Abstract
Elevated semicarbazide-sensitive amine oxidase (SSAO) activity has been observed in several human conditions, eg, diabetes, and it has been speculated that SSAO contributes to the development of vasculopathies associated with this disease. To investigate in vivo consequences of elevated expression of SSAO in vascular tissues, we have developed a transgenic model for overexpression of human SSAO in mice. A smooth muscle-specific promoter, smooth muscle α-actin promoter 8 (SMP8) was used. Transgenic expression of human SSAO in tissues with a high content of smooth muscle cells was confirmed by Northern blot analysis. Enzymatic analysis of homogenates from transgenic tissues showed elevated levels of SSAO activity compared to non-transgenic littermates. Furthermore, when plasma SSAO activity was analyzed, much higher activity was detected compared to plasma from control mice, indicating that plasma SSAO may originate from smooth muscle cells. Histopathological evaluation of aorta and renal artery from transgenic mice revealed an abnormal structure of the elastin tissue. Instead of the regularly folded elastic laminae normally found in tunica media of sacrificed mice, the elastic laminae were straight and unfolded with irregularly arranged elastic fibers, forming tangled webs, between the intercalating elastic laminae. These alterations of the elastin structures suggest that overexpression of SSAO has led to a reduced elasticity of the arteries. Moreover, the mean femoral arterial pressure of the SMP8 SSAO transgenic mice was significantly lower in comparison to non-transgenic littermates. This suggests that the transgenic mice have a defect in their ability to regulate blood pressure.
Semicarbazide-sensitive amine oxidases (SSAO) (E.C.1.4.3.6) constitute a diverse group of copper-dependent enzymes found in both prokaryotes and eukaryotes. 1 In mammals, SSAO exists in a membrane-bound form located mainly in adipose tissue, vascular and visceral smooth muscle cells, and in endothelial cells. Additionally, there is a soluble form of SSAO circulating in blood. For a long time, interest has been focused on the origin of plasma SSAO, the subcellular distribution of the enzyme, the regulation of enzyme activity, and above all, the primary physiological function of the enzyme. 2
SSAO seems to be an enzyme with diverse functions. For example, it appears to play a role in transporting glucose into adipocytes via the glucose transporter GLUT4, 3 and into smooth muscle cells via GLUT1. 4 Moreover, SSAO seems to be involved in the regulation of adipocyte homeostasis, 5 and it is known to be a vascular adhesion molecule (VAP-1), which mediates lymphocyte binding to endothelial cells. 6,7
While amine oxidases are capable of deaminating amines with the production of an aldehyde, ammonia, and hydrogen peroxide, 8 doubt still surrounds the identity of the most important endogenous substrates for these enzymes. At present, methylamine and aminoacetone head the list of endogenous substrates for SSAO (reviewed in 7 ). The deaminated products of these monoamines are formaldehyde and methylglyoxal, respectively, as well as hydrogen peroxide and ammonia, which are all potentially cytotoxic agents. Formaldehyde and methylglyoxal are highly reactive aldehydes that have the capacity to promote unwanted protein cross-linking. Methylglyoxal may also exacerbate formation of advanced glycation end-products. 9
High catalytic activity of plasma SSAO has been linked to several human pathologies, such as diabetes, atherosclerosis, heart failure, and chronic liver disease. 10-15 Due to the production of potentially cytotoxic agents, it has been speculated that excessive SSAO activity could directly initiate endothelial injury. By in vitro studies, it has been shown that deamination of methylamine to formaldehyde is toxic to cultured human endothelial cells, and that SSAO inhibitors were able to prevent this toxicity. 16 The in vivo SSAO-mediated formation of formaldehyde has been studied autoradiographically in mice, revealing that deposits of this product are most prominent in adipose tissue, excretion glands, spleen, small intestines and kidney. 17
The association between elevated plasma SSAO activity and diabetes is particularly prominent in diabetes complicated by vasculopathies, such as retinopathy or nephropathy 15,18-20 and it has been speculated that the enzyme is involved in accelerating the development of such vascular complications. Boomsma et al 13 reported high SSAO activity in diabetic patients at first clinical diagnosis, indicating that high SSAO activity precedes the appearance of vascular complications.
Transgenic expression in mouse models can be used to assess gene function in different tissues. To investigate the possible contribution of elevated SSAO catalytic activity in vasculopathies, a promoter for specific expression in smooth muscle cells of the adult mouse was preferred. Such a model is available through the use of the mouse smooth muscle cell α-actin promoter, 21 whose expression becomes restricted to smooth muscle cells during late fetal maturation. 21,22
Here we describe a mouse model that overexpresses human SSAO in smooth muscle cells. These transgenic animals display a large increase in SSAO catalytic activity in serum and several tissues. The mice also exhibit differences in the elastic structures of arteries, determined by histological examination, and lowered blood pressure, compared to non-transgenic littermates. We suggest that this model is useful for investigating the importance of SSAO in the development of vascular abnormalities.
Materials and Methods
Transgene Construction
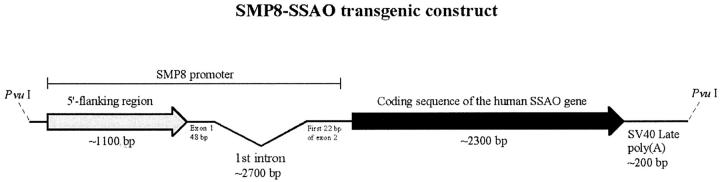
Polymerase chain reaction (PCR) was used to amplify the mouse smooth muscle α-actin promoter region SMP8 21 from genomic DNA isolated from the (C57B1/6×CBA)F1 mouse strain, using primers 5′-GCC GCG ATC GCA CAC CAT AAA ACA AGT GCA TGA GCC-3′ and 5′-GGG GAA TTC TAG CTG GAG CAG CGT CTC AGG G-3′ containing the restriction enzyme cleavage sites SgfI and EcoRI, respectively, for cloning. The coding sequence of human SSAO (GenBank Accession Number NM 003734) was amplified from human aorta QUICK-Clone cDNA (Clontech Laboratories, Palo Alto, CA), using primers 5′-CCG GAA TTC CAA CGC GTC CAT GAA CCA GAA GAC AAT CCT CGT G-3′ and 5′-CCC CCA AGC TTG TCG ACT CAC TAG TTG TGA GAG AGA AGC CCC CCC-3′ containing the EcoRI and SalI sites respectively, for cloning. The SMP8 fragment was inserted between the SgfI and EcoRI sites of the mammalian expression vector pCI-neo (Promega Corporation, Madison, WI), upstream of the coding sequence of human SSAO previously cloned between the EcoRI and SalI sites (Joakim Nilsson, unpublished). This plasmid was designated pBV219. The final transgene construct (designated SMP8-SSAO; ∼6500 bp), flanked by PvuI sites, contains the mouse smooth muscle α-actin promoter (SMP8), followed by the entire coding sequence of the human SSAO gene and the SV40 late polyA signal downstream of the SSAO sequence (Figure 1) ▶ . A SMP8-SSAO linearized fragment was isolated from pBV219 by digestion with PvuI and separated by 1% agarose gel electrophoresis. Purification was performed using a QIAquick Gel Extraction Kit (Qiagen GmbH, Hilden, Germany), followed by CHROMA SPIN-400 columns (Clontech Laboratories) equilibrated in injection buffer (10 mmol/L Tris-HCl, pH 7.5, 0.1 mmol/L ethylenediaminetetraacetic acid). The purified SMP8-SSAO fragment was diluted with injection buffer to ∼2 ng/μl.
Figure 1.
SMP8-SSAO transgenic construct. Linear map of the SMP8-SSAO construct comprising the mouse smooth muscle α-actin promoter SMP8 and the coding sequence of the human SSAO gene, followed by the SV40 late polyadenylation signal.
Generation of Transgenic Mouse Lines
The mouse colony was kept in a maintained barrier facility. The isolated transgenic construct described in Figure 1 ▶ was injected into the pronuclei of 1-cell stage embryos obtained from C57B1/6 and CBA matings (Charles River Laboratories, Uppsala, Sweden). Injected eggs were transferred to the oviduct of pseudo-pregnant female mice. 23 Transgenic founder mice were identified by PCR of tail biopsy lysates. A small piece of the tail biopsy (1 to 2 mm) was incubated in lysis buffer with proteinase K 23 overnight. After inactivation of proteinase K and centrifugation to remove tissue debris, the supernatant was used for PCR. For the generation of PCR products the following primers were used: 5′-CCA GCT CCT TAA CTT GTG ATC CTC C-3′ and 5′-CAC GAG GAT TGT CTT CTG GTT CAT G-3′.
Preparation and Analysis of RNA
For RNA expression analysis, Northern blot was performed. For each transgenic line, adult F1 mice were dissected and RNA prepared from bladder, stomach, and liver using the RNA-Trizol extraction kit. 24 Non-transgenic littermate tissues were used as negative controls. Hybridization to membranes was performed using a 32P-labeled SSAO probe consisting of the sequence corresponding to amino acids 1–763 of the SSAO protein.
Enzymatic Activity of SSAO
The enzyme activity assay is based on a protocol that is a modification of a method described by McCaman. 25 Briefly, serum or 5% (w/v) tissue homogenate was sonicated with a Branson sonifier B-15 cell disrupter. Fifty microliters of each sample were pre-incubated with clorgyline and L-deprenyl at a final concentration of 0.05 and 0.025 mmol/L, respectively, to inhibit MAO-A and -B activities. The SSAO inhibitor hydralazine at a final concentration of 0.2 mmol/L was added to give blank activity samples. The enzymatic activity was determined by addition of the 14C-labeled substrate benzylamine to the tissue homogenate reaching a final concentration of 0.07 mmol/L. The reaction was terminated by addition of 30 μl of 1 mol/L HCl after 20 minutes incubation at 37°C. The 14C-labeled benzaldehyde product was extracted with toluene: ethylacetate 1:1 (v/v) and quantified in a Packard Tri-Carb 1900CA liquid scintillation analyzer. Results are expressed as nanomoles of benzaldehyde per milliliter per hour for serum and nanomoles of benzaldehyde per milligram of protein per hour for tissue homogenate, where the protein concentrations have been determined. 26 SSAO activity in adipose tissue is presented as nanomoles per milligram of wet weight per hour.
Tissue Preparation for Histological and Morphological Analysis
For histological analyses, mice were sacrificed by cervical dislocation and tissues dissected and placed in 4% paraformaldehyde in PBS over night. After tissue dehydration, paraffin blocks were prepared using standard histological procedures and sectioned using a microtome. Serial sections through kidney and aorta from lines 12, 56, and 86 were stained with hematoxylin and eosin (H&E) for standard histology as well as with Weigert and Van Gieson’s picric acid-fuchsine stain 27 to visualize elastin and collagen fibers, respectively. The sections were inspected for morphological abnormalities and signs of vascular damage.
To further assess morphological abnormalities in vascular tissues, elastin-stained sections (4 μm) of proximal thoracic aorta and renal artery were used. Arteries from animals in two transgenic lines, 12 and 86, were chosen and compared to non-transgenic littermates. The number of elastic laminae in aorta was counted and the thickness of the elastic laminae was measured at five positions for each layer in three sections from each group using a QWIN Leica image analyzer (Leica Imaging Systems LTD, Cambridge, UK).
Mean Femoral Artery Pressure
Five mice per transgenic line and five non-transgenic littermate mice were used for blood pressure measurements. Placing mice in a closed chamber with 3% isoflurane induced anesthesia. Anesthesia was maintained by spontaneous inhalation of 30% O2 and 70% N2, which contained 2 to 3 vol% isoflurane via a nose cone. The mice were placed on a servo regulated heating pad to maintain rectal temperature at 37.5°C. A 27G needle was inserted into the right femoral artery. It was connected to a pressure transducer by a polyethylene catheter, re-used for all animals. Mean arterial pressure and the amplitude of the heart cycle pressure variations were recorded for 10 to 20 minutes until a stable recording was obtained. The average values of a 10-minute period were recorded.
Statistical Analysis
Groups of animals from each transgenic line were compared with non-transgenic littermates using the statistical nonparametric Mann-Whitney test. The results have been considered statistically significant when P was less than 0.05. The statistics were performed using StatView 4.5 software (SAS Institute, Inc., Cary, NC).
Results
Generation of Transgenic SMP8-SSAO Mice
Mouse smooth muscle α-actin promoter fragment SMP8 21 has previously been used for targeted overexpression in smooth muscle cells of transgenic mice, and was thus chosen for overexpression of human SSAO. In addition, the expression pattern with this promoter resembles that of human SSAO, which is expressed in both vascular and visceral smooth muscle cells. 2 The SMP8 region was amplified by PCR from mouse genomic DNA and cloned upstream of the complete coding sequence of human SSAO, previously amplified from human aorta cDNA. Fifteen founder mice were obtained from oocyte injection of the SMP8-SSAO construct depicted in Figure 1 ▶ . Founders were mated to C57B1/6 mice to establish stable lines. Out of these, 10 founders passed the SMP8-SSAO construct to the offspring and were further analyzed for mRNA expression.
Transgenic SSAO RNA Is Expressed in Smooth Muscle Cell-Rich Organs
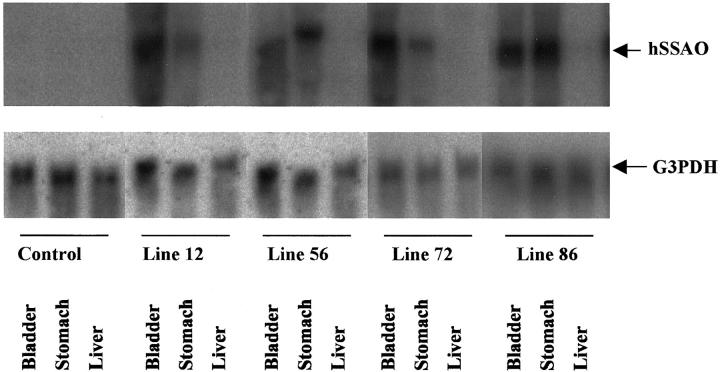
Northern blot analysis was performed to examine transgenic RNA expression. On sacrifice of 2-month-old SSAO transgenic mice and wild-type littermate controls, tissues were dissected for RNA analysis. Bladder and stomach were chosen as examples of smooth muscle cell-rich organs. In contrast, the liver is an organ with only minor contribution of smooth muscle and was therefore used as control tissue. As shown in Table 1 ▶ , SMP8-promoted SSAO RNA was expressed in nine lines in smooth muscle cell-rich organs, whereas liver and non-transgenic tissue were devoid of hybridization signal. Four transgenic lines were chosen for further characterization. Figure 2 ▶ shows that hybridization with a human SSAO cDNA probe resulted in a single band of the expected size (2.3 kb) detected in RNA from bladder and stomach but not from liver. Furthermore, there was no cross-hybridization to endogenous mouse SSAO; no hybridization could be seen in non-transgenic littermate mice using this probe.
Table 1.
Transgenic SSAO Expression in Transgenic and Non-Transgenic Littermate Controls at Two Months of Age
| SMC-SSAO transgenic line | RNA expression | ||
|---|---|---|---|
| Liver | Bladder | Stomach | |
| Non-Transgenic | − | − | − |
| 12 | − | + | + |
| 22 | − | − | − |
| 33 | − | + | + |
| 43 | − | + | + |
| 46 | − | + | + |
| 56 | − | + | + |
| 72 | − | + | + |
| 77 | − | + | + |
| 86 | − | + | + |
| 87 | − | + | + |
Figure 2.
RNA expression analysis of SSAO transgenic mice. Northern blot analysis of SMP8 driven SSAO expression in tissues from 2-month-old transgenic mice of lines 12, 56, 72, and 86. G3PDH hybridization of the membrane was used as a control of RNA loading.
Tissue Distribution of Transgenic SSAO RNA
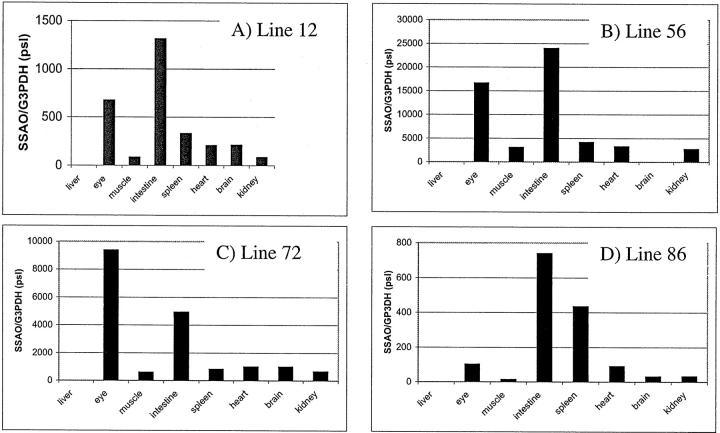
Northern blot analysis was performed as described above on RNA from a panel of tissues to examine the distribution and levels of transgenic SSAO expression in each of the four lines. The intactness and total amount of RNA were verified by hybridization to a cDNA probe for the glyceraldehyde phosphate dehydrogenase (G3PDH) gene. Filters were exposed to Phosphorimager screens and standardization of the SSAO RNA was obtained by scanning the hybridization signal. The ratio of SSAO to G3PDH was calculated using the absence of expression in the liver as a baseline. As shown in Figure 3 ▶ , the expression pattern for the four lines were similar. Apart from bladder and stomach, where robust expression of SMP8-driven SSAO RNA was detected as described above, the highest expression levels were found in the eye and the intestine. Moderate levels of transgenic SSAO were detected in spleen, heart, and brain. An exception was line 86, where there was an unexpectedly high level of transgenic expression in the spleen.
Figure 3.
Tissue distribution of transgenic SSAO RNA. Total RNA from a panel of organs from 2-month-old transgenic and control mice was analyzed by Northern blot analysis. The ratio (arbitrary pixel units) of SSAO RNA to G3PDH, a housekeeping gene, is depicted for line 12 (A), line 56 (B), line 72 (C), and line 86 (D).
SSAO Enzymatic Activity Is Increased in SMP8-SSAO Transgenic Mice
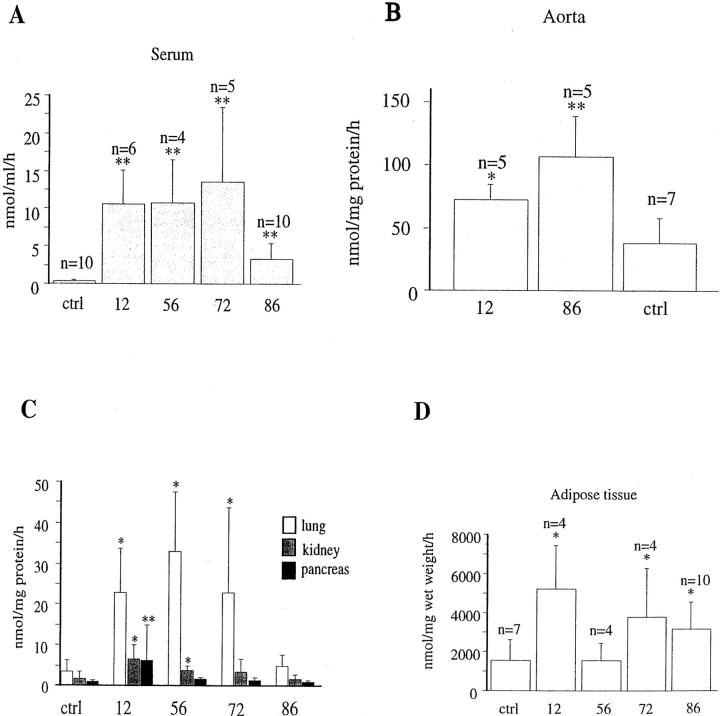
To determine whether the transgenic construct encoding SSAO RNA led to increased levels of SSAO activity, serum and tissue SSAO enzymatic activities were analyzed from mice between 2 and 4 months of age. Figure 4A ▶ shows that the enzymatic activity in serum of all four lines was increased several times above that of serum from littermate control mice. The SSAO activity varied between the different lines. Line 12, 56, and 72 displayed enzymatic activities of 15 to 26 nmol/ml/hour, while in line 86 the increase was less prominent, 4 nmol/ml/hour, as compared to 0.5 nmol/ml/hour for the control mice. Analyses of pancreas, lung, kidney, aorta, and adipose tissues, using the method described above, revealed SSAO activity above control levels in aorta, lung, and adipose tissue, and to a lesser extent also in kidney, whereas no increase was detected in extracts from the pancreas (Figure 4, B to D) ▶ . Data for aorta and adipose tissue is depicted in Figure 4, B and D ▶ , respectively, showing that levels of SSAO activity above non-transgenic littermates were detected in abdominal fat from lines 12, 72, and 86.
Figure 4.
SSAO enzymatic activity in SMP8-SSAO transgenic mouse lines. The enzymatic activity was measured in serum (A) or tissue homogenate from aorta, lung kidney, pancreas, and adipose tissue (B and C) of mice aged 3 to 4 months. The enzymatic activity in serum was elevated in all transgenic lines. B: Line 12, 56, and 72 displayed an increased activity in lung and line 12 and 56 also in kidney. Only line 12 had an elevated activity in pancreas. The SSAO activity in aorta (B) adipose tissue (D) and was higher in line 12 and 86 (** P < 0.01, * P < 0.05).
Phenotypic Characterization of SMP8-SSAO Transgenic Mice
The SSAO transgenic mice appeared essentially normal with no apparent signs of phenotypical alterations. The mice developed normally, were fertile, and produced normal-sized litters with the expected ratio of transgenic versus non-transgenic pups in the first filial (F1) generation. Blood glucose levels were found to be within a normal range, not differing from wild-type littermate control mice (not shown). The only overt phenotypical abnormality was observed in line 72. In this line, transgenic mice were smaller than their non-transgenic littermates and lost their fur in a patchy manner during the first few weeks of life. As this phenotype was not seen in any of the other eight lines, it was considered to be a result of the position of the insertion(s) of the construct into the mouse genome rather than a result of the transgenic expression.
Examination of Microvascular Changes
Acute vascular smooth muscle or endothelial cell injury was not evident in any of the transgenic mice examined. After 24 hours collection of urine, using metabolic cages, an assay for protein in urine was performed and no difference was detected between transgenic mice and their non-transgenic littermates (not shown). Tissue sections of the kidney, a highly vascularized organ, were prepared and stained with the Weigert technique. Additionally, sections from all paraffin blocks were stained with Van Gieson’s picric acid-fuchsine stain 27 for collagen and H&E as counterstain. On histological examination, a slight infiltration of mononuclear leukocytes was observed in the kidney. This may, however, be regarded as an incidental finding since its appearance was not consistent among the transgenic lines. Furthermore, histological examination of retina stained with H&E did not reveal any morphological differences between transgenic and control mice (not shown).
Disorganization of Elastin Architecture of SMP8-SSAO Transgenic Mice in Large Arteries
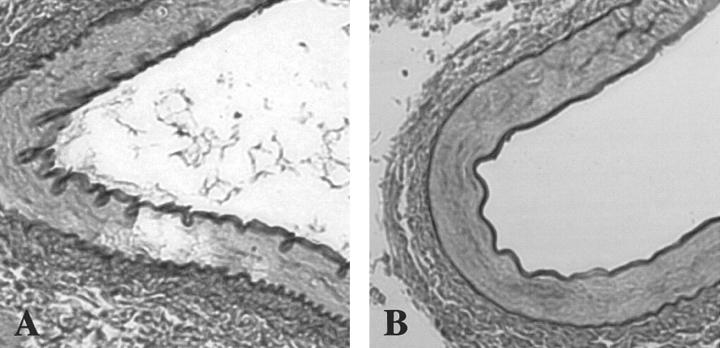
In contrast to the seemingly normal overall phenotype of the SMP8-SSAO transgenic mice, histological evaluation revealed changes at the cellular level. For examination of the consequences of SSAO overexpression in tissues, aorta, renal artery, lung, and kidney were selected. Elastin and collagen staining 27 of sections through paraffin-embedded arteries from transgenic and control mice were used. The aorta is the prime example of a large artery. As such, it contains higher quantities of elastin and collagen than other vessels. Regular folding of the elastica intima and externa in large elastic arteries and in the intercalating elastic laminae in tunica media of the aorta are normally seen in fixed histological sections (Figure 5, A and B) ▶ , and is a result of the absence of blood pressure and contraction of the smooth muscle fibers in blood vessels at death. The SSAO transgenic mice displayed a different morphology. As shown in Figure 5, C and D ▶ , an aorta cross-section from a 4-month-old mouse of line 12 shows profoundly stretched unfolded elastic laminas which suggests an atonic aorta. This phenotype was seen also in older mice (8 to 12 months) of this line and was present in line 56 and 86, where mice at ages 4 and 8 months displayed the same morphology (not shown). This phenotype was never present in wild-type littermate control mice.
Figure 5.
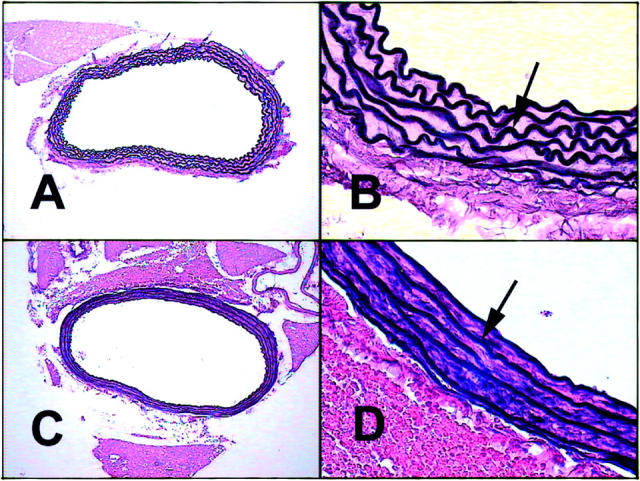
Histopathological evaluation of aortas from transgenic mice expressing human SSAO in smooth muscle cells. Cross-sections through aortas at the thoracic proximal level were stained with the Weigert technique visualizing elastin fibers in black. A: Normal aorta of a non-transgenic littermate control mouse displaying undulated elastic laminas, photographed at ×10 magnification. B: Detail of A showing normal longitudinal folds and wavy elastic laminas (arrow), magnification ×60. C: SMP8-SSAO transgenic mouse of line 12. Aorta lacking the longitudinal folds displays straight elastic laminas photographed at ×10 magnification. D: Detail of C, showing straight elastic laminas at ×60 magnification.
To analyze the renal artery, mice from two transgenic lines (12 and 86) were compared to non-transgenic littermates. A complete undulated appearance, normally seen in tunica intima and externa of arteries, was present in renal artery of control mice (Figure 6A) ▶ . In contrast, the vast majority of transgenic mice (10 of 12) exhibited the same straight unfolded phenotype of the elastic laminas in the renal artery (Figure 6B) ▶ as seen in the aorta. The transgenic lung, despite a large content of elastic tissue, did not exhibit any morphological changes in any of the lines.
Figure 6.
Histopathological evaluation of renal artery from transgenic mice expressing human SSAO in smooth muscle cells. Cross-sections through renal artery were stained with the Weigert technique showing elastin in black. A: The renal artery displaying undulated internal and external elastic laminas from a non-transgenic littermate, with symmetrically arranged elastic fibers in tunica media. B: SMP8-SSAO transgenic mouse of line 12 displaying the same straight phenotype of the elastic laminas as seen in aorta. Both images are photographed at ×60 magnification.
To quantify the changes of elastic fibers, its thickness was measured and the numbers of laminae were counted. The number of elastic laminae was 5–7, and did not differ between transgenic mice and non-transgenic littermates. Line 86 had thicker elastic laminae (3.08 ± 0.1 μm) in comparison with non-transgenic littermates (2.64 ± 0.07 μm). Transgenic mice in line 12, however, did not significantly differ in lamina thickness from control mice (2.34 ± 0.08 μm).
Mean Femoral Artery Pressure
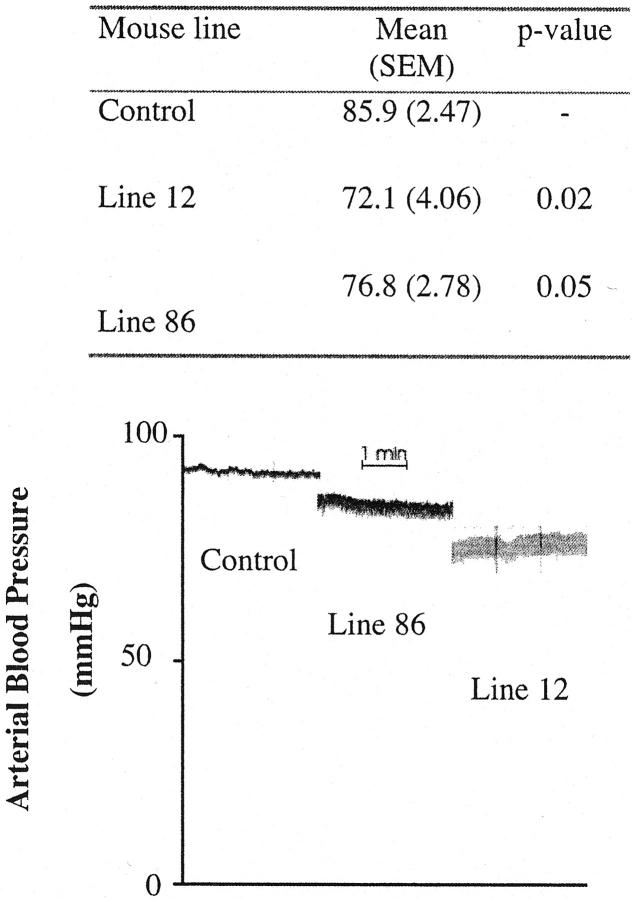
We next performed in vivo studies and measured the femoral artery pressure of anesthetized mice to determine the relevance of the structural changes of elastin fibers described above. When adult transgenic mice (2 to 4 months) of two lines were compared to non-transgenic littermates they were found to have a significantly lower mean arterial pressure (Figure 7) ▶ . For transgenic mice of line 12, the arterial pressure was 84% of the control mice (P < 0.02) and for line 86 the mean pressure mounted to 89% (P < 0.05). From Figure 7 ▶ it can also be seen that the pressure range between systolic and diastolic level is greater in both the transgenic lines than in control mice. While no absolute values of the arterial pulse pressure may be given by this analysis, the relative increase was threefold higher for line 12 and twofold higher for line 86 when compared to non-transgenic littermates.
Figure 7.
Blood pressure measurement of SSAO transgenic mice. Mean arterial blood pressure was measured in the femoral artery in two transgenic lines (line 12 and 86) and in non-transgenic littermates (n = 5). The blood pressure was significantly lower in both transgenic lines (72.1, P = 0.02 and 76.8, P = 0.05, respectively), in comparison to non-transgenic littermates (85.9). The increase in pressure range (an estimate of the pulse pressure) is indicated by the recordings of arterial blood pressure (bottom).
Discussion
We have developed a transgenic model for overexpression of human SSAO. The transgenic construct was successfully transferred to nine mouse lines and out of these, four representative lines were chosen for further characterization. Human SSAO mRNA levels were detected in all smooth muscle-rich tissues investigated, with a corresponding increase of catalytic SSAO activity. The transgenic overexpression did not appear to severely affect the general condition, reproducibility, or life span of the animals. Examination of the retinal vascular bed, urinary protein content, and the kidney microvascular system did not reveal overall pathological changes.
One primary finding in the SSAO transgenic mice is the abnormal changes observed in the elastic fibers of tunica media in the aorta and the renal artery. Normally, when mice are sacrificed, the elastic wall of the blood vessels, ie, the elastic laminae in the tunica media, is folded due to contraction of the vessel wall in the absence of blood pressure.
In the transgenic mice, the lack of longitudinal folds in tunica media of aorta and elastica intima of the renal artery was striking. There is also a severe alteration in the structure of the elastic fibers that normally are arranged in a symmetrical pattern in the smooth muscle layer between the elastic laminas of larger arteries. These findings indicate a reduced elasticity in arteries of mice overexpressing SSAO, as well as an involvement of SSAO in the organization of the elastic fibers.
The abnormal elastic fiber morphology suggests a stiff vessel wall with reduced elasticity, which could result in a higher pulse pressure and also might be an underlying reason for a dysfunction in regulation of the blood pressure. Our data showed that the mean femoral artery pressure was significantly lower in the transgenic mice in comparison to non-transgenic littermates. The results of measurements of the arterial blood pressure also indicated an elevated pulse pressure between transgenic mice and non-transgenic littermates. The stretch receptors are crucial in regulating blood pressure. Stretch receptors are located in the arch of the aorta and it is possible that the baroreceptor reflex is involved in the maintenance of the lowered arterial pressure. In normal individuals, reduced aortic compliance, together with increased pulse pressure, usually means increased aortic and arterial pressure as well, which will result in an increased firing rate of the baroreceptors to decrease the blood pressure. A stiff and rigid aorta, which seems to be a characteristic of SSAO transgenic mice, could explain the lowered arterial pressure. However, it is known that baroreceptors do adapt to a sustained elevated blood pressure within a couple of days, and the increased firing rate as a consequence of high pressure will eventually disappear. Another possibility may therefore be that the transgenic mice are not able to regulate the pressure in the same manner as non-transgenic littermates.
The cellular source of elastic fibers of large arteries are the smooth muscle cells in tunica media, the same cells that overexpress human SSAO in this model. The abnormal elastin architecture in aorta and renal artery of transgenic animals may suggest an involvement of SSAO in the arrangement of elastin in blood vessels. The enzyme lysyl oxidase is known to induce cross-linking of elastin and collagen in connective tissue and it has been speculated that SSAO could have similar properties. 28 Excessive SSAO activity could, by such a mechanism, increase the cross-linking of elastin and thereby change the tertiary structure of the newly synthesized elastin resulting in an atypical morphology, which in turn may lead to a reduced elasticity in blood vessels. Another possible explanation could be that an increased rate of metabolism of methylamine and aminoacetone could generate higher levels of the cytotoxic compounds formaldehyde and methylglyoxal, which in turn could form aldehyde adducts in vascular tissues. 17,29
The source of the soluble form of SSAO found in human plasma is unknown. It has been speculated that plasma SSAO originates from a different gene, or that it is proteolytically cleaved from the membrane-bound form. In plasma from the transgenic mice, we found an elevated SSAO activity, whereas activity in control mice was barely detectable. Our findings might support the hypothesis 30 that SSAO is released into the circulation by proteolytic cleavage near the transmembrane region (shedding), a process which is common for type I and type II membrane proteins. 31 The mechanism by which the soluble form of SSAO enters the circulation still needs to be elucidated and we now might have a tool to specifically examine the transport pathways of SSAO.
An unexpected finding in the present study was the high catalytic activity of SSAO in adipose tissue in transgenic mice from two separate lines generated from different founder animals. Adipose tissue exhibits high SSAO protein content and catalytic activity in wild-type mice 32 and in primary cultures of adipose cells. 5 However, increased SSAO activity in adipose tissue in transgenic mice was not expected since the α-actin SMP8 promoter is smooth muscle-specific and adipose tissue is less vascularized than other tissues. The possibility that plasma SSAO protein, by some yet unknown mechanism, accumulates in adipose tissue will have to be further investigated.
There are several pathological conditions, such as diabetes mellitus, chronic liver disease, and congestive heart failure, where an increased SSAO activity in serum has been observed and it has been suggested that SSAO contributes to the development of vascular disorders, particularly in connection with diabetes. 15,18,33 Human SSAO activity in serum is normally approximately 10-fold higher compared to the activity in serum from mice. The levels of circulating SSAO in the transgenic mice correlates to those reported for human pathologies with an increased SSAO activity. Therefore, with this mouse model we will be able to study the possible involvement of high SSAO levels in the development of vasculopathies. The present transgenic mouse model will also allow us to investigate the effects of SSAO inhibition and may be used for the development of specific inhibitors of human SSAO.
Acknowledgments
We thank Kajsa Axberg for assistance with maintenance of the mouse colony, Ladan Aryan for technical assistance, and Drs. Patrizia Caldirola and Lars Björk for valuable discussions. Dr. Ricardo Feinstein is acknowledged for help with phenotype analysis, and Britta Isaksson for excellent assistance with blood pressure analysis.
Footnotes
Address reprint requests to Karin Forsberg-Nilsson, Department of Medical Biochemistry and Microbiology, Uppsala University, Box 593, Biomedicum, 751 24 Uppsala, Sweden. E-mail: karin.nilsson@imbim.uu.se.
Supported by a grant from the Swedish Medical Research Council (number 4145).
Present address of K. S.: Leica Microsystems AB, 191 27 Sollentuna, Sweden.
Present address of H. G.: Biovitrum AB, 112 76 Stockholm, Sweden.
Present address of K. F.-N.: Department of Medical Biochemistry and Microbiology, Uppsala University, Box 593, Biomedicum, 751 24 Uppsala, Sweden.
References
- 1.Callingham BA, Barraud M: Some properties of semicarbazide-sensitive amine oxidases. J Neurol Transm 1987, 23:37-54 [DOI] [PubMed] [Google Scholar]
- 2.Lewinsohn R: Mammalian monoamine-oxidizing enzymes, with special reference to benzylamine oxidase in human tissues. Braz J Med Biol Res 1984, 17:223-256 [PubMed] [Google Scholar]
- 3.Enrique-Tarancon G, Marti L, Morin N, Lizcano JM, Unzeta M, Sevilla L, Camps M, Palacin M, Testar X, Carpene C, Zorzano A: Role of semicarbazide-sensitive amine oxidase on glucose transport and GLUT4 recruitment to the cell surface in adipose cells. J Biol Chem 1998, 273:8025-8032 [DOI] [PubMed] [Google Scholar]
- 4.El Hadri K, Moldes M, Mercier N, Andreani M, Pairault J, Feve B: Semicarbazide-sensitive amine oxidase in vascular smooth muscle cells: differentiation-dependent expression and role in glucose uptake. Aterioscler Thromb Vasc Biol 2002, 22:89-94 [DOI] [PubMed] [Google Scholar]
- 5.Moldes M, Feve B, Pairault J: Molecular cloning of a major mRNA species in murin 3T3 adipocyte lineage: differentiation-dependent expression, regulation, and identification as semicarbazide-sensitive amine oxidase. J Biol Chem 1999, 274:9515-9523 [DOI] [PubMed] [Google Scholar]
- 6.Salmi M, Kalimo K, Jalkanen S: Induction and function of vascular adhesion protein-1 at sites of inflammation. J Exp Med , 178:2255-2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jalkanen S, Salmi M: Cell surface monoamine oxidses: enzymes in search of a function. EMBO J 2001, 20:3893-3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klinman JP, Mu D: Quinoenzymes in biology. Annu Rev Biochem 1994, 63:299-344 [DOI] [PubMed] [Google Scholar]
- 9.Thormalley P, Langborg A, Minhas H: Formation of glyoxal, methylglyoxal, and 3-DG in the glycation of proteins. Biochem J 1999, 344:109-116 [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsson S, Tryding N, Tufvesson G: Serum monoamine oxidase (MAO) in diabetes mellitus and some other internal diseases. Acta Med Scand 1968, 184:105-108 [DOI] [PubMed] [Google Scholar]
- 11.Boomsma F, van den Meiracker AH, Winkel S, Aanstoot HJ, Batstra MR, Man in’t Veld AJ, Bruining GJ: Circulating semicarbazide-sensitive amine oxidase is raised both in type I (insulin-dependent), in type II (non-insulin-dependent) diabetes mellitus and even in childhood type I diabetes at first clinical diagnosis. Diabetologia 1999, 42:233-237 [DOI] [PubMed] [Google Scholar]
- 12.McEwen CM, Harrison DC: Abnormalities of serum monoamine oxidase (MAO) in chronic congestive heart failure. J Lab Clin Med 1965, 65:546-559 [PubMed] [Google Scholar]
- 13.Boomsma F, van Veldhuisen DJ, de Kam PJ, Man in’t Veld AJ, Mosterd A, Lie KI, Schalekamp MA: Plasma semicarbazide-sensitive amine oxidase is elevated in patients with congestive heart failure. Cardiovasc Res 1997, 33:387-391 [DOI] [PubMed] [Google Scholar]
- 14.McEwen CMJ, Castell DO: Abnormalities of serum monoamine oxidase in chronic liver disease. J Lab Clin Med 1967, 70:36-47 [PubMed] [Google Scholar]
- 15.Meszaros Z, Karadi I, Csanyi A, Szombathy T, Romics L, Magyar K: Determination of human serum semicarbazide-sensitive amine oxidase activity: a possible clinical marker of atherosclerosis. Eur J Drug Metab Pharmacokinet 1999, 24:299-302 [DOI] [PubMed] [Google Scholar]
- 16.Yu P, Zuo D-M: Oxidative deamination of methylamine by semicarbazide-sensitive amine oxidase leads to cytotoxic damage in endothelial cells. Possible consequences for diabetes. Diabetes 1993, 42:594-603 [DOI] [PubMed] [Google Scholar]
- 17.Gronvall JL, Garpenstrand H, Oreland L, Ekblom J: Autoradiographic imaging of formaldehyde adducts in mice: possible relevance for vascular damage in diabetes. Life Sci 1998, 63:759-768 [DOI] [PubMed] [Google Scholar]
- 18.Garpenstrand H, Ekblom J, Backlund LB, Oreland L, Rosenqvist U: Elevated plasma semicarbazide-sensitive amine oxidase (SSAO) activity in type 2 diabetes mellitus complicated by retinopathy. Diabet Med 1999, 16:514-521 [DOI] [PubMed] [Google Scholar]
- 19.Gronvall-Nordquist JL, Backlund LB, Garpenstrand H, Ekblom J, Landin B, Yu PH, Oreland L, Rosenqvist U: Follow-up of plasma semicarbazide-sensitive amine oxidase activity and retinopathy in type 2 diabetes mellitus. J Diabetes Complications 2001, 15:250-256 [DOI] [PubMed] [Google Scholar]
- 20.Boomsma F: Plasma SSAO activity is elevated in diabetes mellitus and correlates with glycosylated haemoglobin. Clinical Science 1995, 88:675-679 [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Niu W, Nikiforov Y, Naito S, Chernausek S, Witte D, LeRoith D, Strauch A, Fagin JA: Targeted overexpression of IGF-1 evokes distinct patterns of organ remodeling in smooth muscle cell tissue beds of transgenic mice. J Clin Invest 1997, 100:1425-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McHugh KM, Crawford K, Lessard JL: A comprehensive analysis of the developmental and tissue-specific expression of the isoactin multigene family in the rat. Dev Biol 1991, 148:442-458 [DOI] [PubMed] [Google Scholar]
- 23.Hogan B, Beddington R, Costantini F, Lacy E: Manipulating the Mouse Embryo: A Laboratory Manual. 2nd ed. 1994:pp 226-250 Cold Spring Harbor Press New York
- 24.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 25.McCaman RE: Microdetermination of catechol-O-methyl transferase in brain. Life Sci 1965, 4:2353-2359 [DOI] [PubMed] [Google Scholar]
- 26.Lowry O, Roesbrough N, Farr A, Randall R: Protein measurement with the Folin phenol reagent. J Biol Chem 1951, 193:265-275 [PubMed] [Google Scholar]
- 27.Mallory FB: Pathological Techniques. 1961. Hafner Publishing Co New York
- 28.Langford SD, Trent MB, Balakumaran A, Boor PJ: Developmental vasculotoxicity associated with inhibition of semicarbazide-sensitive amine oxidase. Toxicol Appl Pharmacol 1999, 155:237-244 [DOI] [PubMed] [Google Scholar]
- 29.Thornalley PJ: Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification: a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol 1996, 27:565-573 [DOI] [PubMed] [Google Scholar]
- 30.Kurkijarvi R, Adams DH, Leino R, Mottonen T, Jalkanen S, Salmi M: Circulating form of human vascular adhesion protein-1 (VAP-1): increased serum levels in inflammatory liver diseases. J Immunol 1998, 161:1549-1557 [PubMed] [Google Scholar]
- 31.Hooper NM, Karran EH, Turner AJ: Membrane protein secretases. Biochem J 1997, 321:265-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raimondi L, Pirisino R, Banchelli G, Ignesti G, Conforti L, Romanelli E, Buffoni F: Further studies on semicarbazide-sensitive amine oxidase activities (SSAO) of white adipose tissue. Comp Biochem Physiol B 1992, 102:953-960 [DOI] [PubMed] [Google Scholar]
- 33.Boomsma F, de Kam PJ, Tjeerdsma G, van den Meiracker AH, van Veldhuisen DJ: Plasma semicarbazide-sensitive amine oxidase (SSAO) is an independent prognostic marker for mortality in chronic heart failure. Eur Heart J 2000, 22:8519-8563 [DOI] [PubMed] [Google Scholar]