Abstract
Factor XIIIa-positive dendrocytes are abundant within the dermis and have been implicated in the pathogenesis of various disorders, including AIDS-related Kaposi’s sarcoma. Purified cultures of factor XIIIa-positive normal dermal dendrocytes have not as yet been achieved. 12E2 is a cloned cell line derived from superficial murine dermis where factor XIIIa-positive dendrocytes are abundant. Subconfluent cultures of 12E2 demonstrate polydendritic cell contours with thin, elongated membranous projections. These cells express Factor XIIIa and VCAM-1 by immunohistochemistry and by Western blot analysis of 12E2 cell lysates. 12E2 cells also constitutively express the Langerhans-cell-related epitope DEC-205, detected by NLDC-145 antibody and the CD80 co-stimulatory molecule, as well as Ia antigen on exposure to interferon-γ. Cells so treated exhibit significant ability to present alloantigens in vitro. 12E2 cells are shown to express mRNA for numerous cytokines, including interleukin (IL)-1α, IL-1β, IL-5, IL-6, IL-7, tumor necrosis factor-α and granulocyte macrophage-colony stimulating factor, by reverse-transcriptase polymerase chain reaction followed by Southern blot hybridization. Microinjection of 12E2 cells, but not 3T3control fibroblasts, into footpads of syngeneic and SCID mice results in lesions that mimic the histology and immunohistochemistry of human Kaposi’s sarcoma. In aggregate, these data indicate that 12E2 cells 1) share lineage characteristics with factor XIIIa-positive dermal dendrocytes, 2) produce mRNA for numerous cytokines and are cytokine responsive to interferon-γ, and 3) behave in vivo in a manner that resembles Kaposi’s sarcoma, a condition known to involve proliferation of human dermal dendrocytes.
Factor XIIIa-positive dermal dendrocytes (FXIIIaDD) are bone marrow-derived spindle cells that are diffusely distributed within the dermis of normal human skin. 1 Discovered in 1986, this distinctive cell type is separate from fibroblasts 2 and expresses the transglutaminase, coagulation Factor XIIIa, known to be important in crosslinking of fibrin and fibronectin. 3 The physiological function of FXIIIaDD has not been established definitively, although roles in wound healing and MHC class II-restricted antigen presentation have been proposed. 4-6 Nickoloff and Griffiths 7 have implicated these cells in the histogenesis and pathogenesis of AIDS-related Kaposi’s sarcoma (KS). KS is an infiltrative angioproliferative spindle cell tumor containing, in addition to proliferating endothelium, numerous FXIIIaDD that, in this pathological setting, also may express the endothelial antigen, vascular cell adhesion molecule-1 (VCAM-1). 8 Experimental studies of the biology of FXIIIaDD and their potential role in KS have been limited by inability to obtain purified cultures of normal FXIIIaDD and paucity of animal models, respectively. However, mouse skin recently has been shown to harbor cells analogous to human FXIIIaDD. 9 In addition, KS-like lesions may develop in murine skin of transgenic animals expressing genes that encode for human immunodeficiency virus (HIV)-associated Tat protein or KS-associated herpesvirus (HHV-8 K1). 10,11
The cell line 12E2 was originally cloned from a murine dermal cell line, DFB1, derived from spindle cells within the superficial dermis that adhered to enzymatically removed epidermal sheets. 12,13 This spatial localization directly beneath the epidermal layer coincides with a known subpopulation of FXIIIaDD, 4 and thus we hypothesized that the 12E2 clone may represent a pure population of murine FXIIIaDD, and as such, have biological relevance to the pathogenesis of KS. Our data indicate that 12E2 cells 1) express FXIIIa, VCAM-1, and inducible class II antigen, 2) produce mRNA for cytokines relevant to human dendritic cells and KS lesions, 3) express a glycoprotein receptor involved in antigen processing and present alloantigen in vitro, and 4) display growth characteristics in vivo similar to spindle cells in human KS.
Materials and Methods
Cell Culture
The murine dermal cell clone, 12E2, was grown at 37°C with 5% CO2 in RPMI 1640 containing 10% heat-inactivated fetal bovine serum (Mediatech, Herndon, VA), 2 mmol/L L-glutamine (Life Technologies, Grand Island, NY), 0.1 mmol/L non-essential amino acids (Life Technologies), 1 mmol/L sodium pyruvate (Life Technologies), 10 mmol/L HEPES (Life Technologies), 1 μg/ml indomethacin (Sigma Chemical, St. Louis, MO), 100 IU penicillin/100 μg/ml streptomycin (Mediatech) and 5 × 10−5 M 2-mercaptoethanol (Sigma) as previously described. 13 Cells were routinely grown in T75 culture flasks and subcultured using 0.025% trypsin/0.01% ethylene diaminetetraacetic acid in Hank’s balanced salt solution (HBSS) at 37°C for 5 to 7 minutes (Mediatech) at approximately 70 to 80% confluency. Murine fibroblast BALB/3T3 (clone A31), keratinocyte PAM212, and endothelial 3B-11 cell lines (ATCC, American Type Culture Collection, Manassas, VA) were grown according to ATCC protocols. In certain experiments, cells were exposed to recombinant murine interferon-γ (IFN-γ; PeproTech Inc, Rocky Hill, NJ) at 500 (12E2 and 3T3) or 5000 IU/ml (12E2) for 48 hours.
12E2 Growth Characteristics
Doubling time was determined by plating 7.5 × 104 cells per T75 flask (1000 cells per cm2) in complete media. Cells were collected by trypsinization from triplicate flasks at 24, 48, 72, and 96 hours and viable cell number using trypan blue exclusion was determined with a hemacytometer. Doubling time was calculated from data generated during logarithmic phase of growth.
Morphology
Cultured cells were evaluated by phase contrast microscopy and conventional transmission electron microscopy. In vivo injected cells were analyzed via hematoxylin and eosin (H&E) staining of paraffin-embedded and/or frozen sections.
Antibodies
Antibodies for immunohistochemistry included 1:25 dilution of rabbit anti-human factor XIIIa and rabbit control (Biogenex, San Ramon, CA), 1:50 dilutions of rat monoclonal anti-murine CD31, CD34, Mac-1 (CD11b), VCAM-1 (CD106), isotype-matched irrelevant control (BD Pharmingen, San Diego, CA), and NLDC-145 antibody (rat monoclonal anti-DEC-205, Bachem Bioscience, King of Prussia, PA), 1:100 dilutions for rat monoclonal anti-murine I-A (b,d,q haplotypes) and panendothelial antibodies (clone MECA-32), hamster monoclonal anti-murine ICAM (CD54), and hamster isotype-matched irrelevant control (BD Pharmingen); 1:50 dilution of rabbit anti-human factor XIIIa (Calbiochem, La Jolla, CA); and 1:25 dilution of mouse monoclonal anti-human CD31 (Dako, Carpinteria, CA).
Immunohistochemistry
12E2 cells were analyzed using BD Falcon culture slides coated with type IV collagen (Sigma) or 5-μm cryostat sections of 12E2-microinjected footpads. Immunohistochemistry was performed using a three-step biotin avidin horseradish peroxidase (HRP) (ABC) system with NovaRed substrate kit (Vector Labs, Burlingame, CA). Briefly, cells or sections were fixed in acetone at −20°C and then incubated with primary monoclonal antibody or isotype-matched control antibody for 1 hour at room temperature (RT). After washing in PBS, a species-specific biotinylated antibody was applied for 30 minutes at RT followed by ABC. For factor XIIIa immunohistochemistry, samples were fixed in 4% paraformaldehyde at 4°C for 10 minutes and then, after washing, treated with 0.1% Triton X-100 (Sigma) for 10 minutes before antibody incubation.
Immunohistochemistry of human KS lesions (n = 5) was performed using paraffin sections as described above after 0.1% trypsin treatment (factor XIIIa) or antigen retrieval with 10 mmol/L citrate buffer, pH 6.0 (CD31). Histological description of typical human KS phenotype was based on review of archival specimens and correlation with published observations. 14,15 Archival conventional histology and immunohistochemistry of 47 cases of human KS 15 were also reviewed for comparison with findings in 12E2 microinjection sites.
Western Blot Analysis
Cell-free lysates were prepared from cultured 12E2, 3T3, and 3B-11 cells, and Western blotting was performed as previously described. 16 Briefly, cells were incubated in lysate buffer [150 mmol/L NaCl, 10 mmol/L Tris-HCl, pH 8.0, containing 1% Triton X-100 and protease inhibitor cocktail (Sigma)] for 1 hour at 4°C and then centrifuged twice at 11,000 × g. Protein concentration of lysate preparations was determined using the Micro BCA Protein Assay Kit per manufacturer’s instructions (Pierce, Rockford, IL). Equivalent amounts of total protein for each preparation were loaded onto a 7.5% Tris-Glycine-SDS gel (Ready Gel, Bio-Rad Laboratories, Hercules, CA) and run at 100V constant voltage. Proteins were electrophoretically transferred to Immobilon-P PVDF membrane and blocked with 5% milk, 10% serum, and 0.1% Tween 20 in PBS (blocking buffer) overnight at 4°C. Membranes then were incubated with primary antibody diluted in blocking buffer for 2 hours at RT, followed by washing in 0.1% Tween 20 in PBS (wash buffer). HRP-conjugated species-specific secondary antibody (Vector) was used for 1 hour at RT. Membranes were washed several times and left overnight in wash buffer at RT. Antibody reactivity was detected by chemiluminescence using an ECL or ECL Plus Western blotting detection kit (Amersham Pharmacia Biotech, Piscataway, NJ) and exposure to X-OMAT film (Eastman Kodak Company, Rochester, NY). Primary antibodies for Western blots included rabbit antiserum to human factor XIIIa (1:5000, Calbiochem), rabbit control antiserum (1:2500, Biogenex), goat polyclonal anti-murine VCAM-1 IgG (1:250, R&D Systems, Minneapolis, MN) and goat polyclonal control IgG (1:35,000; Jackson ImmunoResearch, West Grove, PA). Human plasma-derived FXIII was purchased from Calbiochem and molecular weight markers were from Sigma.
RT-PCR and Southern Blot Analysis
Total RNA was extracted from 12E2 and PAM212 cultures using a total RNA isolation kit (Stratagene, La Jolla, CA) and RT-PCR was performed with first strand synthesis kit (Stratagene) using primers for IL-1α, IL-1β, IL-3, IL-4, IL-5, IL-6, IL-7, TNF-α, granulocyte macrophage-colony stimulating factor (GM-CSF), INF-γ, or β-actin (Cytokine Mapping Amplimer Set; Clontech, Palo Alto, CA) with AmpliTaq DNA polymerase (Perkin Elmer Cetus, Boston, MA). The PCR reaction conditions were 91°C for 5 minutes and 54°C for 1 minute as first denaturing and annealing, followed by 30 cycles of 72°C for 2 minutes, 91°C for 1 minute, and 54°C for 1 minute, and stopped after final elongation at 72°C for 7 minutes. Each product was diluted at 1:2, 1:4, and 1:8 and then electrophoresed in 1.5% agarose gels, transferred to nylon membranes, and hybridized with non-RI labeling probes. The probes were synthesized with cytokine control DNA (Clontech) according to the method by Lo. 17 Samples were detected using a Chemiluminescence Detection System (Applied Biosystems/Tropix, Bedford, MA), scanned by image analyzer and semiquantified as relative density by using NIH Image software.
CFSE Labeling and Detection
Adherent 12E2 cells were washed with PBS containing calcium and magnesium and then incubated with 5 μmol/L carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Inc., Eugene, OR) in PBS containing magnesium and calcium for 15 minutes at 37°C 18 in T75 culture flasks. The CSFE was then removed and the reaction was blocked by the addition of RPMI containing 20% fetal bovine serum (FBS). 12E2 cells were collected by trypsinization 24 hours after CSFE labeling. CSFE-labeled 12E2 cells were detected in cryosections of footpads or cytospin preparations using polyclonal goat anti-fluorescein antibody (Vector) followed by horse anti-goat HRP (Vector) and development with NovaRed substrate kit (Vector).
Footpad Injection of 12E2 or 3T3 Cells
Single-cell suspensions of 12E2 or 3T3 (2 × 106 cells in 50 μl HBSS) were injected intradermally per footpad of syngeneic BALB/c AnCr (n = 8), SCID/NCr (BALB/c background; n = 4) or allogeneic CBA/NCr (n = 6) mice. 3T3 cells were injected into BALB/c AnCr (n = 10) and SCID/NCr (BALB/c background; n = 4). Animals were purchased from the NCI-Frederick Animal Production Area, Frederick, Maryland and used at 6 to 20 weeks of age. Footpads were collected at 1, 2, or 3 weeks.
Tumor Progression and Metastatic Potential of 12E2 Cells
BALB/c AnCr mice were injected with 5 × 106 12E2 cells in 200 μl HBSS either intradermally in the back skin (n = 6) or intraperitoneally (i.p.) (n = 7) (back skin was chosen over footpad in view of increased likelihood of detecting tumorigenic growth at this more compliant site). The skin was shaved and after injection the area surrounding the injection site was marked with indelible ink. These areas were then collected for histological evaluation of tumor growth potential at 6 weeks post-injection. For i.p. injections, liver and lung samples were collected for histological evaluation of metastases 6 weeks after injection.
Flow Cytometry
12E2 cells were grown in complete media or in complete media containing 500 IU/ml IFN-γ (PeproTech) for 48 hours. The cells were then collected (approximately 70% to 80% confluency) by incubation in 0.2% EDTA in PBS for 10 minutes followed by exposure to 0.025% trypsin/0.01% EDTA for 1 to 2 minutes at 37°C. After washing with PBS, cells were resuspended in PBS containing 0.1% BSA and 0.02% sodium azide (FACS buffer). 6 × 105 cells were incubated in 1:50 dilutions of FITC-conjugated anti-CD40, CD80, CD86, or rat IgG2a control antibody (BD Pharmingen) for 30 minutes at 4°C, washed twice with FACS buffer, fixed with 1% paraformaldehyde in PBS for 10 minutes and then washed and resuspended in PBS for flow analysis. For analysis of Ia antigen, cells were treated similarly, except a two-step labeling method was used, using a 1:50 dilution of anti-Ia antibody (BD Pharmingen) followed by a 1:25 dilution of FITC-conjugated donkey anti-rat IgG (Jackson ImmunoResearch, West Grove, PA) for 30 minutes each at 4°C. Two-step labeling control consisted of secondary antibody alone. Fluorescence analysis was performed on a Beckman Coulter XL-MCL analytic cytometer (Beckman Coulter, Miami, FL).
Mixed Lymphocyte Reaction
B10.BR (H-2k) spleen and lymph node cells were RBC lysed, labeled with FITC-conjugated anti-CD4 and anti-CD8 mAbs (BD Pharmingen), incubated with immunomagnetic anti-FITC microbeads (Miltenyi Biotec, Berisch-Gladbach, Germany), and positively selected (≥98% pure) by Vario MACS column system (Miltenyi). Fixed numbers of T cells were plated in quadruplicate with decreasing numbers of irradiated stimulator cells (Balb/C splenocytes; 12E2 and 3T3 cells with and without 48-hour exposure to 500 IU/ml IFN-γ). Proliferation was assessed on day 4 after pulsing with 1 μCi/well [3H]-thymidine (TdR) for 8 hours using a 1205 Betaplate counter (Wallac, Turku, Finland). Data are expressed as mean stimulation index (SI; by dividing stimulator + responder cpm by mean responder-only cpm) as previously described. 19 P values were calculated using Student’s t-test.
Cytometric Bead Array
12E2 culture supernatant was analyzed for cytokine production after 48 hours of culture using a mouse Th1/Th2 cytokine CBA kit (BD Biosciences). Fluorescence analysis was performed using a Beckman Coulter XL-MCL analytic cytometer (Beckman Coulter).
Results
Expression of FXIIIa and VCAM-1 by Cultured 12E2 Cells
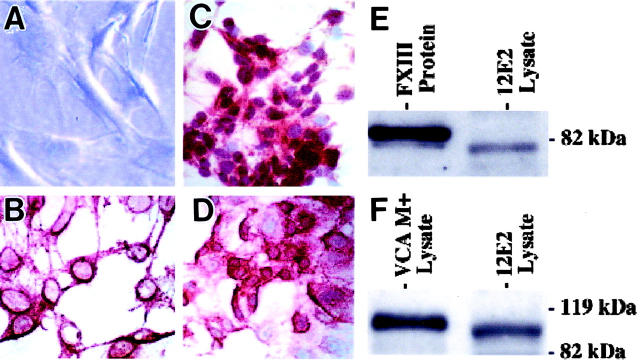
In culture, 12E2 cells attached individually to plastic surfaces and, within 4 to 5 days, formed confluent islands of cells. Trypsinized cell suspensions evaluated during log phase growth revealed a doubling time of approximately 31 hours. 12E2 cells in subconfluent and confluent cultures typically displayed elaborate polydendritic contours by phase contrast microscopy (Figure 1A) ▶ . The dendritic extensions were further highlighted by immunohistochemical staining with NLDC-145, 20,21 a monoclonal antibody to an integral membrane glycoprotein (DEC-205) expressed by antigen-presenting dendritic cells (Figure 1B) ▶ . Dendritic extensions measured up to 40 μm, thus approximating the range of human FXIIIaDD in vivo. 4 There was also uniform reactivity for the dermal dendrocyte-associated transglutaminase, FXIIIa (Figure 1C) ▶ , and for the endothelial-associated glycoprotein, VCAM-1 (Figure 1D) ▶ . Western blot analyses for immunoreactive FXIIIa and VCAM-1 derived from 12E2 cell lysates resulted in bands that closely approximated purified human FXIII (Figure 1E) ▶ and mouse VCAM-1 derived from the SV-40 transformed endothelial cell line, 3B-11 22 (Figure 1F) ▶ , respectively. In contrast to 12E2 cells, cultures of murine 3T3 fibroblasts failed to exhibit polydendritic contours, NLDC-145 antibody reactivity, or significant FXIIIa or VCAM-1 expression by immunohistochemistry or Western blot analyses (data not shown). By transmission electron microscopy, cultured 12E2 cells contained polyribosomes, lysosomes, intermediate filaments, and pinocytotic vesicles. Birbeck granules characteristic of Langerhans cells or other lineage-specific organelles, however, were not demonstrated.
Figure 1.
Phenotypic and immunochemical characterization of 12E2 cells in vitro. A: By phase contrast microscopy, cells with numerous, thin, elongated dendrites characterized subconfluent (depicted here) and confluent cultures. B: Immunohistochemistry for the integral membrane protein (DEC-205) defined by NLDC-145 monoclonal antibody permitted further confirmation of the prominent membranous dendritic extensions of cultured 12E2 cells. C and D: Strong immunoreactivity for FXIIIa (C) and VCAM-1 (D) was restricted primarily to cell bodies, but not dendrites, of 12E2 cells. E and F: Western blot analyses of 12E2 lysates were positive for FXIIIa (E) and VCAM-1 (F). The positive band for FXIIIa was detected at approximately 80 kd and for VCAM-1 at approximately 100 kd. The molecular weight for 12E2-derived FXIIIa approximated that of purified human FXIII (E) and coincided with that previously documented for FXIIIa-derived from murine macrophages. 47 The molecular weight for 12E2-derived VCAM-1 closely approximated that identified from VCAM-1+ lysates of the SV40-transformed murine endothelial cell line that constitutively expresses VCAM-1 (F). Original magnification: ×400 (A to D); NovaRed chromagen used in B to D.
Cytokine mRNA Profiles in 12E2 Cells
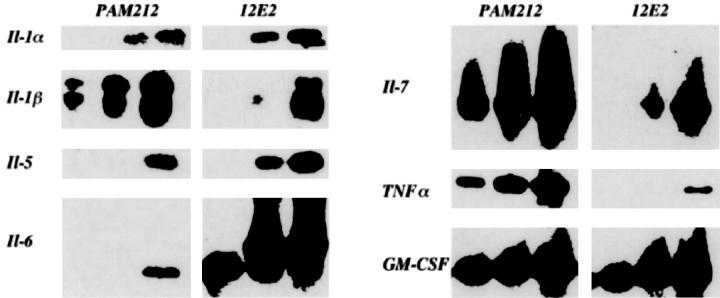
RT-PCR followed by Southern blot analysis of total RNA extracted from 12E2 cells was performed using primers for a panel of cytokines of established relevance to dendritic cells. 23 mRNA was detected for IL-1α and β, IL-5–7, TNF-α, and GM-CSF (Figure 2) ▶ , but not for IL-3, IL-4, and IFN-γ (data not shown). We previously demonstrated IL-7 secretion by 12E2 cells. 13 Screening of supernatants of unstimulated 12E2 cells in culture by cytometric bead array for a panel of candidate-secreted Th1/Th2 cytokines (IL-2, IL-4, IL-5, IFN-γ, and TNF-α) was negative.
Figure 2.
Cytokine mRNA expressed by 12E2 detected by RT-PCR and Southern blot analyses. Each PCR analysis displays three lanes, reflecting increasing concentration of PCR products. Note positive bands for multiple cytokines from 12E2 cells, compared to the cytokine-producing control murine keratinocyte line, PAM212. (mRNA was not detected in 12E2 cells for IL-3, IL-4, and IFN-γ).
Ia Expression and in Vitro Alloantigen Presentation by 12E2 Cells
Cultured 12E2 cells do not express significant class II (Ia) antigen constitutively (Figure 3A) ▶ . We thus examined 12E2 cells for inducible Ia expression after exposure to recombinant IFN-γ. Exposure to 500 IU/ml (Figure 3B) ▶ or 5000 IU/ml (Figure 3C) ▶ induced incremental diffuse expression of Ia, as detected immunohistochemically. The Ia induction by IFN-γ was also confirmed by flow cytometry. Ia was not detected on unstimulated cells (<1%); however, exposure to IFN-γ resulted in Ia expression, which was detected on approximately half the cells (45.5%). 12E2 cells were additionally shown to constitutively express a high level (72%) of the costimulatory molecule CD80 (B7.1) independent of IFN-γ, but not CD86 (B7.2) or CD40. Because 12E2 cells 1) have morphological and immunophenotypic similarities to FXIIIaDD known to present alloantigen, 5,6 2) express DEC-205 integral membrane glycoprotein and the CD80 costimulatory molecule in common with certain antigen-presenting cells, and 3) demonstrate class II antigen on exposure to recombinant IFN-γ, we tested their ability to stimulate alloreactive responder T cells in a classical mixed lymphocyte reaction. As compared to stimulation by control, fully allogeneic BALB/c splenocytes, unstimulated BALB/c-derived 12E2 cells, induced minimal proliferation of allogeneic responder T cells at the highest stimulator/responder ratio (1:4; Figure 3D ▶ ). However, 12E2 cells preconditioned with recombinant IFN-γ and expressing immunoreactive Ia confirmed immunohistochemically in cytospin preparations, exhibited significant allostimulatory activity for stimulator:responder ratios extending to 1:16 when compared to positive control BALB/c splenocytes, (P < 0.027) or IFN-γ -preconditioned 3T3 fibroblast (P < 0.018) (Figure 3D) ▶ .
Figure 3.
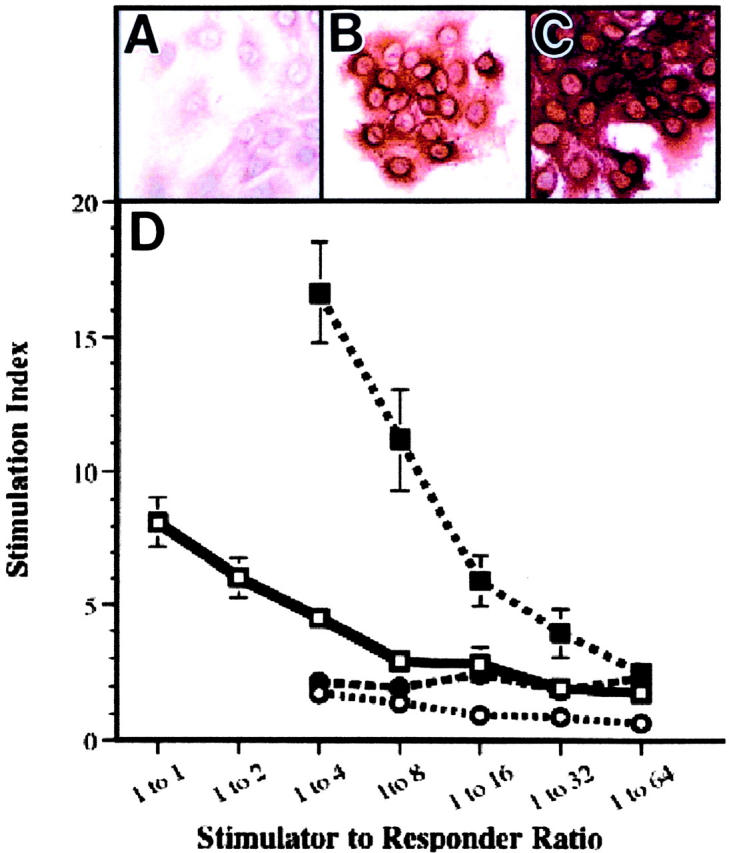
Recombinant IFN-γ-induced Ia expression and allostimulatory activity by 12E2 cells in vitro. A to C: 12E2 cells were negative to weakly positive by immunohistochemistry for Ia (A), whereas exposure to increasing concentrations of recombinant IFN-γ induced progressively intense immunoreactivity (B and C). D: Fixed numbers of naïve B10.BR T cells were combined with decreasing numbers of the respective stimulator cell type (BALB/c-positive control splenocytes (□), unstimulated 12E2 cells (○), 12E2 cells with IFN-γ pretreatment (▪), and IFN-γ-pretreated 3T3-negative control murine fibroblasts (•) to attain the indicated stimulator:responder ratios. Proliferation was measured on day 4 of co-culture and the stimulation index (SI) was calculated (see Materials and Methods). Optimal BALB/c allogeneic control stimulation at higher stimulator:responder cell ratios is shown. However, correlative data could not be generated for 12E2 cells as a result of cell aggregation at these higher ratios. Original magnification, ×400 with NovaRed chromagen (A to C).
Growth Characteristics of 12E2 Cells after Footpad Microinjection
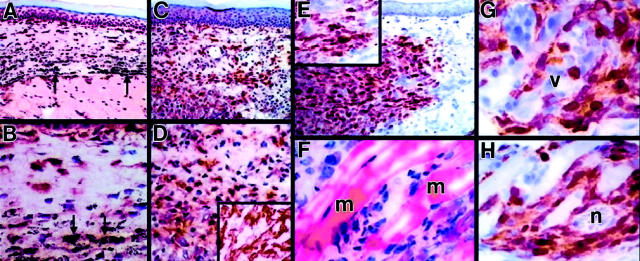
Human KS has a distinctive growth pattern characterized by perineurovascular and infiltrative growth by relatively bland spindle cells composed of FXIIIaDD and associated endothelial cells. 7,14,24 We therefore hypothesized that 12E2 cells might exhibit KS-like growth characteristics in situ. Accordingly, an in vivo microinjection bioassay was developed to assess growth of 12E2 cells introduced into footpads of syngeneic (BALB/c), allogeneic (CBA), or immunodeficient (SCID; BALB/c) mice. As compared to control sites injected with colloidal carbon (Figure 4, A and B) ▶ , 12E2 microinjection sites contained numerous infiltrative FXIIIa-positive cells within 1 week (Figure 4, C and D) ▶ in a pattern that persisted and progressed at 2 and 3 weeks. These FXIIIa-positive cells were proven to have originated from microinjected 12E2 cells, and not from proliferation of native FXIIIaDD, by intracellular fluorochrome labeling of 12E2 cells before injection with CFSE (Figure 4E) ▶ . Correlative conventional histology of adjacent sections confirmed an infiltrative growth pattern of 12E2 cells, with permeation of smooth muscle and collagen bundles (Figure 4F) ▶ . CFSE-positive 12E2 cells were variably immunoreactive with regard to staining intensity and displayed prominent perivascular and perineural growth characteristics (Figure 4, G and H) ▶ . These patterns were seen in all recipient strains, although an inflammatory response was present in the allogeneic recipients. In contrast to FXIIIaDD, fibroblasts are bipolar in culture and do not express FXIIIa. 25 Microinjection of control 3T3 fibroblasts into syngeneic footpads produced well-circumscribed, non-infiltrative dermal nodules composed of spindle cells that were negative for FXIIIa, VCAM-1, and staining by NLDC-145 antibody (data not shown).
Figure 4.
Immunophenotyping and growth characteristics of 12E2 cells in footpad microinjection model at 1 week. A and B: Microinjection of control medium containing inert carbon failed to influence number or distribution of constitutive FXIIIaDD. C, D, and D inset: In contrast, syngeneic footpads microinjected with 12E2 cells produced a lesion composed of closely-aggregated FXIIIaDD. E: CFSE prelabeling of 12E2 cells before injection and detected immunohistochemically demonstrated that the experimentally introduced 12E2 cells, and not recipient (constitutive) FXIIIaDD, accounted for this lesion. CFSE label was of variable intensity within 12E2 cells, consistent with dilution consequent to proliferation (E, inset). F: The in situ growth pattern of 12E2 cells was characterized by interstitial permeation between collagen and smooth muscle fibers by conventional H&E staining. G and H: Immunohistochemistry (here for CFSE) permitted detection of envelopment of dermal blood vessels (G) and nerves (H) by infiltrating 12E2 cells. This pattern differed from that of microinjected 3T3 fibroblasts, which failed to exhibit infiltrative growth. Similar findings were documented in footpads from SCID and allogeneic (CBA) mice, although the latter was associated with a brisk mononuclear cell infiltrate. Arrows indicate particulate carbon deposits (A and B). m, smooth muscle fiber (F); v, vessel (G); n, nerve fiber (H). Original magnifications: ×100 (A, C, and E); ×200 (B and D); ×400 (D (inset), E and inset, and F to H). Chromagen is NovaRed except in F, which is H&E.
Longer term studies (up to 6 weeks) failed to reveal clinical or histological evidence of progression of microinjected 12E2 cells from KS-like infiltrative growth to expansile tumors. Moreover, gross examination at necropsy and serial examination of liver and lung tissue failed to show metastases within this timeframe. Accordingly, for the duration of the study period, lesions induced at 12E2 microinjection sites displayed characteristics most analogous to earlier (non-tumorigenic) stages of KS development.
To examine more precisely the growth characteristics of this 12E2 microinjection model in relationship to typical lesions of human KS, we compared the experimentally induced murine footpad lesions to conventional histological and immunohistochemical data derived from archived (n = 47) 15 and newly prepared (n = 5) cases of human cutaneous KS. 12E2-microinjected footpads and human KS lesions both showed 1) bland infiltrative spindle cell proliferation within the superficial dermis (Figure 5, A and G) ▶ , 2) cellular spindle cell infiltration within the deeper dermis (Figure 5, B and H) ▶ , 3) associated prominence and ectasia of dermal blood vessels (Figure 5, A, B, G and H) ▶ , and 4) immunohistochemical profiles consistent with biphasic proliferation of FXIIIa-positive spindle cells (Figure 5, C and E, I and K) ▶ and CD31-positive blood vessels (Figure 5, D and F, J and L) ▶ , as previously described for human KS. 14,15 The immunophenotype of microinjected 12E2 cells differed somewhat from the preinjection in vitro profiles, with footpad lesions composed of spindle cells that were positive for FXIIIa, VCAM-1, and Mac-1 (NLDC-145-negative), and cultured 12E2 cells positive for FXIIIa, VCAM-1, and NLDC-145 antibody (Mac-1-negative). In both settings, spindle cells were negative for ICAM-1, CD34, and panendothelial cell antigen (MECA-32).
Figure 5.
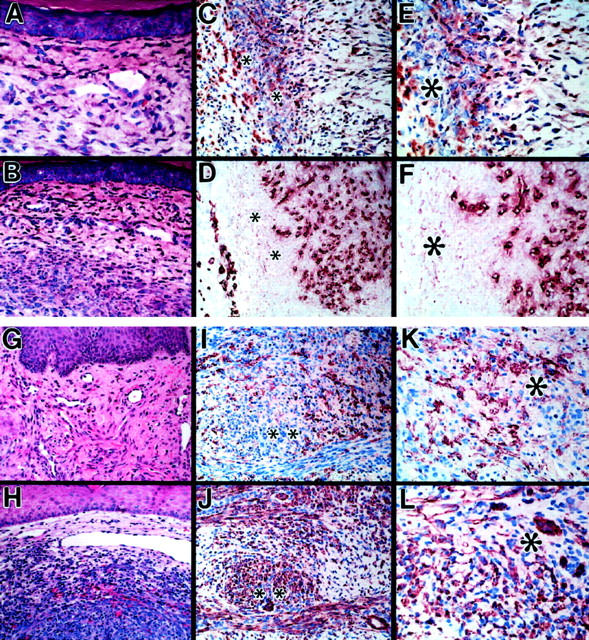
Comparison of lesions produced by 12E2 microinjection model and human Kaposi’s sarcoma. A and B: Conventional histology of 12E2 microinjection sites after 1 week, showing infiltration of superficial (A) and deeper (B) dermal layers by bland spindle cells associated with dilated blood vessels. C to F: Immunohistochemistry of adjacent sections of 12E2 microinjection site (1 week) stained for FXIIIa (C and E) and the endothelial marker, CD31 (D and F), documenting angiogenesis associated with infiltration of 12E2 cells. G and H: Conventional histology of characteristic human KS lesion. I to L: Immunohistochemistry of adjacent sections of typical human KS lesion stained for FXIIIa (I and K) and CD31 (J and L). Note histological and immunohistochemical similarities to the 12E2 microinjection sites (A to F). *Corresponding adjacent foci in C to F, I and J, K, and L FXIIIa/CD31 pairs. Original magnifications: ×400 (A, E to G, K, and L) and ×200 (B to D, H to J). H&E staining in A, B, G and H. NovaRed chromagen in C to F and I to L.
Discussion
Our data demonstrate that 12E2, a cloned dermal cell line, represents the murine equivalent of the human dermal dendrocyte. As such, it is the first available pure population of FXIIIaDD. Like human dermal dendrocytes, 12E2 cells are polydendritic and express the transglutaminase FXIIIa. Moreover, both cytokine-stimulated 12E2 cells and FXIIIaDD derived from normal human skin present antigen in vitro 5,6 . Finally, 12E2 cells proliferate in an infiltrative pattern after intradermal microinjection and express VCAM-1 in vitro and in vivo, both characteristics of FXIIIaDD in human AIDS-related KS, 8 a disorder in which these cells have been pathogenetically implicated. 7
Studies of FXIIIaDD pathobiology have been limited to human tissues as a result of lack of availability of relevant cell lines and of animal models; however, we have recently identified a population of FXIIIa-positive dendritic cells in normal and inflamed murine dermis. 9 When originally isolated, the murine dermal cell line DFB-1 and its clone, 12E2, were considered to be fibroblast-like cells that were normally localized directly beneath the epidermis and that expressed IL-7 potentially responsible for proliferation of the γ/δ subset of T lymphocytes. 12,13 Murine 12E2 cells in vitro intimately envelop certain cloned T cells in a manner similar to cell-cell relationships encountered in vivo between FXIIIaDD and mast cells in subepithelial strata of normal human dermis. 4 These phenotypic similarities between the 12E2 cell line and human FXIIIaDD led to the hypothesis that the former may represent the murine counterpart of the latter cell type. In addition to phenotypic and functional overlap between 12E2 cells and human FXIIIaDD, the striking similarity between AIDS-related human KS, a lesion containing numerous FXIIIaDD, and 12E2 growth characteristics in murine footpad provides further evidence supporting 12E2 cells as a murine counterpart of human FXIIIaDD. Moreover, the demonstration by others that transgenic mice expressing either the HIV-associated tat gene, the KS-associated HHV-8 K1 gene, or the HHV8-encoded chemokine receptor develop spindle cell lesions similar to human KS 10,11,26 indicates that mice harbor requisite skin cells to produce pathology analogous to human KS.
Compared to cells in situ, cultured cells exist in an artificial microenvironment and are frequently activated. Thus, cell cultures may not replicate the in vivo phenotype with complete fidelity. In this regard, it should be noted that human FXIIIaDD express VCAM-1 in KS lesions, 8 but not constitutively, 5,6 as do 12E2 cells in culture. Moreover, normal human FXIIIaDD are positive for class II antigens and Mac-1, 5,6 while 12E2 cells express these markers only after exposure to recombinant IFN-γ or intradermal microinjection, respectively. An unexpected observation was the reactivity of 12E2 cells for NLDC-145 antibody, a marker for murine dendritic cells possessing the ability to process antigen. 20,21 Functional studies reported herein demonstrate that cytokine-stimulated 12E2 cells are capable of presenting alloantigen, and our previous data indicate that, unlike macrophages, 12E2 cells are incapable of uptake of acetylated low-density lipoprotein. 12 In aggregate, these findings are of potential significance in view of recent data that indicate that human FXIIIaDD are capable of antigen processing and presentation. 5,6,27,28
We have developed a murine footpad microinjection bioassay for in situ introduction of cells into the murine dermal microenvironment. This model permitted evaluation of the premise that 12E2 cells may recapitulate the growth characteristics of early human KS, a tumor known to involve FXIIIaDD. Microinjected 12E2 cells, but not 3T3 fibroblasts, demonstrated a perineurovascular and infiltrative pattern of growth remarkably similar to early lesions of human KS. 24 As in human disease, the experimentally induced KS-like lesions in murine footpads expressed FXIIIa and VCAM-1. Additionally, lesions were confirmed to have originated exclusively from CFSE-labeled cultured 12E2 cells. Because CFSE labeling intensity is diluted within progeny of dividing cells, 18 qualitative documentation of variability in staining intensity in adjacent 12E2 cells permitted inference of cell proliferation within recipient dermis.
Human KS has been studied primarily by tissue examination of naturally occurring KS lesions and in experimentally induced lesions that develop in human skin xenografted to SCID mice injected with human herpesvirus-8. 29,30 The growth characteristics of human cutaneous KS are distinctive, with early lesions showing an infiltrative pattern by bland FXIIIaDD. The apparently hyperplastic cells forming these often multifocal lesions proliferate over time, 31,32 eventuating in locally aggressive sarcoma-like tumors that contain a mixture of FXIIIaDD and true endothelial cells. 14,15 Human FXIIIaDD derived from KS lesions have been found to harbor HIV transcripts 33 and infection of human KS cells in vitro with HIV results in production of IL-1β and IL-6, 34 both of which promote growth of human KS spindle cells. 35 Subcutaneous injection of HIV Tat protein and/or basic fibroblast growth factor (bFGF) induces local development of KS-like spindle cell proliferation in mouse skin, with the former synergizing with the latter by promoting increased endothelial growth and invasion. 36 Based on these and other data, Ensoli and Gallo 35 have advanced the central hypothesis that KS pathogenesis involves abnormal production by infected cells of angiogenic cytokines, including, among others, IL-1α, IL-1β, IL-6, GM-CSF, and TNF-α. These factors, in addition to the effects of HIV Tat protein, bFGF, and other angiogenic factors, 37,38 may produce repeated cycles of endothelial stimulation and proliferation that eventuate in cellular transformation and resultant formation of biologically aggressive neoplasms. It is therefore of potential significance that 12E2 cells also express message for IL-1α, IL-1β, IL-6, GM-CSF, and TNF-α. Moreover, microinjection of 12E2 cells was associated with angiogenic proliferation of CD31-positive endothelial cells in a pattern similar to that documented during the natural evolution of human KS. 14,15 Long-term studies are underway to examine the potentiating effects of relevant angiogenic proteins and their inhibitors on the tumorigenic progression and potential for neoplastic transformation in KS-like lesions experimentally induced by microinjected 12E2 cells.
Aside from the importance of FXIIIaDD in the pathogenesis of AIDS-related KS, it has not escaped our notice that the ability of these cells to perform seemingly disparate functions, 39 such as modulation of extracellular matrix via transglutaminase expression (FXIIIa) and antigen presentation, may provide insight into their role in immune-mediated sclerosing disorders such as scleroderma and chronic (sclerodermoid) graft-versus-host disease. In both of these conditions, alloantigen presentation, 40,41 exaggerated healing responses resulting in enhanced collagen deposition, 42,43 and hyperplasia of FXIIIaDD in lesional skin 9,44-46 have been implicated. Recognition of the murine 12E2 cell clone as a relevant analogue for human FXIIIaDD should facilitate further exploration of these and other conditions in which these cells play as yet undefined and potentially central pathogenetic roles.
Footnotes
Address reprint requests to George F. Murphy, M.D., Director, The Jefferson Center for Dermatopathology, Suite 545 Jefferson Alumni Hall, 1020 Locust Street, Philadelphia, PA 19107-6799. E-mail: george.murphy@jefferson.edu.
Supported by the National Institutes of Health grants CA 40358, CA 77401, and HL 55593; grant 14657198 from the Ministry of Education, Science and Culture of Japan; and by Japan Chemical Industry Association (the long-range research initiative).
References
- 1.Cerio R, Griffiths CEM, Cooper KD, Nickoloff BJ, Headington JT: Characterization of factor XIIIa positive dermal dendritic cells in normal and inflamed skin. Br J Dermatol 1989, 121:421-431 [DOI] [PubMed] [Google Scholar]
- 2.Headington JT: The dermal dendrocyte. Callen JP Dahl MV Golitz LE Rassumssen JE Stegmen SJ eds. Advances in Dermatology, 1986, vol 1.:pp 159-171 Year Book Medical Publishers, Illinois [PubMed] [Google Scholar]
- 3.Barry ELR, Mosher DF: Binding and degradation of factor XIIIa by cultured fibroblasts. J Biol Chem 1990, 265:9302-9307 [PubMed] [Google Scholar]
- 4.Sueki H, Whitaker D, Buchsbaum M, Murphy GF: Novel interaction between dermal dendrocytes and mast cells in human skin: implications for hemostasis and matrix repair. Lab Invest 1993, 69:160-172 [PubMed] [Google Scholar]
- 5.Meunier L, Gonzalez-Ramos A, Cooper KD: Heterogeneous populations of class II MHC+ cells in human dermal cell suspensions: identification of a small subset responsible for potent dermal antigen-presenting cell activity with features analogous to Langerhans cells. J Immunol 1993, 151:4067-4080 [PubMed] [Google Scholar]
- 6.Nestle FO, Zheng X-G, Thompson CB, Turka LA, Nickoloff BJ: Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol 1993, 151:6535-6545 [PubMed] [Google Scholar]
- 7.Nickoloff BJ, Griffiths CEM: Factor XIIIa-expressing dermal dendrocytes in AIDS-associated cutaneous Kaposi’s sarcomas. Science 1989, 243:1736-1737 [DOI] [PubMed] [Google Scholar]
- 8.Huang YQ, Friedman-Kien AE, Li JJ, Nickoloff BJ: Cultured Kaposi’s sarcoma cell lines express factor XIIIa, CD14, and VCAM-1, but not factor VIII or ELAM-1. Arch Dermatol 1993, 129:1291-1296 [PubMed] [Google Scholar]
- 9.Yoo YH, Park BS, Whitaker-Menezes D, Korngold R, Murphy GF: Dermal dendrocytes participate in the cellular pathology of experimental acute graft-versus-host disease. J Cutan Pathol 1998, 25:426-434 [DOI] [PubMed] [Google Scholar]
- 10.Vogel J, Hinrichs SH, Reynolds RK, Luciw PA, Jay G: The HIV tat gene induces dermal lesions resembling Kaposi’s sarcoma in transgenic mice. Nature 1988, 335:606-611 [DOI] [PubMed] [Google Scholar]
- 11.Prakash O, Tang Z-Y, Peng X, Coleman R, Gill J, Farr G, Samaniego F: Tumorigenesis and aberrant signaling in transgenic mice expressing the human herpesvirus-8 K1 gene. J Natl Cancer Inst 2002, 94:926-935 [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa S, Hara M, Seki M, Yagita H, Tagami H, Aiba S: Interaction of cutaneous stromal cells and γ/δ T cell receptor (TcR)-positive cells. I. Vγ5−γ/δTcR+ T cells migrating from organ-cultured murine skin proliferate by co-culture with cutaneous stromal cells in the presence of interleukin-2. Eur J Immunol 1993, 23:1705-1710 [DOI] [PubMed] [Google Scholar]
- 13.Aiba S, Nakagawa S, Hara M, Tomioka Y, Deguchi M, Tagami H: Cultured murine dermal cells can function like thymic nurse cells. J Invest Dermatol 1994, 103:162-167 [DOI] [PubMed] [Google Scholar]
- 14.Regezi JA, MacPhail LA, Daniels TE, DeSouza YG, Greenspan JS, Greenspan D: Human immunodeficiency virus-associated oral Kaposi’s sarcoma: a heterogeneous cell population dominated by spindle-shaped endothelial cells. Am J Pathol 1993, 143:240-249 [PMC free article] [PubMed] [Google Scholar]
- 15.Baer S, Radu A, Raymond AK, Kemp B, Murphy GF: AIDS-related Kaposi’s sarcoma has biphasic expression of CD34 and factor XIIIa. Lab Invest 1993, 68:33A8423674 [Google Scholar]
- 16.Kim JC, Whitaker-Menezes D, Deguchi M, Adair BS, Korngold R, Murphy GF: Novel expression of VCAM-1 (CD106) by squamous epithelium in experimental acute graft-versus-host disease. Am J Pathol 2002, 161:763-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo Y-MD, Mehal WZ, Fleming KA: Rapid production of vector-free biotinylated probes using the polymerase chain reaction. Nucl Acids Res 1988, 16:8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruber HE, Leslie KP, Ingram JA, Hanley EN, Jr: Optimization of 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester for labeling human intervertebral disc cells in vitro. Biotech Histochem 2000, 75:118-123 [DOI] [PubMed] [Google Scholar]
- 19.Greenberg SJ, Olanow CW, Dawson DV, Crane B, Roses AD: Autologous mixed lymphocyte reaction in patients with myasthenia gravis: correlation with disease activity. J Immunol 1984, 132:1229-1236 [PubMed] [Google Scholar]
- 20.Kraal G, Breel M, Janse M, Bruin G: Langerhan’s cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med 1986, 163:981-997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swiggard WJ, Mirza A, Nussenzweig MC, Steinman RM: DEC-205, a 205-KDa protein abundant on mouse dendritic cells and thymic epithelium that is detected by the monoclonal antibody NLDC-145: purification, characterization, and N-terminal amino acid sequence. Cell Immunol 1995, 165:302-311 [DOI] [PubMed] [Google Scholar]
- 22.O’Connell KA, Rudmann AA: Cloned spindle and epithelioid cells from murine Kaposi’s sarcoma-like tumors are of endothelial origin. J Invest Dermatol 1993, 100:742-745 [DOI] [PubMed] [Google Scholar]
- 23.De Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Ait-Yahia S, Banchereau J, Liu Y-J, Lebecque S, Caux C: The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol 1998, 160:1666-1676 [PubMed] [Google Scholar]
- 24.Nickoloff BJ, Griffiths CEM: The spindle-shaped cells in cutaneous Kaposi’s sarcoma: histologic simulators include factor XIIIa dermal dendrocytes. Am J Pathol 1989, 135:793-800 [PMC free article] [PubMed] [Google Scholar]
- 25.Bonish BK, Foreman KE, Gutierrez-Steil C, Nickoloff BJ: Phenotype and proliferation characteristics of cultured spindle-shaped cells obtained from normal human skin and lesions of dermatofibroma, Kaposi’s sarcoma, and dermatofibrosarcoma protuberans: a comparison with fibroblasts and endothelial cells of the dermis. J Dermatol Sci 1997, 16:52-58 [DOI] [PubMed] [Google Scholar]
- 26.Yang TY, Chen SC, Leach MW, Manfra D, Homey B, Wiekowski M, Jenh CH, Narula SK, Chensue SW, Lira SA: Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi’s sarcoma. J Exp Med 2000, 191:417-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sontheimer RD: Perivascular dendritic macrophages as immunobiological constituents of the human dermal microvascular unit. J Invest Dermatol 1989, 93:96S-101S [DOI] [PubMed] [Google Scholar]
- 28.Murphy GF, Liu V: The dermal immune system. Bos JD eds. Skin Immune System (SIS). 2nd ed. 1997:pp 347-363 CRC Press, New York
- 29.Foreman KE, Friborg J, Chandran B, Katano H, Sata T, Mercader M, Nabel GJ, Nickoloff BJ: Injection of human herpesvirus-8 in human skin engrafted on SCID mice induces Kaposi’s sarcoma-like lesions. J Dermatol Sci 2001, 26:182-193 [DOI] [PubMed] [Google Scholar]
- 30.Nickoloff BJ, Foreman KE: Etiology and pathogenesis of Kaposi’s sarcoma. Recent Results Cancer Res 2002, 160:332-342 [PubMed] [Google Scholar]
- 31.Kaaya EE, Parravicini C, Sundelin B, Mgaya E, Kitinya J, Lema L, Luande J, Biberfeld P: Spindle cell ploidy and proliferation in endemic and epidemic African Kaposi’s sarcoma. Eur J Cancer 1992, 28:1890-1894 [DOI] [PubMed] [Google Scholar]
- 32.Simonart T, Hermans P, Schandene L, Van Vooren JP: Phenotypic characteristics of Kaposi’s sarcoma tumor cells derived from patch-, plaque- and nodular-stage lesions: analysis of cell cultures isolated from AIDS and non-AIDS patients and review of the literature. Br J Dermatol 2000, 143:557-563 [DOI] [PubMed] [Google Scholar]
- 33.Mahoney SE, Duvic M, Nickoloff BJ, Minshall M, Smith LC, Griffiths CE, Paddock SW, Lewis DE: Human immunodeficiency virus (HIV) transcripts identified in HIV-related psoriasis and Kaposi’s sarcoma lesions. J Clin Invest 1991, 88:174-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang YQ, Li JJ, Kim KS, Nicolaides A, Zhang WG, Le J, Poiesz BJ, Friedman-Kien AE: HIV-1 infection and modulation of cytokine and growth factor expression in Kaposi’s sarcoma-derived cells in vitro. AIDS 1993, 7:317-322 [DOI] [PubMed] [Google Scholar]
- 35.Ensoli B, Gallo RC: AIDS-associated Kaposi’s sarcoma: a new perspective of its pathogenesis and treatment. Proc Assoc Am Physicians 1995, 107:8-18 [PubMed] [Google Scholar]
- 36.Ensoli B, Gendelman R, Markham P, Fiorelli V, Colombini S, Raffeld M, Cafaro A, Chang H-K, Brady JN, Gallo RC: Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi’s sarcoma. Nature 1994, 371:674-680 [DOI] [PubMed] [Google Scholar]
- 37.Samaniego F, Young D, Grimes C, Prospero V, Christofidou-Solomidou M, DeLisser HM, Prakash O, Sahin AA, Wang S: Vascular endothelial growth factor and Kaposi’s sarcoma cells in human skin grafts. Cell Growth Differ 2002, 13:387-395 [PubMed] [Google Scholar]
- 38.Sgadari C, Barillari G, Toschi E, Carlei D, Bacigalupo I, Baccarini S, Pallidino C, Leone P, Bugarini R, Malavasi L, Cafaro A, Falchi M, Valdembri D, Rezza G, Bussolino F, Monini P, Ensoli B: HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi’s sarcoma. Nat Med 2002, 8:225-232 [DOI] [PubMed] [Google Scholar]
- 39.Nestle FO, Nickoloff BJ: A fresh morphological and functional look at dermal dendritic cells. J Cutan Pathol 1995, 22:385-393 [DOI] [PubMed] [Google Scholar]
- 40.Artlett CM, Smith JB, Jimenez SA: Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis. N Engl J Med 1998, 338:1186-1191 [DOI] [PubMed] [Google Scholar]
- 41.Via CS, Rus V, Nguyen P, Linsley P, Gause WC: Differential effect of CTLA4Ig on murine graft-versus-host disease (GVHD) development: CTLA4Ig prevents both acute and chronic GVHD development but reverses only chronic GVHD. J Immunol 1996, 157:4258-4267 [PubMed] [Google Scholar]
- 42.Shulman HM, Sale GE, Lerner KG, Barker EA, Weiden PL, Sullivan K, Gallucci B, Thomas ED, Storb R: Chronic cutaneous graft-versus-host disease in man. Am J Pathol 1978, 92:545-570 [PMC free article] [PubMed] [Google Scholar]
- 43.Rodnan GP, Lipinski E, Luksick J: Skin thickness and collagen content in progressive systemic sclerosis and localized scleroderma. Arthritis Rheum 1979, 22:130-140 [DOI] [PubMed] [Google Scholar]
- 44.Higley H, Persichitte K, Chu S, Waegell W, Vancheeswaran R, Black C: Immunohistochemical localization and serologic detection of transforming growth factor β1: association with type I procollagen and inflammatory cell markers in diffuse and limited systemic sclerosis, morphea, and Raynaud’s phenomenon. Arthritis Rheum 1994, 37:278-288 [DOI] [PubMed] [Google Scholar]
- 45.Gilmour TK, Wilkinson B, Breit SN, Kossard S: Analysis of dendritic cell populations using a revised histological staging of morphoea. Br J Dermatol 2000, 143:1183-1192 [DOI] [PubMed] [Google Scholar]
- 46.Deguchi M, Aiba S, Ohtani H, Nagura H, Tagami H: Comparison of the distribution and numbers of antigen-presenting cells among T-lymphocyte-mediated dermatoses. CD1a+, factor XIIIa+, and CD68+ cells in eczematous dermatitis, psoriasis, lichen planus and graft-versus-host disease. Arch Dermatol Res 2002, 294:297-302 [DOI] [PubMed] [Google Scholar]
- 47.Roth WJ, Fleit HB, Chung SII, Janoff A: Characterization of two distinct transglutaminases of murine bone marrow-derived macrophages: effects of exposure of viable cells to cigarette smoke on enzyme activity. J Leukoc Biol 1987, 42:9-20 [DOI] [PubMed] [Google Scholar]