Abstract
Chemokines play an important role in leukocyte mobilization, hematopoiesis, and angiogenesis. Tissue-specific expression of particular chemokines also influences tumor growth and metastasis. Here, the CC chemokine pulmonary and activation-regulated chemokine (PARC)/CCL18 was measured in pediatric patients with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML). Surprisingly, PARC immunoreactivity was consistently detected in plasma from healthy donors. After purification to homogeneity, the presence of intact PARC (1–69) and processed PARC (1–68) in normal human plasma was confirmed by sequence and mass spectrometry analysis. Furthermore, PARC serum levels were significantly increased in children with T-ALL and prepreB-ALL compared to control serum samples, whereas serum levels in AML and preB-ALL patients were not significantly different from controls. In contrast, the hemofiltrate CC chemokine-1 (HCC-1)/CCL14 was not found to be a biomarker in any of these patients’ strata, whereas the cytokine interleukin-6 (IL-6) was significantly decreased in AML and prepreB-ALL. Stimulated leukocytic cell lines or lymphoblasts from patients produced IL-8/CXCL8 or macrophage inflammatory protein-1α (MIP-1α/CCL3) but not PARC, not even after IL-4 or IL-10 treatment. However, PARC was produced by superantigen or IL-4 stimulated monocytes co-cultured with lymphocytes or lymphoblastic cells. Serum PARC levels thus constitute a novel leukemia marker, possibly reflecting tumor/host cell interactions in the circulation.
Chemokines are chemotactic cytokines that regulate leukocyte migration during inflammatory responses, as well as homeostatic trafficking of lymphocytes and dendritic cells. 1,2 Their primary structure is characterized by the presence of four conserved cysteine residues. Based on the positioning of the two NH2-terminal cysteines, the chemokine family can be structurally divided into CC chemokines and CXC chemokines. Only a few members of both chemokine groups constitutively circulate at high concentration in plasma (eg, the hemofiltrate CC chemokine-1 (HCC-1)/CCL14 and the CXC chemokines platelet factor-4 (PF-4)/CXCL4 and β-thromboglobulin (β-TG)/CXCL7). 3-6 As for many other plasma proteins, their precise biological role remains poorly understood. It is expected that they exert homeostatic functions, although these chemokines have rather weak chemotactic capacities and often need to be proteolytically activated. 5,7 Alternatively, plasma chemokines might influence the formation of new blood vessels (angiogenesis) as was first shown for PF-4. 8 Other members of the CXC chemokine family are also known to be either angiogenic [interleukin-8 (IL-8)/CXCL8] or angiostatic [IFN-γ-inducible protein-10 (IP-10)/CXCL10]. 9 This duality in positive versus negative mutual regulatory activities of chemokines is also eminent in their control of the proliferation of hematopoietic precursors in the bone marrow. 10 For example, the CC chemokine macrophage inflammatory protein-1α (MIP-1α)/CCL3 exerts inhibitory effects on hematopoiesis, 11 whereas the CXC chemokine stromal cell-derived factor-1α (SDF-1α)/CXCL12 has been discovered as a growth factor for pre-B cells. 12
Chemokines activate leukocytes via G-protein coupled receptors, which mediate most of their various biological activities. 13 For some recently discovered chemokines, eg, the CC chemokine pulmonary and activation-regulated chemokine (PARC)/CCL18, which is structurally most closely related to MIP-1α, no receptor has been characterized yet. 14 Enhanced levels of this chemokine were detected in the synovial fluid of arthritis patients and in the ascitic fluid of patients with ovarian carcinoma. 15,16 The broad implication of chemokines in tumor development and metastasis has been evidenced recently by the demonstration of chemokine receptor expression on cell types, other than leukocytes. It has been shown that the CXC chemokine receptor 4 (CXCR4) is expressed on various cancer cell types and is responsible for the metastasis of tumor cells to organs expressing its ligand SDF-1. 17 Since most hematopoietic cells simultaneously express various chemokine receptors, these molecules may not only be implicated in maturation and physiological migration of normal leukocytes but may also influence the pathological development of hematopoietic cancer cells and play a role in their abnormal migration to other organs. For example, we previously found that MIP-1α is a potent autocrine chemoattractant for lymphoma cells and can prevent metastasis of these cells to chemokine expressing organs such as kidney and liver. 18,19 Therefore, we investigated the potential involvement of two other related CC chemokines, PARC and the plasma chemokine HCC-1, in lymphoproliferative diseases.
Materials and Methods
Patients and Patient Samples
From October 1988 to October 2002, 237 patients were admitted for newly diagnosed acute lymphoblastic leukemia (ALL) at the University Hospital of Gent. Treatment was given according to the BFM-based EORTC-58881 and 58951 protocols for children with ALL. During the same time period, 44 patients with acute non-lymphoblastic leukemia (ANLL) were admitted and treated in accordance with the EORTC 58921 protocol for children with acute myeloid leukemia (AML). Classification of leukemia was performed according to French-American-British (FAB) morphological criteria and standard immunophenotyping techniques (European Group for the Immunological Characterization of Leukemias, EGIL). 20
Frozen serum samples from newly diagnosed patients before any transfusion were available from approximately 100 patients. Of these, serum samples from 51 patients were used for more extensive analysis, namely all AML (n = 13) and T-ALL (n = 11) patients and a comparable number of randomly selected precursor B-lineage patients (n = 27; 16 prepreB-ALL, 11 preB-ALL) (Table 1) ▶ . The risk factor (Table 1) ▶ gives an estimation of the leukemic cell mass. 21 The definition of central nervous system (CNS) involvement was based on ≥5 cells/μl cerebrospinal fluid and the presence of lymphoblasts.
Table 1.
Clinical Data of Children with Newly Diagnosed Acute Leukemia
| Total patients in group | AML 13 | T-ALL 11 | PrepreB-ALL 16 | PreB-ALL 11 |
|---|---|---|---|---|
| Sex (male/female) | 8/5 | 11/0 | 11/5 | 4/7 |
| Age | ||||
| <1 years (n) | 1 | 0 | 0 | 0 |
| 1–6 years (n) | 7 | 4 | 9 | 6 |
| ≥6 years (n) | 5 | 7 | 7 | 5 |
| WBC (103/mm3) (mean) | 30.0 | 120.8 | 28.8 | 14.1 |
| % blasts in PB (median) | 27 | 41 | 65 | 28 |
| % blasts in BM (median) | 44 | 66 | 83.5 | 77 |
| Risk factor* | ||||
| <0.8 (n) | NA | 2 | 3 | 5 |
| 0.8–1.2 (n) | NA | 2 | 7 | 5 |
| ≥1.2 (n) | NA | 7 | 6 | 1 |
| Positive corticoresponse† (n) | NA | 10 | 16 | 10 |
| CNS disease‡ (n) | 0 | 1 | 3 | 0 |
| Abnormal karyotype (n) | 12 | 3 | 9 | 2 |
Abbreviations: BM, bone marrow; CNS, central nervous system; n, number of patients; NA, not applicable; PB, peripheral blood; WBC, white blood cells count in peripheral blood.
*The risk factor (RF) was used to estimate the leukemic cell mass and organ infiltration and was calculated by the equation: RF = 0.2 × log (number of blood blasts/μL + 1) + 0.06 liver (cm*) + 0.04 × spleen (cm*) (*below costal margin). 21
†A patient was considered to be responsive to corticosteroid therapy, when after 1 week of treatment, the number of blasts in the peripheral blood was reduced to <103 cells/ml.
‡The definition of central nervous system involvement was based on ≥5 cells/μl cerebrospinal fluid and the presence of lymphoblasts.
Of these 51 patients, 34 were male and 17 female, the difference primarily due to the predominance of males in the T-ALL group (Table 1) ▶ . The median age at diagnosis was 5.5 years (range 7 months to 16 years). In the AML patient group, there were two secondary leukemias: one patient with secondary AML, 9 years after primary diagnosis of ALL, and a patient with monoblastic leukemia, 34 months after diagnosis of inoperable CNS tumor and treated with chemotherapy including VP-16 (total dose 2.4 g/m2 over 4 months, after which complete tumor resection was possible). The FAB classification of the AML patients was as follows: 3 M1, 5 M2, 2 M5a, 1 M5, 1 M6, and 1 M7 patient. Three patients (1 T-ALL, 1 preB-ALL, and 1 prepreB-ALL) had very high PARC levels in their serum (>200 ng/ml). Therefore these three patients were excluded from this study.
In addition to serum samples, frozen samples from bone marrow aspirates, as well as frozen samples of spinal fluid from AML or ALL patients were analyzed for cytokine and chemokine content. Control pediatric sera (n = 21) sampled in the same hospital as where the pediatric leukemia patients were treated, were used to perform statistical analysis. Finally, samples derived from buffy coats of 42 healthy, adult donors at the blood transfusion center of Antwerp were centrifuged to separate the plasma from the cellular fraction. These plasma samples were stored at −20°C until analysis in the PARC and HCC-1 ELISA.
IL-6 Bioassay and Chemokine ELISAs
The growth promoting activity of IL-6 was assayed on the factor-dependent mouse B-cell hybridoma 7TD-1. 22 7TD-1 cells were seeded in microtiter plates (2000 cells/200 μl) in the presence of serial dilutions of an internal standard (10.000 U/ml) of pure IL-6 23 or serum from leukemic patients. Sample potency was evaluated from the cell densities by colorimetric determination of hexosaminidase levels 24 after 4 days of culture, one U/ml of IL-6 corresponding to the dilution associated with half-maximal cell growth. The presence of IL-6 in serum from leukemic patients was confirmed by neutralization of its hybridoma growth activity with a monoclonal antibody directed against natural human IL-6.
Sandwich ELISAs for PARC and HCC-1 were developed with polyclonal antisera (goat anti-human PARC and goat anti-human HCC-1 from R&D Systems, Abingdon, UK; rabbit anti-human PARC and rabbit anti-human HCC-1 from PeproTech, Rocky Hill, NJ). The detection limit of the PARC and HCC-1 ELISA was 0.01 ng/ml and 0.05 ng/ml, respectively. Human monocyte chemotactic protein-1 (MCP-1), IL-8, and MIP-1α immunoreactivities were measured according to a similar procedure. 16 Recombinant human chemokines (PARC and MIP-1α from R&D Systems, HCC-1 from PeproTech) or purified natural chemokine (MCP-1, IL-8) 25 were used as standards. The specificity of the chemokine ELISAs was demonstrated by the lack of cross-reactivity with other chemokines and other potentially cross-reactive agents used as inducers (vide infra induction of cell cultures). All sera samples were diluted at least 1/100 and were analyzed in at least three independent PARC assays. In addition, all patients’ samples were analyzed in the same assay to exclude the possibility that observed differences in PARC levels between patients were due to inter-assay variation.
Purification of PARC and HCC-1 Immunoreactivity from Plasma
A standard procedure, developed in our laboratory for the purification of chemokines from conditioned media, was applied to isolate chemokines from plasma. 26,27 Normal human plasma (2.4 l, pool of six plasma donations at the blood transfusion center of Leuven, Belgium) was first incubated with 0.1 mol/L CaCl2 (0.6 l) for 1 hour at 37°C to allow activation of the clotting cascade. Protein aggregates were subsequently removed by centrifugation. After coagulation, the serum was inactivated for 30 minutes at 56°C before concentration and partial purification by adsorption to silicic acid (Matrex Silica, particle size 35 to 70 μm, pore size 10 nm; Millipore, Bedford, MA). To optimize interaction with silicic acid, the serum was diluted 1/5 in Eagle’s minimum essential medium with Earle’s salts (EMEM; Invitrogen Life Technologies, Merelbeke, Belgium) and stirred with 10 g/L silicic acid at 4°C for 2 hours. The silicic acid was sedimented by centrifugation and washed with phosphate-buffered saline (PBS) containing 1 mol/L NaCl. Adsorbed proteins were eluted at neutral pH in cold PBS, containing 1.4 mol/L NaCl and 50% ethylene glycol. Subsequently, the silicic acid eluate was dialyzed against equilibration/loading buffer (50 mmol/L Tris/HCl, 50 mmol/L NaCl, pH 7.4) before fractionation by heparin-Sepharose affinity chromatography (Amersham Biosciences, Uppsala, Sweden). Proteins were eluted from the column in a linear NaCl gradient (0.05 to 2 mol/L NaCl in the loading buffer; 5 ml fractions). For all fractions, the protein concentration was determined by a Coomassie blue G-250 binding assay using the Bio-Rad commercial kit (Bio-Rad Laboratories, Hercules, CA). For further purification, fractions containing immunoreactivity were prepared for Mono-S cation-exchange fast protein liquid chromatography (FPLC; Amersham Biosciences) by dialysis against 50 mmol/L formate, pH 4.0. A linear NaCl (0 to 1 M) gradient in 50 mmol/L formate, pH 4.0 was used to elute proteins (1 ml fractions). Finally, chemokines were purified to homogeneity by reverse-phase high performance liquid chromatography (RP-HPLC). Samples were injected on a C-8 Aquapore RP-300 column (PerkinElmer, Norwalk, CT) or Resource RPC column (Amersham Biosciences), equilibrated with 0.1% trifluoroacetic acid (TFA) in water and the proteins were eluted with an acetonitrile gradient (0 to 80% in equilibration buffer).
After each purification step, fractions were analyzed for purity by SDS-PAGE under reducing conditions on Tris/tricine gels. 28 Proteins were visualized by silver staining. The relative molecular mass (Mr) markers included in the gels were ovalbumin (Mr 43,000), carbonic anhydrase (Mr 29,000), β-lactoglobulin (Mr 18,400), lysozyme (Mr 14,300) (Gibco/Life Technologies, Paisley, Scotland), and the low molecular mass marker aprotinin (Mr 6500) (Pierce Chemical Co., Rockford, IL).
For mass spectrometry, RP-HPLC fractions (containing minimum 3 μg/ml) were diluted 1/10 in 0.1% acetic acid/50% acetonitrile/50% H2O and sprayed at 5 μl/min in the source of an ESQUIRE ion trap mass spectrometer (Bruker/Daltonic, Bremen, Germany). The average Mr of peptides or proteins was calculated from 1000 or more averaged spectra to increase the accuracy of the mass/charge measurements. NH2-terminal sequence analysis was performed on a Procise 491 cLC protein sequencer (Applied Biosystems, Foster City, CA).
Induction of Cell Cultures for Chemokine Production
The human cell lines THP-1 (monocytic leukemia), HL60 (promyelocytic leukemia), MOLT-4 (acute T lymphoblastic leukemia), MT4 (T-cell lymphoblast), Sup-T1 (non-Hodgkin’s T-cell lymphoma), and Namalwa (Burkitt’s lymphoma) were cultured in RPMI 1640 (BioWhittaker Europe, Verviers, Belgium) enriched with 10% fetal calf serum (FCS; Invitrogen Life Technologies). For induction, these cell lines were seeded in 24-well plates (1.9 cm2; Techno Plastic Products AG, Trasadingen, Switzerland) in RPMI 1640 supplemented with 2% FCS (THP-1 and HL60) or 10% FCS (Sup-T1, MT4, and Namalwa) at 2 × 106 cells/ml. Subsequently, different concentrations of the following inducers were added: phorbol 12-myristate 13-acetate (PMA; Sigma, St. Louis, MO), concanavalin A (ConA; Calbiochem, La Jolla, CA), lipopolysaccharide (LPS; E. coli 0.111.B4; Difco, Detroit, MI), S. aureus enterotoxin A (SEA; Toxin Technology Inc., Sarasota, FL), measles virus [Attenuvax strain, 106.5 50% tissue culture infectious doses/ml (TCID50/ml)], the double-stranded (ds) RNA poly(riboinosinic).poly(ribocytidylic acid) (poly rI:rC; P-L Biochemicals, Milwaukee, WI), recombinant human IFN-γ (Bioferon, Laupheim, Germany), recombinant human IL-4 (PeproTech), recombinant human IL-10 (PeproTech), or pure natural human IL-1β. 23 Supernatants were harvested after 48 hours and stored at −20°C until assay.
Human peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood of single donors as described previously 16 and fractionated into monocytes and lymphocytes by adherence to plastic and magnetic cell sorting, respectively. Monocytes were enriched by seeding PBMC at 2 × 106 cells/well (1 ml/well) in 24-well plates, followed by a 2-hour adhesion period at 37°C. Afterward, non-adherent cells were removed and the adherent cells were washed with EMEM enriched with 2% FCS. Normal blood lymphocytes were isolated using the StemSep T-cell enrichment protocol (StemCell Technologies France, Meylan, France). Subsequently, 105 or 106 freshly isolated autologous lymphocytes or cultured T lymphoblastic cells (Sup-T1 or MOLT-4 in EMEM supplemented with 5% FCS) or the conditioned medium from these T lymphoblastic cells (collected 24 hours after subcultivation of unstimulated cells) were added to the adherent monocyte cultures. The co-cultures were stimulated with 300 ng/ml SEA or 30 ng/ml IL-4. The conditioned media were collected after 48 hours and stored at −20°C until assay.
Statistical Analysis
Data are represented as mean ± SEM, unless otherwise indicated. Statistical analysis was performed using the Mann-Whitney test. The significance of differences with controls is indicated in the figures by asterisks (*, P < 0.05, **, P < 0.01, ***, P < 0.001).
Results
Isolation of Natural PARC Isoforms from Normal Human Plasma
The CC chemokine HCC-1, and the CXC chemokines PF-4 and β-TG are detected at rather high basal levels in plasma. 5,6 This study extends the group of plasma chemokines with PARC. On average (± SD), 22.6 ± 7.8 ng/ml (n = 42) of PARC immunoreactivity was detected in plasma from healthy blood donors (Figure 1) ▶ . For comparison, these plasma samples contained on average (± SD) 85 ± 37 ng/ml (n = 42) of HCC-1, but the HCC-1 levels were scattered over a broader range than the PARC levels in normal adult subjects. This phenomenon was not due to a higher intra- or inter-assay variability of the HCC-1 ELISA, suggesting that PARC and HCC-1 are differently regulated under physiological conditions.
Figure 1.
Distribution of PARC and HCC-1 levels in normal human plasma. PARC and HCC-1 levels in plasma from 42 healthy blood donors were measured by their respective specific ELISAs. To illustrate the distribution of the chemokine levels, individual values are shown as well as box-plot representations. In the latter plot the horizontal bar in the box (between the lower line (=Q1) and the upper line (=Q3) 50% of the data are situated) depicts the median value. The whiskers delimit the range of the data inside the region defined by the lower limit (Q1–1.5(Q3-Q1)) and the upper limit (Q3 + 1.5(Q3-Q1)). Asterisks indicate outliers.
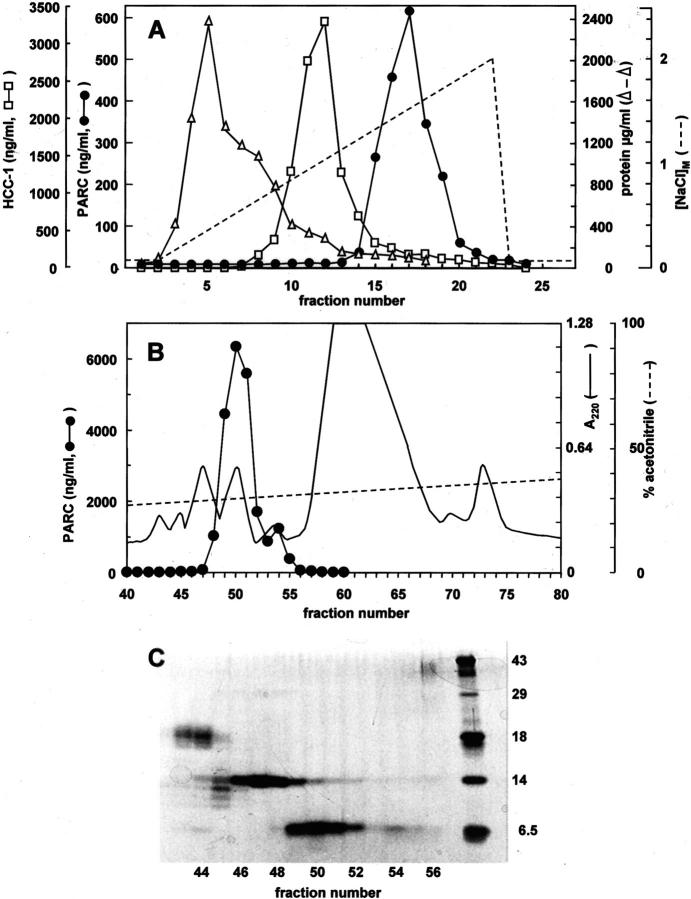
To verify whether the detection of basal PARC plasma levels was due to cross-reactivity with an irrelevant plasma protein or an already known plasma chemokine, we purified the PARC immunoreactivity from 2.4 liters of normal human plasma (pool of six plasma donations) by a four-step chromatographical procedure established in our laboratory. 26 After subsequent adsorption to silicic acid, heparin affinity chromatography and cation-exchange FPLC, RP-HPLC was used as a final purification step (Figure 2) ▶ . Since heparin-Sepharose chromatography (Figure 2A) ▶ allowed fractionation of PARC and HCC-1 immunoreactivity, these two entities were separately purified on FPLC (not shown). Figure 2B ▶ demonstrates that after RP-HPLC fractionation, the PARC immunoreactivity eluted in a single peak at about 30% acetonitrile (fractions 48 to 52), corresponding to a 6.5-kDa protein band (Figure 2C) ▶ . Mass spectrometry revealed the presence of two isoforms of PARC in RP-HPLC fraction 51 (detected average Mr of 7779.8 and 7851.5). The major peak on the mass spectrum (75% of the amount of PARC) corresponded to intact PARC (theoretical Mr of 7851.2) whereas the minor peak (25% of the amount of PARC) contained PARC lacking either the NH2-terminal or COOH-terminal alanine (theoretical Mr of 7780.2). On NH2-terminal amino acid sequence analysis only alanine was detected after the first cycle of capillary Edman degradation, confirming the presence of an intact NH2-terminus for both PARC isoforms. It was therefore concluded that, in addition to intact PARC, a truncated PARC isoform lacking the COOH-terminal alanine was present in the pooled normal plasma.
Figure 2.
Purification of PARC immunoreactivity from normal human plasma. A pool (2400 ml) of six plasma donations was concentrated by adsorption to silicic acid and loaded onto a heparin-Sepharose column (A). Proteins were recovered from this column in a NaCl (0–2 mol/L) gradient (dashed line). All fractions were evaluated for protein content (open triangles) by the Coomassie blue binding assay and analyzed in the PARC (filled circles) and HCC-1 (open squares) ELISAs. After cation-exchange chromatography (not shown) PARC immunoreactivity was subjected to RP-HPLC (B). Proteins were eluted from the C8 column in an acetonitrile (0 to 80%) gradient (dashed line). Absorbance was monitored at 220 nm (solid line). PARC levels were determined in each HPLC fraction by a specific ELISA. HPLC fractions containing PARC immunoreactivity were analyzed by SDS-PAGE (C) under reducing conditions (fractions 43 to 56, 20 μl/lane). The proteins were visualized by silver staining. The right lane shows Mr markers (see Materials and Methods section).
A similar purification procedure for the HCC-1 immunoreactivity (Figure 2A) ▶ , yielded on RP-HPLC a single HCC-1 isoform, with the detected average Mr (8672.9) corresponding to the theoretical Mr (8673.8) of intact HCC-1. The fact that the PARC and HCC-1 immunoreactivities had a different elution pattern in the sequential chromatographical steps and that the purification procedure resulted in the isolation of only the expected chemokines, demonstrates that the two immunotests are specific. In total, about 8 μg of PARC protein was isolated from 1 L of plasma, which corresponds to the amount of PARC immunoreactivity measured in the crude plasma with the assumption that about 50% was the recovered on purification. Thus, PARC is a constitutive chemokine abundantly present in normal human plasma.
Enhanced PARC Levels in Serum of Pediatric Patients with Acute Leukemia
Since high constitutive levels of PARC are present in plasma and since macrophages and dendritic cells are reported as the major cellular source of PARC, we investigated whether this chemokine accumulates in specific organs or body compartments during disease. 15,29,30 Bone marrow aspiration samples, as well as spinal fluid and serum from pediatric patients with acute leukemia were analyzed for the presence of the plasma chemokines PARC and HCC-1, the inflammatory chemokines IL-8 and MCP-1, as well as the cytokine IL-6. In spinal fluids of patients with acute leukemia or other nonmalignant hematological disorders only low levels of PARC (on average <1 ng/ml, n = 80) and HCC-1 (on average <10 ng/ml, n = 80) were detectable compared to those in plasma from healthy donors (Figure 1) ▶ . In addition, neither IL-8 (<0.2 ng/ml, n = 40) nor MCP-1 (<2 ng/ml, n = 40) was measured in these spinal fluids. In bone marrow samples from patients with different acute leukemia types or other nonmalignant hematological diseases, the average PARC (19.2 ± 2.0 ng/ml, n = 51) and HCC-1 (119.6 ± 11.0 ng/ml, n = 51) concentrations were lower or similar to those in control pediatric sera or plasma from healthy donors (Figures 1 and 3) ▶ .
Next, we evaluated serum levels of HCC-1 and PARC in various subtypes of acute leukemia. Figure 3 ▶ demonstrates that PARC serum concentrations in AML (37.8 ± 5.2 ng/ml, n = 13) were comparable to those in control subjects (30.9 ± 3.8 ng/ml, n = 21). However, significantly enhanced PARC levels were found in ALL patients (55.2 ± 6.1, P = 0.004, n = 38). Within this group, the serum of patients with T- and prepreB-ALL contained significantly higher PARC levels (85.2 ± 14.4 ng/ml, n = 11, P = 0.0005 for T-ALL and 44.7 ± 4.6 ng/ml, n = 16, P = 0.01 for prepreB-ALL), whereas preB-ALL serum (40.5 ± 9.5 ng/ml, n = 11) did not show a statistically significant increase in PARC compared to control pediatric serum (30.9 ± 3.8 ng/ml). In contrast to the PARC serum levels, the concentration of serum HCC-1 did not statistically differ between various patient groups (Figure 3) ▶ . Instead, the serum concentration of IL-6 was significantly decreased in AML (6.5 ± 1.8 U/ml, P = 0.0005) and prepreB-ALL (9.4 ± 3.1 U/ml, P = 0.001), whereas IL-6 levels in preB-ALL (16.9 ± 5.6 U/ml) and T-ALL (14.8 ± 3.1 U/ml) were similar to those in control pediatric sera (21.2 ± 2.6 U/ml). Taken together, these data indicate that the chemokine PARC, but not HCC-1, is up-regulated during acute T and prepreB-leukemia, whereas the cytokine IL-6 is down-regulated predominantly in AML.
Figure 3.

Cytokine and chemokine serum levels in childhood acute leukemia. PARC, HCC-1, and IL-6 levels in serum were measured by specific ELISA (PARC and HCC-1) or bioassay (IL-6). In this study 13 AML, 11 T-ALL, 16 prepreB-ALL, and 11 preB-ALL patients (Table 1) ▶ , as well as a matched pediatric control group of patients from the same hospital were included. The horizontal bars indicate the median cytokine concentration for each group. Statistical analysis was performed using the Mann-Whitney test. Significance levels for differences in PARC or IL-6 serum concentration between a patient group and the control group are indicated at the top of the panels.
Cellular Sources of PARC
In view of the strikingly increased PARC levels in serum from ALL, but not AML patients, we investigated whether the tumor cells in the circulation could be a cellular source of PARC. We therefore tried to stimulate PARC production by adding different cytokines or cytokine inducers to in vitro cultured leukemic cell lines. Figure 4 ▶ shows that the myeloid cell line THP-1 failed to produce PARC constitutively or after stimulation with bacterial (LPS), viral (dsRNA), plant products (ConA), or endogenous mediators (IFN-γ, IL-1, IL-4, or IL-10). In contrast, these myeloid cells produced significant amounts of IL-8 on stimulation with either LPS or PMA. Similarly, no PARC immunoreactivity could be detected in supernatants from B or T lymphoblastoid cell lines (Namalwa, MT4, Sup-T1) (Figure 4 ▶ and data not shown).
Figure 4.
PARC is not produced by leukocytic cell lines. Cultures of the human monocytic leukemia cell line THP-1 (top panel) and the acute lymphoblastic leukemia cell line MT4 (bottom panel) were stimulated for 48 hours with measles virus (MV; 105.2 TCID50/ml), the dsRNA polyrI:rC (PIC; 10 μg/ml), LPS (50 μg/ml), PMA (10 ng/ml), ConA (10 μg/ml), IL-1 (100 U/ml), IFN-γ (20 ng/ml), IL-4 (30 ng/ml), IL-10 (100 ng/ml) or were left untreated (Co). The PARC (black histograms), IL-8 (open histograms), and MIP-1α (hatched histograms) concentrations were determined by specific ELISAs, and the mean ± SEM was calculated from at least two independent experiments. Significant differences from controls, determined by the Mann-Whitney test, are indicated by asterisks (**, P < 0.01).
In addition to chemokine induction by soluble inducers, it has become increasingly documented that cell-cell interactions may be a stimulus of chemokine production. 31 Therefore it was verified whether the enhanced PARC levels in T-ALL could be generated within the vascular lumen as a result of cell contact between circulating lymphoblasts and monocytes. For this purpose, freshly isolated adherent monocytes and autologous lymphocytes from healthy donors were co-cultured at different densities and PARC production was evaluated. Figure 5 ▶ documents that intercellular contacts were not sufficient to elicit PARC production. However, when a second signal, known to induce PARC in PBMC (IL-4 or SEA), 15 was added to the co-cultures, PARC production was further enhanced in the presence of lymphocytes (upper panel of Figure 5 ▶ ). Furthermore, cultured T cells (Sup-T1 and MOLT-4) or T cell line-conditioned medium also stimulated IL-4-treated adherent monocytes and a significantly increased production of PARC was observed compared to IL-4 induced adherent monocytes alone (lower panels of Figure 5 ▶ and Table 2 ▶ ). The fact that PARC production was also significantly increased after stimulation of adherent monocytes with conditioned medium and IL-4 or IL-10 (Table 2 ▶ and data not shown), demonstrates that cell-cell contact is not essential for PARC production. Furthermore, these experiments indicate that the conditioned media contain soluble factors other than IL-4 and IL-10 that enhance PARC release by monocytes.
Figure 5.

Increased PARC levels in co-cultures of adherent monocytes with lymphocytes or lymphoblasts. Different cell concentrations (105 or 106 cells/ml) of freshly isolated lymphocytes (n = 7) or cultured T lymphoblastic cells (n = 6 for Sup-T1, n = 4 for MOLT-4) were added to cultures of adherent blood monocytes in the absence or presence of 300 ng/ml SEA or 30 ng/ml IL-4 to stimulate chemokine production. After 48 hours, cell supernatants were collected and analyzed for PARC content by a specific ELISA. Significant differences from control cultures (adherent monocytes alone in the presence of inducer), determined by the Mann-Whitney test, are indicated by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Freshly isolated lymphocytes or cultured T lymphoblastic cells alone failed to produce significant amounts of PARC, either unstimulated or after addition of IL-4 or SEA (not shown).
Table 2.
PARC Production after Stimulation of Adherent Monocytes with T-Cell Conditioned Medium
| Inducers* | Sup-T1 | MOLT-4 | |||
|---|---|---|---|---|---|
| Co-stimulus | Cytokine | PARC (ng/ml)† | P value‡ | PARC (ng/ml)† | P value‡ |
| Buffer | none | 0.5 ± 0.2 | — | 0.2 ± 0.1 | — |
| IL-4 | 0.9 ± 0.5 | — | 0.4 ± 0.2 | — | |
| 105 cells | none | 1.2 ± 0.5 | 0.25 | 0.3 ± 0.1 | 0.1 |
| IL-4 | 5.2 ± 1.7 | 0.028 | 1.9 ± 0.4 | 0.008 | |
| CM (1/2) | none | 2.4 ± 1.0 | 0.047 | 0.5 ± 0.2 | 0.063 |
| IL-4 | 6.1 ± 1.9 | 0.016 | 1.7 ± 0.4 | 0.012 | |
| CM (1/20) | none | 0.8 ± 0.3 | 0.59 | 0.2 ± 0.1 | 0.71 |
| IL-4 | 3.4 ± 0.9 | 0.076 | 1.1 ± 0.4 | 0.06 | |
*To stimulate PARC production, adherent monocytes were treated with dilution buffer, 105 Sup-T1 cells or MOLT-4 cells or conditioned medium (CM, diluted 1/2 or 1/20) from these T-cell lines in combination with the cytokine IL-4 (30 ng/ml) or the dilution buffer. Cultured T lymphoblastic cells alone failed to produce significant amounts of PARC, either unstimulated or after addition of IL-4 (not shown).
†After 48 hours, cell supernatants were collected and analyzed for their PARC content by a specific ELISA. Values indicate the mean (± SEM) PARC concentration from 5 (Sup-T1) or 7 (MOLT-4) experiments.
‡P values, calculated by the Mann-Whitney test, indicate differences between co-stimulated and control cultures (adherent monocytes in the presence of the corresponding inducer, ie, dilution buffer or IL-4).
Discussion
PARC was independently cloned by several groups and therefore simultaneously designated dendritic cell chemokine-1 (DC-CK1) and alternative macrophage activation-associated CC chemokine-1 (AMAC-1). 14,29,30 PARC is either constitutively expressed or induced in monocytes/macrophages and dendritic cells and chemoattracts T and B lymphocytes. 14,29,30,32,33 PARC has a high structural similarity (64% identical amino acids) to the well characterized CC chemokine MIP-1α and shares its capacity to suppress proliferation of subsets of immature myeloid progenitor cells. 11,34 So far, PARC expression has been reported in unrelated pathologies, such as atherosclerosis, 35 hypersensitivity pneumonitis, 36 allergic contact hypersensitivity, 37 arthritis, 15 and ovarian carcinoma. 16 In this study, PARC was unexpectedly detected as a constitutive plasma chemokine, present at about 20 ng/ml. Characterization of the plasma-derived PARC immunoreactivity revealed the occurrence of two PARC protein isoforms that differ in COOH-terminal processing. In contrast, NH2-terminal processing was previously observed for PARC isolated from stimulated PBMC, but no functional characterization was possible, since these natural isoforms could not be separated chromatographically. 15 In general, COOH-terminal processing of chemokines is biologically less relevant than NH2-terminal cleavage, the latter of which can drastically affect the chemotactic activity. 38 Indeed, the plasma chemokines β-TG and HCC-1 need to be proteolytically cleaved at their NH2-terminus to become the biologically active chemoattractants, neutrophil activating peptide-2 (NAP-2) and HCC-1(9–74), respectively. 5,7 However, only intact and hence inactive HCC-1 was isolated from normal plasma in this study. The evaluation of differences in HCC-1 processing between healthy controls and patients, must wait until an ELISA that discriminates between different NH2-terminal-truncated HCC-1 isoforms becomes available.
The structural relationship of PARC with the stem cell inhibitory chemokine MIP-1α, its presence in various unrelated diseases, and the high levels in the blood circulation stimulated us to investigate the presence of PARC in proliferative hematological diseases. It was found that serum PARC levels were significantly elevated in pediatric ALL patients compared to control pediatric samples (Figure 3) ▶ . In particular, T- and prepreB-ALL patients had on average 85 and 45 ng/ml of PARC in their serum on diagnosis, corresponding to a significant increase above control serum levels (31 ng/ml). In contrast, no significant changes in HCC-1 levels were observed in sera from AML, T-ALL, preB-ALL, and prepreB-ALL patients. Remarkably, three of the 13 AML patients were characterized by the M5 differentiation stage, but had normal PARC serum levels. Furthermore, the serum concentration of the hematopoietic growth factor IL-6 was significantly decreased in the AML and prepreB-ALL groups. Conflicting results on IL-6 levels in serum from leukemic patients have been reported. Depending on the AML subtype, the number of peripheral blood and bone marrow blasts or the occurrence of fever, enhanced serum levels of IL-6 have been reported for adult AML patients. 39 Based on in vitro data, increased IL-6 levels in these patients could be the result of autocrine IL-6 secretion by the AML cells. 40 However, Gao et al 41 demonstrated that cytokine production (including IL-6 production) by freshly isolated AML cells differed, compared to cultured leukemic cells, indicating that in vitro findings cannot always be extrapolated to the in vivo situation. Furthermore, it was demonstrated that AML cells produce soluble IL-6 receptor, which might explain the reduced serum levels of biologically active IL-6 detected in our study. 42,43
Since tumor cells are often a good cellular source of chemokines, 44 it was verified whether leukocytic cell lines produce PARC in response to various endogenous or exogenous stimuli. It was found that THP-1, HL60, Sup-T1, and MT4 cells did not secrete a detectable amount of PARC after stimulation with the cytokines IL-1, IL-4, IL-10, or IFN-γ, nor after induction with virus, dsRNA, endotoxin, enterotoxin, phorbol ester, or lectin. For comparison, IL-8 was significantly induced in the myeloid cell lines THP-1 and HL60 by phorbol ester and endotoxin, whereas the former inducer also enhanced MIP-1α production in the lymphoblastoid cell lines Namalwa and MT4. In view of the reported expression of PARC mRNA in THP-1 cells 30 stimulated with IL-4 and phorbol ester and of PARC protein production by PBMC induced with IL-4 or enterotoxin, 15 the lack of PARC protein production by myeloid cell lines in vitro was rather unexpected. This indicates that the tumor cells are probably not the cellular source of PARC and hence are not directly responsible for the enhanced levels of PARC in ALL patients. Indeed, we performed induction experiments with T-ALL lymphoblasts from two patients, either from peripheral blood or bone marrow and although IL-8 was produced, no PARC protein was induced even after a prolonged incubation and treatment with various stimuli (data not shown). We could also not find a correlation between the PARC concentrations and the number of blast cells in the peripheral blood or bone marrow of the ALL and AML patients (data not shown). We then verified whether PARC was released from the bone marrow. The bone marrow PARC levels in leukemic patients were similar to those in serum and their spinal fluid was devoid of any PARC (data not shown). Furthermore, we did not observe a difference in PARC protein expression by immunocytochemistry of bone marrow mononuclear cells from leukemic patients compared to control bone marrow (data not shown). This suggests that not the bone marrow as lymphoid organ, but rather the periphery is the primary source of PARC, whereas its absence in the spinal fluid may be explained by an intact blood-brain barrier. To verify whether leukemic cells or soluble factors produced by these cells can stimulate PARC production by monocytes/macrophages or dendritic cells, freshly isolated lymphocytes, cultured lymphoblast cells, or conditioned medium thereof were added to cultures of adherent peripheral blood monocytes in the presence of IL-4 or SEA. In the presence of 105 or 106 lymphocytes or cultured T lymphoblastoid cells, the PARC production by monocytes/macrophages stimulated by IL-4 or SEA was significantly enhanced (3- to 15-fold). A similar enhancement in PARC levels in these cultures was reached after addition of T-cell line-conditioned medium. This indicates that the tumor cells might (indirectly) affect the PARC levels in leukemia, maybe by stimulating alveolar macrophages or dendritic cells, cell types that are known to be good PARC producers. 14,29,30,33,36,45
Some leukemic cells produce high amounts of chemokines, eg, MIP-1α and MIP-1β, are secreted by adult T-cell leukemia cells. 46 Ghia et al 47 demonstrated that malignant B-cell precursors secrete macrophage-derived chemokine (MDC)/CCL22 and thymus and activation-regulated chemokine (TARC)/CCL17 after CD40 ligation, but not PARC. It has been shown that autocrine production of chemokines can influence the migration of lymphoma cells and their metastasis to specific tissues. 19 Furthermore, evidence has accumulated in recent years that local chemokine production affects the selective invasion of the organ by cancer cells. 48 This was first proven for breast carcinoma cells 17 but also hematological tumor cells infiltrate tissues expressing chemokines that trigger the corresponding receptors on these malignant cells. For example, infiltration of lymphoid organs by adult T-cell leukemia cells and chronic lymphocytic leukemia (CLL) cells correlates with higher expression levels of CCR7 on the leukemic cells. 49,50 One of the ligands of this receptor is abundantly produced by high endothelial venules of lymph nodes and Peyer’s patches. 51 In addition, CCR4 expression by adult T-cell leukemia cells may account for frequent metastasis to skin and lymph nodes. 52 Expression of CCR3 in CD30-positive cutaneous T-cell lymphoma might play a role in the attraction and retention of CD30-positive malignant T cells to the skin. 53 AML, B-ALL, and CLL cells express the SDF-1 receptor CXCR4. 54-56 A high expression level of CXCR4 on ALL cells was strongly predictive for extramedullary organ involvement in affected children. 57 In adult patients with B-CLL, CXCR4 expression probably mediates marrow infiltration by neoplastic B cells. 56 In addition, cell-cell contacts between SDF-1-producing marrow cells and malignant B cells are thought to rescue the latter cells from apoptosis. 58 Taken together, these findings suggest that chemokines and their receptors govern homing and survival of hematological tumor cells. Whether the elevated PARC levels in childhood T-ALL in our study could account for a role for PARC as chemoattractant or survival-promotor still needs to be revealed. Differential chemokine receptor expression may be a valuable tool to distinguish between different subtypes of T-cell non-Hodgkin’s lymphoma. 59 Similarly, CXCR3 is differentially expressed in a subset of B-cell lymphomas and could function as a marker of B-CLL. 60,61 The results presented in this study suggest that elevated serum PARC levels could also constitute a novel leukemia marker. The presence of this CC chemokine in plasma from healthy persons is intriguing. However, the absence of PARC in the mouse system and the fact that its receptor is still not identified, complicates the full functional characterization of this chemokine. Detection of its presence under various pathological conditions might, however, facilitate the delineation of the precise role of PARC.
Acknowledgments
We thank Miss J. Geerinck and K. Swerts from the University Hospital (Gent) for providing the patient samples. The editorial help of D. Brabants and the technical support of R. Conings, J.-P. Lenaerts, and W. Put is greatly appreciated. Finally, we are grateful for the buffy coats received from the Blood Transfusion Centers of Antwerp and Leuven.
Footnotes
Address reprint requests to Jo Van Damme, Laboratory of Molecular Immunology, Rega Institute for Medical Research, University of Leuven, Minderbroedersstraat 10, B-3000 Leuven, Belgium. E-mail: Jozef.VanDamme@rega.kuleuven.ac.be.
Supported by the Fund for Scientific Research of Flanders (FWO-Vlaanderen), the Concerted Research Actions (GOA) of the Regional Government of Flanders, the InterUniversity Attraction Pole initiative of the Belgian Federal Government (IUAP), the Cancer Research Foundation of Fortis AB, Belgium, The Belgian Federation against Cancer and the Quality of Life Program of the European Community.
S.S. and P.P. are senior research assistants of the F.W.O.-Vlaanderen.
References
- 1.Loetscher P, Moser B, Baggiolini M: Chemokines and their receptors in lymphocyte traffic and HIV infection. Adv Immunol 2000, 74:127-180 [DOI] [PubMed] [Google Scholar]
- 2.Gerard C, Rollins BJ: Chemokines and disease. Nat Immunol 2001, 2:108-115 [DOI] [PubMed] [Google Scholar]
- 3.Deuel TF, Keim PS, Farmer M, Heinrikson RL: Amino acid sequence of human platelet factor-4. Proc Natl Acad Sci USA 1977, 74:2256-2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begg GS, Pepper DS, Chesterman CN, Morgan FJ: Complete covalent structure of human β-thromboglobulin. Biochemistry 1978, 17:1739-1744 [DOI] [PubMed] [Google Scholar]
- 5.Brandt E, Petersen F, Ludwig A, Ehlert JE, Bock L, Flad HD: The β-thromboglobulins and platelet factor-4: blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J Leukoc Biol 2000, 67:471-478 [DOI] [PubMed] [Google Scholar]
- 6.Schulz-Knappe P, Mägert HJ, Dewald B, Meyer M, Cetin Y, Kubbies M, Tomeczkowski J, Kirchhoff K, Raida M, Adermann K, Kist A, Reinecke M, Sillard R, Pardigol A, Uguccioni M, Baggiolini M, Forsmann W-G: HCC-1, a novel chemokine from human plasma. J Exp Med 1996, 183:295-299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detheux M, Ständker L, Vakili J, Münch J, Forssmann U, Adermann K, Pöhlmann S, Vassart G, Kirchhoff F, Parmentier M, Forssmann W-G: Natural proteolytic processing of hemofiltrate CC chemokine 1 generates a potent CC chemokine receptor (CCR)1 and CCR5 agonist with anti-HIV properties. J Exp Med 2000, 192:1501-1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maione TE, Gray GS, Petro J, Hunt AJ, Donner AL, Bauer SI, Carson HF, Sharpe RJ: Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science 1990, 247:77-79 [DOI] [PubMed] [Google Scholar]
- 9.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, Shan S-Y, Roczniak S, Shanafelt AB: The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 1995, 270:27348-27357 [DOI] [PubMed] [Google Scholar]
- 10.Broxmeyer HE, Kim CH: Regulation of hematopoiesis in a sea of chemokine family members with a plethora of redundant activities. Exp Hematol 1999, 27:1113-1123 [DOI] [PubMed] [Google Scholar]
- 11.Graham GJ, Wright EG, Hewick R, Wolpe SD, Wilkie NM, Donaldson D, Lorimore S, Pragnell IB: Identification and characterization of an inhibitor of haemopoietic stem cell proliferation. Nature 1990, 344:442-444 [DOI] [PubMed] [Google Scholar]
- 12.Nagasawa T, Kikutani H, Kishimoto T: Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci USA 1994, 91:2305-2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy PM, Baggiolini M, Charo IF, Hébert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA: International union of pharmacology. XXII: nomenclature for chemokine receptors. Pharmacol Rev 2000, 52:145-176 [PubMed] [Google Scholar]
- 14.Hieshima K, Imai T, Baba M, Shoudai K, Ishizuka K, Nakagawa T, Tsuruta J, Takeya M, Sakaki Y, Takatsuki K, Miura R, Opdenakker G, Van Damme J, Yoshie O, Nomiyama H: A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1 α/LD78 α and chemotactic for T lymphocytes, but not for monocytes. J Immunol 1997, 159:1140-1149 [PubMed] [Google Scholar]
- 15.Schutyser E, Struyf S, Wuyts A, Put W, Geboes K, Grillet B, Opdenakker G, Van Damme J: Selective induction of CCL18/PARC by staphylococcal enterotoxins in mononuclear cells and enhanced levels in septic and rheumatoid arthritis. Eur J Immunol 2001, 31:3755-3762 [DOI] [PubMed] [Google Scholar]
- 16.Schutyser E, Struyf S, Proost P, Opdenakker G, Laureys G, Verhasselt B, Peperstraete L, Van de Putte I, Saccani A, Allavena P, Mantovani A, Van Damme J: Identification of biologically active chemokine isoforms from ascitic fluid and elevated levels of CCL18/pulmonary and activation-regulated chemokine in ovarian carcinoma. J Biol Chem 2002, 277:24584-24593 [DOI] [PubMed] [Google Scholar]
- 17.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A: Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410:50-56 [DOI] [PubMed] [Google Scholar]
- 18.Wang JM, Chertov O, Proost P, Li J-J, Menton P, Xu L, Sozzani S, Mantovani A, Gong W, Schirrmacher V, Van Damme J, Oppenheim JJ: Purification and identification of chemokines potentially involved in kidney-specific metastasis by a murine lymphoma variant: induction of migration and NFκB activation. Int J Cancer 1998, 75:900-907 [DOI] [PubMed] [Google Scholar]
- 19.Menten P, Saccani A, Dillen C, Wuyts A, Struyf S, Proost P, Mantovani A, Wang JM, Van Damme J: Role of the autocrine chemokines MIP-1α and MIP-1β in the metastatic behavior of murine T-cell lymphoma. J Leukoc Biol 2002, 72:780-789 [PubMed] [Google Scholar]
- 20.Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, van’t Veer MB: Proposals for the immunological classification of acute leukemias: European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia 1995, 9:1783-1786 [PubMed] [Google Scholar]
- 21.Reiter A, Schrappe M, Ludwig W-D, Hiddemann W, Sauter S, Henze G, Zimmermann M, Lampert F, Havers W, Niethammer D, Odenwald E, Ritter J, Mann G, Welte K, Gadner H, Riehm H: Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients: results and conclusions of the multi-center trial ALL-BFM 86. Blood 1994, 84:3122-3133 [PubMed] [Google Scholar]
- 22.Van Snick J, Cayphas S, Vink A, Uyttenhove C, Coulie PG, Rubira MR, Simpson RJ: Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci USA 1986, 83:9679-9683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Damme J, Van Beeumen J, Decock B, Van Snick J, De Ley M, Billiau A: Separation and comparison of two monokines with lymphocyte-activating factor activity: IL-1β and hybridoma growth factor (HGF): identification of leukocyte-derived HGF as IL-6. J Immunol 1988, 140:1534-1541 [PubMed] [Google Scholar]
- 24.Landegren U: Measurement of cell numbers by means of the endogenous enzyme hexosaminidase: applications to detection of lymphokines and cell surface antigens. J Immunol Methods 1984, 67:379-388 [DOI] [PubMed] [Google Scholar]
- 25.Van Damme J, Decock B, Conings R, Lenaerts J-P, Opdenakker G, Billiau A: The chemotactic activity for granulocytes produced by virally infected fibroblasts is identical to monocyte-derived interleukin-8. Eur J Immunol 1989, 19:1189-1194 [DOI] [PubMed] [Google Scholar]
- 26.Struyf S, Wuyts A, Van Damme J: Purification, sequencing, and synthesis of cytokines and chemokines. Balkwill F eds. Cytokine Molecular Biology, A Practical Approach 2000:pp 73-88 Oxford University Press Oxford
- 27.Struyf S, Proost P, Lenaerts J-P, Stoops G, Wuyts A, Van Damme J: Identification of a blood-derived chemoattractant for neutrophils and lymphocytes as a novel CC chemokine, regakine-1. Blood 2001, 97:2197-2204 [DOI] [PubMed] [Google Scholar]
- 28.Schägger H, von Jagow G: Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 1987, 166:368-379 [DOI] [PubMed] [Google Scholar]
- 29.Adema GJ, Hartgers F, Verstraten R, de Vries E, Marland G, Menon S, Foster J, Xu Y, Nooyen P, McClanahan T, Bacon KB, Figdor CG: A dendritic-cell-derived CC chemokine that preferentially attracts naive T cells. Nature 1997, 387:713-717 [DOI] [PubMed] [Google Scholar]
- 30.Kodelja V, Müller C, Politz O, Hakij N, Orfanos CE, Goerdt S: Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1α with a Th2-associated expression pattern. J Immunol 1998, 160:1411-1418 [PubMed] [Google Scholar]
- 31.Lukacs NW, Strieter RM, Elner V, Evanoff HL, Burdick MD, Kunkel SL: Production of chemokines, interleukin-8, and monocyte chemoattractant protein-1 during monocyte: endothelial cell interactions. Blood 1995, 86:2767-2773 [PubMed] [Google Scholar]
- 32.Guan P, Burghes AHM, Cunningham A, Lira P, Brissette WH, Neote K, McColl SR: Genomic organization and biological characterization of the novel human CC chemokine DC-CK-1/PARC/MIP-4/SCYA18. Genomics 1999, 56:296-302 [DOI] [PubMed] [Google Scholar]
- 33.Lindhout E, Vissers JLM, Hartgers FC, Huijbens RJF, Scharenborg NM, Figdor CG, Adema GJ: The dendritic cell-specific CC-chemokine DC-CK1 is expressed by germinal center dendritic cells and attracts CD38-negative mantle zone B lymphocytes. J Immunol 2001, 166:3284-3289 [DOI] [PubMed] [Google Scholar]
- 34.Broxmeyer HE, Kim CH, Cooper SH, Hangoc G, Hromas R, Pelus LM: Effects of CC, CXC, C, and CX3C chemokines on proliferation of myeloid progenitor cells, and insights into SDF-1-induced chemotaxis of progenitors. Ann NY Acad Sci 1999, 872:142-162 [DOI] [PubMed] [Google Scholar]
- 35.Reape TJ, Rayner K, Manning CD, Gee AN, Barnette MS, Burnand KG, Groot PHE: Expression and cellular localization of the CC chemokines PARC and ELC in human atherosclerotic plaques. Am J Pathol 1999, 154:365-374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pardo A, Smith KM, Abrams J, Coffman R, Bustos M, McClanahan TK, Grein J, Murphy EE, Zlotnik A, Selman M: CCL18/DC-CK-1/PARC up-regulation in hypersensitivity pneumonitis. J Leukoc Biol 2001, 70:610-616 [PubMed] [Google Scholar]
- 37.Goebeler M, Trautmann A, Voss A, Bröcker E-B, Toksoy A, Gillitzer R: Differential and sequential expression of multiple chemokines during elicitation of allergic contact hypersensitivity. Am J Pathol 2001, 158:431-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Struyf S, Proost P, Van Damme J: Regulation of the immune response by the interaction of chemokines and proteases. Adv Immunol 2003, 81:1-45 [DOI] [PubMed] [Google Scholar]
- 39.Thomas X, Hirschauer C, Troncy J, Assouline D, Joly M-O, Fiere D, Archimbaud E: Serum interleukin-6 levels in adult acute myelogenous leukemia: relationship with disease characteristics and outcome. Leuk Lymphoma 1997, 24:291-300 [DOI] [PubMed] [Google Scholar]
- 40.Schuringa J-J, Wierenga AT, Kruijer W, Vellenga E: Constitutive Stat3, Tyr705, and Ser727 phosphorylation in acute myeloid leukemia cells caused by the autocrine secretion of interleukin-6. Blood 2000, 95:3765-3770 [PubMed] [Google Scholar]
- 41.Gao X-Z, Bi S, Copra H, Devemy E, Venugopal P, Li B, Hsu W-T, Loew J, Galvez A, Gregory S, Yang J, Horvath E, Preisler HD: Cytokine gene activity in AML cells in vivo in patients. Leuk Res 1998, 22:429-438 [DOI] [PubMed] [Google Scholar]
- 42.Boayue KB, Gu L, Yeager AM, Kreitman RJ, Findley HW: Pediatric acute myelogenous leukemia cells express IL-6 receptors and are sensitive to a recombinant IL-6-Pseudomonas exotoxin. Leukemia 1998, 12:182-191 [DOI] [PubMed] [Google Scholar]
- 43.Saily M, Koistinen P, Pulkki K, Zheng A, Savolainen ER: Acute myeloblastic leukaemia cells produce soluble interleukin 6 receptor by a mechanism of alternative splicing. Cytokine 1998, 10:860-867 [DOI] [PubMed] [Google Scholar]
- 44.Van Damme J, Proost P, Lenaerts J-P, Opdenakker G: Structural and functional identification of two human, tumor-derived monocyte chemotactic proteins (MCP-2 and MCP-3) belonging to the chemokine family. J Exp Med 1992, 176:59-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vulcano M, Struyf S, Scapini P, Cassatella M, Bernasconi S, Bonecchi R, Calleri A, Penna G, Adorini L, Luini W, Mantovani A, Van Damme J, Sozzani S: Unique regulation of CCL18 production by maturing dendritic cells. J Immunol 2003, 170:3843-3849 [DOI] [PubMed] [Google Scholar]
- 46.Tanaka Y, Mine S, Figdor CG, Wake A, Hirano H, Tsukada J, Aso M, Fujii K, Saito K, van Kooyk Y, Eto S: Constitutive chemokine production results in activation of leukocyte function-associated antigen-1 on adult T-cell leukemia cells. Blood 1998, 91:3909-3919 [PubMed] [Google Scholar]
- 47.Ghia P, Transidico P, Veiga JP, Schaniel C, Sallusto F, Matsushima K, Sallan SE, Rolink AG, Mantovani A, Nadler LM, Cardoso AA: Chemoattractants MDC and TARC are secreted by malignant B-cell precursors following CD40 ligation and support the migration of leukemia-specific T cells. Blood 2001, 98:533-540 [DOI] [PubMed] [Google Scholar]
- 48.Wang JM, Deng X, Gong W, Su S: Chemokines and their role in tumor growth and metastasis. J Immunol Methods 1998, 220:1-17 [DOI] [PubMed] [Google Scholar]
- 49.Hasegawa H, Nomura T, Kohno M, Tateishi N, Suzuki Y, Maeda N, Fujisawa R, Yoshie O, Fujita S: Increased chemokine receptor CCR7/EBI1 expression enhances the infiltration of lymphoid organs by adult T-cell leukemia cells. Blood 2000, 95:30-38 [PubMed] [Google Scholar]
- 50.Till KJ, Lin K, Zuzel M, Cawley JC: The chemokine receptor CCR7 and α4 integrin are important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood 2002, 99:2977-2984 [DOI] [PubMed] [Google Scholar]
- 51.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT: A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA 1998, 95:258-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshie O, Fujisawa R, Nakayama T, Harasawa H, Tago H, Izawa D, Hieshima K, Tatsumi Y, Matsushima K, Hasegawa H, Kanamaru A, Kamihira S, Yamada Y: Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood 2002, 99:1505-1511 [DOI] [PubMed] [Google Scholar]
- 53.Kleinhans M, Tun-Kyi A, Gilliet M, Kadin ME, Dummer R, Burg G, Nestle FO: Functional expression of the eotaxin receptor CCR3 in CD30-positive cutaneous T-cell lymphoma. Blood 2003, 101:1487-1493 [DOI] [PubMed] [Google Scholar]
- 54.Möhle R, Schittenhelm M, Failenschmid C, Bautz F, Kratz-Albers K, Serve H, Brugger W, Kanz L: Functional response of leukaemic blasts to stromal cell-derived factor-1 correlates with preferential expression of the chemokine receptor CXCR4 in acute myelomonocytic and lymphoblastic leukaemia. Br J Haematol 2000, 110:563-572 [DOI] [PubMed] [Google Scholar]
- 55.Bradstock KF, Makrynikola V, Bianchi A, Shen W, Hewson J, Gottlieb DJ: Effects of the chemokine stromal cell-derived factor-1 on the migration and localization of precursor-B acute lymphoblastic leukemia cells within bone marrow stromal layers. Leukemia 2000, 14:882-888 [DOI] [PubMed] [Google Scholar]
- 56.Burger JA, Burger M, Kipps TJ: Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood 1999, 94:3658-3667 [PubMed] [Google Scholar]
- 57.Crazzolara R, Kreczy A, Mann G, Heitger A, Eibl G, Fink FM, Möhle R, Meister B: High expression of the chemokine receptor CXCR4 predicts extramedullary organ infiltration in childhood acute lymphoblastic leukaemia. Br J Haematol 2001, 115:545-553 [DOI] [PubMed] [Google Scholar]
- 58.Burger JA, Kipps TJ: Chemokine receptors and stromal cells in the homing and homeostasis of chronic lymphocytic leukemia B cells. Leuk Lymphoma 2002, 43:461-466 [DOI] [PubMed] [Google Scholar]
- 59.Jones D, O’Hara C, Kraus MD, Perez-Atayde AR, Shahsafaei A, Wu L, Dorfman DM: Expression pattern of T-cell-associated chemokine receptors and their chemokines correlates with specific subtypes of T-cell non-Hodgkin lymphoma. Blood 2000, 96:685-690 [PubMed] [Google Scholar]
- 60.Jones D, Benjamin RJ, Shahsafaei A, Dorfman DM: The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood 2000, 95:627-632 [PubMed] [Google Scholar]
- 61.Trentin L, Agostini C, Facco M, Piazza F, Perin A, Siviero M, Gurrieri C, Galvan S, Adami F, Zambello R, Semenzato G: The chemokine receptor CXCR3 is expressed on malignant B cells and mediates chemotaxis. J Clin Invest 1999, 104:115-121 [DOI] [PMC free article] [PubMed] [Google Scholar]