracellular matrix of hypertrophic cartilage showed a strong signal for both proteins (Figure 3, D to F) ▶ . In agreement with the literature, 28 all active osteoblasts laying down osteoid matrix as well as matrix itself displayed strong immunoreactivity for both proteins while osteocytes and the mineralized matrix associated showed no positive staining (Figure 3, G to I) ▶ . RANKL and OPG immunoreactivities were further detected in skeletal muscle in 100% myocytes (Figure 3, J to L) ▶ . Thus, in cartilage, bone and skeletal muscle, OPG and RANKL were co-localized and mainly expressed in the cytoplasmic domain of the cells.
Figure 4.
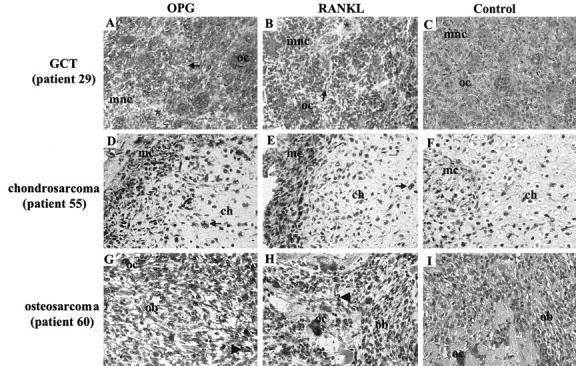
Immunolocalization of OPG and RANKL in tumoral bone pathologies, GCT, chondrosarcoma and osteosarcoma. Immunostaining of OPG (A, D, G) and RANKL (B, E, H). Non-immune negative controls are represented in (C, F, I). In GCT (A, B, C), multinucleated osteoclast-like giant cells (oc) exhibit positive immunostaining for OPG (A) and RANKL (B) except for a few number of osteoclast-like cells which did not express both factors (*). Similarly to osteoclast-like cells, most of mononucleated cells (mnc) were positive for both factors except a small number of them (→). OPG staining was mainly cytoplasmic whereas RANKL was immunolocalized both in cytoplasm and nucleus. In chondrosarcoma (D, E, F), part of the chondrocytes (ch) were immunoreactive for OPG (D) and RANKL (E) as well as mesenchymal cells (mc) of fibrous septa. Positive chondrocyte: →. In osteosarcoma (G, H, I), OPG (G), and RANKL (H) were also co-localized in osteoblast-like cells (ob) and in multinucleated osteoclasts (oc). Osteoblast-like cells moderately expressed RANKL in contrast to OPG. Positive ob: ▸. Original magnification, ×40.
Table 4.
Immunoreactivity Results (IR) of Health and Pathological Osteoarticular Tissues to Anti-OPG and Anti-RANKL Antibody
| Group | Patient and/or tissues | IR anti-OPG (% of positive cells, localization) | IR anti-RANKL (% of positive cells, localization) |
|---|---|---|---|
| Control | Cartilage, chondrocytes | − | − |
| extracellular matrix: nonhypertrophic cartilage | + | + | |
| hypertrophic cartilage | +++ | +++ | |
| Bone, osteoblasts | +++ (100, cytoplasm) | +++ (100, cytoplasm) | |
| osteocytes | − | − | |
| unmineralized matrix | +++ | +++ | |
| mineralized matrix | − | − | |
| Skeletal muscle, | |||
| myocytes | +++ (100, cytoplasm) | ++ (100, cytoplasm, cell membrane) | |
| mesenchymal cells | +++ (100, cytoplasm) | +++ (100, cytoplasm) | |
| Giant Cell Tumors | 28, stromal cells | +++ (100, cytoplasm) | ++ (100, cytoplasm, nucleus) |
| osteoclast-like | +++ (98, cytoplasm) | ++ (98, cytoplasm, nucleus) | |
| 29, stromal cells | +++ (98, cytoplasm) | +++ (98, cytoplasm, nucleus) | |
| osteoclast-like | +++ (98, cytoplasm) | +++ (98, cytoplasm, nucleus) | |
| 30, stromal cells | ++ (98, cytoplasm) | + (75, nucleus) | |
| osteoclast-like | ++ (98, cytoplasm) | + (25/cytoplasm, 75/nucleus) | |
| Primary benign bone tumors | 38, chondromyxoid fibroma | +++ (75, cytoplasm) | +++ (75, cytoplasm) |
| 41, fibrous dysplasia | |||
| osteoblasts | +++ (100, cytoplasm) | ++ (100, cytoplasm) | |
| osteocytes | − | − | |
| mesenchymal cells | +++ (100, cytoplasm) | + (100, cytoplasm) | |
| 55, chondrosarcoma | |||
| chondrocytes | +++ (50, cytoplasm) | ++ (30, cytoplasm) | |
| mesenchymal cells | +++ (75, cytoplasm) | +++ (98, cytoplasm) | |
| extracellular matrix | − | − | |
| Tumors of the soft tissues | 49, liposarcoma | + (100, cytoplasm) | + (100, cytoplasm) |
| Primary malignant bone tumors | 60, osteosarcoma | ||
| osteoblast-like | ++ (100, cytoplasm) | + (50, cytoplasm) | |
| osteocytes | ++ (100, cytoplasm) | − | |
| osteoclasts | +++ (100, cytoplasm) | +++ (100, cytoplasm) | |
| 62, osteosarcoma | |||
| osteoblast-like | ++ (100, cytoplasm) | + (100, cytoplasm) | |
| osteocytes | ++ (100, cytoplasm) | − | |
| osteoclasts | ++ (100, cytoplasm) | ++ (100, cytoplasm) |
−, absence of or negligible signal; +, weak signal; ++, moderate signal; +++, strong signal.
Figure 3.
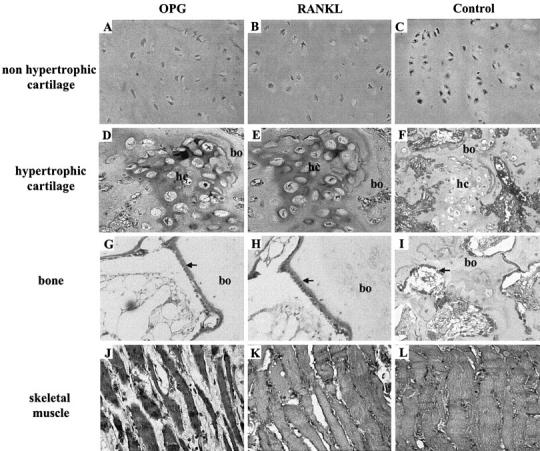
Immunolocalization of OPG and RANKL in healthy bone, cartilage, and skeletal muscle. Immunostaining of OPG (A, D, G, J) and RANKL (B, E, H, K) in cartilage (A to F), bone (G to I) and skeletal muscle (J to L). Non-immune negative controls are represented in (C, F, I, L). OPG and RANKL are colocalized in the healthy tissues analyzed. Chondrocytes were negative for both factors (A, B, D, E) in contrast to active osteoblasts (G and H) and myocytes (J and K). Extracellular matrix were weakly positive for OPG and RANKL in non hypertrophic cartilage (A and B) and strongly positive in hypertrophic cartilage (hc) (D and E). Osteoid matrix (→) was strongly immunoreactive both for OPG and RANKL (G and H). bo, bone. Original magnification, ×40.
In GCT, most of the multinucleated osteoclast-like giant cells exhibited a weak or moderate immunostaining for RANKL (GCT 28 and 30) (Table 4) ▶ except for GCT 29, where osteoclast-like cells expressed a strong signal for RANKL (Figure 4, B and C) ▶ . OPG is more expressed than RANKL but presented similar immunolocalization (Figure 4, A and C) ▶ . In all samples studied, occasional (2%) osteoclast-like cells lacked both RANKL and OPG protein expression. A moderate to strong staining was also observed in the mononucleated stromal cells and a minority (2%) of these cells was negative for OPG or RANKL. If OPG staining is essentially localized to the cytoplasm, RANKL expression is observed either in the cytoplasmic domain and in nucleus (Figure 4, A to C ▶ ; Table 4 ▶ ).
In chondrosarcoma (patient 55), half of chondrocytes were strongly positive for OPG and 30% moderately stained for RANKL (Figure 4, D to F) ▶ . This expression pattern persisted in 98% of the mesenchymal cells located in fibrous septa of the tumors. In contrast to healthy cartilage, extracellular matrix was negative for both proteins (Table 4) ▶ .
Two PBBTs were analyzed for RANKL and OPG protein expression. Thus, 75% of cells of chondromyxoid fibroma (patient 38) showed positive immunostaining for RANKL and OPG. Similarly, OPG and RANKL immunolabeling were detected in fibrous dysplasia (patient 41). In this case, as healthy bone tissue, osteoblasts displayed immunostaining for both proteins whereas osteocytes were negative. However, RANKL and OPG were moderately detected in liposarcoma (patient 49), a tumor of soft tissues (Table 4) ▶ .
In primary malignant bone tumors such as osteosarcoma, RANKL and OPG were also colocalized except in osteocytes (Table 4) ▶ . Osteoblasts expressed higher OPG immunostaining than RANKL which is weakly expressed (Table 4 ▶ ; Figure 4, G to I ▶ ). If RANKL was not detected in osteocytes, these cells were moderately positive for OPG. Osteoclasts, located in bone remodeling area, were strongly positive for both RANKL and OPG.
Thus, OPG and RANKL proteins are systematically colocalized as well in osteoblasts as in osteoclasts, except in osteocytes of both cases of osteosarcoma studied. Cytoplasmic domain was the main site of immunoreactivity except in GCT where cytoplasm and nucleus were both positive for RANKL.
Discussion
Focal bone loss caused by osteolysis is a critical etiological component of human diseases such as primitive bone tumors, bone metastases, and erosive arthritis. Another common clinical condition characterized by inflammatory bone loss is periprosthetic osteolysis, which results in implant failure. In all cases and mainly for tumoral processes, osteolysis is responsible for a high morbidity. Pain, a common sign of these pathologies, is relevant of most of bone benign tumors and of 70% of bone metastases. 29 Pathological fractures are revealing in more than 80% of essential cysts 30 as well as in 15% of bone metastases. Epidural spinal cord compression and malignant hypercalcemia in secondary bone tumors are also frequently observed. Because bone remodeling is the result of osteoblast and osteoclast activities, pathological osteolysis must be considered as a consequence of a disturbance in the mechanisms that govern the communications between both cell types. The host response to the foreign agents is the major cause of osteolysis during aseptic loosening 31 as well as in tumor development. 2,3 Wear debris and tumor cells initiate an inflammatory response that leads to the recruitment of activated osteoclasts and then to bone resorption. Moreover, tumor cells create a favorable environment for their own growth. The inflammatory response and the tumor environment lead to the establishment of a vicious circle between bone cells and tumor cells or between bone cells, wear debris, and immune cells. Thus, the bulk of evidence suggests that the vicious cycle established acts by co-opting the physiological mechanisms that normally favor bone resorption.
Numerous soluble factors have already been involved in the osteolytic vicious circle. Thus, parathyroid hormone-related peptide (PTHrP), directly produced by tumor cells, accelerate bone resorption and release stored growth factors such as TGF-β from the mineralized bone matrix which in turn maintain the tumor growth. 32 The discovery of the RANKL/OPG system in osteoclast biology has led numerous investigators to evaluate its involvement in osteolysis and to demonstrate that RANKL/OPG system play a pivot role between osteoblasts and osteoclasts. 13,14 In the present work, we show that RANKL and its natural inhibitors, OPG, are expressed (as mRNA and proteins) in most of osteolytic pathologies (tumoral and non-tumoral) and are colocalized in all samples studied. Moreover, the RANKL/OPG ratio is increased in severe osteolysis both at the transcript and serum protein levels. In the present study, serum OPG levels weakly fluctuate in osteolytic pathologies according to the work of Lipton et al which did not find any modification of OPG levels in 145 patients with solid tumors. 22 On the opposite, RANKL is submitted to strong modulation in osteolytic pathologies compared to the control group. To our knowledge, these data on serum RANKL levels are the first available in the literature and cannot be compared to already referenced series. Thus, serum RANKL concentrations are increased about 6-fold in bone metastases, 5-fold in primitive malignant bone tumors and in tumors associated with severe osteolysis. When the ratio RANKL/OPG is considered, it is significantly increased in bone metastases and in tumor associated with severe osteolysis. Similar data are obtained for RANKL/OPG mRNA. These data demonstrate 1) that tumor cells induce an imbalance in the RANKL/OPG system in favor of RANKL, not only in development foci of bone tumors but also in systemic circulation, and 2) that this ratio is significantly higher in severe osteolysis. Such imbalance has been already envisaged for myeloma cells which modify human bone marrow environment and induce osteoclastogenesis. 24 Huang et al 23 and Roux et al 24 showed that RANKL is involved in the tumor cell-induced osteoclast-like cell formation in GCT and that GCT strongly expressed RANK. These authors did not find any correlation of RANKL and OPG expression with the Enneking Clinical Stage. 30 The strong expression of the osteoclastic inhibitor OPG in osteolytic GCT can be explained by a defensive reaction of the tissues against osteoclastic activation. Thus, the tumor microenvironment can release high levels of OPG to counterbalance the high RANKL concentration produced by tumors cells. OPG acts in this case as a decoy receptor of RANKL and must be considered as a protector of bone. 5 In severe osteolysis (grade 3 in the present work), it is suggested that the high levels of RANKL released by tumor cells cannot be compensated by the OPG production. This hypothesis is strengthened by the observations of Alvarez et al 33 who demonstrate that serum OPG level is higher in patients with Paget’s disease than in healthy control subjects, and is decreased by treatment with bisphosphonate, in contrast to RANKL, which is not modified. In this study, OPG may reflect a protective mechanism of the skeleton to compensate increased bone resorption by inhibiting osteoclast formation and bone resorbing activity in GCT. Similar protective mechanism has been envisaged in osteoporosis. 34 In this context, serum OPG level is relatively constant in patients, and all disturbances of RANKL production results in bone remodeling pathology. This result strengthens the interest to study RANKL and OPG together through the RANKL/OPG ratio.
In human osteogenic osteosarcoma, RANKL signal is weakly expressed by osteoblasts in contrast to OPG (Figure 4 ▶ ; Table 4 ▶ ). In this pathology, the imbalance of RANKL/OPG is also disturbed and results both in bone apposition and resorption. The osteogenesis induced by osteosarcoma cells can be explained by a predominance of OPG production toward RANKL. These data are in agreement with those obtained on the transgenic mice overexpressing OPG that present a severe osteopetrosis. 5 However, the ratio RANKL/OPG still significantly increased compared to the control, resulting in a balance in favor of RANKL and then in bone resorption. Therefore, we cannot also exclude another osteoclastic activation via growth factors released by osteoblast-like or immune cells. Thus, cytokines such as interleukin-1 or TNF-α act on bone cells independently of RANKL and would impinge on osteoclast activity independent of RANKL and OPG. 35,36
The immunohistochemistry of chondrosarcoma revealed that a part of tumor chondrocytes presented both RANKL and OPG immunoreactivity (Figure 4) ▶ in contrast to healthy cartilage, where all chondrocytes were negative (Figure 3) ▶ . The role of the RANKL/OPG system in cartilage is still unclear and the expression of RANKL/OPG/RANK at the protein level in chondrocytes is controversed and depends on the differentiation level of the cells. Recently, Komuro et al 37 demonstrate that the expression of OPG and RANKL are detected in the superficial zone of normal cartilage and in cultured chondrocytes. However, in their model, RANKL does not activate chondrocytes. In our study, only part of the tumoral chondrocytes were positive for both proteins and we hypothesized an involvement in osteoclast recruitment, as suggested by Huang et al 38 in chondroblastoma.
RANKL and OPG are systematically colocalized in osteoarticular tissues. In light of the above discussion concerning the compensatory role of OPG on RANKL, this co-localization appears justified. The positive expression of RANKL in osteoclasts can be easily explained by an internalization of this ligand after binding to its specific receptor RANK, located on the cell membrane. The negative 2% of multinucleated cells revealed a heterogeneity of the giant cells in GCT. The presence of inflammatory macrophage polykaryon lacking RANK expression may explain this heterogeneity in the response. However, the immunolocalization of OPG in osteoclast (GCT, osteosarcoma) is very surprising although OPG is considered as a decoy receptor of RANKL inhibiting its binding to RANK. 5 Differentiation-based hypothesis can be suggested to explain these observations. First, the presence of a membrane-bound form of OPG has been reported in dendritic cells and may correspond to either a matrix-bound and/or a transmembrane form of the protein. 39 Thus, the transmembrane form of OPG could be expressed by osteoclasts. Second, more recently, Standal et al 40 presented evidence that myeloma cells internalize and degrade OPG through its binding to syndecan-1, the major heparan sulfate proteoglycan expressed on myeloma cells. OPG possesses a highly basic heparin-binding domain, making interactions with heparin and heparan sulfates possible, suggesting a new control mechanism for OPG biological activity. Proteoglycans expressed on the osteoclast membrane could be responsible for the internalization of OPG alone or associated with RANKL into the osteoclasts. However, the strong signal observed for RANKL, especially on the nucleus membrane of osteoclasts in GCT, but not in osteosarcoma, is another surprising observation. The possible presence of a modified ligand of RANKL on GCT osteoclasts must be envisaged and needs to be further characterized.
Recently, soluble RANKL/OPG have been proposed as a predictive survival index of multiple myeloma, where serum levels of RANKL are elevated in 121 patients and correlated with bone disease. 41 The RANKL/OPG ratio is also increased and correlated with markers of bone resorption, osteolytic lesions, and markers of disease activity. The authors have then generated an index based on these factors, which divided the patients into three risk groups. The low-risk group had 96% probability of survival at five years while the intermediate-risk and the high-risk group had a probability of survival of 52% and 0%, respectively. In the present study, RANKL/OPG ratio is increased in severe osteolysis, mainly in primitive bone tumors and in bone metastases. To define the veracity of such an index in assessing and managing patients with severe osteolysis, an extended population of patients suffering from severe osteolysis must be monitored.
Footnotes
Address reprint requests to Dr. Dominique Heymann, Laboratoire de Physiopathologie de la Résorption Osseuse et Thérapie des Tumeurs Osseuses Primitives. EE 99–01, Faculté de Médecine, 1 rue Gaston Veil, 44035 Nantes Cedex, France. E-mail: dominique.heymann@sante.univ-nantes.fr.
Supported by a Contrat de Recherche Stratégique (CReS) of INSERM (number 4CR06F), by a grant from the French Ministry of Research and Technology (ACI “Technologies pour la Santé”, number TS/02 2 0044), and by a grant from the Loire-Atlantique Committee of the Ligue Contre le Cancer.
References
- 1.Heymann D, Rousselle AV: gp130 cytokine family and bone cells. Cytokine 2000, 12:1465-1468 [DOI] [PubMed] [Google Scholar]
- 2.Goltzman D, Karaplis AC, Kremer R, Rabbani SA: Molecular basis of the spectrum of skeletal complications of neoplasia. Cancer 2000, 88:2903-2908 [DOI] [PubMed] [Google Scholar]
- 3.Goltzman D: Osteolysis and cancer. J Clin Invest 2001, 107:1219-1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouin F, Moreau A, Guicheux J, Passuti N, Heymann D: Mechanism of tumor-induced osteolysis. Rev Chir Orthop 1999, 85:58-68 [PubMed] [Google Scholar]
- 5.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, : Amgen EST ProgramBoyle WJ: Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997, 89:309-319 [DOI] [PubMed] [Google Scholar]
- 6.Tsuda E, Goto M, Michizuki S, Yano K, Kobayashi F, Morinaga T, Hisgashio K: Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun 1997, 234:137-142 [DOI] [PubMed] [Google Scholar]
- 7.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T: Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 1998, 95:3597-3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ: Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93:165-176 [DOI] [PubMed] [Google Scholar]
- 9.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM: Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 1999, 402:304-309 [DOI] [PubMed] [Google Scholar]
- 10.Burgess TL, Qian Y, Kaufman S, Ring BD, Van G, Capparelli C, Kelley M, Hsu H, Boyle WJ, Dunstan C, Hsu S, Lacey DL: The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol 1999, 145:527-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ: Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA 1999, 96:3540-3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, Gomibuchi T, Yano K, Shima N, Washida N, Tsuda E, Morinaga T, Higashio K, Ozawa H: Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun 1998, 247:610-615 [DOI] [PubMed] [Google Scholar]
- 13.Theill LE, Boyle WJ, Penninger JM: RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol 2002, 20:795-823 [DOI] [PubMed] [Google Scholar]
- 14.Grimaud E, Rédini F, Heymann D: Osteoprotegerin: a new agent for the treatment of bone disease. Drug Discov Today 2002, 6:1241-1242 [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Dai J, Lin DL, Smith P, Strayhorn C, Mizokami A, Fu Z, Westman J, Keller ET: Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest 2001, 107:1235-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown JM, Corey E, Lee ZD, True LD, Yun TJ, Tondravi M, Vessela RI: Osteoprotegerin and RANK ligand expression in prostate cancer. Urology 2001, 57:611-616 [DOI] [PubMed] [Google Scholar]
- 17.Nagai M, Kyakumoto S, Sato N: Cancer cells responsible for humoral hypercalcemia express mRNA encoding a secreted form of ODF/TRANCE that induces osteoclast formation. Biochem Biophys Res Commun 2000, 269:532-536 [DOI] [PubMed] [Google Scholar]
- 18.Huang L, Cheng YY, Chom LTC, Zheng MH, Kumta SM: Tumour cells produce receptor activator of NF-kB ligand (RANKL) in skeletal metastases. J Clin Pathol 2002, 55:877-878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, Gillepsie MT: Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 1999, 140:4451-4458 [DOI] [PubMed] [Google Scholar]
- 20.Chikatsu N, Takeuchi Y, Tamura Y, Fukumoto S, Yano K, Tsuda E, Ogata E, Fujita T: Interactions between cancer and bone marrow cells induce osteoclast differentiation factor expression and osteoclast-like cell formation in vitro. Biochem Biophys Res Commun 2000, 267:632-637 [DOI] [PubMed] [Google Scholar]
- 21.Good CR, O’Keefe RJ, Puzas JE, Schwarz EM, Rosier RN: Immunohistochemistry study of receptor activator of nuclear kappa-B ligand (RANK-L) in human osteolytic bone tumors. J Surg Oncol 2002, 79:174-179 [DOI] [PubMed] [Google Scholar]
- 22.Lipton A, Ali SM, Leitzel K, Chinchilli V, Witters L, Engle L, Holloway D, Bekker P, Dunstan C: Serum osteoprotegerin levels in healthy controls and cancer patients. Clin Cancer Res 2002, 8:2306-2310 [PubMed] [Google Scholar]
- 23.Huang L, Xu J, Wood D, Zheng MH: Gene expression of osteoprotegerin ligand, osteoprotegerin, and receptor activator of NFkB in giant cell tumor of bone. Am J Pathol 2000, 156:761-767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roux S, Amazit L, Meduri G, Guichon-Mantel A, Milgrom E, Mariette X: RANK (receptor activator of nuclear factor κB) and RANK ligand are expressed in giant cell tumors of bone. Am J Clin Pathol 2002, 117:210-216 [DOI] [PubMed] [Google Scholar]
- 25.Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barille S: Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood 2001, 98:3527-3533 [DOI] [PubMed] [Google Scholar]
- 26.Campanacci M, Baldini N, Boriani S, Sudanese A: Giant cell tumor of bone. J Bone Joint Surg 1987, 69A:106-114 [PubMed] [Google Scholar]
- 27.Wittrant Y, Couillaud S, Theoleyre S, Dunstan C, Heymann D, Rédini F: Osteoprotegerin differentially regulates protease expression in osteoclast cultures. Biochem Biophys Res Commun 2002, 293:38-44 [DOI] [PubMed] [Google Scholar]
- 28.Kartogiannis V, Zhou H, Horwood NJ, Thomas RJ, Hards DK, Quinn JMW, Niforas P, Ng KW, Matrin TJ, Gillespie MT: Localization of RANKL (receptor activator of NFκB ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone 1999, 25:525-534 [DOI] [PubMed] [Google Scholar]
- 29.Conroy T, Mallissard L, Dartois D, Luporsi E, Stines J, Chardot C: Histoire naturelle et évolution des métastases osseuses: a propos de 429 observations. Bull Cancer 1988, 75:845-857 [PubMed] [Google Scholar]
- 30.Enneking WF: Staging benign lesions. Enneking WF eds. Musculoskeletal Tumour Surgery 1983, vol 1.:pp 69-88 Churchill Livingstone New York [Google Scholar]
- 31.Jacobs JJ, Shanbhag A, Glant T, Black J, Galante JO: Wear debris in total joint replacements. J Am Acad Orthop Surg 1994, 2:212-220 [DOI] [PubMed] [Google Scholar]
- 32.Guise TA: Molecular mechanisms of osteolytic bone metastases. Cancer 2000, 88:2892-2898 [DOI] [PubMed] [Google Scholar]
- 33.Alvarez L, Peris P, Guanabens N, Vidal S, Ros I, Pons F, Fitella X, Monegal A, Munoz-Gomez J, Ballesta AM: Serum osteoprotegerin and its ligand in Paget’s disease of bone: relationship to disease activity and effect of treatment with bisphosphonate. Arthritis Rheum 2003, 43:824-828 [DOI] [PubMed] [Google Scholar]
- 34.Ueland T, Brixen K, Mosekilde L, Mosekilde L, Flyvbjerg A, Bollers J: Age-related in cortical bone content of insulin-like growth factor binding protein (IGFBP)-3, IGFBP-5, osteoprotegerin, and calcium in postmenopausal osteoporosis: a cross-sectional study. J Clin Endocrinol Metab 2003, 88:1014-1018 [DOI] [PubMed] [Google Scholar]
- 35.Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ: TNFα potently activates osteoclasts, through a direct act independent of and strongly synergistic with RANKL. Endocrinology 2002, 143:1108-1118 [DOI] [PubMed] [Google Scholar]
- 36.Kudo O, Fujikawa Y, Itonaga I, Sabokbar A, Torisu T, Athanasou NA: Proinflammatory cytokine (TNFα/IL-1α) induction of human osteoclast formation. J Pathol 2002, 198:220-227 [DOI] [PubMed] [Google Scholar]
- 37.Komuro H, Olee T, Kuhn K, Quach J, Brinson DC, Shikhman A, Valbracht J, Creighton-Achermann L, Lotz M: The osteoprotegerin/receptor activator of nuclear factor κB/receptor activator of nuclear factor κB ligand system in cartilage. Arthritis Rheum 2001, 44:2768-2776 [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Cheng YY, Chow LTC, Zheng MH, Kumta SM: Receptor activator of NF-kB ligand (RANKL) is expressed in chondroblastoma: possible involvement in osteoclastic giant cell recruitment. J Clin Pathol 2003, 56:116-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yun TJ, Chaudhary PM, Shu GL, Frazer JK, Ewings MK, Schwartz SM, Pascual V, Hood LE, Clark EA: OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J Immunol 1998, 161:6113-6121 [PubMed] [Google Scholar]
- 40.Standal T, Seidel C, Hjertner O, Plesner T, Sanderson RD, Waage A, Borset M, Sundan A: Osteoprotegerin is bound, internalized, and degraded by multiple myeloma cells. Blood 2002, 100:3002-3007 [DOI] [PubMed] [Google Scholar]
- 41.Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis Viniou N, Yataganas X, Goldman JM, Rahemtulla A: Soluble receptor activator of nuclear factor κB Ligand (RANKL)/osteoprotegerin (OPG) ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood 2003, 102:1064-1069 [DOI] [PubMed] [Google Scholar]