Abstract
A key feature of recovery from liver fibrosis is hepatic stellate cell (HSC) apoptosis, which serves the dual function of removing the major source of neomatrix and tissue inhibitors of metalloproteinases thereby facilitating matrix degradation. The mechanisms regulating HSC apoptosis remain undefined but may include the interaction of nerve growth factor (NGF) with its receptor, p75, on HSC. In this study, by TaqMan polymerase chain reaction in situ hybridization and immunohistochemistry, we demonstrate that NGF is expressed by hepatocytes during fibrotic injury. Peak hepatocyte expression of NGF (48 hours after CCl4 injection) coincides with maximal rate of apoptosis of HSC by terminal dUTP nick-end labeling staining. Addition of recombinant NGF to HSC in tissue culture causes a dose-dependent increase in apoptosis. NGF regulates nuclear factor (NF)-κB activity, reducing p50/p65 binding detected by electromobility shift assay and reduced NF-κB CAT reporter activities from both basal unstimulated levels and after NF-κB induction by tumor necrosis factor. In each case, a relative reduction in NF-κB binding was associated with a significant increase in caspase 3 activity. These data provide evidence that NGF is expressed during fibrotic liver injury and may regulate number of activated HSCs via induction of apoptosis.
There is now a wealth of evidence supporting a critical role for the hepatic stellate cell (HSC) as the major mediator of the fibrotic reaction in the context of chronic liver injury. 1,2 In the presence of a fibrotic stimulus, these cells undergo a phenotypic change termed “activation” in which they express α-smooth muscle actin (α-SMA), become myofibroblast-like, and enter the cell cycle. Activated HSCs are now recognized as the major mediators of fibrosis both through secretion of interstitial collagens and matrix-degrading metalloproteinases and their tissue inhibitors. We and others have recently demonstrated that liver fibrosis is a dynamic process. 3-16 In vivo, experimental models of biliary and parenchymal injury that resulted in hepatic fibrosis were demonstrated to undergo spontaneous resolution with matrix remodeling throughout a 4- to 6-week recovery period. 16-18
A key feature of the process of recovery was apoptosis of the activated HSCs. 16-18 The mechanisms responsible for this apoptosis were not determined in these studies but are likely to result from withdrawal of survival factor(s) and/or induction by specific ligand(s). Moreover, we have recently demonstrated that experimental induction of HSC apoptosis, using the fungal metabolite, gliotoxin, results in a diminution of fibrosis in the context of chronic CCl4 intoxication in the rat. 19 Taken together, these data suggest that stellate cell apoptosis is an important future target for therapies.
One potential factor mediating stellate cell apoptosis is the neurotrophin, nerve growth factor (NGF). Although NGF has well-documented cytoprotective effects on neuronal tissue, there is evidence to suggest that it is proapoptotic in cells of nonneuronal origin. Indeed, we have recently demonstrated that HSCs express the low-affinity NGF receptor, p75, and have presented data suggesting that HSCs undergo apoptosis in tissue-culture models in response to stimulation with recombinant murine NGF. 20
Although we have demonstrated the expression of low-affinity NGF receptors (p75) in activated HSCs during experimental liver injury in rats and pathological fibrosis and cirrhosis in human liver biopsies, to date no one has demonstrated expression of one of the major ligands for this receptor (NGF) in the liver. 21,22 Clearly, to understand the relevance and importance of the NGF-p75 system in regulating HSC apoptosis, a cellular source of NGF must be identified within the liver and demonstrated to express NGF in a biologically relevant time frame. Furthermore, because the effect of NGF on apoptosis is influenced by concurrent stimulation of the high-affinity NGFR (TrKA), the presence and locality of this receptor is pivotal to interpreting any potential role of NGF in vivo.
The myofibroblast-like activated stellate cell phenotype is associated with activation of the transcription factor NF-κB. 23,24 Moreover, fibrosis is frequently associated with inflammation, which is accompanied by increased levels of inflammatory cytokines such as tumor necrosis factor (TNF)-α. TNF-α stimulation of cells is also associated with the activation of the NF-κB. In its inactive state, NF-κB is constitutively present in the cytoplasm as a p50/p65 heterodimer bound to its inhibitory protein IκB. On activation IκB is degraded permitting the nuclear translocation of the NF-κB complex where gene transcription is then regulated. In activated HSCs, three NF-κB DNA-binding activities have been described. As well as a p50/p65 heterodimer, gel shift assays suggest the presence of a p65/p65 homodimer and a further complex demonstrating rapid mobility that we have recently identified as CBF1. Although the p50/p65 and p65/p65 dimers have positive regulatory activity to NF-κB-regulated genes, CBF1 has the potential to be inhibitory. Considerable evidence suggests that NF-κB may have a cytoprotective role inhibiting apoptosis in response to proapoptotic stimuli. This hypothesis has been supported by observations in RelA knockout mice 25 and in experimental models using both stellate cells and glomerular mesangial cells in which IκB levels are regulated. 24,26 In these models, when NF-κB activation is prevented or inhibited, cells are predisposed to apoptosis and undergo enhanced apoptosis in response to TNF-α. Regulation of NF-κB activation, therefore, represents a potential mechanism through which NGF may influence HSC fate.
In this study, we demonstrate evidence that NGF is expressed in liver injury and that this expression is by hepatocytes and is associated with apoptosis of HSCs. In addition, we demonstrate evidence that NGF, not only regulates HSC apoptosis but also reduces activation of the p50/p65 NF-κB heterodimer.
Materials and Methods
Acute CCL4 Model
Acute self-limiting liver injury characterized by stellate cell activation was induced in cohorts of male C57Bl-6 mice (22 to 25 g) (n = 21). CCl4 was administered at 1.6 g of CCl4 per kg by oral gavage. After a single dose, animals were sacrificed and their livers harvested for analysis at 28 hours (n = 6), 48 hours (n = 7), and 96 hours (n = 8). In addition, cohorts of between five and six animals were treated with olive oil vehicle to act as a control for each time point. After harvesting, livers were divided with lobes and fixed in formalin for routine histology, or were snap-frozen for immunohistochemistry and protein and mRNA analysis.
HSC Isolation
HSCs were isolated and culture-activated in the presence of serum on uncoated tissue-culture plastic exactly as previously described. Cells were passaged as previously described. 16-18
Histological Analysis
Formalin-fixed liver sections were embedded in paraffin and sectioned. Hematoxylin and eosin staining was performed in these thin sections for histological analysis.
Immunohistochemistry
Fresh-harvested liver tissues were snap-frozen in OCT. Fourteen-μm cryosections were collected on Superfrost-plus glass slides, baked for 20 minutes at 50°C, and then allowed to adhere further at 4°C. Immunofluorescent staining was performed on these sections using the following antibodies: mouse anti-desmin (MAB1698; Chemicon Inc., Temecula, CA) at 1:100 dilution, rabbit anti-type 1 collagen (AB765P, Chemicon Inc.) at 1:200 dilution, mouse anti-α-SMA (M0851; DAKO Corp., Carpinteria, CA) at 1:50 dilution. Cy3-conjugated secondary antibodies from Jackson Immunochemical Inc. were used to detect the signals. Sections were then fixed in 4% paraformaldehyde for 20 minutes before mounting.
Seven examples of inflamed human liver (alcoholic steatohepatitis) were analyzed for NKF expression by immunohistochemistry. Sections (3 to 4 μm) of formalin-fixed, paraffin-embedded liver biopsies were deparaffinized and rehydrated before blocking endogenous peroxidase activity with 0.6% hydrogen peroxide in methanol. Tissue sections were blocked with serum from the Vectastain Universal Quick Kit before adding 0.6 ng/μl of NGF antibody or 0.6 ng/μl of rabbit immunoglobulin (negative control) and incubating overnight at 4°C. The Vectastain (Peterborough, UK) Universal Quick Kit was used as the secondary detection system and visualization occurred with diaminobenzidine substrate. Tissue sections were counterstained with Mayer’s hemalum and blued in Scott’s tap water before dehydrating and mounting in DPX (Poole, Dorset, UK) and coverslips.
Terminal dUTP Nick-End Labeling (TUNEL) Stain for Apoptosis and Dual-TUNEL and α-SMA Staining
TUNEL detection reagents were purchased from Roche Molecular Biochemicals (Indianapolis, IN), and used according to the manufacturer’s instruction. Signals were revealed by AP-colorimetric substrate nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate. In dual labeling, TUNEL detection reaction was performed after α-SMA immunofluorescent staining.
TaqMan mRNA Analysis
RNAs were isolated from snap-frozen liver tissues using RNAzolB (Tel Test Inc., Friendswood, TX). cDNAs were synthesized using MMLV-RT from Invitrogen Corp. (Carlsbad, CA). Fifty ng of total cDNA were used for each TaqMan polymerase chain reaction (PCR) using the TaqMan Gold PCR kit from Roche Molecular Systems Inc. (Indianapolis, IN).
In Situ Hybridization
33P-UTP-labeled sense and anti-sense RNA probes were generated as described. 27 Radioactive in situ hybridization was performed as described previously. 28
Nuclear Extraction
Nuclear extracts were prepared from cells using a modified version of the protocol described by Dignam and colleagues. 29 In brief, cells (107) were harvested into 2 ml of ice-cold phosphate-buffered saline by centrifugation at 2500 rpm. The pellets were resuspended into 50 μl of Dignam buffer A containing 0.2% Nonidet P-40 and 0.5 mmol/L 4-(2-aminoethyl)benzenesulfonyl fluoride. The lysate was centrifuged at 8000 rpm for 10 seconds to pellet the Nonidet P-40-insoluble material, and the supernatant was removed. The pellet was resuspended into 50 μl of Dignam buffer C, incubated on ice for 10 minutes with occasional vortexing to disrupt the nuclear membranes. Extracts were centrifuged for 1 minute at 8000 rpm, the supernatants were removed, and the pellets were discarded. The protein content of the nuclear extracts was determined using the Bio-Rad DC assay kit (Bio-Rad, Hercules, CA).
Electrophoretic Mobility Shift Assay
Nuclear extracts (5 μg) were analyzed by electrophoretic mobility shift assay as described previously with some modification. 30 The double-stranded wild-type NF-κB (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) oligonucleotides were obtained from Promega (Chilworth, UK) and radiolabeled with [γ32P]ATP (Amersham Pharmacia Biotech, Bucks, UK) by incubation with 10 U of T4 polynucleotide kinase (Promega). After DNA-binding reaction and electrophoresis, gels were dried and autoradiographed. To assess the specificity of the reaction, competition assays were performed with 100-fold excess of unlabeled NF-κB. The unlabeled probes were added to the binding reaction 5 minutes before the addition of the labeled probe. Supershift assays were performed to characterize the transcription factors binding to the NF-κB sequence exactly as previously described. 31
Chloramphenicol Acetyltransferase (CAT) Assays
HSCs were transfected with plasmid DNA (10 μg/106 cells) using the Effectine transfection kit (Qiagen, Crawley, UK) according to the manufacturer’s instructions and left in contact with the cells for 48 hours. Cells were serum-deprived for 24 hours before transfection. NF-κB-dependent transcription was monitored using a CAT reporter gene ligated downstream of a metallothionein promoter and four consensus NF-κB-binding sites (pJG21). 23 CAT assays were performed as described. 30 In brief, cell extracts were prepared by repeated freeze-thaw cycles and normalized for protein content using the Bio-Rad DC protein assay, and CAT activities were determined using [14C]chloramphenicol (Amersham Pharmacia Biotech) and acetyl-CoA (Sigma). Acetylated products were separated by thin-layer chromatography and quantitated by phosphor imaging. CAT activities were normalized to the amount of DNA taken up by cultures, determined using a modification of Hirt’s assay. 31
Ribonuclease Protection Assay
We undertook ribonuclease protection analysis for caspase 1 and 3 on HSC RNA. Total RNA was extracted from HSCs using the RNeasy method (Qiagen) after serum deprivation, with or without NGF (10 ng/ml) treatment. Ribonuclease protection assay for caspase mRNAs was undertaken as previously described. 17 Riboprobes were transcribed using the rAPO-1 multiprobe template (Ambion, Oxon, UK) according to the manufacturer’s instructions. The probe template contained an L32 housekeeping gene cDNA that was used as a control for sample loading.
Caspase 3 Assays
HSCs were serum-deprived for 24 hours and treated with NGF and or TNF for up to 24 hours. Cells were then lysed by freeze-thawing in a buffer containing 25 mmol/L HEPES, 5 mmol/L ethylenediaminetetraacetic acid, 2 mmol/L dithiothreitol, 0.1% CHAPS, 0.5 mmol/L Pefabloc, 0.1 mg/ml leupeptin, and 0.1 mg/ml pepstatin. The lysate was centrifuged at 12,000 × g for 10 minutes (4°C) and the supernatant subjected to caspase measurements using the CaspACE assay system (Promega) following the instructions provided by the manufacturer. In brief, caspase 3 substrate, Ac-DEVD was labeled with the chromophore p-nitro-analide (pNA). A colorimetric substrate (Ac-DEVD-pNA) releases free pNA from the substrate on cleavage by DEVDase. Free pNA produces a yellow color that was monitored by a photometer at 405 nm after 4 hours of incubation at 37°C. The amount of yellow color produced on cleavage is proportional to the amount of DEVDase activity present in the sample.
DNA Laddering Experiments
Activated HSCs were cultured in 75-cm2 tissue-culture flasks and serum-deprived for 24 hours, followed by treatment with NGF and/or TNF for a further 24 hours. The supernatants containing nonadherent cells that had lifted from the monolayer were recovered and flick-spun (12,000 × g for 10 seconds). The DNA was extracted from the resulting pellet using the DNeasy method (Qiagen) according to the manufacturer’s instructions. This was ethanol-precipitated overnight, dried, and resuspended in 4 μl of water. This was then electrophoresed on a 1× Tris-buffered ethanolamine-agarose gel containing ethidium bromide (200 ng/ml) and visualized by UV fluorescence.
Results
Hepatocytes Express NGF during Profibrotic Liver Injury
By TaqMan PCR, hepatic levels of NGF were determined in cohorts of mice after a single dose of CCl4. The relative abundance of mRNA transcripts for NGF demonstrated a clear induction after liver injury that coincided with the death and regeneration of injured hepatocytes (Figure 1) ▶ . In situ hybridization was undertaken to determine the site of NGF expression. No expression was observed in normal control livers but strong expression was seen at 28- and 48-hour time points after administration of CCl4 over hepatocytes in acinar zones 2 and 3. The signal was exclusively observed over hepatocytes and was associated with areas of hepatocellular injury. By 96 hours expression was observed focally and only in a few hepatocytes adjacent to areas of injury (Figure 2A) ▶ . Controls, hybridized with a sense transcript for NGF demonstrated no signal (data not shown). To validate the results obtained with the rodent model with respect to human liver disease, seven examples of alcoholic steatohepatitis were immunostained for NGF. In six of these cases, there was an established cirrhosis, the other showed evidence of bridging fibrosis. In each case, strong staining of the hepatocytes was observed in areas of damage, hepatocyte degeneration, and architectural distortion (Figure 2B) ▶ . The population kinetics of activated (α-SMA-positive) HSCs and liver myofibroblasts closely mirrored the expression of NGF in the treated murine livers determined histologically and by TaqMan analysis of α-SMA mRNA (Figure 3) ▶ . Furthermore, when α-SMA-positive activated HSC apoptosis was quantified by counting after dual labeling with TUNEL and α-SMA, the peak expression of NGF was found to coincide with the maximum rate of HSC apoptosis (Figure 4) ▶ .
Figure 1.
NGF mRNA was quantified by TaqMan PCR of total RNA from rat livers after a single dose of CCl4 as described in Materials and Methods. The levels of liver NGF mRNA show a dramatic increase at 28 and 48 hours after CCL4 intoxication before dropping by 96 hours to levels comparable with olive oil-treated controls.
Figure 2.

A: In situ hybridization for NGF was undertaken as described in Materials and Methods. Representative sections (from left to right) of: an olive oil-fed control animal at 28 hours, a CCl4-fed animal at 28 hours, a CCl4-fed animal at 48 hours, and a CCl4-fed animal at 96 hours. Top: Dark-field images (×10) showing expression of NGF at 28 and 48 hours in CCl4-fed animals over acinar zones 2 and 3. Middle: The corresponding H&E images (×10). Bottom: H&E images at higher magnification (×40) showing NGF mRNA (black silver grains) in hepatocytes. At 28 and 48 hours, clear evidence of NGF mRNA expression in damaged and regenerating hepatocytes is visible (arrows). B: Immunohistochemistry for NGF of needle biopsies from patients with alcoholic steatohepatitis was undertaken as described in Materials and Methods. After detection NGF was localized to the cytoplasm of hepatocytes in the region of maximal damage and architectural distortion. Nonimmune IgG controls were uniformly negative. A representative example of the staining for NGF in human hepatocytes in situ is presented in the figure.
Figure 3.
The population kinetics of activated HSCs after a single dose of CCl4 was determined by TaqMan PCR for α-SMA as described in Materials and Methods. By TaqMan analysis of α-SMA mRNA, peak expression was observed at 48 hours after a single dose of CCl4. Concurrent histological analysis of liver sections demonstrated enhanced numbers of α-SMA-positive cells at 48 hours after intoxication (see Figure 4a ▶ ).
Figure 4.
Apoptosis in α-SMA-positive cells was quantified after a single dose of CCl4 after dual labeling with α-SMA and TUNEL as described in Materials and Methods. a: Representative liver sections taken from animals sacrificed at 28 hours (left), 48 hours (middle), and 96 hours (right) after dosing and stained with α-SMA (top row; original magnifications, ×10) or TUNEL (bottom row; original magnifications, ×10). Inset: α-SMA and TUNEL dual staining demonstrates the presence of α-SMA-positive cells that are undergoing apoptosis. The number of apoptotic HSCs defined by being TUNEL-positive and α-SMA-positive (black bars) were quantified over four random ×10 fields from each liver section and the results are represented graphically in b. There is a clear increase in apoptotic activated HSCs that coincides with the peak NGF mRNA expression at 48 hours. The numbers of TUNEL-positive, α-SMA-negative cells in each field are represented by gray bars.
Expression of TrkA Is Not Observed during Liver Injury
No expression of TrkA was detected by in situ hybridization or TaqMan PCR during the acute CCl4 injury model (data not shown).
Recombinant Human NGF Induces a Dose-Dependent Increase in Rat HSC Apoptosis Even in the Presence of Serum
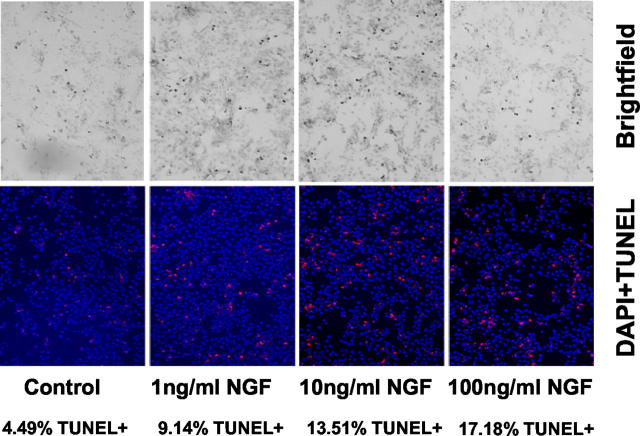
HSCs activated by culture on uncoated tissue culture plastic in the presence of serum were exposed to nerve NGF in increasing concentration. After 4,6-diamidino-2-phenylindole and TUNEL staining, cells demonstrated clear evidence of increased apoptosis in response to increasing concentrations of NGF (Figure 5) ▶ .
Figure 5.
Incubation of culture-activated HSCs with NGF was undertaken as described in Materials and Methods. After 24 hours of exposure to NGF in the presence of 10% serum, a clear increase in apoptosis was observed after staining with 4,6-diamidino-2-phenylindole and TUNEL. The number of apoptotic cells were counted as described in Materials and Methods and are recorded below each relevant panel.
NGF Up-Regulates Caspase Activity and the Expression of Caspase 1 and Caspase 3 mRNAs in HSC
Protected RNA fragments for caspases 1 and 3 and the housekeeping gene L32 were quantitated after electrophoretic resolution by phosporimager. To control for sample loading, the relative levels of caspase mRNA were normalized to those of L32. An elevation in caspase 1 (2.6×) and in caspase 3 (3×) mRNAs relative to those observed in control cells maintained in serum-free conditions alone were observed in serum-free cultures of rat HSCs treated with NGF (10 ng/ml) for 24 hours (data representative of two independent experiments). Incubation of HSCs with NGF in serum-free conditions for 24 hours was associated with an increase in caspase 3 activity relative to control cells left in serum-free conditions (Figure 6) ▶ .
Figure 6.
Stellate cells were washed in serum-free media before being incubated with NGF for 24 hours as described in Materials and Methods. After 24 hours of exposure to NGF, cells were harvested and subjected to assessment of caspase 3 activity. Caspase activity was increased threefold in cells exposed to NGF compared to untreated (con) cells maintained in serum-free conditions.
NGF and TNF-α Administered Concurrently Are Potent Stimuli of HSC Apoptosis
Caspase 3 activity was used to quantify the HSC apoptotic response to stimulation with TNF-α and NGF both separately and in combination. In rat HSCs, caspase 3 activity was significantly elevated relative to serum-free control cultures by treatment with NGF or TNF for 24 hours (5.1 ± 1.5-fold and 5.7 ± 1.2-fold, respectively; n = 4, P < 0.05 by Mann-Whitney test). Throughout the same period a combination of NGF and TNF resulted in a greater caspase 3 activity (10.4 ± 3.1-fold control activity; n = 4, P < 0.05 by Mann-Whitney test). Addition of selective caspase 3 inhibitor ZvadFMK reduced the effect of TNF and NGF on caspase 3 activity (2.7 ± 1.2-fold and 2.3 ± 1.0-fold control, respectively), caspase 3 activity first became detectable above baseline in NGF-treated HSCs after 3 hours. Cycloheximide used as a positive control in each experiment, resulted in a pronounced increase in caspase activity (25.5 ± 0.5-fold control) (Figure 7) ▶ .
Figure 7.
Activated HSCs were exposed to NGF and TNF either separately or in combination, as described in Materials and Methods. In addition, concurrent samples maintained solely in serum-free conditions (C) and treated with cycloheximide (CX) were used as negative and positive controls, respectively. In a further experiment, the caspase 3 inhibitor ZVAD was added to parallel wells. After exposure cells were harvested and caspase activity determined as described in Materials and Methods. Relative to control [untreated cells (C)] treatment with both NGF (N) and TNF (T) alone in serum-free conditions resulted in a significant increase in caspase activity. The combination of NGF with TNF (N & T) resulted in the largest increase in caspase activity assay. A significant increase was also seen in cells treated with cycloheximide to induce apoptosis. Concurrent treatment of each of these conditions by co-incubation with the caspase 3 inhibitor ZVAD resulted in an inhibition of the increase in caspase activity assay despite treatment with NGF and TNF separately or in combination or treatment with cycloheximide. Results are expressed as fold control (untreated serum-free conditions) which was given the arbitrary value of 1 (*, P < 0.05 by Mann-Whitney test).
The extent of DNA laddering, a further marker of apoptosis, correlated with the data obtained from the caspase 3 assays. Laddering was demonstrated in DNA extracted from cells treated with TNF and NGF. There was no detectable DNA laddering in serum-free parallel control (Figure 8) ▶ . Laddering was also evident in cells treated with either NGF alone or NGF and TNF concurrently (Figure 8) ▶ .
Figure 8.
Cells treated with NGF and TNF, both separately and in combination, were harvested and the DNA extracted and subjected to electrophoresis as described in Materials and Methods. In comparison to control serum-free-treated cells (lane 1), treatment with NGF (10 ng/ml) alone (lane 2), TNF (10 ng/ml) (lane 3), and TNF and NGF (10 ng/ml each) in combination (lane 4), resulted in cellular apoptosis and oligonucleosomal fragmentation of cellular DNA.
NGF and TNF Differentially Regulate NF-κB Activation
By electrophoretic mobility shift assay, NF-κB-binding activity was detected and elevated by TNF in activated, but not quiescent HSCs. In activated rat HSCs, treatment with TNF-α (30 minutes, 10 ng/ml in serum-free conditions) simulated an increase in NF-κB binding determined by electrophoretic mobility shift assay (n = 5) (Figure 9) ▶ . In contrast to the effect of TNF-α, NGF (30 minutes, 10 ng/ml in serum-free conditions) was found to decrease NF-κB (p50/p65)-binding activity relative to control in the same cultures (n = 5) (Figure 9) ▶ . A combination of TNF-α and NGF (both 10 ng/ml in serum-free conditions) resulted in NF-κB binding comparable to untreated controls and below that seen with TNF-α stimulation alone (Figure 9) ▶ . TNF-α elevated nuclear p50/p65 binding in a relatively selective manner in comparison to CBF1 binding. Moreover, although treatment with NGF resulted in a reduction in NF-κB binding, as with TNF, the regulation was predominantly of the p50/p65 complex. Similar results were obtained using activated primary cultures of human HSCs. The identities of the P50/p65 and CBF1 complexes were all confirmed in supershift experiments. The pattern observed was identical to that we have already described. 31 The down-regulation of NF-κB by NGF is a rapid event. Onset of the reduction in NF-κB binding was within 15 minutes, with maximum inhibition between 30 and 60 minutes (data not shown).
Figure 9.
Electromobility shift assay using an NF-κB consensus oligonucleotide was used to determine the relative expression of the p65/50 heterodimer and the CBF1-binding complexes (Figure 9) ▶ . Cells treated with NGF demonstrated a decrease in binding of p50/p65 heterodimer. In contrast, the negative regulatory element CBF1 was unaltered. Incubation of HSCs for TNF-α resulted in up-regulation in the p50/p65 heterodimer relative to controls. As with NGF expression of CBF1 remained unaltered. In response to a combination of NGF and TNF relative to untreated controls, p50/p65 heterodimer demonstrated no change and expression of CBF1 remained unchanged. However, relative to TNF treatment alone, treatment with TNF and NGF resulted in a diminution in the binding of the p50/p65 heterodimer. Data are representative of five independent experiments.
NGF and TNF-α Differentially Regulate Activity of a NF-κB CAT Reporter
The NF-κB-driven (pJG21) CAT reporter construct was used to determine net NF-κB-regulated activities after stimulation with NGF. By this method, basal activity was observed at a similar level to that previously reported in activated HSCs. 23 This basal activity was regulated by treatment with NGF and TNF in activated HSCs. CAT activities (expressed as mean ± SD of fold control; n = 4 for each condition) were NGF, 0.44 ± 0.09; TNF, 1.55 ± 1.11; NGF and TNF, 0.8 ± 0.11; pBLCAT3, empty vector control, 0.16 ± 0.04 (Figure 10) ▶ . The decrease by NGF and increase by TNF over untreated controls were both significant (P < 0.01 by Mann-Whitney test). Furthermore, although the combination of TNF and NGF did not result in a significant change in basal CAT activity relative to untreated controls, a decrease in CAT activity was observed relative to TNF-α treatment alone (Figure 10) ▶ .
Figure 10.
Cells were transfected with the PJG21 reporter plasmid as described in Materials and Methods and incubated with TNF and NGF separately and in combination in serum-free conditions. NF-κB reporter activity is expressed in a graph form as fold change relative to untreated control cells, which were given the arbitrary value of 1. Whereas incubation with TNF caused an increase in NF-κB reporter activity, a significant decrease was observed relative to control after incubation with NGF. Concurrent incubation with NGF and TNF resulted in a NF-κB reporter activity, not significantly different from untreated control, but reduced relative to cells treated with TNF alone. Transfection with an empty reporter construct (PBL CAT3) resulted in a negligible reporter activity (n = 4; **, P < 0.01 by Mann-Whitney relative to untreated control).
Discussion
We have previously demonstrated the presence of p75 on HSCs and have demonstrated that activation of this receptor by NGF results in apoptosis. 20 In this study, we have demonstrated that NGF is produced in the liver by hepatocytes during experimental injury. Thus, we have demonstrated a novel paracrine loop whereby the injured and regenerating epithelium directly influences the survival of cells mediating the mesenchymal fibrotic response. The clear effect of NGF in inducing HSC apoptosis, even in the presence of serum suggests that HSC p75 stimulation is a potent proapoptotic mechanism effective even in the presence of soluble proinflammatory and survival signals. Finally, we have gone on to demonstrate that regulation of the NF-κB system is a major potential mechanism mediating control of HSC apoptosis in response to p75 stimulation.
A critical step to understanding the potential relevance of the NGF-p75 pathway in regulating stellate cell apoptosis was to determine a cellular source on NFG in the context of liver injury. Using a combination of molecular techniques we have demonstrated quite conclusively that NGF is expressed by hepatocytes in areas of damage and regeneration during experimental CCl4-induced liver injury. Moreover, in this model, in which a careful chronology of cell population kinetics can be studied, the liver expression of NGF parallels the peak period of stellate cell apoptosis after a single dose of CCl4. Taken together, these data strongly suggest a role for hepatocyte-derived NGF mediating loss of activated HSCs via induction of apoptosis during self-limiting liver injury. These data provide cogent evidence for a novel model of the regulation of apoptosis in the mesenchymal cells mediating the fibrotic response after inflammatory injury. In this model, expression of NGF by the epithelial cell type (in this case, the hepatocyte) may directly regulate apoptosis of the wound healing myofibroblast (in this case, activated HSCs) and, therefore, represents a potential factor determining resolution of the fibrotic response.
To further investigate the axis and determine the relevance of our findings in rodent models to human liver disease, we immunostained a panel of liver biopsies from patients with alcoholic steatohepatitis. In each example, intense staining for NGF was observed in areas of hepatocyte injury. Clearly only limited parallels can be drawn between steatohepatitis and CCl4-induced liver injury. Nevertheless, this data provides cogent evidence that in injured liver, NGF is expressed and released by hepatocytes. In neuronal models, apoptosis mediated by NGF binding to p75 occurs in the absence of TrkA receptor stimulation. Whereas, co-expression and concurrent stimulation of the TrkA receptor leads to an inhibition of p75 death signaling and a proliferative response. 32 Previous work has suggested that TrkA is not detected in normal liver tissue by immunohistochemistry. 21,22 Our data supports this conclusion in that we have consistently failed to demonstrate the presence of TrkA receptors on HSCs by Western blotting 20 or as reported here, in normal or experimentally injured liver by highly sensitive TaqMan PCR and in situ hybridization. The importance that the livers were negative for TrKA mRNA at each stage of the injury is underscored by the observation that its expression may be cell cycle related. 33 Taken together these data suggest that activated HSCs solely express p75 and further suggest that the major HSC response to NGF is the initiation of apoptosis.
Our results demonstrate that the intracellular signaling of apoptosis in response to NGF stimulation results in a down-regulation of translocation of the activated p50/p65 heterodimer with resulting decrease in NF-κB activation and associated caspase 3 activation. A further key observation in this study is that in HSC, TNF-α, and NGF differentially regulate the activation of the p50/p65 heterodimer. In contrast, neither appear to regulate activation of CBF1. We have exhaustively characterized the identity of the three major NF-κB-binding complexes (p66:p65, p50:p65, and CBF-1) in previous studies. In conjunction with these previous studies, 23,34 we can now construct a model in which HSC activation is associated with increased nuclear translocation of both positive and negative transcriptional regulatory proteins that bind to NF-κB. These data provide direct evidence that the transcriptional regulation of the survival and phenotype of the HSC is a finely balanced process resulting in the net effects of positive and negative regulatory elements responsive to both survival and proapoptotic stimuli.
We went on to study the response of HSCs to the effects of NGF treatment with TNF-α treatment in serum-free conditions. In this model, both NGF and TNF-α stimulation demonstrate clear regulation of apoptosis determined by cell morphology; and an increase in caspase activity and DNA laddering. Further support for the critical role of NGF in eliciting apoptosis in HSC is lent by the observation that caspase 3 mRNA expression is increased by NGF stimulation. This result was unexpected because the execution phase of apoptosis, mediated by the caspase cascade, results from activation of pre-existing intracellular enzyme stores. 35 Nevertheless, our observation provides powerful evidence that NGF enhances the propensity of HSC to undergo apoptosis by both increasing the production of the machinery cell death in addition to stimulating its activation.
Caspase 3 is the critical downstream factor of the cell death process and its activation is associated with apoptosis. 35 The link between caspase 3 and NF-κB is complex. It has previously been found that IκBα can be cleaved in vitro within its amino terminus by caspase 3. 36 Moreover caspase 3 has been demonstrated to mediate cleavage of p65/p65 and p50/p65. 37 Together these data suggest that the regulation of NF-κB is downstream of that of caspase 3. From the experiments presented in this report, it would seem that in HSC, the converse applies, because NF-κB regulation is rapid (minutes) and returns to pretreatment level within 3 hours but that caspase 3 activity follows a longer time course, first becoming detectable above baseline after 3 hours.
There is now a wealth of evidence suggesting NF-κB transcriptionally regulates apoptosis in a variety of cell types. NF-κB is known to regulate the induction of genes involved in cell survival such as CIAP-1, CIAP-2, and XIAP. TNF up-regulates NF-κB binding, which appears to promote survival. Cells deficient in NF-κB function display increased sensitivity to the cytocidal and apoptotic effect of TNF and have a much lower dependence on treatment with protein synthesis inhibitors for exhibiting such effect. 24,26,38,39 We, therefore, proposed that NGF-mediated inhibition of NF-κB would increase the propensity of TNF-treated HSC to undergo apoptosis. Moreover, because it has previously been demonstrated that NF-κB inhibition via an IκB super repressor predisposes HSC to TNF-α-induced apoptosis, we proposed that this NGF-mediated effect would be associated with a down-regulation of NF-κB binding even in the presence of TNF. Concurrent administration of TNF and NGF resulted in reduction of nuclear translocation of the p65/p65 heterodimer relative to controls treated with TNF-α only. This treatment was associated with a significant increase in caspase 3 activity relative to that observed in untreated cells and those treated with NGF and TNF-α alone, suggesting that the ligands are indeed additive in their proapoptotic effect and that NGF stimulation may predispose HSCs to TNF-induced apoptosis. This observation may simply result from the simultaneous activation of two related receptor groups, with convergence and amplification of their respective intracellular pathways. Alternatively or additionally the relative reduction in p50/p65 activation resulting from concurrent NGF and TNF stimulation may critically alter the balance of pro- and anti-apoptotic stimuli within HSCs and consequently enhance the apoptotic response. This latter model is attractive, particularly because activity of the negative NF-κB-regulating protein CBF-1 is not altered by NGF. Regardless of the precise mechanism, this data suggests that the release of NGF in the context of an inflammatory liver injury is likely to result in an enhanced HSC apoptotic response.
The data presented in this study provide cogent evidence that NGF regulates HSC apoptosis and the release of NGF by hepatocytes during liver injury may represent an important paracrine loop limiting the HSC-dependent fibrotic response. The action of NGF down-regulates nuclear translocation of the p50/p65 NF-κB heterodimer without regulating CBF-1 which predisposes the cell to apoptosis. Our results suggest that an approach based on p75 stimulation, either by NGF or an homologous peptide, represents an important potential therapeutic approach to the treatment of liver fibrosis.
Footnotes
Address reprint requests to Dr. J. P. Iredale, Professor of Hepatology, Liver Research Group, IIR Division, School of Medicine, Southampton General Hospital, Tremona Rd., Southampton SO16 6YD, UK. E-mail: jpi@soton.ac.uk.
Supported by the Medical Research Council (UK) (project grant to D. A. M. and J. P. I.; a Clinical Senior Fellowship to J. P. I.; and Cooperative Group Grant to J. P. I., R. C. B., and D. A. M.); the Children’s Liver Disease Foundation (to J. P. I. and R. C. B.); and the Wessex Medical Trust.
References
- 1.Friedman SL: The cellular basis of hepatic fibrosis: mechanisms and treatment strategies. N Engl J Med 1993, 328:1828-1835 [DOI] [PubMed] [Google Scholar]
- 2.Geert AB: History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis 2001, 21:311-335 [DOI] [PubMed] [Google Scholar]
- 3.Iredale JP, Murphy G, Hembry RM, Friedman SL, Arthur MJP: Human hepatic lipocytes synthesize tissue inhibitor of metalloproteinases-1 (TIMP-1): implications for regulation of matrix degradation in liver. J Clin Invest 1992, 90:282-287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJP: Expression of tissue inhibitor of metalloproteinases-1 and -2 is increased in fibrotic human liver. Gastroenterology 1996, 110:821-831 [DOI] [PubMed] [Google Scholar]
- 5.Iredale JP, Winwood PJ, Kawser CA, Arthur MJP: Lipocyte proliferation in the C. parvum model of macrophage-induced liver injury is associated with expression of 72kDa type IV collagenase/gelatinase. Knook DL Wisse E eds. Cells of the Hepatic Sinusoid 1993, vol 4.:pp 105-108 Kupffer Cell Foundation Leiden [Google Scholar]
- 6.Herbst H, Heinrichs O, Schuppan D, Milani S, Stein H: Temporal and spatial patterns of transin/stromelysin RNA expression following toxic injury in rat liver. Virchows Arch B Cell Pathol 1991, 60:295-300 [DOI] [PubMed] [Google Scholar]
- 7.Arthur MJP: Matrix degradation in the liver. Surrenti C Casini A Milani S Pinzani M eds. Fat-Storing Cells and Liver Fibrosis. 1994:pp 110-127 Kluwer Academic Publishers, Dordrecht
- 8.Li JJ, Kim CI, Leo MA, Mak KM, Rojkind M, Lieber CS: Polyunsaturated lecithin prevents acetaldehyde-mediated hepatic collagen accumulation by stimulating collagenase activity in cultured lipocytes. Hepatology 1992, 15:373-381 [DOI] [PubMed] [Google Scholar]
- 9.Iredale JP, Benyon RC, Arthur MJP, Ferris WF, Alcolado R, Winwood PJ, Clark N, Murphy G: Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology 1996, 24:176-184 [DOI] [PubMed] [Google Scholar]
- 10.Hovell CJ, Benyon RC, Pawley SJ, Iredale JP, Arthur MJP: Membrane-type matrix metalloproteinase (MTMMP) is upregulated in liver fibrosis. J Hepatol 1997, 26:133 [Google Scholar]
- 11.Iredale JP, Goddard S, Murphy G, Benyon RC, Arthur MJP: Tissue inhibitor of metalloproteinase-1 and interstitial collagenase expression in autoimmune chronic active hepatitis and activated human hepatic lipocytes. Clin Sci 1995, 89:75-81 [DOI] [PubMed] [Google Scholar]
- 12.Murawaki Y, Yamamoto H, Kawasaki H, Shima H: Serum tissue inhibitor of metalloproteinases in patients with chronic liver disease and with hepatocellular carcinoma. Clin Chim Acta 1993, 218:47-58 [DOI] [PubMed] [Google Scholar]
- 13.Muzzillo DA, Imoto M, Fukuda Y, Koyama Y, Saga S, Nagai Y, Hayakawa T: Clinical evaluation of serum tissue inhibitor of metalloproteinases-1 levels in patients with liver diseases. J Gastroenterol Hepatol 1993, 8:437-441 [DOI] [PubMed] [Google Scholar]
- 14.Murawaki Y, Kawasaki H, Burkhardt H: Serum collagenase activity in chronic liver diseases. Pathol Res Pract 1994, 190:929-933 [DOI] [PubMed] [Google Scholar]
- 15.Okazaki I, Maruyama K: Collagenase activity in experimental hepatic fibrosis. Nature 1974, 252:49-50 [DOI] [PubMed] [Google Scholar]
- 16.Murphy FR, Issa R, Zhou X, Rhatnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP: Inhibition of apoptosis of activated hepatic stellate cells by TIMP-1 is mediated via effects on MMP inhibition: implications for reversibility of liver fibrosis. J Biol Chem 2002, 277:11069-11076 [DOI] [PubMed] [Google Scholar]
- 17.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJP: Mechanisms of spontaneous resolution of rat liver fibrosis: hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest 1998, 102:538-549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Issa R, Williams E, Trim N, Kenall T, Arthur MJP, Reichen J, Benyon RC, Iredale JP: Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut 2001, 48:548-557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright MC, Issa R, Smart DE, Trim N, Murray GI, Primrose JN, Arthur MJP, Iredale JP, Mann DA: Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology 2001, 121:685-698 [DOI] [PubMed] [Google Scholar]
- 20.Trim N, Morgan S, Evans M, Issa R, Fine D, Afford S, Wilkins B, Iredale J: Hepatic stellate cells express the low affinity nerve growth factor receptor p75 and undergo apoptosis in response to nerve growth factor stimulation. Am J Pathol 2000, 156:1235-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibayama E, Koizumi H: Cellular localization of the Trk neurotrophin receptor family in human non-neuronal tissues. Am J Pathol 1996, 148:1807-1818 [PMC free article] [PubMed] [Google Scholar]
- 22.Koizumi H, Morita M, Mikami S, Shibayama E, Uchikoshi T: Immunohistochemical analysis of TrkA neutrophin receptor expression in human non-neuronal carcinomas. Pathol Int 1998, 48:93-101 [DOI] [PubMed] [Google Scholar]
- 23.Elsharkawy AM, Wright MC, Hay RT, Arthur MJP, Hughes T, Bahr MJ, Degitz K, Mann DA: Persistent activation of nuclear factor-κB in cultured rat hepatic stellate cells involves the induction of potentially novel rel-like factors and prolonged changes in the expression of IκB family proteins. Hepatology 1999, 30:761-769 [DOI] [PubMed] [Google Scholar]
- 24.Hellerbrand C, Jobin C, Licato LL, Sartor RB, Brenner DA: Cytokines induce NF-κB in activated but not in quiescent rat hepatic stellate cells. Am J Physiol 1998, 275:G269-G278 [DOI] [PubMed] [Google Scholar]
- 25.Beg AA, Sha WA, Bronson RT, Ghosh S, Baltimore D: Embryonic lethality and liver degeneration in mice lacking the Re1A component of NF-kappa B. Nature 1995, 376:167-170 [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama H, Savill JS, Kitamura M, Zhao L, Stylianou EJ: Selective sensitization to tumor necrosis factor-alpha-induced apoptosis by blockade of NF-kappaB in primary glomerular mesangial cells. J Biol Chem 1999, 274:19532-19537 [DOI] [PubMed] [Google Scholar]
- 27.Melton DA, Kreig PA, Rebagliati MR, Maniatis T, Zinn K, Green MR: Efficient in vitro synthesis if biologically active RNA and RNA hybridisation probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res 1984, 12:7035-7056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips HS, Hains JM, Laramee GR, Rosenthal A, Winslow JW: Widespread expression of BDNF- but not NT3 by target areas of basal forebrain cholinergic neurons. Science 1990, 250:290-294 [DOI] [PubMed] [Google Scholar]
- 29.Dignam JD, Lebovitz RM, Roeder RG: Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 1983, 11:1475-1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bahr MJ, Vincent K, Arthur MJP, Fowler AV, Smart DE, Wright MC, Clark IM, Benyon RC, Iredale JP, Mann DA: Control of the tissue inhibitor of metalloproteinases-1 promoter in culture-activated rat hepatic stellate cells: regulation by activator protein-1 DNA binding proteins. Hepatology 1999, 29:839-848 [DOI] [PubMed] [Google Scholar]
- 31.Hirt B: Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol 1967, 26:365-369 [DOI] [PubMed] [Google Scholar]
- 32.Urdiales JL, Becker E, Andrieu M, Thomas A, Jullien J, van Grunsven LA, Menut S, Even GI, Martín-Zanca D, Rudkin BB: Cell cycle phase-specific surface expression of nerve growth factor receptors TrkA and p75NTR. Neuroscience 1998, 18:6767-6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thornberry NA, Lazebnik Y: Caspases: enemies within. Science 1998, 281:1312-1316 [DOI] [PubMed] [Google Scholar]
- 34.Oakley F, Mann J, Ruddell RG, Pickford J, Weinmaster G, Mann DA: Basal expression of Ikappa B alpha is controlled by the mammalian transcriptional repressor RBP-J (CBF-1) and its activator notch 1. J Biol Chem 2003, 278:24359-24370 [DOI] [PubMed] [Google Scholar]
- 35.Barkett M, Xue D, Horvitz HR, Gilmore TD: Phosphorylation of IkappaB-alpha inhibits its cleavage by caspase CPP32 in vitro. J Biol Chem 1997, 272:29419-29422 [DOI] [PubMed] [Google Scholar]
- 36.Ravi R, Bedi A, Fuchs EJ, Bedi A: CD95(Fas)-induced caspase-mediated proteolysis of NF-κB. Cancer Res 1998, 58:882-886 [PubMed] [Google Scholar]
- 37.Wallach C: Cell death induction by TNF: a matter of self control. Trends Biochem Sci 1997, 22:107-109 [DOI] [PubMed] [Google Scholar]
- 38.Bhakar AL, Roux PP, Lachance C, Kryl D, Zeindler C, Barker PA: The p75 neurotrophin receptor (p75NTR) alters tumor necrosis factor-mediated NF-kappaB activity under physiological conditions, but direct p75NTR-mediated NF-kappaB activation requires cell stress. J Biol Chem 1999, 274:21443-21449 [DOI] [PubMed] [Google Scholar]
- 39.Klefstrom J, Arighi E, Littlewood TD, Saksela E, Evan GI, Alitalo K: Induction of TNF-sensitive cellular phenotype by c-Myc involves p53 and impaired NF-κB activation. EMBO J 1997, 16:7382-7392 [DOI] [PMC free article] [PubMed] [Google Scholar]