Abstract
The tumor level of plasminogen activator inhibitor-1 (PAI-1) is an informative biochemical marker of a poor prognosis in several cancer types. However, the tumor biological functions of PAI-1 and the identity of PAI-1-expressing cells are controversial. With the aim of immunohistochemically localizing PAI-1 in formalin-fixed, paraffin-embedded invasive ductal breast carcinoma samples, we raised new polyclonal antibodies against PAI-1 from different expression systems. The antibodies were affinity purified by absorption on immobilized preparations of PAI-1 different from those used for immunization. The specificity of the antibodies was ensured by immunoblotting analysis. In immunohistochemistry, the staining pattern obtained with the antibodies showed a good correlation with the PAI-1 mRNA expression pattern. In all 25 cases analyzed, PAI-1 immunoreactivity was predominantly localized in fibroblast-like cells. Double-immunofluorescence analyses showed co-expression of PAI-1 and α-smooth muscle actin in these cells, suggesting that they are myofibroblasts. PAI-1 was also seen in some myoepithelial cells surrounding occasional foci of ductal carcinoma in situ (9 of 25), some endothelial cells (8 of 25), some cancer cells (3 of 25), and some mast cells (6 of 25). In conclusion, we have provided a robust immunohistochemical procedure for detection of PAI-1 and shown that the majority of the PAI-1-expressing cells in invasive ductal breast carcinomas are myofibroblasts.
One of the proteolytic enzyme systems involved in the degradation of extracellular matrix during tumor growth, invasion, and metastasis is the urokinase-type plasminogen activator (uPA) system. 1-4 . uPA catalyzes the conversion of the inactive zymogen plasminogen to the active broad-spectrum protease plasmin, which is able to degrade many extracellular proteins, eg, fibrin and laminin. 5,6 uPA-directed activation of plasminogen occurs mainly on the cell surface after concomitant binding of uPA to its specific receptor, uPAR, and of plasminogen to proteins with C-terminal lysines. 7 The primary inhibitor of uPA is the serpin plasminogen activator inhibitor-1 (PAI-1). 8,9
The hypothesis that uPA promotes tumor growth and spread was originally based on observations with cell culture and animal tumor models. 1 The hypothesis has been supported by quantification of uPA protein in extracts of primary tumors, including breast carcinomas, demonstrating that high levels of uPA are correlated with a poor prognosis. 10,11 The hypothesis of a causal role of uPA-catalyzed plasminogen activation and plasmin proteolytic activity in primary tumor growth, local invasion, and/or metastasis was recently strongly supported by studies with tumors growing on mice with targeted disruption of the uPA or plasminogen genes, 3 including a study with a genetically induced mammary carcinoma. 12
It was therefore unexpected that tumors were found to contain higher amounts of the uPA inhibitor PAI-1 than the corresponding normal tissue and particularly that a high PAI-1 level in tumors was correlated with poor prognosis in several cancer types, including breast cancer, 11,13 being an even better prognostic marker than uPA. 14 In addition, the value of PAI-1 as a predictor of poor prognosis in breast cancer is independent of tumor size and of estrogen receptor status, 15 and the prognostic value of combined measurement of uPA and PAI-1 levels in tumor extracts is independent of the prognostic value of HER2 status. 16 It has been suggested that the combined measurement may be of value for planning of individualized cancer therapy. 17
Despite the prognostic value of PAI-1, the precise tumor biological functions of PAI-1 are not known. Studies with animal tumor models have failed to give a consistent picture. A high level of PAI-1 expression by human or murine cancer cells growing on nude mice was reported to be associated with impairment of tumor growth, invasion, and/or metastasis. 18-20 Injections of PAI-1 protein into immunodeficient mice bearing transplanted human tumors led to either inhibition of tumor growth 21 or stimulation of tumor growth at low PAI-1 level injected and inhibition of tumor growth at high PAI-1 level injected. 22 Overexpression of PAI-1 by transgenic hosts did not affect the growth or metastasis of a transplanted murine melanoma. 23 In work with PAI-1 gene-deficient mice, transplanted murine transformed keratinocytes needed host PAI-1 for tumor invasion and vascularization, 24,25 whereas a genetically induced mammary carcinoma was unaffected by PAI-1 gene deficiency with respect to tumor growth, vascularization, and metastasis. 26 Thus, PAI-1 may have diverse functions in animal tumor models, depending on the cell type expressing PAI-1, the level of expression, and the biology of the tumor model used. Nevertheless, using PAI-1 gene-deficient mice in in vivo and ex vivo angiogenesis model systems, 22,24,25,27,28 physiological concentrations of PAI-1 were consistently found to have a proangiogenic effect, suggesting that PAI-1 may enhance tumor growth and/or invasion by stimulating angiogenesis.
Important for understanding the role of PAI-1 in human cancer is identification of the PAI-1-expressing cells. Such information may also be valuable for selection of relevant animal tumor models for experimental studies of the role of PAI-1, and may even provide information of additional prognostic value. There are several reports on the localization of PAI-1 in primary breast carcinoma, but a consistent picture cannot be drawn. 29-37 The aim of this study was to develop polyclonal antibodies that could be used on routinely processed paraffin-embedded breast cancer samples. Careful affinity purification steps were used to provide two specific high-affinity antibody preparations that showed identical staining patterns, which were in good agreement with the pattern obtained with a monoclonal antibody against PAI-1 as well as the expression pattern of PAI-1 mRNA obtained by in situ hybridization.
Materials and Methods
Tumor Samples
Tumor specimens from 25 patients with primary invasive ductal breast carcinoma (7 grade I, 11 grade II, 7 grade III) registered in the Danish Breast Cancer Cooperative Group were obtained from the Department of Pathology, Copenhagen University Hospital, Copenhagen, Denmark. The tumor specimens were fixed in 10% buffered formalin for 24 hours at room temperature and paraffin-embedded. Sections from each of these paraffin blocks were used for histopathological analysis, and confirmed the presence of cancer tissue within the samples. Frozen tissue specimens were obtained from eight invasive ductal carcinomas and were used for protein extraction. The study was approved by the Regional Scientific-Ethical Committee for Aarhus (j.nr. 1991/2106) and the Regional Scientific-Ethical Committee for Copenhagen and Frederiksberg (j.nr. KF 01-456/93).
Antibodies against PAI-1
A rabbit polyclonal anti-PAI-1 antibody was generated after repeated immunizations with recombinant human PAI-1 expressed in Pichia pastoris. 38 The IgG fraction (AB-1) was purified from the rabbit serum by chromatography on a protein A-Sepharose column. The IgG fraction was then passed six times through a Sepharose column with immobilized recombinant human PAI-1 expressed in Escherichia coli. 39 The sixth run-through, which was depleted for most anti-PAI-1 IgG, was used as negative control IgG and named AB-1D. An affinity-purified anti-PAI-1 IgG preparation (AB-1A) was prepared by eluting the column with a buffer of 0.1 mol/L glycin, pH 2.5, and 1 mol/L NaCl. As evaluated by enzyme-linked immunosorbent assay, the reactivity of AB-1A toward PAI-1 40 was increased by a factor of 3.3 compared to the starting material, whereas AB-1D showed an ∼500-fold reduction in PAI-1 reactivity. In brief, this enzyme-linked immunosorbent assay was performed as follows: wells were coated with HT-1080 PAI-1 and a dilution series of fractions of anti-PAI-1 IgG and anti-PAI-1-depleted IgG were added. The captured antibodies were detected with horseradish-peroxidase-conjugated swine anti-rabbit antibodies and a peroxidase reaction.
Another polyclonal anti-PAI-1 antibody preparation was raised against PAI-1 purified from HT-1080 cells. 40 This IgG preparation will be referred to as AB-2. Affinity purification was done as for AB-1A, but using a Sepharose column with immobilized P. pastoris PAI-1. This affinity-purified anti-PAI-1 antibody preparation (AB-2A) showed a fivefold increased reactivity compared with the starting material. The sixth run-through (AB-2D) had an ∼150-fold reduced PAI-1 reactivity. A mouse monoclonal anti-PAI-1 antibody, clone 380, was purchased from American Diagnostica (Greenwich, CT).
Antibodies against uPA and tPA
A rabbit polyclonal antibody raised against uPA from Serono, Aubonne, Switzerland, was a gift from the late Dr. I. Clemmensen (DakoCytomation, Glostrup, Denmark). IgG was purified from the rabbit serum by the use of protein A-Sepharose. The uPA immunogen appeared pure on Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and we previously used absorption against the immunogen for preparing an antibody to be used as a negative control. 32 To improve the specificity of the negative control preparation, the uPA immunogen was affinity-purified by chromatography on an anti-uPA monoclonal antibody immobilized on Sepharose. 41 Another Sepharose column was made containing this affinity-purified uPA preparation, and the protein A-purified anti-uPA IgG fraction was passed over the column until the reactivity against uPA had been reduced more than 100-fold. This run-through IgG preparation will be referred to as “polyclonal antibodies depleted of uPA-reactivity.” Polyclonal anti-tPA IgG was a gift from the late Dr. I. Clemmensen (DakoCytomation, Denmark).
Immunoblotting Analysis
Tumors were taken from −80°C and homogenized immediately in 0.1 mol/L Tris, pH 8.1, 0.5% Triton X-100, 10 mmol/L ethylenediaminetetraacetic acid, and 10 μg/ml aprotinin (10 μl/mg tissue) with a Ultraturrax with a S 25 N8G head (24,000 rpm) at 4°C. The homogenate was centrifuged at 10,000 × g for 10 minutes to remove cell debris and nuclei. The supernatants will be referred to as “tumor extracts.” Several pools, each consisting of extracts of 5 to 10 tumors, were analyzed by immunoblotting analysis. Samples of the pools, each corresponding to ∼200 μg of total protein, were subjected to SDS-PAGE and transferred electrophoretically to polyvinylidene difluoride filters (Immobilon-P; Millipore, Bedford, MA). 32 The filters were incubated with affinity-purified polyclonal anti-PAI-1 antibodies AB-1A and AB-2A, polyclonal antibodies depleted of PAI-1 reactivity AB-1D and AB-2D, polyclonal anti-uPA antibodies, polyclonal anti-tPA antibodies, polyclonal antibodies depleted of uPA immunoreactivity, or preimmune IgG. The primary antigen-antibody reactions were visualized with peroxidase-conjugated swine anti-rabbit antibodies (P217, DakoCytomation) and the ECL system (Amersham Pharmacia, Uppsala, Sweden).
Immunoperoxidase Staining
Paraffin sections (3- to 4-μm-thick sections of formalin-fixed and paraffin-embedded tissue samples) were deparaffinized in xylene and ethanol. For retrieval of PAI-1 antigen, sections were boiled in a microwave oven in 10 mmol/L Tris containing 0.5 mmol/L EGTA (pH 9.0, TEG buffer) for 15 minutes. 42,43 We could not retrieve PAI-1 antigen by boiling in citrate buffer, pH 6.0, or by proteolytic digestion with 0.05% pronase (S2013, DakoCytomation) in Tris-buffered saline (TBS) buffer, pH 7.2, for 15 minutes at 37°C. The sections were left for 20 minutes at room temperature and then incubated with 2% H2O2 in 99% ethanol for 20 minutes to block endogenous peroxidase activity. Sections were washed in TBS containing 0.5% Triton X-100 (TBS-T) for 30 minutes, and incubated with Protein Block (X909, DakoCytomation) for 10 minutes to reduce unspecific adhesion of the antibodies. The affinity-purified rabbit polyclonal antibodies against PAI-1, as well as the anti-PAI-1-depleted IgG preparations, were diluted in Antibody Diluent (S809, DakoCytomation) and incubated at 0.5 μg/ml (if not otherwise stated) overnight at 4°C in a humidifying chamber, followed by ∼1 hour at room temperature. Primary antibodies were detected with anti-rabbit IgG-horseradish peroxidase-conjugated polymers (EnVision rabbit reagent, K4003; DakoCytomation). Each antibody incubation step was followed by thorough washes in TBS-T. Finally, sections were developed for 15 minutes with either 3-amino-9-ethylcarbazole (Sigma A-5754) or NovaRed (Vector Laboratories, Burlingame, CA), resulting in red staining. Finally Mayer’s hematoxylin was used for nuclear counterstaining. The monoclonal antibody against PAI-1 (clone 380) was used in some experiments. Antigen retrieval was in this case performed by boiling in citrate buffer, pH 6.0, for 10 minutes. 31 Clone 380 and a monoclonal antibody against trinitrophenyl hapten (TNP 44 ) used as negative control (both antibodies are IgG1 subtype), were both used at 5 μg/ml and incubated overnight at 4°C and detected with Envision mouse reagent (K4003, DakoCytomation), followed by tyramine signal amplification using biotinyl tyramine (NEN, Boston, MA) according to the manufacturer’s instructions. All other steps were as for the rabbit polyclonal antibodies specified above.
Frozen sections (5-μm cryostat sections) were immediately dried on a heating plate at 60°C for 2 minutes and fixed in neutral-buffered formalin overnight at 4°C. After thorough washes in water, the sections were heat-treated at 99°C for 3 minutes in TEG-buffer using a Micromed T/T microwave processor (Milestones, Sorisol, Italy) and subsequently retempered for 30 minutes at room temperature. Endogenous peroxidase was blocked by 15 minutes of incubation in 1% H2O2. Sections were incubated with 0.5 μg/ml of AB-2A for 2 hours and subsequently with EnVision rabbit reagent for 30 minutes at room temperature. Sections were developed with NovaRed chromogene as specified above for the paraffin sections.
Double-Immunofluorescence Analysis
Four-μm paraffin sections were hydrated and boiled in TEG buffer as specified above. Primary antibodies against PAI-1 were incubated at 0.5 μg/ml together with monoclonal antibodies (all obtained from DakoCytomation) against α-smooth muscle actin (α-SMA, clone 1A4) diluted 1:400, CD34 (QBend10) diluted 1:200, cytokeratin-17 (CK-17, clone E3) diluted 1:50, CD68 (clonePG-M1) diluted 1:50, and mast cell tryptase (clone AA1) diluted 1:200, and incubated overnight at 4°C. The polyclonal antibodies were detected with Cy3-conjugated goat anti-rabbit IgG (1:200) and the mouse monoclonal antibodies with fluorescein isothiocyanate-conjugated goat-anti-mouse IgG (1:200), both obtained from Jackson Immunoresearch, West Grove, PA. Sections were mounted with anti-fade mounting medium (DakoCytomation). Digital images were obtained with a conventional fluorescence microscope equipped with a Coolsnap charge-coupled device camera (Rober Scientific-Photometrics, Tucson, AZ). Digital image overlays were processed using MetaMorph software (Universal Imaging Corporation, Downingtown, PA).
In Situ Hybridization
Two nonoverlapping cDNA subclones (phPAI-04, phPAI-05) for human PAI-1 were described previously. 45 35S-Labeled anti-sense and sense RNA probes were generated by in vitro transcription 46 and the in situ hybridization performed essentially as described. 46 In brief, 3-μm paraffin sections were deparaffinized in xylene, hydrated through graded ethanol solutions, and digested with protease K (5 μg/ml) for 5 minutes at 44°C. Sections were then dehydrated and the 35S-labeled probes (2 × 106 cpm/slide) incubated overnight at 55°C in a humidified chamber. Sections were washed with agitation at 55°C with a buffer of 15 mmol/L sodium citrate, pH 7.0, 0.15 mol/L NaCl (SSC) containing 0.1% SDS and 10 mmol/L dithiothreitol for 10 minutes in 2× SSC, for 10 minutes in 0.5× SSC, and for 10 minutes in 0.2× SSC. Sections were then RNase A-treated (20 μg/ml) for 10 minutes at 44°C to remove nonspecifically bound riboprobe. Subsequent wash was performed in 0.2× SSC as specified above. Sections were dehydrated in graded ethanol solutions containing 300 mmol/L of ammonium acetate and soaked into an autoradiographic emulsion (Ilford), exposed for 7 to 10 days and finally developed. Sections were counterstained with hematoxylin and eosin.
Combined in Situ Hybridization and Immunohistochemical Analysis for α-SMA
Combined immunohistochemistry and in situ hybridization were performed essentially as described. 46 In brief, 3-μm paraffin sections were pretreated by boiling in 10 mmol/L sodium citrate, pH 6.0, in a microwave oven for 10 to 12 minutes. The sections were allowed to chill for 20 minutes at room temperature and then transferred to TBS. Primary antibodies were diluted in TBS containing 0.25% bovine serum albumin and 1% RNase inhibitor (Roche, Basel, Switzerland) and incubated with the sections for 2 hours at room temperature, using a monoclonal antibody (clone 1A4, DakoCytomation) against α-SMA, and a monoclonal antibody against CD68 (clone PG-M1, DakoCytomation) both diluted 1:200. The primary antibodies were detected with EnVision mouse. The incubation steps were followed by washes with autoclaved TBS. Sections were developed with diaminobenzidine for 7 to 10 minutes, and immediately dehydrated for in situ hybridization, which was performed as described above, using the anti-sense PAI-1 probe (phPAI-1-05). Finally, the sections were counterstained with hematoxylin.
Results
Polyclonal Anti-PAI-1 Antibodies
Two polyclonal antibody (IgG) preparations, AB-1 and AB-2, were obtained from antiserum generated by immunization of rabbits with human PAI-1 expressed either recombinantly by P. pastoris or by HT-1080 cells. To remove antibodies against possible contaminating proteins, the anti-PAI-1 IgG preparations were affinity-purified with PAI-1 from a source different from that used for immunization. Thus, AB-1 and AB-2 were affinity-purified on Sepharose-immobilized human PAI-1 expressed recombinantly in E. coli and P. pastoris, respectively, to give AB-1A and AB-2A. Sixth run-through fractions, AB-1D and AB-2D, from each of the affinity purification columns were prepared. Being depleted for anti-PAI-1 IgG, these fractions were used as negative controls.
Immunoblotting Analysis
The specificity of the polyclonal anti-PAI-1 antibodies was tested by immunoblotting analysis of tumor extracts (Figure 1) ▶ . Blots were incubated with AB-1A, AB-1D, AB-2A, and AB-2D. AB-1A and AB-2A reacted specifically with a band co-migrating with PAI-1, and bands co-migrating with the SDS-resistant uPA-PAI-1 and tPA-PAI-1 complexes, respectively, as these bands were absent in blots analyzed with AB-1D and AB-2D. Supplementing the tumor extracts with purified HT-1080 PAI-1 protein led to enhancement of the bands co-migrating with PAI-1, uPA-PAI-1 complex, and tPA-PAI-1 complex and to appearance of two other bands (PAI-1d and PAI-1h in Figure 1 ▶ ), one representing a PAI-1 degradation product and one with high Mr, probably representing an SDS-resistant complex of PAI-1 with another protease. The bands co-migrating with uPA-PAI-1 and tPA-PAI-1 complexes also reacted with anti-uPA and anti-tPA IgG, respectively, but not with the corresponding negative controls (data not shown). The blots also displayed some nonspecific background and some aggregates at the top of the gels, and the detection of bands in the Mr 50,000 to 60,000 region of the gel was hampered by the presence of relative large amounts of serum albumin in the extracts. Similar results were obtained with AB-1 and AB-2, but the signals were clearly weaker than those obtained with the affinity-purified preparations AB-1A and AB-2A. There were no obvious differences between the two affinity-purified antibody preparations in immunoblotting analysis (data not shown). The results show that a large fraction of the PAI-1 in the tumors appears in the extracts as complexes with uPA and tPA, but clearly suggest that AB-1, AB-1A, AB-2, and AB-2A react specifically with PAI-1 in the tumors.
Figure 1.
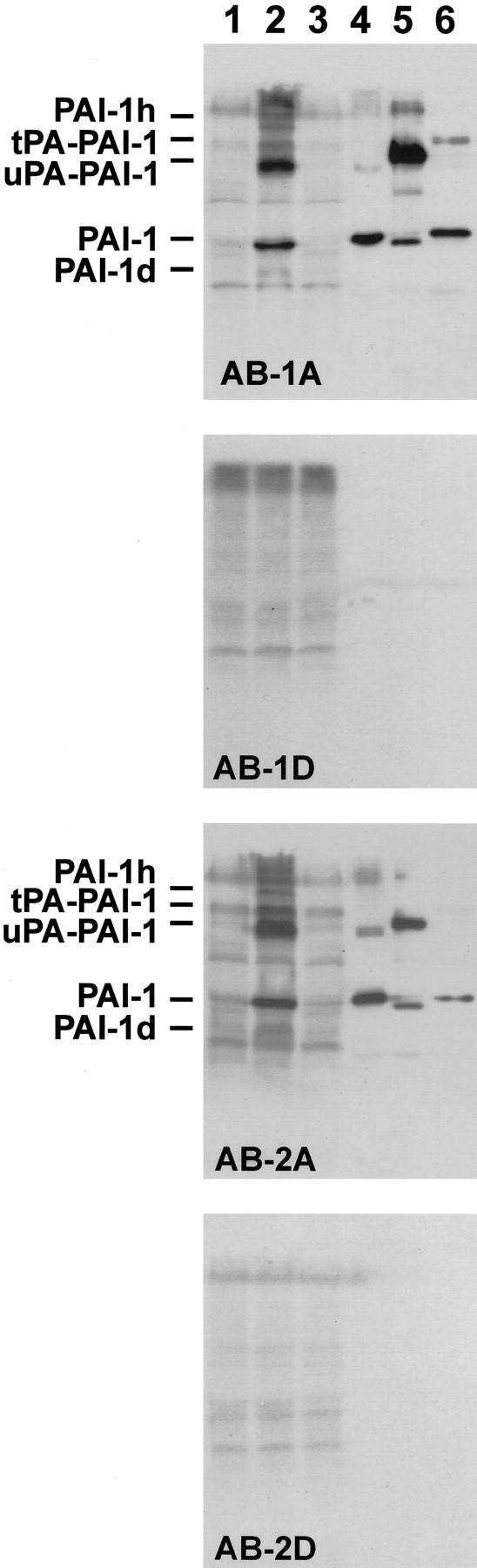
Immunoblotting analysis of the reactivity of anti-PAI-1 antibodies with tumor extracts. The following samples were subjected to SDS-PAGE: a portion of a pool of tumor extracts from eight tumors, corresponding to ∼200 μg of total protein without additions (lane 1); tumor extract supplemented with 25 ng of purified HT 1080 PAI-1 (lane 2); tumor extract supplemented with 50 ng of purified uPA (lane 3); 25 ng of purified PAI-1 (lane 4); 100 ng of purified uPA-PAI-1 complex (lane 5); or 100 ng of purified tPA-PAI-1 complex (lane 6). Blots of the gel lanes on polyvinylidene difluoride filters were prepared and analyzed with AB-1A, AB-1D, AB-2A, or AB-2D, as indicated. The migration of PAI-1, uPA-PAI-1 complex, and tPA-PAI-1 complex are indicated to the left. Two additional bands, appearing after supplementing tumor extracts with purified PAI-1 (lane 2), are labeled PAI-1d and PAI-1h. Please note that the preparations of uPA-PAI-1 complex and tPA-PAI-1 complex contain some free PAI-1, which in the case of the uPA-PAI-1 complex is in the form of reactive center-cleaved PAI-1, migrating slightly faster than native PAI-1. The slowly migrating band in the PAI-1 preparation in lane 4 is aggregated PAI-1.
Specificity of the Anti-PAI-1 Antibodies in Immunohistochemical Analysis
Adjacent sections from six breast carcinomas were analyzed with AB-1A, AB-2A, AB-1D, and AB-2D. PAI-1 immunoreactivity was observed with both AB-1A and AB-2A in all six cases. A virtually identical staining pattern was observed with AB-1A and AB-2A (Figure 2) ▶ . However, the AB-2A preparation appeared to give strong staining of the positive cells and little background staining, whereas the staining obtained with AB-1A varied more in intensity and gave a staining of the border of the sections in some of the tumors not seen with AB-2A. The anti-PAI-1-depleted IgG fraction, AB-1D, showed little or no staining of the PAI-1-positive cells detected with AB-1A. AB-2D showed little or no reactivity toward the PAI-1-positive cells detected with AB-2A, but reacted with extracellular material in vascular and glandular lumens as well as some necrotic foci present in some of the samples (Figure 2) ▶ . PAI-1 immunoreactivity was seen with both antibody preparations in several different cell types, including fibroblast-like cells, endothelial cells, cancer cells, myoepithelial cells, and some stromal mononuclear cells (Figure 3 ▶ , see below). We also tested the monoclonal antibody clone 380, previously used to stain PAI-1 in human breast cancer tissue. 31,37 A moderate staining intensity was obtained with clone 380 after tyramide signal amplification. The staining pattern obtained with this antibody was similar but slightly weaker than that obtained with AB-2A. Thus, staining was seen of fibroblast-like stromal cells, some endothelial cells, myoepithelial cells associated with foci of ductal carcinoma in situ, and some stromal mononuclear cells. A monoclonal antibody against TNP, which is of the same IgG subclass as clone 380 and incubated at the same concentration, showed no staining (data not shown). We also tested the AB-2A in cryostat sections obtained from frozen samples from tumors also tested in paraffin sections. In one of the samples, AB-2A stained fibroblast-like cells and myoepithelial cells. In the other sample only fibroblast-like cells were positive in agreement with the cell types stained in the paraffin sections (Figure 2, e and f) ▶ .
Figure 2.
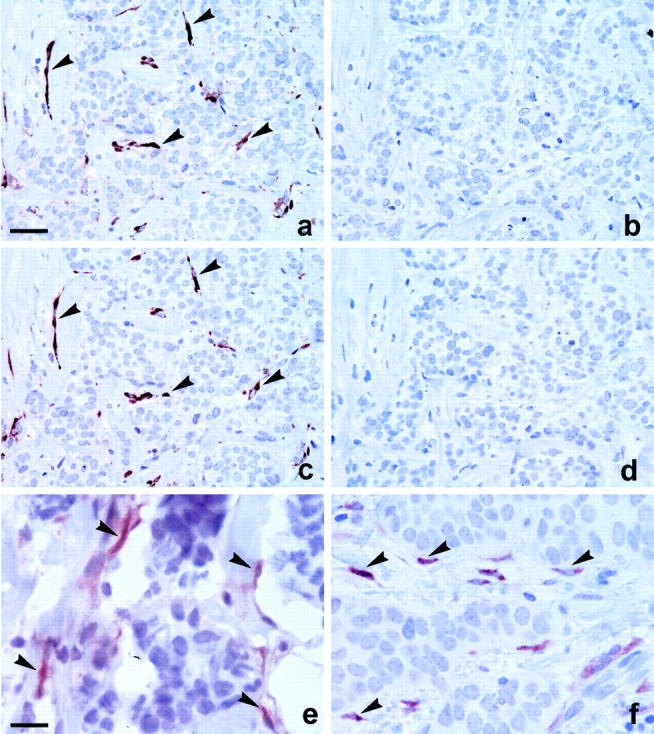
Immunoperoxidase staining with affinity-purified anti-PAI-1 polyclonal antibodies in human breast cancer tissue. Four adjacent paraffin sections were analyzed immunohistochemically for PAI-1 with affinity-purified anti-PAI-1 IgG, AB-1A (a), and AB-2A (c), and anti-PAI-1-depleted IgG, AB-1D (b), and AB-2D (d). PAI-1 immunoreactivity obtained with AB-1A and AB-2A was seen in the very same (stromal) cells (arrows), whereas no staining was seen with AB-1D and AB-2D (b, d). The weak background staining observed with AB-1A (a) is related to the difficulties with this antibody preparation generally staining intensely toward the border of the sections. A cryostat section (e) and a paraffin section (f) from the same tumor were incubated with AB-2A. PAI-1 immunoreactivity is seen in stromal cells in both sections (arrows) and no staining is seen in cancer cells. Scale bars: ∼80 μm (a–d); ∼20 (e, f).
Figure 3.
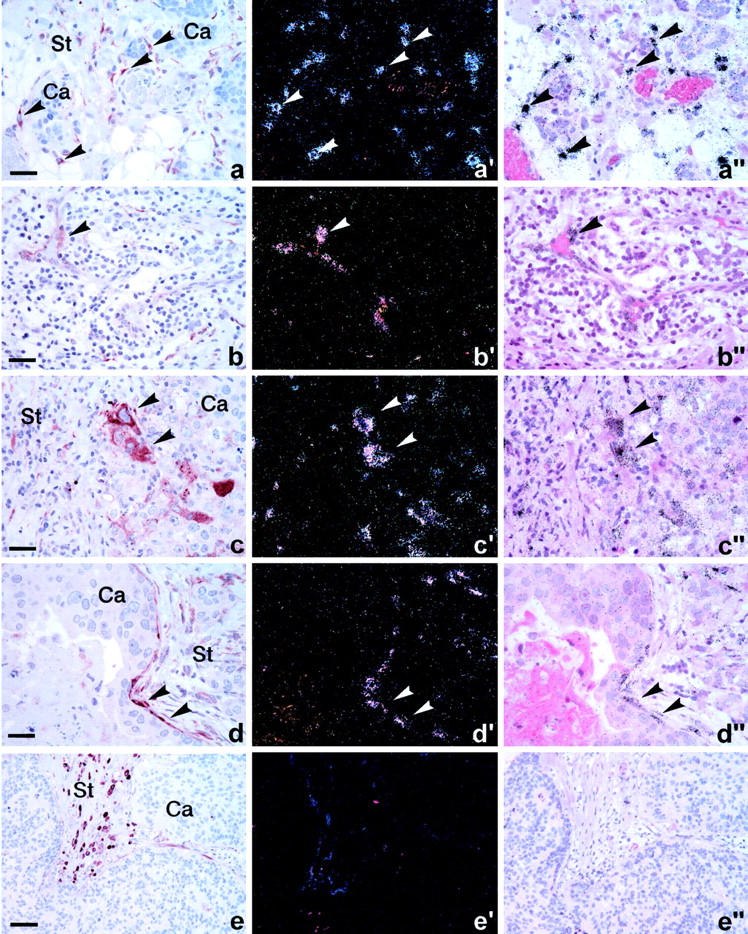
Expression of PAI-1 protein and mRNA in various cell types in human breast cancer. Two adjacent sections were processed for PAI-1 immunoperoxidase staining using rabbit polyclonal affinity purified anti-PAI-1 IgG, AB-2A (a–e) and in situ hybridization with a 35S-labeled PAI-1 anti-sense probe (a′–e′, a″–e″) shown in dark-field (a′–e′) and bright-field (a″–e″) illumination. PAI-1 immunoreactivity and mRNA are seen in the very same cells, as indicated by arrows in fibroblast-like cells surrounding cancer cell (Ca) nodules (arrows in a), vascular endothelial cells (arrows in b), cancer cells (arrows in c), and myoepithelial cells (arrows in d). No PAI-1 mRNA signal (e′–e″) is detected in the PAI-1 immunoreactive stromal mononuclear cells (e) identified as mast cells. The cancer cell compartment is indicated by Ca and the stromal compartment by St. Scale bars: ∼75 μm (a–d); ∼150 μm (e).
To further evaluate the immunohistochemical staining, we compared the localization of PAI-1 immunoreactivity obtained with AB-1A and AB-2A with that of PAI-1 mRNA in eight samples, including the six samples studied above. In all eight samples, we observed co-localization of PAI-1 immunoreactivity with its mRNA in fibroblast-like cells, endothelial cells, cancer cells, as well as myoepithelial cells (Figure 3) ▶ . PAI-1 immunoreactivity and mRNA expression was seen in fibroblast-like cells in all eight cases, endothelial cells in three cases, cancer cells in one case, and myoepithelial cells surrounding occasional carcinoma in situ foci in three cases. However, the staining of stromal mononuclear cells observed in immunohistochemical analysis and identified in a considerable number in two of the cases was not accompanied by PAI-1 mRNA expression.
Because AB-1A showed some background staining especially toward the border of the sections, we chose not to use it for the more extensive immunohistochemical studies described below. Because the staining pattern obtained with AB-2A was more reproducible and correlated well with the staining pattern obtained with clone 380 and with the PAI-1 mRNA expression pattern, we chose the AB-2A preparation for the further studies.
Localization of PAI-1 Immunoreactivity in 25 Cases of Invasive Ductal Breast Cancer
Twenty-five cases of invasive ductal carcinoma including those described above were analyzed with the AB-2A and AB-2D preparations. PAI-1 immunoreactivity was seen in fibroblast-like cells in all 25 cases. These PAI-1-positive cells were seen both in central tumor areas and in the periphery, but generally the number of PAI-1-positive cells increased toward the very periphery of the carcinoma. A few endothelial cells were PAI-1-positive in eight of the cases and were seen in central areas as well as in the tumor periphery. Myoepithelial cells surrounding foci of carcinoma in situ located together with invasive carcinoma in nine of the cases often showed distinct PAI-1 immunoreactivity. One case had many (∼20%) PAI-1-positive cancer cells, particularly in the tumor periphery, and two of the cases had a few positive cancer cells (∼1%). PAI-1-positive stromal mononuclear cells were seen in a considerable number (more than 10 in a tissue section) in six of the cases incidentally located within in the tumor stroma.
Characterization of the PAI-1-Expressing Cells with Specific Cell-Type Markers
The specific identity of the different PAI-1-expressing cell types could to a certain extent be determined by morphological criteria, eg, endothelial cells of vascular structures, myoepithelial cells, and foci of cancer cells. A closer characterization of the PAI-1-expressing cells was done by double immunofluorescence of sections incubated with AB-2A together with antibodies against α-SMA, CD34, CD68, CK-17, or mast cell tryptase. In breast cancer, α-SMA is expressed by myofibroblasts, vascular smooth muscle cells, and myoepithelial cells. 47,48 CD34 is a marker of endothelial cells and CD68 (clone PG-M1 49 ) is specifically expressed in macrophages. CK-17 is a marker of myoepithelial cells. 50 PAI-1 immunoreactivity was co-expressed with α-SMA in the majority of the PAI-1-positive fibroblast-like cells (Figure 4 ▶ ; a to f) in all of six samples tested. Many of the α-SMA-positive myofibroblasts were PAI-1-negative, indicating that PAI-1 is expressed in a subpopulation of myofibroblasts. The PAI-1/α-SMA-positive cells surrounding cancer cell nodules were generally CK-17-negative and therefore not myoepithelial cells, but in three of the six cases, PAI-1 and CK-17 (and α-SMA) were co-expressed, indicating PAI-1 expression in myoepithelial cells in carcinoma in situ foci (results not shown). CD34 immunoreactivity was seen in a few PAI-1-positive stromal cells in two of the six cases (Figure 4 ▶ ; g, h, i), but most CD34-immunoreactive capillaries were PAI-1-negative (Figure 4 ▶ ; j, k, l). Because many of the CD68-positive macrophages showed autofluorescence, we could not with this method clarify whether PAI-1 is expressed in macrophages and thereby relate our observations to those of Bianchi and colleagues 31 who reported PAI-1-positive macrophages in 13 of 15 cases of invasive breast cancer. Using combined immunohistochemistry for CD68 and in situ hybridization for PAI-1 mRNA, we could however not detect PAI-1 mRNA in the CD68-positive macrophages in the eight cases tested, but we could detect PAI-1 mRNA in α-SMA-positive myofibroblasts in all eight cases (Figure 5) ▶ . The PAI-1-positive stromal mononuclear cells observed in 6 of the 25 cases were recognized as a small subpopulation of mast cells by double immunofluorescence performed on two of the six cases using an antibody against mast cell tryptase (Figure 4 ▶ ; m, n, o). These co-expression studies indicate that the PAI-1-expressing stromal fibroblast-like cells in breast cancer primarily are myofibroblasts. The mast cells express PAI-1 immunoreactivity and a few endothelial cells and myoepithelial cells express PAI-1 mRNA and immunoreactivity.
Figure 4.
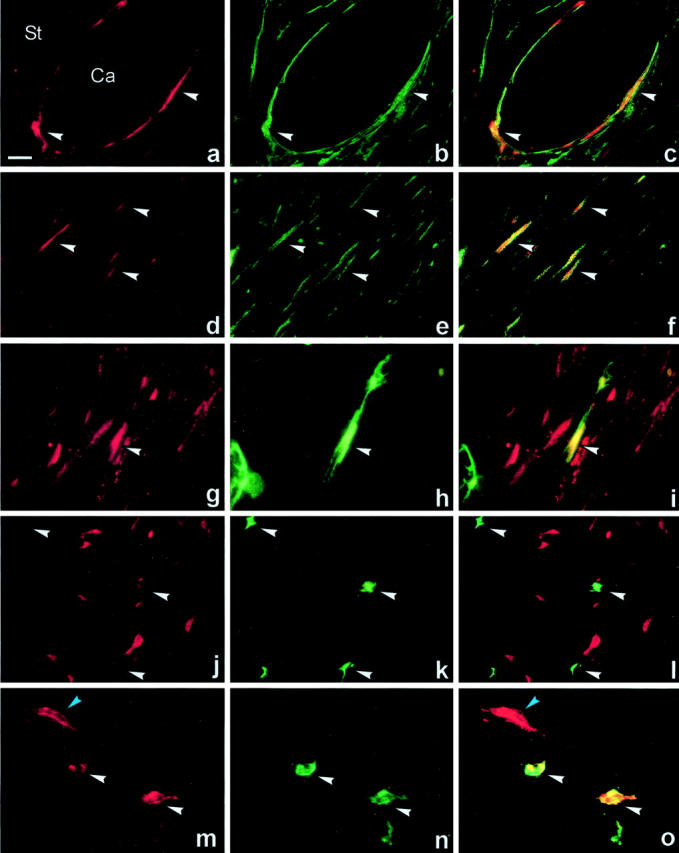
Double immunofluorescence of PAI-1 with α-SMA, CD34 and mast cell tryptase immunoreactivities in human breast cancer. Paraffin sections were incubated with polyclonal anti-PAI-1 antibodies (AB-2A) together with a monoclonal antibody to α-SMA (a–f), monoclonal antibody to CD34 (g–l), or monoclonal antibody against mast cell tryptase (m–o). The polyclonal antibodies were recognized with Cy3-conjugated goat anti-rabbit (a, d, g, j, m) and the monoclonal antibodies with FITC-conjugated goat anti-mouse (b, e, h, k, n). Image overlays of Cy3 and FITC signals were processed (c, f, i, l, o). PAI-1 immunoreactivity is co-expressed with α-SMA immunoreactivity in myofibroblasts surrounding cancer cell (Ca) nodules (arrows in a–c, yellow color in c), and in myofibroblasts located in stromal tissue with multiple myofibroblast (arrows in d–f, yellow color in f). Only a few PAI-1-positive cells were CD34-positive (arrows in g–i, yellow color in i), but most CD34-positive capillary endothelial cells were negative (arrows in j–l). Strong PAI-1 immunoreactivity is seen in two mast cell tryptase-positive cells (white arrows in m–o) close to a PAI-1-positive myofibroblast (blue arrow in m and o). Scale bar, ∼30 μm.
Figure 5.
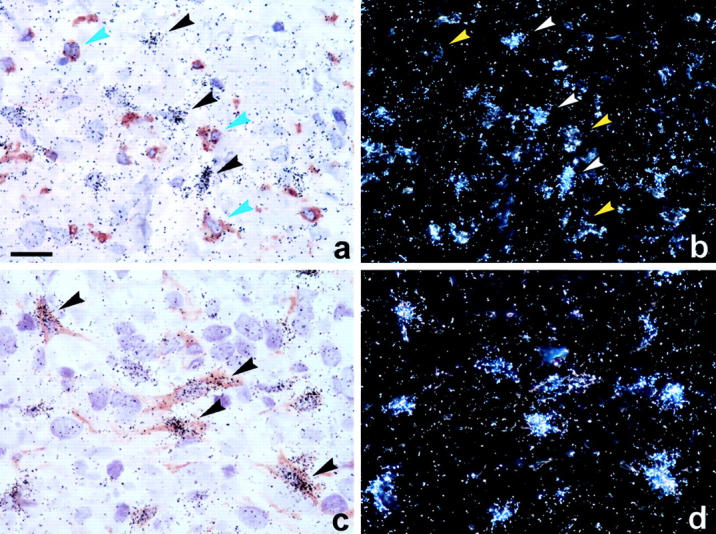
Double labeling for PAI-1 mRNA and CD68 or α-SMA immunoreactivities in human breast cancer. Sections were first processed for immunohistochemical staining of CD68 (a, b) or α-SMA (c, d; brown color, left), and then for PAI-1 mRNA in situ hybridization [black silver grains in bright-field illumination (a, c) and light grains in dark-field illumination (b, d)]. No PAI-1 mRNA signal (black arrows in a, white arrows in b) is detected in CD68-positive macrophages cells (blue arrows in a, yellow arrows in b), but is clearly seen in the α-SMA-positive myofibroblasts (arrows in c). Scale bar, ∼20 μm.
Discussion
Previously, a number of studies 29-37 have reported on immunohistochemical analysis of PAI-1 in human breast carcinomas. However, it has been surprisingly difficult to reach a consensus on the localization of PAI-1 because PAI-1 immunoreactivity was reported to be present solely or mainly in cancer cells by some authors, 29,30 mainly in the stromal fibroblast-like cells by others, 31 or both in cancer cells and stromal fibroblast-like cells by still others. 32-37 The difficulty in reaching conclusive results should be seen in relation to the low amounts of PAI-1 in breast tumors, in extracts in the order of magnitude of 10 ng/mg total protein. This fact renders the requirement for strict specificity controls particularly pertinent. The aim of the present study was to generate new polyclonal antibodies that could be used for immunohistochemical detection of PAI-1 in routinely processed, formalin-fixed and paraffin-embedded breast cancer samples, to overcome the ambiguities appearing from the previous reports. Two different rabbit polyclonal antibodies, AB-1 and AB-2, were raised against PAI-1 purified from two different sources. Affinity-purified IgG preparations, AB-1A and AB-2A, respectively, were prepared by the use of immobilized recombinant PAI-1 from sources different from those used for producing the antigens. The advantage of this affinity purification procedure is that it reduces the risk of antibodies against other human proteins in the antibody preparation. Run-through fractions from the affinity columns, depleted for anti-PAI-1 IgG, were used as negative controls. In immunoblotting analysis of tissue extracts, the antibodies reacted specifically with PAI-1, uPA-PAI-1 complex, and tPA-PAI-1 complex. By immunohistochemistry, we obtained very similar and intense staining patterns with the two affinity-purified anti-PAI-1 preparations, however, AB-1A showed some background staining especially toward the border of the sections. The anti-PAI-1-depleted IgG preparations showed no or little staining of the cells that were stained with the affinity-purified anti-PAI-1 antibodies. In addition, a monoclonal anti-PAI-1 antibody gave a staining pattern in agreement with that obtained with the AB-2A but showed slightly weaker staining intensity. Moreover, we found virtually identical localizations of PAI-1 immunoreactivity and PAI-1 mRNA, the latter being identified by in situ hybridizations with two PAI-1 mRNA anti-sense probes. One exception was the PAI-1-immunoreactive stromal mononuclear cells that were stained with all three anti-PAI-1 antibodies, but in which no PAI-1 mRNA was detected. Based on this wide spectrum of specificity controls, we concluded that AB-2A provides an unambiguous detection of the PAI-1 protein, while we could not exclude that AB-1A gave some nonspecific background staining.
In total, we analyzed 25 cases of invasive ductalcarcinoma with AB-2A. By morphological criteria, PAI-1 immunoreactivity was in all cases (25 of 25) seen in fibroblast-like cells, some endothelial cells (8 of 25), some myoepithelial cells surrounding carcinoma in situ foci (9 of 25), small subpopulations of cancer cells (3 of 25), and in some stromal mononuclear cells (6 of 25). Using double-immunofluorescence analysis with AB-2A together with antibodies against cell-type-specific markers, including α-SMA for myofibroblast, CD34 for endothelial cells, and CK-17 for myoepithelial cells, we could conclude that the predominant PAI-1-expressing cell type in breast cancer tissue is the myofibroblast. This conclusion was supported by the observation that many PAI-1 mRNA-positive cells were α-SMA-positive. There was co-expression of PAI-1 and CD34 in some obvious vascular structures, but only in few stromal fibroblast-like cells, and most CD34-positive capillaries were PAI-1-negative. None of the cell-specific markers, including α-SMA, CD34, CK-17, or the macrophage-specific marker CD68/PG-M1 stained the PAI-1-positive stromal mononuclear cells. The morphological characteristics of this cell population suggested that they could be mast cells, and this was confirmed by double-immunofluorescence staining using an antibody against mast cell tryptase. Because some macrophages showed autofluorescence, we used combined in situ hybridization and immunohistochemistry in an attempt to clarify whether some of the PAI-1-expressing stromal cells were macrophages, but we were unable to detect PAI-1 mRNA in CD68-positive cells.
A main difference between our conclusions and most of the previous reports is our finding of a less abundant presence of PAI-1 in carcinoma cells. In a recent immunohistochemical study of uPA in breast cancer tissue, 51 it was reported that the use of some negative control monoclonal antibodies as well as some anti-uPA monoclonal antibodies resulted in unspecific staining of cancer cells under certain methodological circumstances. Such immunohistochemical difficulties could account for the cancer cell staining described in the previous reports, in which no isotype-matched negative control antibody was used. Bianchi and colleagues 31 did use an isotype-matched negative control monoclonal antibody in parallel with the clone 380 anti-PAI-1 antibody. In 15 of 20 invasive carcinomas positive for PAI-1, only 2 showed staining of many cancer cells and 10 of only few cancer cells. PAI-1 immunoreactivity was found primarily in fibroblasts, but also in macrophages (13 of 15), endothelial cells (5 of 15), and in myoepithelial cells in carcinoma in situ lesions, mostly based on morphological criteria. Their findings are in good agreement with ours. However, a noteworthy disagreement is that Bianchi and colleagues 31 reported a high frequency of cases (13 of 15) with expression of PAI-1 in macrophages. This disagreement may be explained by the fact that we measured PAI-1 expression at the mRNA level with in situ hybridization, whereas Bianchi and colleagues 31 used double immunolabeling with the clone 380 anti-PAI-1 antibody and a macrophage-specific anti-CD68 monoclonal antibody. In the method used by Bianchi and colleagues 31 it is unclear whether cross-reaction of the detecting antibodies toward the anti-PAI-1 and anti-CD68 mouse monoclonal antibodies was prevented. If not, false-positive PAI-1 staining in all CD68-positive cells would be expected.
Because several studies of mice with targeted disruption of the PAI-1 gene have pointed to a role of PAI-1 in angiogenesis, 22,24,25,27,28 and because high levels of PAI-1 measured in tumor extracts is associated with poor prognosis, it could be presumed that vascular cells in the breast cancer tissue expressed the majority of the PAI-1 antigen. However, our analyses showed that only a few of the PAI-1-positive cells were endothelial cells. Whether the eight cases with evident PAI-1 immunoreactivity in endothelial cells could be associated with particular vascularized or aggressive carcinomas is uncertain. Thus, our findings do not exclude a proangiogenic effect of PAI-1 in human breast cancer, but it does not support the assumption that this is the main function. It is relevant to note that the prognostic values of estimates of angiogenesis and the PAI-1 level in breast cancer were recently reported to be independent. 52
In all 25 cases, PAI-1 was detected in fibroblast-like cells, most of which were found to be α-SMA-positive. α-SMA is not confined to myofibroblasts but is also expressed in vascular smooth muscle cells and myoepithelial cells. Because the PAI-1-positive fibroblast-like cells were not directly associated with vessels, we could exclude that they were vascular smooth muscle cells. High PAI-1 expression was seen in cells, which immediately surrounded invasive cancer cell nodules and could be misinterpreted as myoepithelial cells. However, myoepithelial cells are not associated with invasive breast cancer and the nodular foci were clearly different from carcinoma in situ foci. Furthermore, we conclusively excluded (with few exceptions, see below) that the majority of the PAI-1-positive cells directly associated with cancer cell nodules could be myoepithelial cells, because they were negative for the myoepithelial cell marker CK-17. We therefore concluded that the predominant PAI-1-expressing cell type is the myofibroblast. Myofibroblasts are modified fibroblasts that may constitute up to 80% of the interstitial cells in the breast cancer stroma, whereas they are essentially absent from normal breast. 48,53 Subpopulations of breast cancer-associated myofibroblasts were previously demonstrated to express uPA 51,54,55 and collagenase-3. 46 In addition, other matrix metalloproteases (MMPs), such as gelatinase-2, stromelysin-3, and MT1-MMP, and MMP inhibitors, such as TIMP-2 and TIMP-3, have all been detected in fibroblast-like cells that may well be myofibroblasts. 46,56,57 Myofibroblasts also secrete a variety of extracellular matrix components, and the desmoplastic stroma contains increased amounts of hyaluronan, chondroitin sulfate, collagen I, fibrin, fibronectin, tenascin, and thrombospondin, as compared to normal breast. 56,58 The concomitant synthesis by myofibroblasts of extracellular matrix proteins, proteases, and their inhibitors suggests a function of these cells in remodeling of the tumor stroma extracellular matrix.
In 9 of the 25 carcinomas investigated, we observed foci of carcinoma in situ, and PAI-1 immunoreactivity was detected in some of the myoepithelial cells surrounding these foci. The myoepithelial cell layer is the basal cell layer of the mammary gland. During breast carcinogenesis, the myoepithelial cells rarely undergo malignant transformation, but remain as a continuous basal cell layer of the carcinoma in situ lesion. The myoepithelium is considered a natural suppressor of tumor invasion by producing basement membrane components and by providing a cellular barrier for the underlying cancer cells. 59 Myoepithelial cells may release protease inhibitors such as PAI-1, as suggested from this study, and tissue inhibitor of metalloproteinases-1 (TIMP-1), which may be protecting the basement membrane against proteolytic activity. 46,59 Interestingly, Bianchi and colleagues 31 reported that PAI-1 was detected in myoepithelial cells in two of nine pure carcinoma in situ lesions.
In 6 of the 25 carcinomas, we identified a considerable number of PAI-1-expressing stromal mononuclear cells identified as a subpopulation of mast cells. Zhao and colleagues 37 and Christensen and colleagues 32 also reported PAI-1 immunostaining in cells in human breast cancer samples that morphologically were characterized as mast cells. PAI-1 can be expressed by mast cells following stimulation, but is not detected in resting mast cells. 60,61 Cho and colleagues 60 showed that the PAI-1 mRNA level was increased dramatically after 3 hours of stimulation of resting mast cells, but was decreased again after 24 hours, whereas the PAI-1 protein within the mast cell granules remained more than 150-fold increased after 24 hours. Such a relationship between PAI-1 mRNA and protein levels may explain the lack of detectable PAI-1 mRNA and the presence of PAI-1 protein in mast cells observed here. Factors regulating de novo synthesis of PAI-1 in mast cells in breast cancer remain to be elucidated.
In 3 of the 25 cases, some cancer cells expressed PAI-1 protein and mRNA. PAI-1 produced by malignant cells could restrict the invasive capacity of those cells by inhibiting plasminogen activation.
Thus, as discussed above, for each of the cell types shown here to express PAI-1, a PAI-1 function may be suggested on the basis of general knowledge about the functions of these cell types. Furthermore, as the PAI-1 present in tumor extracts must originate from the cell types identified as PAI-1 expressing, the association between a poor prognosis and a high PAI-1 level in tumor extracts could suggest a function of PAI-1 in the cell type with the predominant expression, ie, the myofibroblast, in tumor growth and spread. A possible lack of association or even adverse association with prognosis of PAI-1 produced by some of the other cell types may be overridden by the more predominant expression by the myofibroblasts, and future immunohistochemical studies may reveal the prognostic significance of PAI-1 expressed by this and each of the other cell types found to express PAI-1. In line with this idea, Dublin and colleagues 36 reporting PAI-1 in both stromal fibroblast-like cells and cancer cells, noted a correlation between fibroblast expression and poor prognosis. Alternatively, the association between a high PAI-1 level in myofibroblasts and a poor prognosis could merely reflect a carcinogenesis-induced increase in the PAI-1 expression, varying with the aggressiveness of the tumor, the expressed PAI-1 being without functions related to the malignant phenotype. It may be possible to design animal tumor models for studying the functions of PAI-1 expression by each specific cell type. Expression of PAI-1 by stromal cells, which may well be myofibroblasts, has been reported in some of the models previously used to study PAI-1’s role in cancer. 24-26 A genetically induced murine mammary tumor, in which PAI-1 was expressed by stromal cells, grew and metastasized as well on PAI-1 gene-deficient mice as on wild-type mice. 26 Further studies with this and similar models may elucidate whether the association between a high PAI-1 level and a poor prognosis in human breast cancers is because of the PAI-1 level just reflecting aggressiveness, or whether PAI-1 has a causal function in tumor growth and spread, which in the model was taken over by other protease inhibitors with an overlapping function.
Conclusively, we have shown that PAI-1 is present in myofibroblasts and in some cases also in endothelial cells, myoepithelial cells, mast cells, and cancer cells in the breast carcinomas. The multiplicity of the cell types expressing PAI-1 points to a multifunctional role in the tumors. The immunohistochemical technique developed in the present study appears well suited for analysis of larger series of tumors and evaluation of a possible prognostic significance of expression by certain cell types. A possible perspective is that immunohistochemical detection of PAI-1 could be implemented in the routine histopathology together with, eg, estrogen receptor and HER2, and may thereby provide additional prognostic information aimed at individualized therapy.
Acknowledgments
We thank Dr. Jon Askaa, Dako, Denmark, for immunization of rabbits with P. pastoris PAI-1; Anni Christensen, Birthe Hermansen, Pia Gottrup Knudsen, and Charlotte Lønborg for expert technical assistance; and John Post for excellent photographic assistance.
Footnotes
Address reprint requests to Birgitte Vrou Offersen, Department of Experimental Clinical Oncology, Aarhus University Hospital, Nørrebrogade 44, Bldg. 5, DK-8000 Aarhus C, Denmark. E-mail: bvo@oncology.dk.
Supported by Aarhus University, the Danish Cancer Society, the Weimann Foundation, the Danish Cancer Research Foundation, the NOVO-Nordisk Foundation, and by the European Commission (QLG1-CT-2000-0111131).
B. V. O. and B. S. N. contributed equally to this study.
References
- 1.Dano K, Andreasen PA, Grondahl-Hansen J, Kristensen P, Nielsen LS, Skriver L: Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res 1985, 44:139-266 [DOI] [PubMed] [Google Scholar]
- 2.Mignatti P, Rifkin DB: Biology and biochemistry of proteinases in tumor invasion. Physiol Rev 1993, 73:161-195 [DOI] [PubMed] [Google Scholar]
- 3.Andreasen PA, Egelund R, Petersen HH: The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci 2000, 57:25-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreasen PA, Kjoller L, Christensen L, Duffy MJ: The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 1997, 72:1-22 [DOI] [PubMed] [Google Scholar]
- 5.Bugge TH, Kombrinck KW, Flick MJ, Daugherty CC, Danton MJ, Degen JL: Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell 1996, 87:709-719 [DOI] [PubMed] [Google Scholar]
- 6.Chen ZL, Strickland S: Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell 1997, 91:917-925 [DOI] [PubMed] [Google Scholar]
- 7.Behrendt N, Stephens R: The urokinase receptor. Fibrinolysis Proteolysis 1998, 12:191-204 [Google Scholar]
- 8.Andreasen PA, Georg B, Lund LR, Riccio A, Stacey SN: Plasminogen activator inhibitors: hormonally regulated serpins. Mol Cell Endocrinol 1990, 68:1-19 [DOI] [PubMed] [Google Scholar]
- 9.Wind T, Hansen M, Jensen JK, Andreasen P: The molecular basis for anti-proteolytic and non-proteolytic functions of plasminogen activator inhibitor-1: roles of the reactive centre loop, the shutter region, the flexible joint region, and the small serpin fragment. Biol Chem 2002, 383:21-36 [DOI] [PubMed] [Google Scholar]
- 10.Duffy MJ, Reilly D, O’Sullivan C, O’Higgins N, Fennelly JJ, Andreasen P: Urokinase-plasminogen activator, a new and independent prognostic marker in breast cancer. Cancer Res 1990, 50:6827-6829 [PubMed] [Google Scholar]
- 11.Janicke F, Schmitt M, Graeff H: Clinical relevance of the urokinase-type and tissue-type plasminogen activators and of their type 1 inhibitor in breast cancer. Semin Thromb Hemost 1991, 17:303-312 [DOI] [PubMed] [Google Scholar]
- 12.Bugge TH, Lund LR, Kombrinck KK, Nielsen BS, Holmback K, Drew AF, Flick MJ, Witte DP, Dano K, Degen JL: Reduced metastasis of Polyoma virus middle T antigen-induced mammary cancer in plasminogen-deficient mice. Oncogene 1998, 16:3097-3104 [DOI] [PubMed] [Google Scholar]
- 13.Grondahl-Hansen J, Christensen IJ, Rosenquist C, Brunner N, Mouridsen HT, Dano K, Blichert-Toft M: High levels of urokinase-type plasminogen activator and its inhibitor PAI-1 in cytosolic extracts of breast carcinomas are associated with poor prognosis. Cancer Res 1993, 53:2513-2521 [PubMed] [Google Scholar]
- 14.Schmitt M, Harbeck N, Thomssen C, Wilhelm O, Magdolen V, Reuning U, Ulm K, Hofler H, Janicke F, Graeff H: Clinical impact of the plasminogen activation system in tumor invasion and metastasis: prognostic relevance and target for therapy. Thromb Haemost 1997, 78:285-296 [PubMed] [Google Scholar]
- 15.Duffy MJ: Urokinase plasminogen activator and its inhibitor, PAI-1, as prognostic markers in breast cancer: from pilot to level 1 evidence studies. Clin Chem 2002, 48:1194-1197 [PubMed] [Google Scholar]
- 16.Zemzoum I, Kates RE, Ross JS, Dettmar P, Dutta M, Henrichs C, Yurdseven S, Hofler H, Kiechle M, Schmitt M, Harbeck N: Invasion factors uPA/PAI-1 and HER2 status provide independent and complementary information on patient outcome in node-negative breast cancer. J Clin Oncol 2003, 21:1022-1028 [DOI] [PubMed] [Google Scholar]
- 17.Harbeck N, Schmitt M, Kates RE, Kiechle M, Zemzoum I, Janicke F, Thomssen C: Clinical utility of urokinase-type plasminogen activator and plasminogen activator inhibitor-1 determination in primary breast cancer tissue for individualized therapy concepts. Clin Breast Cancer 2002, 3:196-200 [DOI] [PubMed] [Google Scholar]
- 18.Ma D, Gerard RD, Li XY, Alizadeh H, Niederkorn JY: Inhibition of metastasis of intraocular melanomas by adenovirus-mediated gene transfer of plasminogen activator inhibitor type 1 (PAI-1) in an athymic mouse model. Blood 1997, 90:2738-2746 [PubMed] [Google Scholar]
- 19.Soff GA, Sanderowitz J, Gately S, Verrusio E, Weiss I, Brem S, Kwaan HC: Expression of plasminogen activator inhibitor type 1 by human prostate carcinoma cells inhibits primary tumor growth, tumor-associated angiogenesis, and metastasis to lung and liver in an athymic mouse model. J Clin Invest 1995, 96:2593-2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Praus M, Collen D, Gerard RD: Both u-PA inhibition and vitronectin binding by plasminogen activator inhibitor 1 regulate HT1080 fibrosarcoma cell metastasis. Int J Cancer 2002, 102:584-591 [DOI] [PubMed] [Google Scholar]
- 21.Jankun J, Keck RW, Skrzypczak-Jankun E, Swiercz R: Inhibitors of urokinase reduce size of prostate cancer xenografts in severe combined immunodeficient mice Cancer Res 1997, 57:559-563[published erratum appears in Cancer Res 1998 Jan 1;58(1):179]. [PubMed] [Google Scholar]
- 22.McMahon GA, Petitclerc E, Stefansson S, Smith E, Wong MK, Westrick RJ, Ginsburg D, Brooks PC, Lawrence DA: Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. J Biol Chem 2001, 276:33964-33968 [DOI] [PubMed] [Google Scholar]
- 23.Eitzman DT, Krauss JC, Shen T, Cui J, Ginsburg : Lack of plasminogen activator inhibitor-1 effect in a transgenic mouse model of metastatic melanoma. Blood 1996, 87:4718-4722 [PubMed] [Google Scholar]
- 24.Bajou K, Noel A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, Skobe M, Fusenig NE, Carmeliet P, Collen D, Foidart JM: Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med 1998, 4:923-928 [DOI] [PubMed] [Google Scholar]
- 25.Bajou K, Masson V, Gerard RD, Schmitt PM, Albert V, Praus M, Lund LR, Frandsen TL, Brunner N, Dano K, Fusenig NE, Weidle U, Carmeliet G, Loskutoff D, Collen D, Carmeliet P, Foidart JM, Noel A: The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin. Implications for antiangiogenic strategies. J Cell Biol 2001, 152:777-784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almholt K, Nielsen BS, Frandsen TL, Brunner N, Dano K, Johnsen M: Metastasis of transgenic breast cancer in plasminogen activator inhibitor-1 gene deficient mice. Oncogene 2003, 22:4389-4397 [DOI] [PubMed] [Google Scholar]
- 27.Lambert V, Munaut C, Noel A, Frankenne F, Bajou K, Gerard R, Carmeliet P, Defresne MP, Foidart JM, Rakic JM: Influence of plasminogen activator inhibitor type 1 on choroidal neovascularization. EMBO J 2001, 15:1021-1027 [DOI] [PubMed] [Google Scholar]
- 28.Devy L, Blacher S, Grignet-Debrus C, Bajou K, Masson V, Gerard RD, Gils A, Carmeliet G, Carmeliet P, Declerck PJ, Noel A, Foidart JM: The pro- or antiangiogenic effect of plasminogen activator inhibitor 1 is dose dependent. EMBO J 2002, 16:147-154 [DOI] [PubMed] [Google Scholar]
- 29.Sumiyoshi K, Baba S, Sakaguchi S, Urano T, Takada Y, Takada A: Increase in levels of plasminogen activator and type-1 plasminogen activator inhibitor in human breast cancer: possible roles in tumor progression and metastasis. Thromb Res 1991, 63:59-71 [DOI] [PubMed] [Google Scholar]
- 30.Jankun J, Merrick HW, Goldblatt PJ: Expression and localization of elements of the plasminogen activation system in benign breast disease and breast cancers. J Cell Biochem 1993, 53:135-144 [DOI] [PubMed] [Google Scholar]
- 31.Bianchi E, Cohen RL, Dai A, Thor AT, Shuman MA, Smith HS: Immunohistochemical localization of the plasminogen activator inhibitor-1 in breast cancer. Int J Cancer 1995, 60:597-603 [DOI] [PubMed] [Google Scholar]
- 32.Christensen L, Wiborg Simonsen AC, Heegaard CW, Moestrup SK, Andersen JA, Andreasen PA: Immunohistochemical localization of urokinase-type plasminogen activator, type-1 plasminogen-activator inhibitor, urokinase receptor and alpha(2)-macroglobulin receptor in human breast carcinomas. Int J Cancer 1996, 66:441-452 [DOI] [PubMed] [Google Scholar]
- 33.Costantini V, Sidoni A, Deveglia R, Cazzato OA, Bellezza G, Ferri I, Bucciarelli E, Nenci GG: Combined overexpression of urokinase, urokinase receptor, and plasminogen activator inhibitor-1 is associated with breast cancer progression: an immunohistochemical comparison of normal, benign, and malignant breast tissues. Cancer 1996, 77:1079-1088 [DOI] [PubMed] [Google Scholar]
- 34.Umeda T, Eguchi Y, Okino K, Kodama M, Hattori T: Cellular localization of urokinase-type plasminogen activator, its inhibitors, and their mRNAs in breast cancer tissues. J Pathol 1997, 183:388-397 [DOI] [PubMed] [Google Scholar]
- 35.Ferrier CM, de Witte HH, Straatman H, van Tienoven DH, van Geloof WL, Rietveld FJ, Sweep CG, Ruiter DJ, van Muijen GN: Comparison of immunohistochemistry with immunoassay (ELISA) for the detection of components of the plasminogen activation system in human tumour tissue. Br J Cancer 1999, 79:1534-1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dublin E, Hanby A, Patel NK, Liebman R, Barnes D: Immunohistochemical expression of uPA, uPAR, and PAI-1 in breast carcinoma: fibroblastic expression has strong associations with tumor pathology. Am J Pathol 2000, 157:1219-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao H, Morimoto T, Sasa M, Tanaka T, Izumi K: Immunohistochemical expression of uPA, PAI-1, cathepsin D and apoptotic cells in ductal carcinoma in situ of the breast. Breast Cancer 2002, 9:118-126 [DOI] [PubMed] [Google Scholar]
- 38.Rodenburg KW, Kjoller L, Petersen HH, Andreasen PA: Binding of urokinase-type plasminogen activator-plasminogen activator inhibitor-1 complex to the endocytosis receptors alpha2-macroglobulin receptor/low-density lipoprotein receptor-related protein and very-low-density lipoprotein receptor involves basic residues in the inhibitor. Biochem J 1998, 329:55-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen JK, Wind T, Andreasen PA: The vitronectin binding area of plasminogen activator inhibitor-1, mapped by mutagenesis and protection against an inactivating organochemical ligand. FEBS Lett 2002, 521:91-94 [DOI] [PubMed] [Google Scholar]
- 40.Munch M, Heegaard C, Jensen PH, Andreasen PA: Type-1 inhibitor of plasminogen activators. Distinction between latent, activated and reactive centre-cleaved forms with thermal stability and monoclonal antibodies. FEBS Lett 1991, 295:102-106 [DOI] [PubMed] [Google Scholar]
- 41.Nielsen LS, Hansen JG, Skriver L, Wilson EL, Kaltoft K, Zeuthen J, Dano K: Purification of zymogen to plasminogen activator from human glioblastoma cells by affinity chromatography with monoclonal antibody. Biochemistry 1982, 21:6410-6415 [DOI] [PubMed] [Google Scholar]
- 42.Ferrier CM, van Geloof WL, de Witte HH, Kramer MD, Ruiter DJ, van Muijen GN: Epitopes of components of the plasminogen activation system are re-exposed in formalin-fixed paraffin sections by different retrieval techniques. J Histochem Cytochem 1998, 46:469-476 [DOI] [PubMed] [Google Scholar]
- 43.Floridon C, Nielsen O, Holund B, Sweep F, Sunde L, Thomsen SG, Teisner B: Does plasminogen activator inhibitor-1 (PAI-1) control trophoblast invasion? A study of fetal and maternal tissue in intrauterine, tubal and molar pregnancies. Placenta 2000, 21:754-762 [DOI] [PubMed] [Google Scholar]
- 44.Shulman M, Wilde CD, Kohler G: A better cell line for making hybridomas secreting specific antibodies. Nature 1978, 276:269-270 [DOI] [PubMed] [Google Scholar]
- 45.Pyke C, Kristensen P, Ralfkiaer E, Eriksen J, Dano K: The plasminogen activation system in human colon cancer: messenger RNA for the inhibitor PAI-1 is located in endothelial cells in the tumor stroma. Cancer Res 1991, 51:4067-4071 [PubMed] [Google Scholar]
- 46.Nielsen BS, Rank F, Lopez JM, Balbin M, Vizoso F, Lund LR, Dano K, Lopez-Otin C: Collagenase-3 expression in breast myofibroblasts as a molecular marker of transition of ductal carcinoma in situ lesions to invasive ductal carcinomas. Cancer Res 2001, 61:7091-7100 [PubMed] [Google Scholar]
- 47.Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G: A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 1986, 103:2787-2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sappino AP, Skalli O, Jackson B, Schurch W, Gabbiani G: Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer 1988, 41:707-712 [DOI] [PubMed] [Google Scholar]
- 49.Falini B, Flenghi L, Pileri S, Gambacorta M, Bigerna B, Durkop H, Eitelbach F, Thiele J, Pacini R, Cavaliere A: PG-M1: a new monoclonal antibody directed against a fixative-resistant epitope on the macrophage-restricted form of the CD68 molecule. Am J Pathol 1993, 142:1359-1372 [PMC free article] [PubMed] [Google Scholar]
- 50.Guelstein VI, Tchypysheva TA, Ermilova VD, Litvinova LV, Troyanovsky SM, Bannikov GA: Monoclonal antibody mapping of keratins 8 and 17 and of vimentin in normal human mammary gland, benign tumors, dysplasias and breast cancer. Int J Cancer 1988, 42:147-153 [DOI] [PubMed] [Google Scholar]
- 51.Nielsen BS, Sehested M, Duun S, Rank F, Timshel S, Rygaard J, Johnsen M, Dano K: Urokinase plasminogen activator is localized in stromal cells in ductal breast cancer. Lab Invest 2001, 81:1485-1501 [DOI] [PubMed] [Google Scholar]
- 52.Hansen S, Overgaard J, Rose C, Knoop A, Laenkholm AV, Andersen J, Sorensen FB, Andreasen PA: Independent prognostic value of angiogenesis and the level of plasminogen activator inhibitor type 1 in breast cancer patients. Br J Cancer 2003, 88:102-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ronnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ: The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest 1995, 95:859-873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nielsen BS, Sehested M, Timshel S, Pyke C, Dano K: Messenger RNA for urokinase plasminogen activator is expressed in myofibroblasts adjacent to cancer cells in human breast cancer. Lab Invest 1996, 74:168-177 [PubMed] [Google Scholar]
- 55.Frandsen TL, Holst-Hansen C, Nielsen BS, Christensen IJ, Nyengaard JR, Carmeliet P, Brunner N: Direct evidence of the importance of stromal urokinase plasminogen activator (uPA) in the growth of an experimental human breast cancer using a combined uPA gene-disrupted and immunodeficient xenograft model. Cancer Res 2001, 61:532-537 [PubMed] [Google Scholar]
- 56.De Wever O, Mareel M: Role of myofibroblasts at the invasion front. Biol Chem 2002, 383:55-67 [DOI] [PubMed] [Google Scholar]
- 57.Egeblad M, Werb Z: New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002, 2:161-174 [DOI] [PubMed] [Google Scholar]
- 58.Ronnov-Jessen L, Petersen OW, Bissell MJ: Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 1996, 76:69-125 [DOI] [PubMed] [Google Scholar]
- 59.Sternlicht MD, Kedeshian P, Shao ZM, Safarians S, Barsky SH: The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res 1997, 3:1949-1958 [PubMed] [Google Scholar]
- 60.Cho SH, Tam SW, Demissie-Sanders S, Filler SA, Oh CK: Production of plasminogen activator inhibitor-1 by human mast cells and its possible role in asthma. J Immunol 2000, 165:3154-3161 [DOI] [PubMed] [Google Scholar]
- 61.Wojta J, Kaun C, Zorn G, Ghannadan M, Hauswirth AW, Sperr WR, Fritsch G, Printz D, Binder BR, Schatzl G, Zwirner J, Maurer G, Huber K, Valent P: C5a stimulates production of plasminogen activator inhibitor-1 in human mast cells and basophils. Blood 2002, 100:517-523 [DOI] [PubMed] [Google Scholar]


