Abstract
Excessive cell-mediated tissue contraction after injury can lead to morbid contractile scarring in the body. In the eye this can cause blindness because of posterior capsule opacification, proliferative vitroretinopathy, failure of glaucoma filtration surgery, and corneal haze. During repair, transforming growth factor (TGF)-β and connective tissue growth factor (CTGF) genes are co-ordinately expressed. Although TGF-β and CTGF stimulate new matrix deposition, their role and regulation during contractile scarring is unknown. In this study, an in vitro model of collagen matrix contraction culminating from tractional forces generated by fibroblasts showed that both TGF-β1 and CTGF-stimulated contraction. Using a specific anti-sense oligodeoxynucleotide to CTGF the procontractile activity of TGF-β1 was found to be mediated by CTGF. During contraction fibroblasts produced similar levels of matrix metalloproteinases (MMPs)-2 and -9 with TGF-β1 or CTGF and a modest increase in MMP-1 with CTGF only (indicated by zymography and enzyme-linked immunosorbent assay). The requirement of MMPs for contraction was demonstrated using a broad-spectrum synthetic inhibitor. This study demonstrates a new function for CTGF in mediating matrix contraction by fibroblasts involving MMPs and suggests a novel regulatory mechanism for TGF-β-stimulated contraction. Inhibition of CTGF activity or gene transcription could be a suitable target for anti-scarring therapies.
Co-ordinated phenotypic changes, extracellular matrix deposition, and remodeling by a variety of cells are central to tissue formation during embryogenesis, development, and repair. Perturbations in control of the cascade of tissue generation are likely to result in embryonic tissue malformation, fibrotic disorders, and abnormal wound healing.
The tissue repair process is regulated by a number of peptides including cytokines and growth factors. Transforming growth factor (TGF)-β mRNA and protein is increased in sites of tissue repair 1,2 and is a potent stimulator of connective tissue formation. 3-5 TGF-β up-regulates fibroblast proliferation 6 and extracellular matrix synthesis 7,8 and reduces matrix degradation after injury. 9
During tissue repair and early development, TGF-β gene expression is co-ordinately regulated with that of connective tissue growth factor (CTGF). 1,10 CTGF is a cysteine-rich, heparin-binding protein 11 whose gene expression is strongly induced by TGF-β in fibroblasts. 1,12 A 1-hour exposure to TGF-β is sufficient to induce CTGF gene transcription for up to 36 hours in fibroblasts. 13 A novel TGF-β response element controls transcription in both human and murine CTGF promoters. 13 These observations suggest that CTGF is a downstream mediator of TGF-β activity in fibroblasts. 13 Indeed, CTGF mediates a number of TGF-β-stimulated wound healing functions, 14 including collagen synthesis during granulation tissue formation. 15
Contraction and remodeling of matrix are important elements of the tissue repair process, 16 however excessive contraction induces pathological scarring in a variety of tissues including parts of the eye, skin, liver, and kidney. 17-21 For example, CTGF mRNA has been found in plaques of human cataracts, the major blinding disease, and the highly contractile membranes of posterior capsule opacification. 22 Wound contraction is governed by a combination of two fibroblast-driven mechanisms; tractional forces generated by fibroblasts migrating into a wound, 23-25 and differentiation of fibroblasts into a highly contractile myofibroblast phenotype. 26,27
Using an in vitro model representing matrix contraction culminating from generation of tractional forces by fibroblasts, 24,28 we have recently shown that matrix metalloproteinases (MMPs) are produced by Tenon’s capsule fibroblasts during this process in response to serum and that they are essential for contraction. 29 Using a similar model we have previously shown that in response to serum retinal pigment epithelial cells can also contract collagen matrix in an MMP-dependent manner. 30 This has important implications for our understanding of the mechanism of proliferative vitreoretinopathy.
The MMPs are essential for turnover of matrix during homeostasis and pathology. 31 Increasing evidence suggests that members of the MMP family are also involved in many processes besides matrix degradation, including recruitment of stem and progenitor cells from the bone marrow niche 32 and cleavage of growth factors including CTGF. 33 Furthermore, using an in vivo model of subconjunctival wound healing we found that application of a broad spectrum MMP inhibitor significantly reduced matrix deposition, contraction, and ultimately scarring in the rabbit. 34
TGF-β strongly induces matrix contraction involved in scarring. 35-37 However, involvement of CTGF in the regulation of matrix contraction either independently or via control of TGF-β activity has not been addressed despite the co-expression of these growth factors during wound healing. Our study demonstrates a new function for CTGF in mediating matrix contraction by fibroblasts via utilization of MMPs and also indicates a novel regulatory mechanism for TGF-β-mediated contraction. This data suggests that CTGF could be a suitable target for anti-scarring therapies in a number of pathologies throughout the body using agents that inhibit CTGF activity or CTGF gene expression.
Materials and Methods
Growth Factors
Recombinant human CTGF was prepared as previously described using a baculovirus expression system. 14 Human recombinant TGF-β1 was purchased from R&D Systems (Oxford, UK).
Neutralizing Antibody to CTGF
Anti-human CTGF IgG-neutralizing antibodies were prepared as described previously by injecting recombinant human CTGF into goats, isolating total IgG fraction with protein G Sepharose chromatography, followed by CTGF affinity column chromatography. 15
Anti-Sense Oligodeoxynucleotides to CTGF
The human, mouse, and rat CTGF mRNA genes were analyzed for unique, nonrepetitive, 20 mer nucleotide sequences with high GC contents that would minimize self-hybridization and provide stability of the oligodeoxynucleotide mRNA complex. A total of 81 nucleotide sequences were selected, and 20 mer anti-sense oligodeoxynucleotides were synthesized with thioate ester linkages replacing the phosphate ester linkages, 2′-O-methoxyethyl ribose groups coupled at base positions 1 to 5 and 16 to 20, and 5-methylcytosine substituted for all cytosines. The anti-sense oligodeoxynucleotides were tested for the ability to reduce CTGF mRNA levels using a cell culture-screening assay. 38,39 Briefly, mouse endothelial cells (bEND.3; American Type Culture Collection, Manassas, VA) were grown to confluence and then treated with 150 nmol/L of oligodeoxynucleotide and 10 μg/ml of lipofectin (Life Technologies, Inc, Rockville, MD) for 4 hours. The cells were washed and grown for 24 hours then total RNA was isolated from the cells by guanidine isothiocyanate and phenol-chloroform extraction. CTGF mRNA expression was measured by Northern blotting. The gel blots were stripped and reprobed for expression of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase to confirm equal RNA loading. The CTGF mRNA levels from the Northern blot analysis were expressed as a percentage of the levels of CTGF mRNA in control cells, which were treated with lipofectin only, after normalization to glyceraldehyde-3-phosphate dehydrogenase mRNA levels. The oligodeoxynucleotide with the sequence, GCC-AGA-AAG-CTC-AAA-CTT-GA, corresponds to nucleotides 987 to 1007 of the human CTGF mRNA, reduced the level of mouse CTGF mRNA in bEND cells by 86%, and was selected as the CTGF anti-sense oligodeoxynucleotide. A scrambled mismatch control 20 mer that contained a random mix of all four bases was used as a control to assess specificity and toxicity of the phosphorothioate oligodeoxynucleotide.
MMP Inhibitor
The broad-spectrum MMP inhibitor GM 6001 40,41 (a generous gift from Glycomed, CA) was dissolved in dimethyl sulfoxide to give a stock concentration of 10 mmol/L. The stock was diluted in culture medium such that 1% (v/v) dimethyl sulfoxide was the maximum concentration cells were exposed to during experiments. A structural analog of GM 6001 (GM 6001-negative control; CN Biosciences UK, Nottingham, UK) with no MMP inhibitory activity was treated similarly and used as a negative control.
Cell Culture
Human fibroblasts were harvested and cultured from corneas donated, with informed consent, from Moorfields Eye Hospital Eye Bank as previously described. 42 Briefly, fibroblasts were isolated from corneal tissue explants and cultured with Dulbecco’s modified Eagle’s medium (Sigma Chemical Company, Poole, Dorset, UK) supplemented with 10% w/v newborn calf serum, 2 mmol/L l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (all supplied by Gibco Life Technologies, Paisley, Scotland, UK). For experimentation fibroblasts were used at passage two or three.
Contraction Model
Free-floating relaxed fibroblast-populated collagen gels were prepared with modifications of the method of Bell and colleagues, 28 as previously described. 42 These gels are an in vitro representation of contraction resulting from tractional forces generated by fibroblasts migrating through matrix such as a wound. 24,28 Triplicate serum-free fibroblast-populated collagen gels were prepared in 48-well tissue culture plates. The gels contained 2.5 mg/ml of type I rat-tail collagen (Sigma Chemical Company, Poole, Dorset, UK) and 1 × 105 fibroblasts per ml of gel mixture. After gelation of the collagen solution, each gel was fed with 500 μl of test medium and detached from the well. Contracting gels were digitally photographed (Casio Computer Co. Ltd., Japan) and the gel areas were calculated in pixels using image analysis software (Image Tool; The University of Texas Health Science Center in San Antonio (UTHSCSA), San Antonio, TX). To achieve reproducible values with respect to magnification and camera resolution, the camera was mounted on a retort stand and every photograph was taken from the same specified distance. The tissue-culture plate, containing the gels to be photographed, was mounted on a light box in the same position on each occasion. Conditioned medium from contracting fibroblast-populated collagen gels was collected from each treatment group and stored at −20°C in siliconized Eppendorfs until analyzed.
Growth Factor Stimulation of Contraction
To assess matrix contraction, gels were fed with concentrations of TGF-β1 or CTGF (0 to 250 ng/ml) in serum-free Dulbecco’s modified Eagle’s medium supplemented with 1% bovine serum albumin before release.
CTGF Inhibition during Contraction
To inhibit CTGF gene transcription in the presence of TGF-β1, fibroblast-populated collagen gels were fed with serum-free Dulbecco’s modified Eagle’s medium containing 1% (w/v) bovine serum albumin and 25 ng/ml recombinant human TGF-β1 together with concentrations of anti-sense oligodeoxynucleotides to CTGF or a scrambled sequence control (0 to 100 μmol/L) before release. After 24 hours of contraction, media was removed and replenished with fresh test media. To assess the effect of inhibition of CTGF gene transcription on basal levels of contraction gels fed with serum-free medium only plus concentrations of oligodeoxynucleotides to CTGF and the scrambled control were also included. To inhibit CTGF protein activity in the presence of TGF-β1, gels were treated as above, and CTGF-neutralizing antibody (10 μg/ml) or an IgG control were added.
CTGF Enzyme-Linked Immunosorbent Assay (ELISA)
CTGF was measured in cultured cells using a capture sandwich ELISA with biotinylated and nonbiotinylated affinity-purified goat polyclonal antibodies to human CTGF. Briefly, a flat-bottom ELISA plate (96-well; Costar, Cambridge, MA) was coated with 50 μl of goat anti-human CTGF antibody (which recognizes predominantly epitopes in the N-terminal half of the CTGF molecule) at a concentration of 10 μg/ml in phosphate-buffered saline (PBS)/0.02% sodium azide for 1 hour at 37°C. The wells were washed four times and incubated with 300 μl of blocking buffer (PBS/0.02% sodium azide/1% bovine serum albumin) for 1 hour at room temperature. The wells were washed again four times and 50 μl of recombinant human CTGF standard or sample was added and incubated at 37°C for 1 hour. After washing, 50 μl of biotinylated goat anti-human CTGF was added at a concentration of 2 μg/ml and incubated at room temperature in the dark then 50 μl of alkaline phosphatase-conjugated streptavidin (Zymed, South San Francisco, CA) was added at a 1:1000 dilution and incubated at room temperature for 1 hour after washing. The wells were washed again and incubated with 100 μl of alkaline-phosphatase substrate solution (1 mg/ml p-nitrophenyl phosphate; Sigma, St. Louis, MO) in sodium carbonate/bicarbonate buffer/0.02% sodium azide, pH 9.6) until the reaction developed. Absorbance readings were taken at 405 nm using a microplate reader (Molecular Devices, Sunnyvale, CA). The values for CTGF concentration were normalized for total protein content in the sample using bicinchoninic acid protein assay reagent (Pierce Chemical, Rockford, IL).
Zymography
MMP activity in conditioned medium was demonstrated by gelatin zymography (10% zymogram gelatin gels), using the manufacturer’s buffers and instructions (Mini Cell; Invitrogen, Groningen, The Netherlands). Briefly samples were diluted in sample buffer (1:1) and electrophoresed through gelatin-impregnated zymogram gels at 150 V for 90 minutes. Kaleidoscope molecular weight markers (BioRad, Hemel Hempstead, UK) were also included. The gels were incubated at room temperature in renaturing buffer for 30 minutes then washed in developing buffer for a further 30 minutes. Fresh developing buffer was added and the gels were incubated for 16 hours at 37°C. Zymograms were stained with 0.5% Coomassie blue (BioRad) for 90 minutes before destaining until clear bands of MMP activity appeared against a blue background. The sum intensity of the bands was calculated using a Kodak Digital Science electrophoresis documentation and analysis system 120 (Eastman Kodak Co., Rochester, NY).
MMP-1 ELISA
Total MMP-1 was measured in undiluted conditioned media using a Biotrak human two-site sandwich ELISA (Amersham Pharmacia Biotech UK Limited, Buckinghamshire, UK) in accordance with the manufacturer’s instructions. Briefly, samples and standards were adsorbed onto duplicate wells of a microtiter plate precoated with anti-MMP-1 antibody. A secondary polyclonal antibody to MMP-1 was added and subsequently quantified using the horseradish-peroxidase conjugate/tetramethylbenzidine (TMB) substrate detection system.
MMP Inhibition during Contraction
To assess the involvement of MMPs in fibroblast-mediated matrix contraction, collagen gels were fed with 25 ng/ml of TGF-β1 or CTGF plus 1% bovine serum albumin together with concentrations of the broad-spectrum MMP inhibitor GM 6001 or the control molecule (0 to 100 μmol/L) before release.
Cell Viability
Fibroblast viability in the presence of each test medium was monitored using a kit incorporating the reagent WST-1 according to the manufacturer’s instructions (Boehringer Mannheim Diagnostics and Biochemicals, Lewes, UK). Briefly, fibroblasts were seeded into triplicate wells of 96-well tissue-culture plates, at a density of 2.5 × 103 per well in Dulbecco’s modified Eagle’s medium supplemented with 10% newborn calf serum, and cultured for 4 hours. The medium was aspirated and replaced with 100 μl of test medium. At time 0 and 4 days 10 μl of WST-1 reagent was added to each well and the plate was incubated at 37°C for 2 hours. The absorbance, related to the number of viable cells converting the reagent to colored formazan crystals, was read at 450 nm.
Statistical Analysis
Experiments were repeated at least three times. One-way analysis of variance was performed using computer software (SPSS for Windows; SPSS Inc., Chicago, IL). The observed significance levels were adjusted with the Bonferroni test for multiple comparisons. A P value of <0.05 was considered significant.
Results
TGF-β1 and CTGF Stimulate Fibroblast-Mediated Matrix Contraction
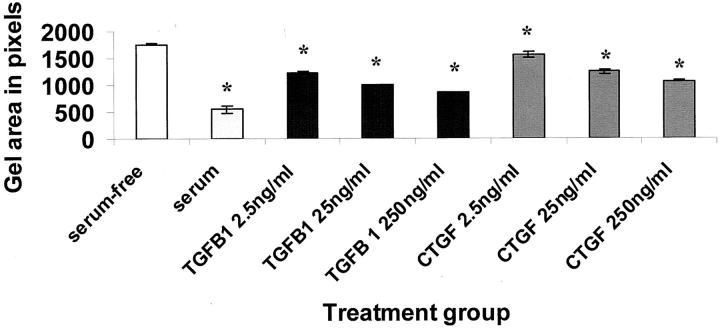
Remodeling and contraction of newly deposited extracellular matrix is an integral part of wound healing. To determine whether CTGF could stimulate fibroblast-mediated matrix contraction, fibroblast-populated collagen gels were used as an in vitro model of wound matrix contraction. TGF-β1 stimulated matrix contraction by fibroblasts in a dose-dependent manner, as did CTGF (P < 0.001; Figure 1 ▶ ). This indicated a new function for CTGF in regulating fibroblast activity.
Figure 1.
TGF-β1 and CTGF stimulated fibroblast-mediated collagen matrix contraction. Fibroblast-populated collagen gel areas, measured in pixels, are shown after 3 days of culture in the presence of concentrations of TGF-β1 or CTGF. Serum-free medium and medium containing 10% (v/v) serum were used as negative and positive controls, respectively. Both TGF-β1 and CTGF stimulated contraction in a concentration-dependent manner. The error bars represent the SEM. The asterisk indicates a significant difference (P < 0.05) from the serum-free control.
Inhibition of CTGF Gene Transcription Reduces TGF-β1-Stimulated Contraction
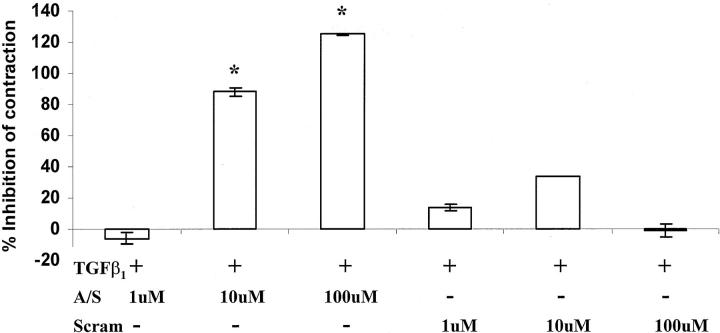
Because CTGF can mediate a number of TGF-β-stimulated functions in fibroblasts, 15,43,44 we hypothesized that CTGF may also mediate TGF-β1-stimulated matrix contraction. To test this, the fibroblast-populated collagen gels were cultured with TGF-β1 together with concentrations of anti-sense oligodeoxynucleotides to CTGF or with a scrambled CTGF oligodeoxynucleotide sequence control. As shown in Figure 2 ▶ , increasing the concentration of the CTGF anti-sense oligodeoxynucleotide from 1 to 100 μmol/L progressively reduced the percentage inhibition of contraction relative to the degree of contraction stimulated by TGF-β1 (P < 0.001). Furthermore, the highest dose of anti-sense oligodeoxynucleotide (100 μmol/L) reduced gel contraction to a level less than that of serum-free medium, suggesting that there is a basal level of expression of CTGF by fibroblasts in the absence of exogenously added TGF-β1. Addition of a scrambled 20 mer did not significantly reduce gel contraction stimulated by TGF-β1. These results suggest that CTGF mediates gel contraction stimulated by TGF-β1 in fibroblast-populated collagen matrix, and also suggests that there is a low, basal level of CTGF synthesis by fibroblasts even in the absence of exogenously added TGF-β1.
Figure 2.
The addition of anti-sense oligodeoxynucleotides to CTGF significantly inhibited fibroblast-mediated collagen contraction in the presence of 25 ng/ml of TGF-β1 after 2 days of culture. The data are plotted as the percentage inhibition of contraction relative to the degree of contraction stimulated by TGF-β1 only. Statistical analysis of the raw data (actual gel areas) indicated that concentrations of 10 and 100 μmol/L of anti-sense oligodeoxynucleotide to CTGF significantly inhibited matrix contraction compared to gels treated with TGF-β1 only. Although the scrambled sequence control seemed to have some inhibitory effect, it was not found to be statistically significant. The error bars represent the SEM. The asterisks indicate statistically significant differences (P < 0.05).
Inhibition of CTGF Gene Transcription Reduces Basal Levels of Contraction
Under serum-free conditions, anti-sense oligodeoxynucleotides to CTGF also significantly inhibited fibroblast-mediated matrix contraction by day 3 (P < 0.05; Figure 3 ▶ ). However, unlike the complete inhibition of contraction seen with the highest dose of anti-sense oligodeoxynucleotides to CTGF in cells primed with TGF-β1, less than half (44%) of contraction was inhibited under serum-free conditions.
Figure 3.
The addition of anti-sense oligodeoxynucleotides to CTGF significantly inhibited basal levels of fibroblast-mediated collagen contraction under serum-free conditions after 2 days of culture. The data are plotted as the percentage inhibition of contraction relative to the degree of contraction stimulated under serum-free conditions alone. Statistical analysis of the raw data (actual gel areas) indicated that concentrations of 1 and 100 μmol/L of anti-sense oligodeoxynucleotide to CTGF significantly inhibited matrix contraction compared to serum-free cultured gels. The scrambled sequence control had no significant effect. The error bars represent the SEM. The asterisks indicate statistically significant differences (P < 0.05).
Neutralization of CTGF Protein TGF-β1-Stimulated Matrix Contraction
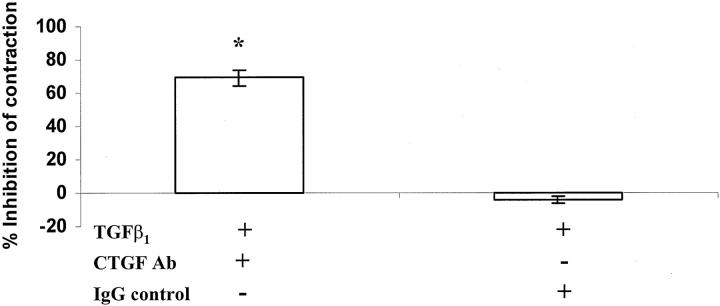
Addition of affinity-purified IgG antibodies that neutralize CTGF also significantly reduced TGF-β1-stimulated matrix contraction (P < 0.001; Figure 4 ▶ ), again supporting the premise that CTGF mediates promotion of matrix contraction by fibroblasts in response to TGF-β1.
Figure 4.
The addition of CTGF-neutralizing antibody significantly inhibited fibroblast-mediated collagen contraction in the presence of 25 ng/ml of TGF-β1 after 7 days of culture. Again the data are plotted as the percentage inhibition of contraction relative to the degree of contraction stimulated by TGF-β1 only. The IgG control had no significant effect on contraction. The error bars represent the SEM. The asterisk indicates a statistically significant difference (P < 0.05).
Anti-Sense Oligodeoxynucleotide-Inhibited CTGF mRNA Expression in bEND.3 Cells
The anti-sense oligodeoxynucleotides were tested for the ability to reduce CTGF mRNA levels using the bEND cell culture-screening assay (Figure 5) ▶ . The oligodeoxynucleotide with the sequence, GCC-AGA-AAG-CTC-AAA-CTT-GA, corresponding to nucleotides 987 to 1007 of the human CTGF mRNA, reduced the level of mouse CTGF mRNA in bEND cells by 86%, and was selected as the CTGF anti-sense oligodeoxynucleotide.
Figure 5.
Anti-sense oligodeoxynucleotides (MOE gapmers at 150 nmol/L) were tested for the ability to reduce CTGF mRNA levels using the bEND cell culture-screening assay. The oligodeoxynucleotide, with the sequence GCC-AGA-AAG-CTC-AAA-CTT-GA, corresponds to nucleotides 987 to 1007 of the human CTGF mRNA, reduced the level of mouse CTGF mRNA in bEND cells by 86%, and was selected as the CTGF anti-sense oligodeoxynucleotide. This is represented by the 12th bar from the left (the oligo with the lowest percent control value). The error bars represent the range from two experiments.
Anti-Sense Oligodeoxynucleotide Inhibited CTGF Protein Release in Human Fibroblasts
To assess if anti-sense oligodeoxynucleotide reduced CTGF gene transcription in human corneal fibroblasts, levels of CTGF protein in conditioned media was measured by ELISA. Increasing concentrations of the CTGF anti-sense 20 mer (1, 10, and 100 μmol/L) progressively decreased CTFG protein levels in conditioned media samples collected 24 hours (Figure 6 ▶ , open boxes) or 48 hours (Figure 6 ▶ , shaded boxes) after addition of TGF-β1 and the 20 mer. Addition of the scrambled sequence oligodeoxynucleotide (100 μmol/L) did not reduce the level of CTGF protein induced by TGF-β1 at 24 hours (threefold increase) and 48 hours (fivefold increase), indicating the effect of the anti-sense oligodeoxynucleotide was not because of nonspecific inhibition of protein synthesis.
Figure 6.
CTGF anti-sense oligodeoxynucleotide reduced CTGF protein produced by fibroblasts in the presence of TGF-β1. To determine the specificity of CTGF gene down-regulation, conditioned medium collected from contracting gels fed with 25 ng/ml of TGF-β1 or TGF-β1 plus concentrations of anti-sense oligodeoxynucleotide to CTGF or scrambled sequence control were assayed by ELISA at 24 and 48 hours to determine the levels of CTGF protein. At 24 hours all concentrations of the CTGF anti-sense oligodeoxynucleotide reduced CTGF protein in the presence of TGF-β1 (open boxes). By 48 hours the 1 μmol/L concentration was no longer potent (shaded boxes). The scrambled control sequence had no effect. The error bars represent SEM.
Gelatinases Are Produced during Fibroblast-Mediated Matrix Contraction
Members of the MMP family are up-regulated during a number of tissue-remodeling events such as embryogenesis and development, metastasis, and wound healing. 31,45,46 To determine whether MMPs were produced during in vitro contraction of collagen by fibroblasts, conditioned medium collected from contracting gels was evaluated by gelatin zymography (Figure 7) ▶ . Bands of proteolytic activity corresponded to the molecular weights of pro-MMP-9 (92 kd) and pro- and active MMP-2 (72 and 66 kd) were detected in all samples of conditioned media from gels that were incubated in serum-free medium (Figure 7A ▶ , lane 1) or medium supplemented with increasing concentrations of TGF-β1 (Figure 7A ▶ , lanes 2 to 5) or CTGF (Figure 7A ▶ , lanes 6 to 9). However, the levels of pro- or active MMP-2 in conditioned media did not change substantially, even though gel contraction increased with increasing concentrations of TGF-β1 (Figure 7B) ▶ or CTGF (Figure 7C) ▶ . The only exception was a decrease in active MMP-2 at the highest concentration of TGF-β1. The faint band corresponding to the molecular weight of pro-MMP-9 was not strong enough to accurately measure using the image analysis software.
Figure 7.
MMPs were produced during fibroblast-mediated collagen matrix contraction. Conditioned media collected from gels contracting in the presence of concentrations of TGF-β1 or CTGF for 3 days were analyzed by zymography for the presence of MMPs (A). The lanes represent MMP activity in medium collected from gels treated with: serum-free medium (lane 1), TGF-β1 at 0.25 ng/ml (lane 2), 2.5 ng/ml (lane 3), 25 ng/ml (lane 4), and 250 ng/ml (lane 5) and CTGF at 0.25 ng/ml (lane 6), 2.5 ng/ml (lane 7), 25 ng/ml (lane 8), and 250 ng/ml (lane 9). The top faint band corresponds to the molecular weight of pro-MMP-9, the middle band to pro-MMP-2, and the bottom band to active MMP-2. No stimulation above basal levels (lane 1) with either growth factor was detected. After semiquantitative image analysis of the bands, TGF-β1 appeared to have an inhibitory effect on active MMP-2 activity (B) while CTGF had little effect (C).
MMP-1 Is Produced during Fibroblast-Mediated Matrix Contraction
MMP-1 (collagenase) is capable of cleaving the triple helix structure of type I collagen 47 making it thermally unstable and susceptible to further proteolysis by gelatinases. Because MMP-1 may facilitate fibroblast-mediated matrix contraction by cleavage of collagen, the total amount of MMP-1 released into conditioned media during 48 hours of contraction was measured by ELISA. As shown in Figure 8A ▶ , addition of TGF-β1 (2.5, 25, and 250 ng/ml) did not alter levels of MMP-1 compared to the average level present in serum-free conditioned medium. In contrast, CTGF at 2.5 and 25 ng/ml induced a modest, yet statistically significant increase (P < 0.05), in MMP-1 levels.
Figure 8.
Using an ELISA measuring total MMP-1 protein in conditioned medium collected on day 3, it was found that TGF-β1 had no significant effect on MMP-1 protein production during collagen gel contraction (A). CTGF induced a modest, yet statistically significant increase in MMP-1 protein (P < 0.05) at the concentrations indicated by the asterisks (B). The error bars represent SEM.
Broad Spectrum MMP Inhibition Reduces TGF-β1 and CTGF-Stimulated Matrix Contraction by Fibroblasts
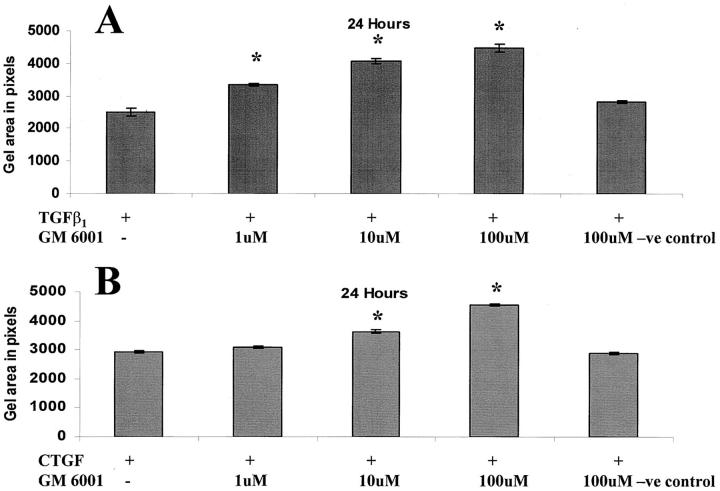
To determine whether MMPs were required for CTGF and/or TGF-β1-stimulated matrix contraction by fibroblasts, the broad-spectrum MMP inhibitor GM 6001 was added to the culture medium. MMP inhibition was found to significantly reduce both TGF-β1 and CTGF stimulated matrix contraction in a dose-dependent manner (P < 0.001; Figure 9 ▶ ). Addition of the inactive structural analogue of GM 6001 did not reduce contraction, and evaluation of metabolic activity using the WST-1 indicator of cell viability, showed that GM 6001 was not toxic to fibroblasts.
Figure 9.
Addition of the broad-spectrum MMP inhibitor, GM 6001, significantly (P < 0.05) reduced collagen contraction in a dose-dependent manner in gels cultured with 25 ng/ml of TGF-β1 (A) or 25 ng/ml of CTGF (B). The negative control analog of GM 6001 had no effect. The asterisks indicate significant differences in gel areas between growth factor-treated and growth factor plus MMP inhibitor-treated groups. The error bars represent the SEM.
Discussion
A series of highly orchestrated events lead to the deposition, remodeling, and contraction of matrix that occurs during the normal process of tissue repair after injury. Previous reports demonstrate that TGF-β plays a significant role in this process. 16 However, excessive matrix deposition and contraction induced by elevated levels of TGF-β can lead to pathological scarring. 48-53 As a result the TGF-β family has become a target for modulating the wound-healing response. This strategy has been shown to work clinically, 54 however, the control of scarring is still incomplete. Understanding the control mechanisms involved in TGF-β-mediated events should facilitate the development of new therapeutic strategies to control aberrant wound healing such as scarring.
Like TGF-β, CTGF acts as a fibroblast chemoattractant and mitogen and also stimulates production of extracellular matrix components. 11,14,43 The potential role of CTGF in fibroblast-mediated matrix contraction is currently unknown. Our data demonstrate CTGF, like TGF-β1, stimulates collagen matrix contraction in a dose-dependent manner in the absence of other exogenous growth factors. This indicates a potential new regulatory role for CTGF in the process of wound healing. After injury, resting fibroblasts become activated and start to migrate into the wound, generating tractional forces leading to matrix contraction. Fibroblasts become orientated parallel to the wound bed and along expected lines of stress. 26,27,55 The cells acquire focal adhesions, stress fibers, and extracellular fibronectin fibrils 55 and acquire a proto-myofibroblast phenotype. 27 The model used in our experiments is thought to mimic this process in vitro. 56 These new data expands the known activities of CTGF by demonstrating that CTGF is sufficient to induce fibroblast-mediated collagen matrix contraction.
The CTGF gene is transcriptionally activated by TGF-β, 10 and is known to mediate a variety of TGF-β-stimulated fibroblast activities. 15,43,44 However, no studies have previously looked at CTGF in relation to TGF-β-mediated wound contraction. This study shows for the first time that the potent stimulatory effects of TGF-β on fibroblast matrix contraction can be regulated by another growth factor, ie, CTGF. Our anti-sense oligodeoxynucleotide study clearly demonstrated that contraction of relaxed gels by TGF-β1-stimulation could be negated by inhibiting CTGF gene transcription and that this occurred without toxicity to the cells. Inhibition of CTGF protein activity using a neutralizing antibody corroborated these findings.
TGF-β1 stimulates the differentiation of fibroblasts (or protomyofibroblasts) into myofibroblasts that are characterized by de novo expression of α-smooth muscle actin, increased expression of ED-A fibronectin, increased expression of stress fibers, and increasingly complex formation of focal adhesions. 27 However, for TGF-β1 to achieve the transition from fibroblast to myofibroblast via the Smad signaling pathway, 57 mechanical tension must also be applied to the cells. 55,58 Our contraction model confirmed these findings because very few cells with assembled α-smooth muscle actin stress fibers, ie, myofibroblasts were observed (unpublished data). This indicates a role for TGF-β1 in the generation of tractional forces by fibroblasts in a collagen matrix under the control of CTGF. In fact, recent data have shown that CTGF activates TGF-β signals by direct binding in the extracellular space. 59 Perhaps this mechanism is involved in complex process of TGF-β-mediated matrix contraction. Our data has very important implications for TGF-β biology as it demonstrates a new and alternative control mechanism for this procontractile influence of TGF-β on fibroblasts. Interestingly, in the absence of any exogenous stimulation by growth factors, inhibition of CTGF gene expression was not as potent. This suggests that although CTGF still seems to be involved it is likely that alternative signaling pathways are being used by the cells in the absence of exogenous or in the presence of autocrine levels of TGF-β1. This data further supports the hypothesis that CTGF is an important mediator of TGF-β1-stimulated collagen matrix contraction by fibroblasts.
The requirement of MMP activity for the penetration and movement of a number of cell types through ECM has been identified. 30,60-62 These reports suggest that movement of cells through ECM and subsequent matrix contraction (processes involved in scarring) may involve MMPs. We recently demonstrated that broad-spectrum MMP inhibition reduced matrix deposition, contraction, and scarring in a rabbit model of subconjunctival wound healing. 34 Furthermore, we recently reported that fibroblast-mediated matrix contraction induced by cellular tractional forces is dependent on MMP activity. 29 In those in vitro experiments the fibroblasts were responding to serum. In the current study we investigated the potential involvement of MMPs in TGF-β1 and CTGF-mediated collagen matrix contraction. During contraction TGF-β1 did not up-regulate the levels of the gelatinases MMP-2 and MMP-9 or the collagenase MMP-1. Yet basal levels of MMPs were required for contraction because the presence of TGF-β1 was not sufficient to stimulate contraction in the face of broad-spectrum MMP inhibition. In contrast CTGF induced a modest increase in MMP-1 protein production during contraction, which was reduced by MMP inhibition. Although the increase in MMP-1 by CTGF was found to be statistically significant, the potential biological significance of this finding is yet to be established. CTGF has recently been shown to increase MMPs -2, -3, -9, and -14 in vascular endothelial cells possibly for initiation of angiogenesis during early hypoxia. 63 The interactions between these growth factors and MMPs during fibroblast-mediated matrix contraction are clearly complex and the subject of further investigation. These data do however indicate a novel feature of both TGF-β1 and CTGF function in the utilization of MMPs for fibroblast-mediated matrix contraction.
Using gene array technology, CTGF gene expression was found to be stress-responsive. 64 A fourfold to sixfold increase was found in the mRNA levels for CTGF from fibroblasts cultured in tethered collagen gels, which induce mechanical stress on the cells, compared to fibroblasts cultured in free-floating relaxed gels as in our experiments. The authors also suggested that TGF-β alone is not sufficient to induce full induction of CTGF expression as the application of neutralizing antibodies to TGF-β could not negate CTGF expression induced by mechanical stress. 64 Excessive contraction of extracellular matrix by myofibroblasts is thought to play a key role in fibrotic pathologies. It has previously been shown that CTGF did not mediate TGF-β induction of the myofibroblast phenotype when fibroblasts were cultured in the presence of the growth factor on plastic. 65 However, the cells were not studied within a matrix under mechanical stress. To further dissect the mechanisms by which CTGF may contribute to matrix contraction, which is thought to occur via a combination of fibroblast migration into a wound and myofibroblast contraction within the wound, 18 the ability of CTGF to induce the myofibroblast phenotype under mechanical stress is currently being investigated in our laboratory.
Our present data has highlighted several novel functions of CTGF; stimulation of fibroblast-mediated collagen matrix contraction, utilization of MMPs for this contraction and the regulation of TGF-β1-mediated matrix contraction. These findings help to further our understanding of CTGF and its important interactions with TGF-β during events crucial to wound healing. The data are also important clinically as they suggest CTGF may be a suitable therapeutic target for the control of contractile scarring.
Footnotes
Address reprint requests to Julie T. Daniels, Ph.D., Epithelial Repair and Regeneration Group, Wound Healing Research Unit, Divisions of Pathology and Cell Biology, Institute of Ophthalmology, Bath St., London EC1V 9EL, UK. E-mail: j.daniels@ucl.ac.uk.
Supported by the Royal National Institute for the Blind, UK (to J. T. D., P. T. K.); the National Institutes of Health (grant number NEI EYO 5587 to G. S. S., T. D. B.); the Eranda Foundation (to J. T. D.); the Hayman Trust (to J. T. D.); the Moorfields Eye Hospital Special Trustees (to J. T. D.); and the Moorfields Eye Hospital Executive Trust (to J. T. D., P. T. K.).
The views presented represent those of the authors and not necessarily those of the funding bodies.
References
- 1.Igarashi A, Okochi H, Bradham DM, Grotendorst GR: Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell 1993, 4:637-645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine JH, Moses HL, Gold LI, Nanney LB: Spatial and temporal patterns of immunoreactive transforming growth factor beta 1, beta 2, beta 3 during excisional wound repair. Am J Pathol 1993, 143:368-380 [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts AB, Sporn MB: Physiological actions and clinical applications of transforming growth factor beta (TGF-B). Growth Factors 1993, 8:1-9 [DOI] [PubMed] [Google Scholar]
- 4.Wahl SM: Transforming growth factor beta in inflammation. J Immunol 1992, 12:61-74 [DOI] [PubMed] [Google Scholar]
- 5.Raghow R: Role of transforming growth factor B in repair and fibrosis. Chest 1991, 99:s61-s65 [DOI] [PubMed] [Google Scholar]
- 6.Battegay EJ, Raines EW, Seifert RA, Bowen-Pope DF, Ross R: Transforming growth factor beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine platelet-derived growth factor loop. Cell 1990, 63:515-524 [DOI] [PubMed] [Google Scholar]
- 7.Ignotz R, Massague J: Transforming growth factor beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem 1986, 261:4337-4345 [PubMed] [Google Scholar]
- 8.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehri JR, Fauci AS: Transforming growth factor type B: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in-vitro. Proc Natl Acad Sci USA 1986, 83:4167-4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laiho M, Saksela O, Andreasen PA, Keski-Oja J: Enhanced production and extracellular deposition of the endothelial-type plasminogen activator inhibitor in cultured human lung fibroblasts by transforming growth factor B. J Cell Biol 1986, 103:2403-2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grotendorst GR, Okochi H, Hayashi N: A novel transforming growth factor-B response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ 1996, 7:469-480 [PubMed] [Google Scholar]
- 11.Bradham DM, Igarashi A, Potter RL, Grotendorst GR: Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol 1991, 114:1285-1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soma Y, Grotendorst GR: TGF-beta stimulates primary human skin fibroblast DNA synthesis via an autocrine production of PDGF-related peptides. J Cell Physiol 1989, 140:246-253 [DOI] [PubMed] [Google Scholar]
- 13.Grotendorst GR: Connective tissue growth factor: a mediator of TGF-B action on fibroblasts. Cytokine Growth Factor Rev 1997, 8:171-179 [DOI] [PubMed] [Google Scholar]
- 14.Frazier KS, Williams S, Kothapalli D, Klapper H, Grotendorst GR: Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol 1996, 107:404-411 [DOI] [PubMed] [Google Scholar]
- 15.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR: Connective tissue growth factor mediates transforming growth factor-beta-induced collagen synthesis: down-regulation by cAMP. FASEB 1999, 13:1774-1786 [PubMed] [Google Scholar]
- 16.Clark RAF, Henson PM: Chapter 17. The Molecular and Cellular Biology of Wound Repair. 1988:pp 373-394 Plenum Press, New York
- 17.Daniels JT, Occleston NL, Crowston JG, Cordeiro MF, Alexander RA, Wilkins M, Porter R, Brown RA, Khaw PT: Understanding and controlling the scarring response: the contribution of histology and microscopy. Microsc Res Tech 1998, 42:317-333 [DOI] [PubMed] [Google Scholar]
- 18.Nedelec B, Ghahary A, Scott PG, Tredget EE: Control of wound contraction: basic and clinical features. Hand Clinics 2000, 16:289-302 [PubMed] [Google Scholar]
- 19.Bissell DM: Chronic liver injury. Exp Mol Med 2001, 33:179-190 [DOI] [PubMed] [Google Scholar]
- 20.Zeisberg M, Strutz F, Muller GA: Renal fibrosis: an update. Curr Opin Nephrol Hypertens 2001, 10:315-320 [DOI] [PubMed] [Google Scholar]
- 21.Wong TT, Sethi C, Daniels JT, Limb GA, Murphy G, Khaw PT: Matrix metalloproteinases in disease and repair processes in the anterior segment. Surv Ophthalmol 2002, 47:239-256 [DOI] [PubMed] [Google Scholar]
- 22.Wunderlich K, Pech M, Eberle AN, Mihatsch M, Flammer J, Meyer P: Expression of connective tissue growth factor (CTGF) mRNA in plaques of human anterior subcapsular cataracts and membranes of posterior capsule opacification. Curr Eye Res 2000, 21:627-636 [PubMed] [Google Scholar]
- 23.Harris AK, Stopak D, Wild P: Fibroblast traction as a mechanism for collagen morphogenesis. Nature 1981, 290:249-251 [DOI] [PubMed] [Google Scholar]
- 24.Ehrlich HP, Rajaratnam JB: Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue Cell 1990, 22:407-417 [DOI] [PubMed] [Google Scholar]
- 25.Eastwood M, McGrouther DA, Brown RA: A culture force monitor for measurement of contraction forces generated in human dermal fibroblast cultures: evidence for cell-matrix mechanical signalling. Biochim Biophys Acta 1994, 1201:186-192 [DOI] [PubMed] [Google Scholar]
- 26.Gabbiani G, Ryan GB, Majno G: Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971, 27:549-550 [DOI] [PubMed] [Google Scholar]
- 27.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA: Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature 2002, 3:349-363 [DOI] [PubMed] [Google Scholar]
- 28.Bell E, Ivarsson B, Merrill C: Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA 1979, 76:1274-1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniels JT, Cambrey AD, Occleston NL, Garrett Q, Tarnuzzer RW, Schultz GS, Khaw PT: Matrix metalloproteinase inhibition modulates fibroblast-mediated contraction and production in vitro. Invest Ophthalmol Vis Sci 2003, 44:1104-1110 [DOI] [PubMed] [Google Scholar]
- 30.Sheridan CM, Occleston NL, Hiscott P, Kon CH, Khaw PT, Grierson I: Matrix metalloproteinases: a role in the contraction of vitreo-retinal scar tissue. Am J Pathol 2001, 159:1555-1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birkedal-Hansen H: Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol 1995, 7:728-735 [DOI] [PubMed] [Google Scholar]
- 32.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S: Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 2002, 109:625-637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y: Matrix metalloproteinases cleave connective tissue growth factor and reactivate activity of vascular endothelial growth factor. J Biol Chem 2002, 277:36288-36295 [DOI] [PubMed] [Google Scholar]
- 34.Wong TTL, Mead AL, Khaw PT: Matrix metalloproteinase inhibition modulates post-operative scarring following experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci 2003, 44:1097-1103 [DOI] [PubMed] [Google Scholar]
- 35.Montesano R, Orci L: Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA 1988, 85:4894-4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jester JV, Petroll WM, Cavanagh HD: Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res 1999, 18:311-356 [DOI] [PubMed] [Google Scholar]
- 37.Van der Boom R, Wilmink JM, O’Kane S, Wood J, Ferguson MW: Transforming growth factor beta levels during second intention healing are related to the different course of wound contraction in horses and ponies. Wound Repair Regener 2002, 10:188-194 [DOI] [PubMed] [Google Scholar]
- 38.Dean NM, McKay R, Condon TP, Bennett CF: Inhibition of protein kinase C-alpha expression in human A549 cells by antisense oligonucleotides inhibits induction of intercellular adhesion molecule 1 (ICAM-1) mRNA by phorbol esters. J Biol Chem 1994, 23:16416-16424 [PubMed] [Google Scholar]
- 39.McGraw K, McKay R, Miraglia L, Boggs RT, Pribble JP, Muller M, Geiger T, Fabbro D, Dean NM: Antisense oligonucleotide inhibitors of isozymes of protein kinase C: in vitro and in vivo activity, and clinical development as anti-cancer therapeutics. Anti-Cancer Drug Design 1997, 12:315-326 [PubMed] [Google Scholar]
- 40.Fini ME, Cui TY, Mouldovan A, Grobelny D, Galardy RE, Fisher SJ: An inhibitor of the matrix metalloproteinases synthesized by rabbit corneal epithelium. Invest Ophthalmol Vis Sci 1991, 32:2997-3001 [PubMed] [Google Scholar]
- 41.Grobelny D, Poncz L, Galardy RE: Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry 1992, 31:7152-7154 [DOI] [PubMed] [Google Scholar]
- 42.Daniels JT, Khaw PT: Temporal stimulation of corneal fibroblast wound healing activity by differentiating epithelium in vitro. Invest Ophthalmol Vis Sci 2000, 41:3754-3762 [PubMed] [Google Scholar]
- 43.Kothapalli D, Frazier K, Grotendorst GR: TGF-B induces anchorage-independent growth of NRK fibroblasts via the synergistic action of CTGF-dependent and CTGF-independent signalling pathways. Cell Growth Differ 1997, 8:61-68 [PubMed] [Google Scholar]
- 44.Kothapalli D, Hayashi N, Grotendorst GR: Inhibition of TGF-beta stimulated CTGF gene expression and anchorage independent growth by elevation of intracellular cAMP. FASEB J 1998, 12:1151-1161 [DOI] [PubMed] [Google Scholar]
- 45.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal Hansen B, DeCarlo A, Engler JA: Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 1993, 4:197-250 [DOI] [PubMed] [Google Scholar]
- 46.Brinkerhoff CE, Matrisian LM: Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol 2002, 4:207-214 [DOI] [PubMed] [Google Scholar]
- 47.Gross J, Nagai Y: Specific degradation of the collagen molecule by tadpole collagenolytic activity. Proc Natl Acad Sci USA 1965, 54:1197-1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kulozik M, Hogg A, Lankat-Buttgereit B, Krieg T: Co-localisation of TGF-B and type I procollagen mRNA in tissue sections of patients with systemic disease. J Clin Invest 1990, 86:917-922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagy P, Schaff Z, Lapis K: Immunohistochemical detection of TGFB1 in fibrotic liver disease. Hepatology 1991, 14:269-273 [PubMed] [Google Scholar]
- 50.Kagami S, Border WA, Ruoslahti E, Noble NA: Coordinated expression of beta 1 integrins, and transforming growth factor-beta induced matrix proteins in glomerulonephritis. Lab Invest 1993, 69:68-76 [PubMed] [Google Scholar]
- 51.O’Kane S, Ferguson MW: Transforming growth factor betas and wound healing. Int J Biochem Cell Biol 1997, 29:63-78 [DOI] [PubMed] [Google Scholar]
- 52.Cordeiro MF, Reichel MB, Gay JA, D’Esposita F, Alexander RA, Khaw PT: Transforming growth factor-beta1, -beta2, and -beta3 in vivo: effects on normal and mitomycin C-modulated conjunctival scarring. Invest Ophthalmol Vis Sci 1999, 40:1975-1982 [PubMed] [Google Scholar]
- 53.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S: Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res 2000, 19:113-129 [DOI] [PubMed] [Google Scholar]
- 54.Siriwardena D, Khaw PT, King AJ, Donaldson ML, Overton BM, Migdal C, Cordeiro MF: Human antitransforming growth factor beta (2) monoclonal antibody—a new modulator of healing in trabeculectomy: a randomized placebo controlled clinical study. Ophthalmology 2002, 109:427-431 [DOI] [PubMed] [Google Scholar]
- 55.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G: Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 2001, 159:1009-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grinnell F: Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 1994, 124:401-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Massague J, Wotton D: Transcriptional control by the TGF-B/Smad signalling system. EMBO J 2000, 19:1745-1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G: Transforming growth factor beta 1 induces alpha-smooth actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993, 122:103-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abreu JG, Ketpura NI, Reversade B, Robertis EM: Connective tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol 2002, 8:599-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA: Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res 1994, 75:539-545 [DOI] [PubMed] [Google Scholar]
- 61.Leppert D, Waubant E, Galardy R, Bunnett NW, Hauser SL: T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol 1995, 154:4379-4389 [PubMed] [Google Scholar]
- 62.Terada T, Okada Y, Nakanuma Y: Expression of matrix proteinases during human intrahepatic bile duct development. A possible role in biliary cell migration. Am J Pathol 1995, 147:1207-1213 [PMC free article] [PubMed] [Google Scholar]
- 63.Kondo S, Kubota S, Shimo T, Nishida T, Yosimichi G, Eguchi T, Sugahara T, Takigawa M: Connective tissue growth factor increased by hypoxia may initiate angiogenesis in collaboration with matrix metalloproteinases. Carcinogenesis 2002, 23:769-776 [DOI] [PubMed] [Google Scholar]
- 64.Schild C, Trueb B: Mechanical stress is required for high-level expression of connective tissue growth factor. Exp Cell Res 2002, 274:83-91 [DOI] [PubMed] [Google Scholar]
- 65.Folger PA, Zekaria D, Grotendorst GR, Masur SK: Transforming growth factor beta-stimulated connective tissue growth factor expression during corneal myofibroblast differentiation. Invest Ophthalmol Vis Sci 2001, 42:2534-2541 [PubMed] [Google Scholar]