Abstract
Preterm birth is a leading cause of neonatal morbidity and mortality. Despite a growing body of evidence correlating inflammation with preterm birth, the signal transduction pathways responsible for the emptying of the uterus in the setting of intrauterine inflammation has not been elucidated. We now report a unique, reproducible mouse model of localized intrauterine inflammation. This model results in 100% preterm delivery with no maternal mortality. Using our model, we also show that platelet-activating factor is a crucial mediator of both inflammation-induced preterm birth and fetal demise. Using C3H/HeJ mice, we demonstrate that toll-like receptor-4 (TLR-4) plays a role in lipopolysaccharide-induced preterm birth but not in inflammation-induced fetal death. Immunohistochemistry studies demonstrate the presence of the platelet-activating factor receptor in both endometrial glands and smooth muscle in uterine tissues. Molecular studies demonstrate the differential expression of platelet-activating factor receptor and TLR-4 in uterine and cervical tissue throughout gestation. Quantitative polymerase chain reaction revealed an up-regulation of TLR-4 in the fundal region of the uterus in response to intrauterine inflammation. The use of this model will increase our understanding of the significant clinical problem of inflammation-induced preterm birth and will elucidate signal transduction pathways involved in an inflammatory state.
Preterm delivery is a leading cause of neonatal morbidity and mortality. In the last decade, there has been a growing body of evidence correlating preterm birth with inflammation and/or infection in the uterus. While most women with preterm labor will not demonstrate signs of systemic illness or inflammation, greater than 85% of pregnancies delivered at less than 28 weeks gestation will have evidence of localized inflammation as evidenced by the presence of histological chorioamnionitis. 1 While the presence of an inflammatory response is evident in many patients with preterm labor, an active infectious process is not apparent as amniotic fluid cultures are usually negative in these patients. 2,3 Further confirming the lack of an active infectious process in most cases of preterm labor is that antibiotic therapy in animal or human trials has not been proven to significantly improve outcome. 4-6 Despite a growing association between inflammation and preterm birth, the mechanism by which intrauterine inflammation results in the emptying of the uterus remains unclear.
Platelet-activating factor (PAF) may be an important mediator in the signal transduction pathways leading to inflammation-induced preterm birth. PAF, 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine, is a potent phospholipid mediator with diverse biological properties. 7,8 The majority of PAF is synthesized from glycerophosphocholins, but it can also be synthesized via a “remodeling” pathway that involves cytosolic phospholipase A2. 8 PAF is degraded by PAF-acetylhydrolase (PAF-AH). 9,10 Mediators of inflammation decrease levels and transcription of PAF-AH. 8,10 PAF elicits its diverse effects through activation of a G-protein coupled receptor (the PAF receptor PAFR). 7,11 Transgenic and knockout mice for the PAF receptor have provided essential information as to the crucial roles of PAF. Studies with PAFR-/- mice have demonstrated that PAF is a direct mediator of circulatory collapse and activation of polymorphonuclear cells (PMNs), while PAFR transgenic mice are more sensitive to lipopolysaccharide (LPS)-induced shock. 12,13
PAF has been implicated in diverse pathophysiologic events, including roles in sepsis, pancreatitis, and necrotizing enterocolitis. 14-18 The proinflammatory properties of PAF are also evident during parturition. PAF stimulates cytokine production and infiltration of PMNs in the cervix at time of parturition. 19-21 Cytokines and endotoxin can decrease the release of PAF-AH from uterine decidual cells. 22,23 Further evidence implicating PAF in parturition is noted by decreased systemic levels of PAF-AH and increased levels of PAF in animals as parturition occurs. 24,25 Unlike other inflammatory mediators (such as cytokines or LPS itself), PAF is a direct uterotonic agent. 26-30 Finally, PAF has also been implicated directly in preterm parturition because the phospholipid is elevated in the amniotic fluid of patients with preterm labor who ultimately deliver preterm. 31,32
Because PAF can be produced as a result of an infectious or inflammatory stimulus, it is important to understand the role of bacterial products in the signal transduction pathways leading to the production of PAF in a model of localized intrauterine inflammation. In recent years, toll-like receptor-4 (TLR-4) has been discovered as an innate immune recognition receptor essential to LPS signaling. 33,34 Gram-negative bacterial cell wall components activate proinflammatory signal transduction pathways on stimulation of TLR-4. 35,36 Perhaps in an effort to augment the inflammatory response, LPS appears to up-regulate TLR-4 expression. 37 The expression and regulation of TLR-4 in gestational tissues and their roles in LPS-induced preterm birth have not been elucidated.
In an attempt to increase our understanding of the clinical problem of preterm birth, animal models have been created. Many of these models involve the systemic administration of an inflammatory agent. 38,39 While these manipulations provoke preterm delivery, they also produce maternal morbidity and even mortality, unlike the most common clinical situation. Therefore, these animal models more closely mimic preterm birth in the setting of maternal sepsis, pyelonephritis, or overwhelming pneumonia. Different models are needed to more closely approximate localized intrauterine inflammation to investigate the signal transduction pathways essential to inflammation-induced preterm birth.
The first aim of this study was to develop a reproducible mouse model of localized inflammation that is not associated with maternal mortality. The second aim was to determine whether PAF is a crucial mediator of both inflammation-induced preterm delivery and fetal death. The third aim was to determine whether the TLR-4 receptor was necessary for LPS-induced preterm delivery. The fourth aim was to characterize the expression of PAFR and TLR-4 in both the uterus and cervix of the pregnant mouse.
Materials and Methods
Animals
CD-1 outbred, timed-pregnant mice were purchased from Charles River Laboratories (Wilmington, MA). Timed-pregnant CH3/HeJ (endotoxin-resistant, TLR-4 mutants) were obtained from The Jackson Laboratory (Bar Harbor, ME). Animals were shipped on day 8–12 after mating. Animals were acclimated in our facility for 3 to 7 days before use in these experiments. All of the experiments were performed in accordance with the National Institutes of Health guidelines on laboratory animals and with approval from the committees on Animal Use and Care from the University of Chicago and the University of Pennsylvania.
Mouse Model of Localized Intrauterine Inflammation
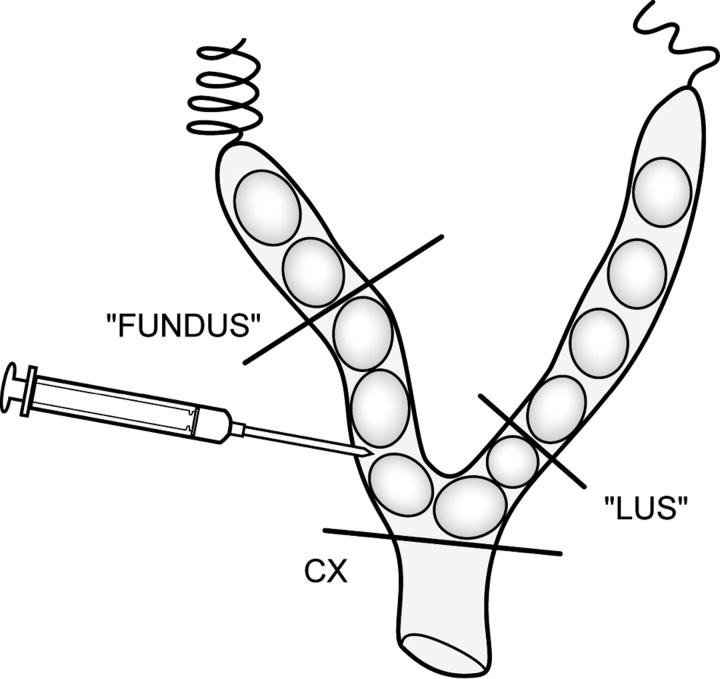
Surgery was performed on day 15 of gestation, which is 79% of the CD-1 gestation. CD-1 animals normally deliver pups on day 19 to 20 of gestation. Isoflurane anesthesia was used with an induction chamber (Vet Equip, Pleasanton, CA). Continuous isoflurane/oxygen anesthesia was supplied by a mask that fits over the mouse’s face. After deep anesthesia was reached, a minilaporatory was performed in the lower abdomen. The right uterine horn was exposed through the incision to allow visualization of the first two gestational sacs (those most proximal to the cervix). LPS (250 μg/mouse) (Sigma, St. Louis, MO) was then infused into the uterus between the lower two gestational sacs with care not to enter the amniotic cavity (n = 16) (Figure 1) ▶ . Sterile saline was then applied to the exposed uterus, after which the uterus was returned to the abdomen. The fascia was closed with a continuous vicryl suture and the skin was closed with staples (Autoclips; Clay Adams, from Fisher Scientific). The entire procedure varied between 2 and 5 minutes per mouse. The animals recovered in individual cages and the majority of animals were moving within 10 minutes of completion of the procedure. Control animals received no anesthesia and no intrauterine infusion while sham animals received anesthesia and intrauterine saline. Animals were observed closely for any signs of morbidity (piloerection, decreased movement), vaginal bleeding, and/or preterm delivery (pups present in the cage).
Figure 1.
A schematic representation of the bicornuate mouse uterus with gestational sacs in each horn. For the model, LPS was infused into the lower right uterine horn. For the molecular studies, the uterus was divided into three regions: fundus, LUS, and cervix (CX).
For studies with CD-1 mice, we used LPS from Escherichia coli (L2280), which was phenol-extracted and had <3% endotoxin protein by the Lowry method (Sigma). For studies with TLR-4 mutant mice, we used two different preparations of LPS: L2880 and L4525, which is purified by phenol extraction by ion-exchange chromatography and had only 0.2% protein by the Lowry method (Sigma), thereby eliminating the possibility of contamination with other endotoxin proteins.
PAF as a Mediator of Inflammation-Induced Preterm Birth
To determine whether a PAF antagonist could prevent inflammation-induced preterm birth, CV-6209 (Biomol, Plymouth Meeting, PA) was administered intraperitoneally 1 hour before intrauterine LPS administration in various doses (3 to 6 mg/kg). CV-6209 is a PAF antagonist that is structurally similar to PAF and acts as a competitive antagonist.
To investigate if PAF alone could induce preterm parturition, a stable PAF analog (mcPAF; Sigma) was administered into the uterine lumen. Using the techniques as previously described with infusion of intrauterine LPS, we infused 10 to 40 μg/mouse of PAF into the uterine lumen (n = 9). All animals were observed for any signs of maternal morbidity or mortality.
Documentation of Preterm Delivery and Fetal Viability
All animals were observed for 48 hours after intrauterine infusion with LPS or saline. At 48 hours, all animals were placed under deep anesthesia and laparotomy was performed. The uterine horns were opened. Preterm delivery was defined by delivery of at least one pup before this time point and empty uterine horns at repeat laparotomy. At the time of laparotomy, 48 hours after intrauterine infusion, the number of live and dead pups in each horn was recorded. Intrauterine fetal deaths (IUFDs) were identified by white discoloration, markedly smaller fetal size, and lack of blood flow in the umbilical cord. Mothers were kept alive during the exploration of the uterine horns and documentation of live pups to avoid confounding effects caused by maternal death.
Expression and Localization of PAFR and Expression and Regulation of TLR-4
Quantitative PCR
To quantify the expression of PAFR and TLR-4 throughout gestation, real-time quantitative polymerase chain reaction (PCR) experiments were performed. RNA was harvested using trizol and cDNA was generated using random hexamers. Primers were designed using the Primer Express 1.5 software (PE Applied Biosystems, Foster City, CA). The primer set was empirically tested to determine the maximal concentration of primers that could be used to produce specific amplification of the target sequence in the absence of primer dimer amplification. Quantitative PCR reactions were carried out using equivalent dilutions of each cDNA sample, the fluorescent indicator SYBR green, an empirically determined concentration of each primer and the Applied Biosystems Model 7900 sequence detector PCR machine (PE Applied Biosystems). To verify that only a single PCR product was generated for the amplified transcript, the multicomponent data for each sample was subsequently analyzed using the Dissociation Curves 1.0 program (PE Applied Biosystems). To account for differences in starting material, quantitative PCR was also carried out for each cDNA sample using primers to mouse 18S RNA. These quantitative PCR reactions defined a threshold cycle (Ct) of detection for the target and 18S in each cDNA sample. An arbitrary value of template was assigned to the highest standard and corresponding values to the subsequent dilutions and these relative values were plotted against the Ct value determined for each dilution to generate a standard curve. The relative abundance of the target was divided by the relative abundance of 18S in each sample to generate a standardized abundance for the target transcript. All samples were analyzed in duplicate. The average for each duplicate was obtained. At least three samples were evaluated for each study group. Statistical analysis was performed by one-way analysis of variance and pair-wise comparison by Student-Newman-Keuls (SNK) method. P < 0.05 was considered statistically significant.
Differential expression of contraction-associated proteins between the upper and lower segments of the uterus has been demonstrated. 40 Therefore, to investigate whether PAFR and/or TLR-4 are differentially regulated based on uterine location, we harvested uterine tissue from the fundus and lower uterine segment (LUS) (Figure 1) ▶ . Fundal tissue was defined as the uterine tissue surrounding the two uppermost gestational sacs adjacent to the fallopian tube. LUS tissue was the uterine tissue surrounding the two most distal gestational sacs adjacent to the cervix. The cervix was dissected from the uterus and vagina and debrided of adipose tissue.
We investigated the expression of these proteins in the cervices and uteri from animals on day 15 of gestation (which is 79% of a CD-1 mice gestation), day 15 of gestation 6 hours after intrauterine LPS, day 19 of gestation (which is considered term gestation), postpartum (PP) and in the non-pregnant (NP) animal. For each treatment group, uterine and cervical tissue was harvested from 3 to 6 dams to control for biological variability. The LUS and fundus were taken from the right uterine horn.
Immunohistochemistry
To determine the location and expression of PAFR in the gravid uterus and cervix, tissues were harvested from day 15 control mice, flash frozen, and then prepared for immunohistochemistry studies. Tissues were embedded in paraffin and then transverse sections were obtained across the lumen. Immunohistochemistry was performed using the Vectastain Elite ABC kit (Vector Laboratories, Burlington, CA). Currently, commercially available antibodies to the PAF receptor are not useful for studies with mouse tissue. We developed a polyclonal antibody raised to the mouse PAFR (Genemed Synthesis, San Francisco, CA). Uterine sections were also taken from uteri of pregnant PAFR-/- mice (a generous gift from Professors Shimizu and Ishii, University of Tokyo) to confirm the specificity of our antibody. 12 Immunohistochemistry was performed with this antibody at a dilution of 1:200.
Western Blots
To determine the protein expression of PAFR and TLR-4 protein, Western blot analyses was performed using tissue homogenates from NP, day 15 and day 19 pregnant uteri from CD-1 mice. Uterine tissue was removed with the animal under anesthesia. The tissues were washed in sterile saline, flash frozen, and stored at −70 until protein was harvested. For the PAFR studies, uterine tissues from pregnant PAFR-/- mice were also used to confirm the validity of our antibody. LUS and fundal tissues were homogenized in protein extract buffer (Pierce Biotechnology, Inc., Rockford, IL). The amount of crude protein present in each sample was determined using the BCA protein assay (Pierce Biotechnology, Inc., Rockford, IL). Forty micrograms of protein were mixed with 2X sodium dodecyl sulfate (SDS) sample buffer and subjected to SDS-polyacrylamide gel electrophoresis. The separated proteins were transferred electrophoretically to polyvinylidene difluoride membranes. Membranes were blocked in Tris-buffered saline with 5% nonfat dried milk powder for 2 hours. For the TLR-4 studies, a polyclonal antibody to the mouse TLR-4 was used (sc 12511; Santa Cruz Biotechnology, Santa Cruz, CA) in a dilution of 1:200 for 4 hours. For PAFR studies, Western blots were performed using the PAFR antibody at a 1:200 dilution and incubated overnight. Blots were developed by the enhanced chemifluoresence (ECF) system (Amersham Biosciences). Protein expression for each blot was quantified using the NIH imaging software.
Results
Intrauterine Inflammation Results in Preterm Delivery
With our model of intrauterine infusion of LPS, we were able to cause a 100% preterm delivery rate within 24 hours. All animals delivered non-viable pups (as is consistent with E15–16 of gestation) within 20 hours of intrauterine LPS administration. Forty-eight hours after intrauterine LPS, there were no remaining live pups. These results were consistent in repetitive trials with all animals receiving intrauterine LPS delivering preterm (n = 16). We observed no maternal mortality in this model. Maternal morbidity, while a subjective parameter, was minimal with only a small number of animals demonstrating piloerection and lethargy before delivery of pups.
We investigated whether LPS induced preterm birth via the activation of TLR-4. Using the same amount and type of LPS (L2880) as used with CD-1 mice, we observed a 40% preterm delivery rate (n = 10) in the C3H/HEJ, TLR-4 mutant mice. All of the C3H/HEJ animals which did not deliver preterm had at least one IUFD in the uterine horns 48 hours after intrauterine LPS treatment. Because commercial preparations of LPS contain sufficient endotoxin protein to activate other inflammatory pathways not involving TLR4, we also performed these studies with enhanced purified LPS (L4524). 35 None of the C3H/HEJ animals delivered preterm, but 66% of animals had at least one IUFD in the uterine horns 48 hours after intrauterine LPS (n = 3) (Table 1) ▶ .
Table 1.
Preterm Parturition and Fetal Viability Based on Mouse Strain and Treatment
| Mouse strain | Treatment(s) | n | Preterm delivery rate (%) | Mean number of live pups per undelivered dam at 48 hours | Mean number of IUFDS per undelivered dam at 48 hours |
|---|---|---|---|---|---|
| CD-1 | Saline | 8 | 0/8 (0) | 11.5 ± 1.9 | 0.2 ± 0.4 |
| CD-1 | LPS (L2880) 250 μg/mouse | 16 | 16/16 (100) | 0 | NA* |
| CD-1 | PAF (10–40 μg/mouse) | 9 | 4/9 (44) | 7.0 ± 1.4 | 5.5 ± 3.5 |
| CD-1 | LPS (L2880) 250 μg/mouse + PAF antagonists 3–6 mg/kg) | 24 | 10/24 (58) | 6.7 ± 3.2 | 4.9 ± 2.8 |
| C3H/HEJ | LPS (L2880) 250 μg/mouse | 10 | 4/10 (40) | 3.0 ± 3.7 | 2.2 ± 1.0 |
| C3H/HEJ | LPS (L4524) 250 μg/mouse | 3 | 0/3 (0) | 6.3 ± 1.5 | 1.7 ± 1.2 |
*Since all animals receiving LPS alone delivered preterm, there were no remaining live or dead pups in the uterine horns at 48 hours.
A PAF Antagonist Prevents Inflammation-Induced Preterm Delivery and Fetal Demise
Fifty-eight percent of all animals pretreated with the PAF antagonist, CV-6209, before intrauterine LPS (n = 24) delivered preterm. Therefore, a PAF antagonist was able to significantly decrease the incidence of preterm delivery compared to LPS alone (P = 0.003; Fisher’s exact test). To determine whether there was a relationship between the dose of the PAF antagonist and the occurrence of preterm delivery, we analyzed the effect of different doses of CV-6209 on LPS-induced preterm birth. As Table 2 ▶ demonstrates, the results suggest a relationship between the dose of the PAF antagonist and the incidence of preterm birth (P = 0.01; Chi-square for trend).
Table 2.
Effect of a PAF Antagonist on Inflammation-Induced Parturition
| PAF antagonist dose | LPS | Preterm delivery no. | % Preterm delivery |
|---|---|---|---|
| None | 250 μg/mouse | 16/16 | 100 |
| 3 mg/kg | 250 μg/mouse | 5/8 | 63 |
| 4.5 mg/kg | 250 μg/mouse | 5/8 | 63 |
| 6 mg/kg | 250 μg/mouse | 4/8 | 50 |
We also wanted to determine whether pretreatment with a PAF antagonist could reduce the incidence of inflammation-induced fetal death. Forty-eight hours after intrauterine LPS or saline, animals were sacrificed and the number of live pups and IUFDs in each uterine horn was recorded. The mean number of live pups per animal did not differ between control animals (11.3 ± 2.2) and sham animals (11.5 ± 1.9). Because all animals receiving only intrauterine LPS delivered preterm, there were no remaining live pups at 48 hours. In contrast, 100% of the animals pretreated with the PAF antagonist that did not deliver preterm (n = 10) maintained viable fetuses 48 hours after intrauterine infusion of LPS. The mean number of live pups per animal in this group (6.7 ± 3.2) was significantly increased compared to animals receiving LPS alone (P < 0.001; rank sum test).
PAF Induces Preterm Delivery
We administered PAF into the uterine lumen of CD-1 mice on day 15 of gestation (n = 9). Forty-four percent of the mice delivered preterm (4 of 9). Only 33% of mice maintained live pups at 48 hours after intrauterine infusion (3 of 9). Sixty-six percent of mice receiving the highest dose administered (40 μg/mouse) delivered preterm with none of the animals having live pups 48 hours after intrauterine infusion of PAF (Table 1) ▶ .
Expression and Localization of the PAFR
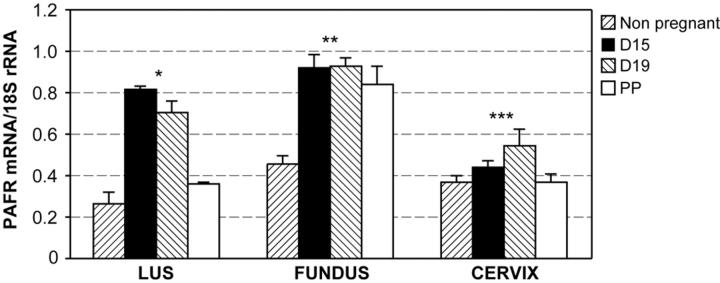
Results from our quantitative PCR experiments revealed that PAFR is expressed in the uterus and in the cervix (Figure 2) ▶ in control mice. PAFR is up-regulated during the latter half of gestation in both the uterus and cervix (Figure 2) ▶ . PAFR mRNA expression declines in the LUS and cervix immediately postpartum but remained elevated in fundal tissue.
Figure 2.
Results from quantitative PCR experiments demonstrating PAFR expression in uterine and cervical tissue throughout mouse gestation. Each bar represents the mean ± SD of message RNA from 3 to 6 specimens for each gestational time point. Expression of the PAFR was significantly different throughout gestation in the LUS, fundus, and cervix (one-way analysis of variance, * P < 0.001 for LUS and fundus, and P = 0.001 for cervix). In the LUS, PAFR expression on day 15 (D15), day 19 (D19), and postpartum (PP) was significantly increased compared to non-pregnant tissues (P < 0.001, P = 0.01, and P = 0.02, respectively). In the fundus, PAFR expression was significantly increased in D15, D19, and PP uterine tissue compared to NP uteri (P < 0.001 for all comparisons). In the cervix, PAFR mRNA was significantly up-regulated in D19 tissues compared to NP cervices (P = 0.001). All pair-wise comparisons were performed using the SNK method.
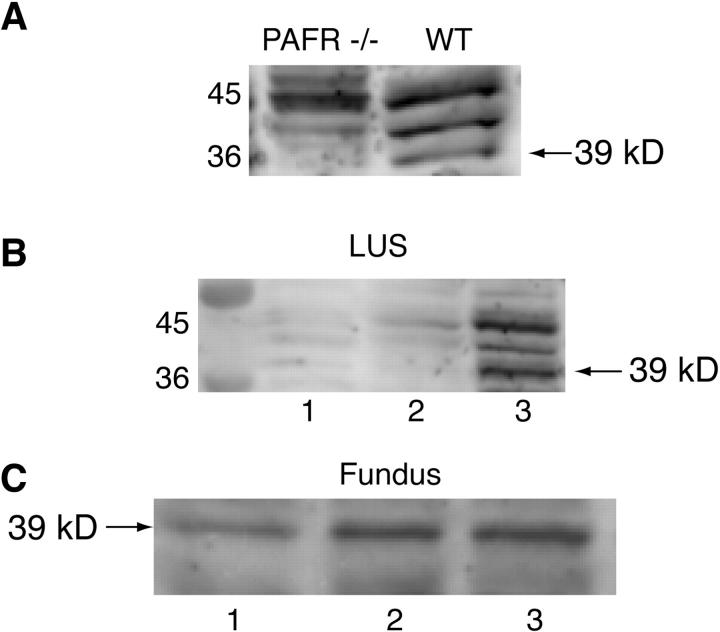
Western blot studies demonstrated the specificity of the polyclonal antibody against the mouse PAFR. Three discrete bands were visualized in pregnant uterine tissue at 36 to 45 kd in Western blots. The expected molecular weight of the PAFR is 39 kd. The uterine tissue from the PAFR knockout mice did not yield a band at 39 kd, while there was a discrete band in the wild-type uterine tissue (Figure 3A) ▶ . Western blot studies demonstrated an up-regulation of the PAFR in LUS at term in CD-1 mice (Figure 3B) ▶ . In the LUS, the up-regulation of the PAFR protein appears to be more dramatic than demonstrated with the quantitative PCR. While an increase in PAFR mRNA was observed in fundal tissues from term uteri compared to NP, the PAFR protein expression in fundal tissues appears to have a more steady-state expression (Figure 3C) ▶ . The differences in the protein studies may be secondary to the fact that both protein and mRNA studies were performed at the same time period. These differences may also indicate posttranslational regulation of the PAFR protein.
Figure 3.
Representative Western blots using the PAFR polyclonal antibody. A: PAFR antibody produces three bands in the 36 to 45 kd range. One of these bands at 39 kd is observed in pregnant uterine tissue from wild-type mice but not in pregnant uterine tissue from PAFR-/- mice. B: PAFR protein expression in the LUS in NP (lane 1), day 15 of gestation (lane 2) and day 19 of gestation (lane 3); a 39-kd band is visualized most prominently in day 19 uterine tissue. C: PAFR protein expression in fundal tissue: lane 1, NP; lane 2, day 15 of gestation; lane 3, day 19 of gestation.
As with the Western blots, the IHC studies demonstrated antigen with our PAFR antibody and no appreciable staining in the tissues from the PAFR knockout mouse (Figure 4, A and B) ▶ . After confirming the specificity of the PAFR antibody, we determined the localization of the PAFR in the gravid uterus. The PAFR appears to be concentrated in the endometrial glands with less intense staining in the myometrium (Figure 4, C and D) ▶ .
Figure 4.
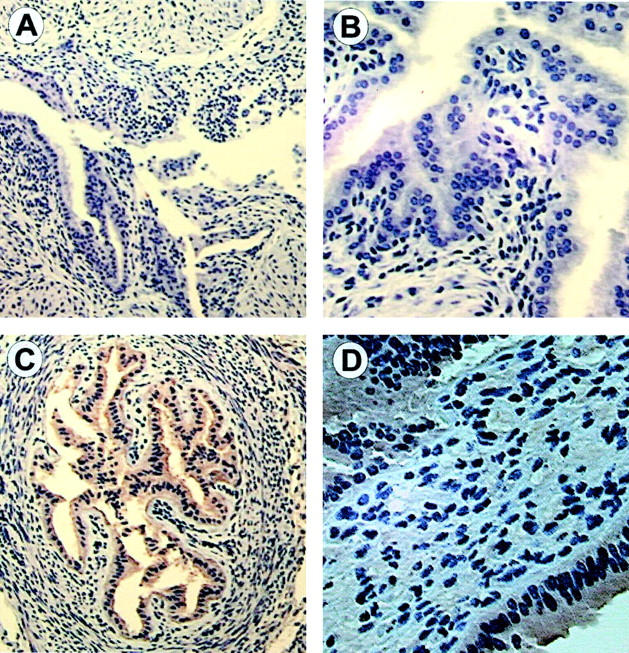
Immunohistochemistry using the PAFR antibody. A: Representative uterine section from PAFR-/- mice (×10). B: Uterine section from PAFR -/- mice at higher magnification (×20) demonstrating no antibody binding. C: Representative section from wild-type mice on day 15 of gestation at ×10 magnification. D: Representative section from CD-1 mice on day 15 of gestation at ×40. Moderate to intense staining is observed in the endometrial glands and smooth muscle, while lighter staining is seen in the smooth muscle only.
Expression and Regulation of TLR-4
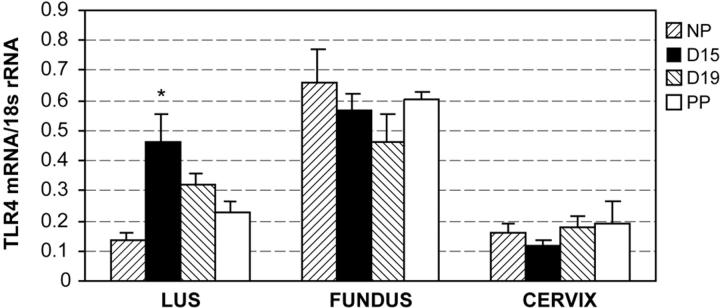
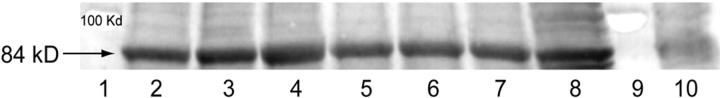
Results from our quantitative PCR experiments demonstrated expression of TLR-4 mRNA in the fundus, LUS, and cervix during mouse gestation. Greatest expression of TLR-4 mRNA was detected in fundal tissues. TLR-4 mRNA was significantly up-regulated in the LUS but not fundus at term compared to the NP state (Figure 5) ▶ . Western blotting confirmed the presence of TLR-4 in gestational tissues and correlated with the TLR-4 mRNA expression. TLR-4 is reported to have a molecular weight of 110 to 120 kd and a molecular weight of 80 to 85 kd in its non-glycosylated form. 41 In these studies, a band at about 80 to 85 kd is visualized in uterine tissue. Studies with a blocking peptide confirmed the specificity of this band in uterine tissue (Figure 6) ▶ .
Figure 5.
Results from quantitative PCR experiments demonstrating TLR-4 mRNA expression in uterine and cervical tissue throughout mouse gestation. Each bar represents the mean ± SD of message RNA from 3 to 6 specimens for each gestational time point. TLR-4 mRNA is significantly up-regulated in LUS throughout gestation (*, one-way analysis of variance, P < 0.001). Using pair-wise comparison, TLR-4 expression on day 15 (D15) and day 19 (D19) was significantly increased from NP controls (P < 0.001 and P = 0.009, respectively; SNK method). There was no significant difference in TLR-4 mRNA expression observed in the fundus or cervix throughout gestation.
Figure 6.
Representative Western blot demonstrating TLR-4 protein expression in uterine tissues. Lane 1, molecular weight standard; lane 2, LUS NP; lane 3, LUS day 15; lane 4, LUS day 19; lane 5, fundus NP; lane 6, fundus day 15; lane 7, fundus day 19; lane 8, mouse heart; lane 9, molecular weight standard; lane 10, mouse heart incubated with antibody plus blocking peptide. An increase in TLR-4 protein expression is observed in LUS between NP and day 19. No significant change in TLR-4 protein is observed in fundal tissue throughout gestation.
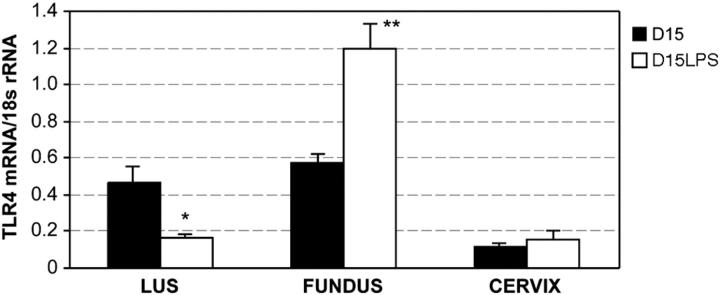
To determine the effect of intrauterine inflammation on the regulation of TLR-4, we investigated the expression of these receptors 6 hours after intrauterine infusion of LPS. Intrauterine inflammation resulted in a two-fold increase in TLR-4 expression in the fundus (Figure 7) ▶ . Interestingly, intrauterine LPS resulted in a down-regulation of TLR-4 mRNA in the LUS with no significant change observed in the cervix.
Figure 7.
Results from quantitative PCR experiments demonstrating TLR-4 mRNA expression in uterine and cervical tissue in response to intrauterine LPS. Each bar represents the mean ± SD of message RNA from 3 to 6 specimens for each gestational time point. Intrauterine LPS resulted in a differential regulation of TLR-4 mRNA in the LUS and fundus. In the LUS, TLR-4 mRNA expression was significantly decreased (*P = 0.006, t-test) while in the fundus, it was significantly increased (**P = 0.002). No significant change in TLR-4 mRNA expression was observed in cervical tissue.
Discussion
In these studies, we describe a reproducible model of localized intrauterine inflammation, which closely mimics the most common clinical presentation of preterm birth. The use of the mouse in this model offers several advantages. The mouse is a relatively inexpensive animal with a short gestational period and reliable fertility. Additionally, the mouse model allows the use of genetic manipulation to discern essential mediators in the pathways leading to preterm birth. The model described here is similar to the mouse model described by Hirsch which instills live or killed E. coli into the uterine horn to induce preterm delivery. 42 Our model demonstrates that LPS, a component of the gram-negative bacteria cell wall, is sufficient to induce an inflammatory state and promote parturition. This model is a powerful tool that allowed us to investigate PAF, a potential mediator of inflammation-induced preterm birth.
Confirming our hypothesis that PAF is a significant mediator of inflammation-induced preterm birth, we demonstrated that PAF itself can induce preterm birth and that a PAF antagonist was able to significantly reduce the incidence of inflammation-induced preterm birth. For these studies, we were interested in the mechanisms leading to the emptying of the uterus in the setting of localized inflammation. Therefore, we dosed the PAF antagonist before infusion of LPS. The ability of a PAF antagonist to prevent preterm birth after the initiation of an inflammatory response has not yet been demonstrated. It is possible that the bioavailability of the PAF antagonist used in these studies limited its effectiveness. The intraperitoneal route of CV-6209 administration is not widely reported and absorption of this drug may be limited by this route of delivery. Intravenous infusion of higher doses of this drug has been shown to elicit transient hypotension in the mice. 43 Therefore, since maternal hypotension could affect fetal viability, we were reluctant to increase the dose of the PAF antagonist for these studies. Other PAF antagonist might be more efficacious in preventing inflammation-induced preterm birth. Other investigators have reported success in other animal models using a recombinant PAF-AH to suppress inflammation with few side effects. 44,45
Recent studies have documented that TLR-4 is required for LPS activation of diverse signal transduction pathways typical of an inflammatory state. 46 However, whether TLR-4 is required for LPS-induced preterm birth has not been established. Our studies with the C3H/HEJ, TLR-4 mutant mice suggest that TLR-4 mediates LPS-induced preterm birth but not fetal demise. We used two different commercial preparations of LPS for these studies. Previous investigations demonstrated that less purified LPS contain bacterial lipoproteins that can activate signal transduction pathways independent of TLR-4. 35 Consistent with these other studies, we found that purified LPS was not able to induce preterm birth in TLR-4 mutant mice. However, inflammation-induced preterm birth in humans probably represents a diverse inflammatory response that does not result solely from the components of the cell wall of gram-negative bacteria. Therefore, the results obtained in the CD-1 mice with the unpurified LPS (less than 3% endotoxin protein) more likely represent the signal transduction pathways triggered in vivo in humans. Variations in the TLR-4 gene have been hypothesized to contribute to certain disease states. 47,48 Genetic variations in the TLR-4 gene in the fetus have recently been reported to be associated with preterm birth. 49 Genetic variations in TLR-4 in the mother may contribute to a propensity or a protective effect to inflammation-induced preterm birth.
Uterine or placental inflammation is also associated with fetal demise. We investigated the ability of the PAF antagonist to prevent fetal demise in animals which did not deliver preterm. We demonstrated that PAF can cause fetal death and that a PAF antagonist significantly preserved fetal viability in the setting of intrauterine inflammation. The majority of IUFDs observed in these animals were in the right lower uterine horn, near the site of LPS injection. We speculate that the presence of the PAF antagonist allowed for a more “controlled or contained” inflammatory response in these animals and that the fetuses further from the site of LPS injection were preserved. Additional studies are required to determine whether exposure to intrauterine inflammation in these fetuses, in the absence of death, results in adverse outcomes. There was a wide variation in the number of live pups per animal in the 10 animals who did not deliver preterm after receiving a PAF antagonist before intrauterine LPS. This may be attributable to naturally occurring genetic and immunological differences among the out-bred strain of mice used in these studies. These studies also demonstrate that isoflurane exposure, sufficient for survival surgery in the gravid mouse, is not associated with fetal death within 48 hours. This is an important finding for continued mouse studies investigating fetal outcomes.
There have been limited studies regarding the expression and localization of the PAFR in gestational tissues. Our studies demonstrate the expression of the PAFR mRNA in both the non-pregnant and pregnant uterus. Interestingly, the latter half of pregnancy is associated with a significant increase in PAFR expression in both the fundus and lower uterine segment. This finding is not surprising in that previous in vitro work has demonstrated an increased sensitivity to PAF in the uterus with increasing gestation. 50 Others have demonstrated an estrogen response element in the PAFR gene and that estrogen can modulate PAF-activated signal transduction pathways. 51,52 The increased circulating estrogen in pregnancy may thus be responsible for the up-regulation of the PAFR.
The unavailability of an antibody to the PAFR has limited studies on the PAFR protein in the mouse. We demonstrate here the specificity of our PAFR antibody with use of PAFR knockout tissues using both Western blot and immunohistochemistry. The immunohistochemistry results demonstrate an abundance of the PAFR in the endometrial glands. The presence of the PAFR in this location may serve to activate both uterotonic (prostaglandins) and inflammatory (interleukin-1, tumor necrosis factor) mediators. The light staining observed in the myometrium may reflect its uterotonic function as stimulation of the PAFR results in activation of the phosphoinositol signaling pathway, calcium influx and smooth muscle contractions. 8,30,53,54
We also demonstrated for the first time the differential expression of TLR-4 mRNA in gestational tissue. TLR-4 is expressed in uterine and cervical tissue and is differentially regulated in uterine tissue in response to intrauterine inflammation. While LPS resulted in a down-regulation of TLR-4 mRNA in the LUS, it caused an up-regulation of TLR-4 mRNA in the fundus. The up-regulation of TLR-4 mRNA in the fundus may be due to a direct LPS effect or may be secondary to other inflammatory mediators generated in response to LPS. The up-regulation of TLR-4 mRNA in fundal tissue may also act as a positive feedback loop to propagate the inflammatory response and may serve to enhance the inflammatory response in the uterus, leading to parturition and hence maternal survival by expulsion of an infected pregnancy. The up-regulation of TLR-4 mRNA observed in the fundus may represent a true increase in receptor expression within uterine tissue or it may reflect an influx of inflammatory cells into this area on stimulation with LPS.
These studies support the hypothesis that LPS, via activation of TLR-4, generates PAF and stimulates multiple signal transduction pathways resulting in preterm parturition. These studies also indicate that there are pathways, independent of TLR-4, that result in fetal death. Whether there are other bacterial products stimulating other TLRs or whether PAF can be generated via a TLR-4-independent pathway requires further investigation.
In conclusion, we have developed a mouse model of localized intrauterine inflammation that will enhance our understanding of inflammation-induced preterm birth and will provide insights into the effect of inflammation on both the mother and the fetus. Since many of the signal transduction pathways involved in the inflammatory cascade are redundant in different biological systems, the elucidation of the interplay between LPS and PAF and their specific receptors will yield essential information for the understanding of not only inflammation-induced preterm birth but also other biological systems affected by proinflammatory pathways. With these studies, we have demonstrated the presence of the PAFR in the pregnant uterus and that PAF is a significant mediator of both inflammation-induced preterm birth and fetal death. This new information on the signal transduction pathways in inflammation-induced preterm birth may broaden therapeutic options in the prevention of preterm birth and inflammation-associated fetal and neonatal morbidity.
Footnotes
Address reprint requests to Michal A. Elovitz, M.D., University of Pennsylvania, Center for Research in Reproduction and Women’s Health, Biomedical Research Building II/III, Room 1353, 421 Curie Boulevard, Philadelphia, Pennsylvania 19104-6142. E-mail: melovitz@obgyn.upenn.edu.
Supported by a Scholarship Award from the Society for Maternal-Fetal Medicine (Washington, DC) and a Women’s Reproductive Health Research Award K12 HD01265 from the University of Pennsylvania.
Research was performed at the University of Pennsylvania and the University of Chicago.
References
- 1.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, Jun JK: The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin-6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000, 183:1124-1129 [DOI] [PubMed] [Google Scholar]
- 2.Rizzo G, Capponi A, Rinaldo D, Tedeschi D, Arduini D, Romanini C: Interleukin-6 concentrations in cervical secretions identify microbial invasion of the amniotic cavity in patients with preterm labor and intact membranes. Am J Obstet Gynecol 1996, 175:812-817 [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Bosquet E, Cerqueira MJ, Dominguez C, Gasser I, Bermejo B, Cabero L: Amniotic fluid glucose and cytokines values in the early diagnosis of amniotic infection in patients with preterm labor and intact membranes. J Matern Fetal Med 1999, 8:155-158 [DOI] [PubMed] [Google Scholar]
- 4.King J, Flenady V: Prophylactic antibiotics for inhibiting preterm labour with intact membranes. Cochrane Database Syst Rev 2002, :CD000246. [DOI] [PubMed] [Google Scholar]
- 5.Debillon T, Gras-Leguen C, Verielle V, Winer N, Caillon J, Roze JC, Gressens P: Intrauterine infection induces programmed cell death in rabbit periventricular white matter. Pediatr Res 2000, 47:736-742 [DOI] [PubMed] [Google Scholar]
- 6.Gibbs RS, Davies JK, McDuffie RS, Jr, Leslie KK, Sherman MP, Centretto CA, Wolf DM: Chronic intrauterine infection and inflammation in the preterm rabbit, despite antibiotic therapy. Am J Obstet Gynecol 2002, 186:234-239 [DOI] [PubMed] [Google Scholar]
- 7.Bito H, Shimzu T: Molecular characterization and physiological functions of PAF receptors. Honn KV Marnett LJ Nigam S Jones RL Wong PY-K eds. Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation and Radiation Injury 2. 1997:pp 215-221 Plenum Press New York [DOI] [PubMed]
- 8.Chao W, Olson MS: Platelet-activating factor: receptors and signal transduction. Biochem J 1993, 292:617-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai H: Platelet-activating factor acetylhydrolase. Prostaglandins Other Lipid Mediat 2002, 68–69:83-94 [DOI] [PubMed] [Google Scholar]
- 10.Cao Y, Stafforini D, Zimmerman GA, McIntyre TM, Prescott SM: Expression of plasma platelet-activating factor acetylhydrolase I transcriptionally regulated by mediators of inflammation. J Biol Chem 1997, 273:4012-4020 [DOI] [PubMed] [Google Scholar]
- 11.Bito H, Honda Z, Nakamura M, Shimizu T: Cloning, expression and tissue distribution of rat platelet-activating-factor-receptor cDNA. Eur J Biochem 1994, 221:211-218 [DOI] [PubMed] [Google Scholar]
- 12.Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, Cao WH, Kume K, Fukuchi Y, Ikuta K, Miyazaki J, Kumada M, Shimizu T: Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med 1998, 187:1779-1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishii S, Nagase T, Tashiro F, Ikuta K, Sato S, Waga I, Kume K, Miyazaki J, Shimizu T: Bronchial hyperreactivity, increased endotoxin lethality and melanocytic tumorigenesis in transgenic mice overexpressing platelet-activating factor receptor. EMBO J 1997, 16:133-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Sun Z, Borjesson A, Haraldsen P, Aldman M, Deng X, Leveau P, Andersson R: Treatment with lexipafant ameliorates the severity of pancreatic microvascular endothelial barrier dysfunction in rats with acute hemorrhagic pancreatitis. Int J Pancreatol 1999, 25:45-52 [DOI] [PubMed] [Google Scholar]
- 15.Thompson W, Coyle S, Van Zee K, Oldenburg H, Trousdale R, Rogy M, Felsen D, Moldawer L, Lowry SF: The metabolic effect of platelet-activating factor antagonism in endotoxemic man. Arch Surg 1994, 120:72-79 [DOI] [PubMed] [Google Scholar]
- 16.Mozes T, Heiligers JP, Tak CJ, Zijlstra FJ, Ben-Efraim S, Saxena PR, Bonta IL: Platelet-activating factor is one of the mediators involved in endotoxic shock in pigs. J Lipid Mediat 1991, 4:309-325 [PubMed] [Google Scholar]
- 17.Caplan MS, Hedlund E, Adler L, Lickerman M, Hsueh W: The platelet-activating factor receptor antagonist WEB 2170 prevents neonatal necrotizing enterocolitis in rats. J Pediatr Gastroenterol Nutr 1997, 24:296-301 [DOI] [PubMed] [Google Scholar]
- 18.Mathiak G, Szewczyk D, Abdullah F, Ovadia P, Rabinovici R: Platelet-activating factor (PAF) in experimental and clinical sepsis. Shock 1997, 7:391-404 [DOI] [PubMed] [Google Scholar]
- 19.Sugano T, Nasu K, Narahara H, Kawano Y, Nishida Y, Miyakawa I: Platelet-activating factor induces an imbalance between matrix metalloproteinase-1 and tissue inhibitor of metalloproteinases-1 expression human uterine cervical fibroblasts. Biol Reprod 2000, 62:540-546 [DOI] [PubMed] [Google Scholar]
- 20.Sugano T, Narahara H, Nasu K, Arima K, Fujisawa K, Miyakawa I: Effects of platelet-activating factor on cytokine production by human uterine cervical fibroblasts. Mol Hum Reprod 2001, 7:475-481 [DOI] [PubMed] [Google Scholar]
- 21.Maul H, Shi L, Marx SG, Garfield RE, Saade GR: Local application of platelet-activating factor induces cervical ripening accompanied by infiltration of polymorphonuclear leukocytes in rats. Am J Obstet Gynecol 2002, 187:829-833 [DOI] [PubMed] [Google Scholar]
- 22.Nahara H, Nikoshioka Y, Johnston JM: Secretion of platelet-activating acetylhydrolase by human decidual macrophages. J Clin Endocrinol Metab 1993, 77:1258-1262 [DOI] [PubMed] [Google Scholar]
- 23.Kawano Y, Narahara H, Johnston JM: Inhibitory effect of interleukin-8 on the secretion of platelet-activating factor acetylhydrolase by human decidual macrophages. J Soc Gynecol Investig 1999, 6:328-332 [DOI] [PubMed] [Google Scholar]
- 24.Maki N, Hoffman D, Johnston JM: Platelet-activating factor acetylhydrolase activity in maternal, fetal and newborn rabbit plasma during pregnancy and lactation. Proc Natl Acad Sci USA 1988, 85:728-732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsubara T, Yasuda K, Johnston JM, Sanezumi M, Okada H, Matsuoka S, Kanzaki H: Platelet-activating factor and PAF acetylhydrolase activity in rat uterus and placenta during the late stages of pregnancy. Biol Reprod 1997, 56:885-890 [DOI] [PubMed] [Google Scholar]
- 26.Tetta C, Montrucchio G, Alloatti G, Roffinello C, Emanuelli G, Benedetto C, Camussi G, Massobrio M: Platelet-activating factor contracts human myometrium in vitro. Proc Soc Exp Biol Med 1986, 183:376-381 [DOI] [PubMed] [Google Scholar]
- 27.Montrucchio G, Alloatti G, Tetta C, Roffinello C, Emanuelli G, Camussi G: In vitro contractile effect of platelet-activating factor on guinea-pig myometrium. Prostaglandins 1986, 32:539-554 [DOI] [PubMed] [Google Scholar]
- 28.Kim BK, Ozaki H, Lee SM, Karaki H: Increased sensitivity of rat myometrium to the contractile effect of platelet-activating factor before delivery. Br J Pharmacol 1995, 115:1211-1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oshiro BT, Monga M, Eriksen NL, Graham JM, Weisbrodt NW, Blanco JD: Endotoxin, interleukin-1β, interleukin-6, or tumor necrosis factor-α do not acutely stimulate isolated murine myometrial contractile activity. Am J Obstet Gynecol 1993, 169:1424-1427 [DOI] [PubMed] [Google Scholar]
- 30.Jeanneton O, Delvaux M, Botella A, Frexinos J, Bueno L: Platelet-activating factor induces a contraction of isolated smooth muscle cells from guinea pig ileum: intracellular pathway involved. J Pharmocol Exp Ther 1993, 267:31-37 [PubMed] [Google Scholar]
- 31.Silver RK, Caplan MS, Kelly AM: Amniotic fluid platelet-activating factor is elevated in patients with tocolytic failure and preterm delivery. Prostaglandins 1992, 43:181-187 [DOI] [PubMed] [Google Scholar]
- 32.Hoffman D, Romero R, Johnston JM: Detection of platelet-activating factor in amniotic fluid of complicated pregnancies. Am J Obstet Gynecol 1990, 162:525-528 [DOI] [PubMed] [Google Scholar]
- 33.Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton MJ, Oikawa M, Qureshi N, Monks B, Finberg RW, Ingalls RR, Golenbock DT: Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest 2000, 105:497-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B: Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998, 282:2085-2088 [DOI] [PubMed] [Google Scholar]
- 35.Tapping RI, Akashi S, Miyake K, Godowski PJ, Tobias PS: Toll-like receptor 4, but not toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J Immunol 2000, 165:5780-5787 [DOI] [PubMed] [Google Scholar]
- 36.Laflamme N, Rivest S: Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. EMBO J 2001, 15:155-163 [DOI] [PubMed] [Google Scholar]
- 37.Faure E, Thomas L, Xu H, Medvedev A, Equils O, Arditi M: Bacterial lipopolysaccharide and IFN-γ induce toll-like receptor 2 and toll-like receptor 4 expression in human endothelial cells: role of NF-κ B activation. J Immunol 2001, 166:2018-2024 [DOI] [PubMed] [Google Scholar]
- 38.Kaga N, Katsuki Y, Obata M, Shibutani Y: Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am J Obstet Gynecol 1996, 174:754-759 [DOI] [PubMed] [Google Scholar]
- 39.Romero R, Mazor M, Tartakovsky B: Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol 1991, 165:969-971 [DOI] [PubMed] [Google Scholar]
- 40.Sparey C, Robson SC, Bailey J, Lyall F, Europe-Finner GN: The differential expression of myometrial connexin-43, cyclooxygenase-1 and -2, and Gs α proteins in the upper and lower segments of the human uterus during pregnancy and labor. J Clin Endocrinol Metab 1999, 84:1705-1710 [DOI] [PubMed] [Google Scholar]
- 41.da Silva Correia J, Ulevitch RJ: MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J Biol Chem 2002, 277:1845-1854 [DOI] [PubMed] [Google Scholar]
- 42.Hirsch E, Saotome I, Hirsh D: A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol 1995, 172:1598-1603 [DOI] [PubMed] [Google Scholar]
- 43.Terashita Z, Imura Y, Takatani M, Tsushima S, Nishikawa K: CV-6209, a highly potent antagonist of platelet-activating factor in vitro and in vivo. J Pharmacol Exp Ther 1987, 242:263-268 [PubMed] [Google Scholar]
- 44.Caplan MS, Lickerman M, Adler L, Dietsch GN, Yu A: The role of recombinant platelet-activating factor acetylhydrolase in a neonatal rat model of necrotizing enterocolitis. Pediatr Res 1997, 42:779-783 [DOI] [PubMed] [Google Scholar]
- 45.Henderson WR, Jr, Lu J, Poole KM, Dietsch GN, Chi EY: Recombinant human platelet-activating factor-acetylhydrolase inhibits airway inflammation and hyperreactivity in mouse asthma model. J Immunol 2000, 164:3360-3367 [DOI] [PubMed] [Google Scholar]
- 46.Matsuguchi T, Musikacharoen T, Ogawa T, Yoshikai Y: Gene expressions of toll-like receptor 2, but not toll-like receptor 4, is induced by LPS and inflammatory cytokines in mouse macrophages. J Immunol 2000, 165:5767-5772 [DOI] [PubMed] [Google Scholar]
- 47.Reindl M, Lutterotti A, Ingram J, Schanda K, Gassner C, Deisenhammer F, Berger T, Lorenz E: Mutations in the gene for toll-like receptor 4 and multiple sclerosis. Tissue Antigens 2003, 61:85-88 [DOI] [PubMed] [Google Scholar]
- 48.Lorenz E, Schwartz DA, Martin PJ, Gooley T, Lin MT, Chien JW, Hansen JA, Clark JG: Association of TLR4 mutations and the risk for acute GVHD after HLA-matched-sibling hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001, 7:384-387 [DOI] [PubMed] [Google Scholar]
- 49.Lorenz E, Hallman M, Marttila R, Haataja R, Schwartz DA: Association between the Asp299Gly polymorphisms in the toll-like receptor 4 and premature births in the Finnish population. Pediatr Res 2002, 52:373-376 [DOI] [PubMed] [Google Scholar]
- 50.Kim B-K, Ozaki H, Lee SM, Karaki H: Increased sensitivity of rat myometrium to the contractile effect of platelet-activating factor before delivery. Br J Pharm 1995, 115:1211-1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mutoh H, Kazuhiko, Sato S, Kato S, Shimizu T: Positive and negative regulations of human platelet-activating factor receptor transcript 2 (tissue-type) by estrogen and TGF-β1. Biochem Biophys Res Commun 1994, 205:1130-1136 [DOI] [PubMed] [Google Scholar]
- 52.Sato S, Kume K, Takan T, Mutoh H, Taketani Y, Shimizu T: Up-regulation of the intracellular Ca signaling and mRNA expression of platelet-activating factor receptor by estradiol in human uterine endometrial cells. Adv Exp Med Biol 1996, 416:95-100 [DOI] [PubMed] [Google Scholar]
- 53.Ahmed A, Sage SO, Plevin R, Shoaibi MA, Sharkey AM, Smith SK: Functional platelet-activating factor receptors linked to inositol lipid hydrolysis, calcium mobilization, and tyrosine kinase activity in the human endometrial HEC-1B cell line. J Reprod Fertil 1994, 101:459-466 [DOI] [PubMed] [Google Scholar]
- 54.Molnar M, Hertelendy F: Platelet-activating factor activates the phosphoinositide cycle and promotes Ca2+ mobilization in human myometrial cells: comparison with PGF2 α. Journal of Matern Fetal Med 1992, 1:1-6 [Google Scholar]