Abstract
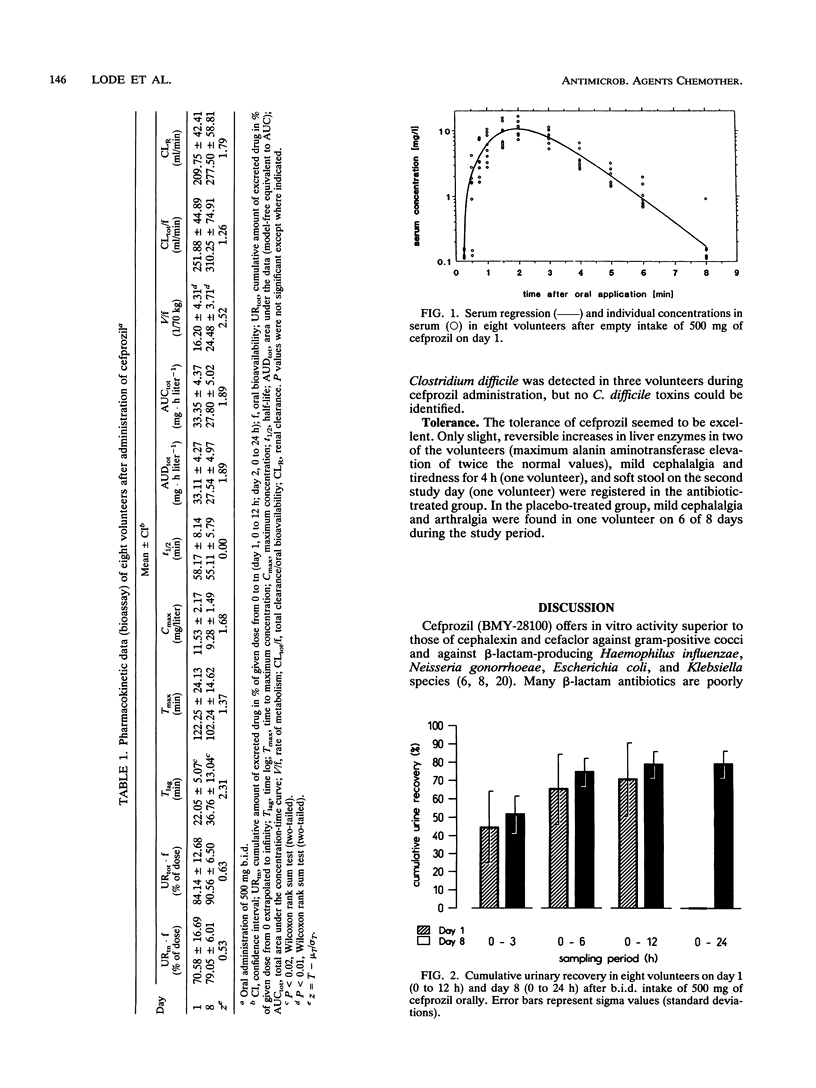
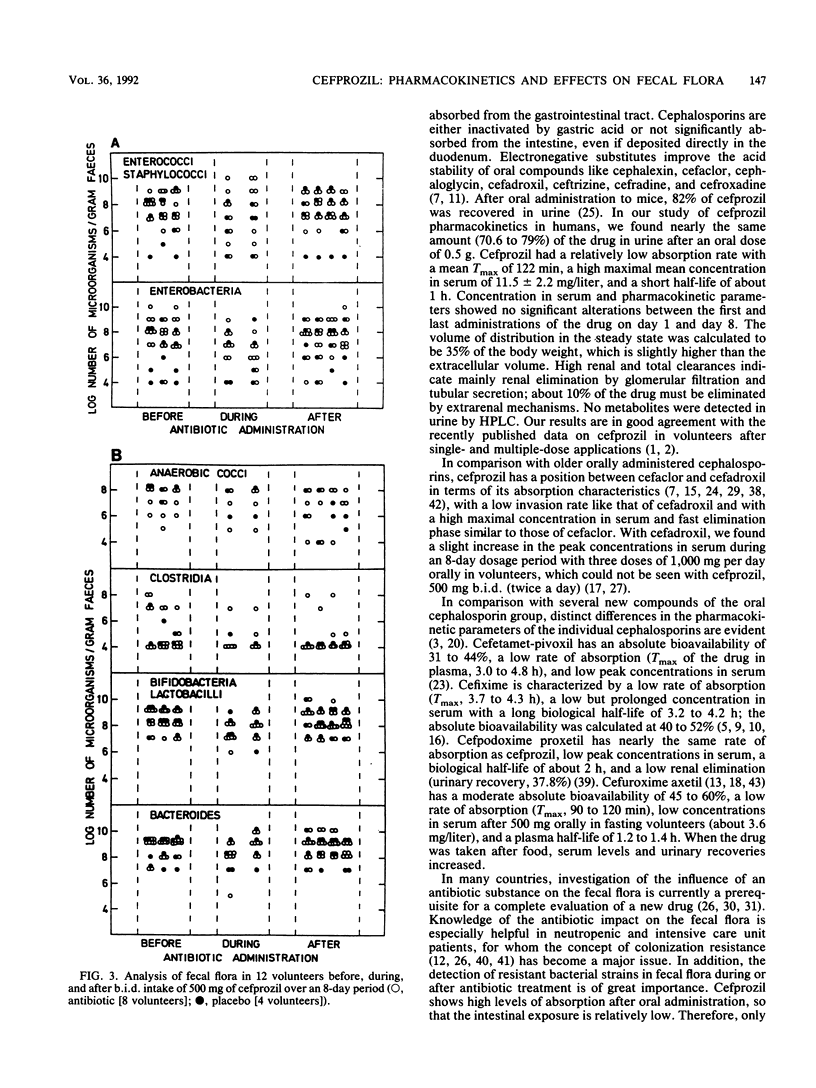
The pharmacokinetics of cefprozil were determined with 12 volunteers (8 received cefprozil and 4 received a placebo) after oral administration of 500 mg every 12 h over an 8-day period in a randomized, double-blind, placebo-controlled design. Concentrations in serum and urine were measured by high-pressure liquid chromatography and bioassay. The pharmacokinetic parameters were calculated on the basis of an open one-compartment model. The mean maximum concentration in serum on day 1 was 11.5 +/- 2.6 mg/liter, and the time to reach maximum concentration was 122.3 +/- 30 min after administration. Bioavailability parameters (area under the concentration-time curve from zero to infinity, maximum concentration of the drug in serum, and urinary recovery) indicated an excellent absorption. No accumulation over the 8-day period was registered. Cefprozil had a short biological elimination half-life of 58 +/- 10 min and a renal clearance of 210 +/- 51 ml/min, indicating high rates of renal excretion and tubular secretion. Analysis of the fecal flora showed an ecological impact of cefprozil on the intestinal microflora, such as a moderate decrease in enterobacteria and a slight increase in enterococci, staphylococci, and bacteroides during the study. The number of all bacterial species was already normalized 4 days after the administration period. The tolerance of cefprozil proved to be excellent; only a slight and reversible increase of liver enzymes (in two volunteers), mild cephalalgia, tiredness, and soft stool were registered during the 8-day period. Cefprozil had excellent absorption, no accumulation over an 8-day period, and only a limited impact on the intestinal microflora.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbhaiya R. H., Shukla U. A., Gleason C. R., Shyu W. C., Wilber R. B., Martin R. R., Pittman K. A. Phase I study of multiple-dose cefprozil and comparison with cefaclor. Antimicrob Agents Chemother. 1990 Jun;34(6):1198–1203. doi: 10.1128/aac.34.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Shukla U. A., Gleason C. R., Shyu W. C., Wilber R. B., Pittman K. A. Comparison of cefprozil and cefaclor pharmacokinetics and tissue penetration. Antimicrob Agents Chemother. 1990 Jun;34(6):1204–1209. doi: 10.1128/aac.34.6.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan T. Pharmacokinetic properties of the cephalosporins. Drugs. 1987;34 (Suppl 2):89–104. doi: 10.2165/00003495-198700342-00008. [DOI] [PubMed] [Google Scholar]
- Borner K., Lode H., Elvers A. Determination of apalcillin and its metabolites in human body fluids by high-pressure liquid chromatography. Antimicrob Agents Chemother. 1982 Dec;22(6):949–953. doi: 10.1128/aac.22.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain D. C., Scully B. E., Hirose T., Neu H. C. The pharmacokinetic and bactericidal characteristics of oral cefixime. Clin Pharmacol Ther. 1985 Nov;38(5):590–594. doi: 10.1038/clpt.1985.229. [DOI] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Comparative antibacterial activity of a new oral cephalosporin, BMY-28100. Antimicrob Agents Chemother. 1987 Mar;31(3):480–483. doi: 10.1128/aac.31.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry J. E. Evaluation of cefaclor. Am J Hosp Pharm. 1981 Jan;38(1):54–58. [PubMed] [Google Scholar]
- Eliopoulos G. M., Reiszner E., Wennersten C., Moellering R. C., Jr In vitro activity of BMY-28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1987 Apr;31(4):653–656. doi: 10.1128/aac.31.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner R. D., Bohaychuk W., Lanc R. A., Haynes J. D., Desjardins R. E., Yacobi A., Silber B. M. Pharmacokinetics of cefixime in the young and elderly. J Antimicrob Chemother. 1988 Jun;21(6):787–794. doi: 10.1093/jac/21.6.787. [DOI] [PubMed] [Google Scholar]
- Faulkner R. D., Fernandez P., Lawrence G., Sia L. L., Falkowski A. J., Weiss A. I., Yacobi A., Silber B. M. Absolute bioavailability of cefixime in man. J Clin Pharmacol. 1988 Aug;28(8):700–706. doi: 10.1002/j.1552-4604.1988.tb03203.x. [DOI] [PubMed] [Google Scholar]
- Fujimoto K., Ishihara S., Yanagisawa H., Ide J., Nakayama E., Nakao H., Sugawara S., Iwata M. Studies on orally active cephalosporin esters. J Antibiot (Tokyo) 1987 Mar;40(3):370–384. doi: 10.7164/antibiotics.40.370. [DOI] [PubMed] [Google Scholar]
- Ginsburg C. M., McCracken G. H., Jr, Petruska M., Olson K. Pharmacokinetics and bactericidal activity of cefuroxime axetil. Antimicrob Agents Chemother. 1985 Oct;28(4):504–507. doi: 10.1128/aac.28.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt D. J., Koch-Weser J. Clinical pharmacokinetics (second of two parts). N Engl J Med. 1975 Nov 6;293(19):964–970. doi: 10.1056/NEJM197511062931905. [DOI] [PubMed] [Google Scholar]
- Griffith R. S. The pharmacology of cephalexin. Postgrad Med J. 1983;59 (Suppl 5):16–27. [PubMed] [Google Scholar]
- Guay D. R., Meatherall R. C., Harding G. K., Brown G. R. Pharmacokinetics of cefixime (CL 284,635; FK 027) in healthy subjects and patients with renal insufficiency. Antimicrob Agents Chemother. 1986 Sep;30(3):485–490. doi: 10.1128/aac.30.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel B., Lode H., Wagner J., Koeppe P. Pharmacokinetics of cefadroxil and cefaclor during an eight-day dosage period. Antimicrob Agents Chemother. 1982 Dec;22(6):1061–1063. doi: 10.1128/aac.22.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S. M., Williams P. E., Ayrton J. Pharmacology of Cefuroxime as the 1-acetoxyethyl ester in volunteers. Antimicrob Agents Chemother. 1984 Jan;25(1):78–82. doi: 10.1128/aac.25.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimdahl A., Nord C. E. Effect of phenoxymethylpenicillin and clindamycin on the oral, throat and faecal microflora of man. Scand J Infect Dis. 1979;11(3):233–242. doi: 10.3109/inf.1979.11.issue-3.11. [DOI] [PubMed] [Google Scholar]
- Kemmerich B., Warns H., Lode H., Borner K., Koeppe P., Knothe H. Multiple-dose pharmacokinetics of ceftazidime and its influence on fecal flora. Antimicrob Agents Chemother. 1983 Sep;24(3):333–338. doi: 10.1128/aac.24.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe P., Hamann C. A program for non-linear regression analysis to be used on desk-top computers. Comput Programs Biomed. 1980 Dec;12(2-3):121–128. doi: 10.1016/0010-468x(80)90058-6. [DOI] [PubMed] [Google Scholar]
- Koup J. R., Dubach U. C., Brandt R., Wyss R., Stoeckel K. Pharmacokinetics of cefetamet (Ro 15-8074) and cefetamet pivoxil (Ro 15-8075) after intravenous and oral doses in humans. Antimicrob Agents Chemother. 1988 Apr;32(4):573–579. doi: 10.1128/aac.32.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause R. M. Koch's postulates and the search for the AIDS agent. Rev Infect Dis. 1984 Mar-Apr;6(2):270–279. doi: 10.1093/clinids/6.2.270. [DOI] [PubMed] [Google Scholar]
- La Rosa F., Ripa S., Prenna M., Ghezzi A., Pfeffer M. Pharmacokinetics of cefadroxil after oral administration in humans. Antimicrob Agents Chemother. 1982 Feb;21(2):320–322. doi: 10.1128/aac.21.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner F., Pursiano T. A., Buck R. E., Tsai Y. H., Chisholm D. R., Misiek M., Desiderio J. V., Kessler R. E. BMY 28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1987 Feb;31(2):238–243. doi: 10.1128/aac.31.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzenmeier G. Darmflora und Chemotherapie. Internist (Berl) 1989 Jun;30(6):362–366. [PubMed] [Google Scholar]
- Lode H., Stahlmann R., Koeppe P. Comparative pharmacokinetics of cephalexin, cefaclor, cefadroxil, and CGP 9000. Antimicrob Agents Chemother. 1979 Jul;16(1):1–6. doi: 10.1128/aac.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The new beta-lactamase-stable cephalosporins. Ann Intern Med. 1982 Sep;97(3):408–419. doi: 10.7326/0003-4819-97-3-408. [DOI] [PubMed] [Google Scholar]
- Nord C. E., Heimdahl A., Lundberg C., Marklund G. Impact of cefaclor on the normal human oropharyngeal and intestinal microflora. Scand J Infect Dis. 1987;19(6):681–685. doi: 10.3109/00365548709117204. [DOI] [PubMed] [Google Scholar]
- Nord C. E., Movin G., Stålberg D. Impact of cefixime on the normal intestinal microflora. Scand J Infect Dis. 1988;20(5):547–552. doi: 10.3109/00365548809032504. [DOI] [PubMed] [Google Scholar]
- Peck C. C., Sheiner L. B., Nichols A. I. The problem of choosing weights in nonlinear regression analysis of pharmacokinetic data. Drug Metab Rev. 1984;15(1-2):133–148. doi: 10.3109/03602538409015060. [DOI] [PubMed] [Google Scholar]
- Scribner R. K., Marks M. I., Finkhouse B. D. In vitro activity of BMY-28100 against common isolates from pediatric infections. Antimicrob Agents Chemother. 1987 Apr;31(4):630–631. doi: 10.1128/aac.31.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollaard E. J., Clasener H. A., van Griethuysen A. J., Janssen A. J., Sanders-Reijmers A. J. Influence of amoxycillin, erythromycin and roxithromycin on colonization resistance and on appearance of secondary colonization in healthy volunteers. J Antimicrob Chemother. 1987 Nov;20 (Suppl B):131–138. doi: 10.1093/jac/20.suppl_b.131. [DOI] [PubMed] [Google Scholar]
- Wise R., Bennett S. A., Dent J. The pharmacokinetics of orally absorbed cefuroxime compared with amoxycillin/clavulanic acid. J Antimicrob Chemother. 1984 Jun;13(6):603–610. doi: 10.1093/jac/13.6.603. [DOI] [PubMed] [Google Scholar]


