Abstract
Our effort to identify novel drug-resistant genes in cyclophosphamide-resistant EMT6 mouse mammary tumors led us to the identification of SPF45. Simultaneously, other groups identified SPF45 as a component of the spliceosome that is involved in alternative splicing. We isolated the human homologue and examined the normal human tissue expression, tumor expression, and the phenotype caused by overexpression of human SPF45. Our analyses revealed that SPF45 is expressed in many, but not all, normal tissues tested with predominant expression in normal ductal epithelial cells of the breast, liver, pancreas, and prostate. Our analyses using tissue microarrays and sausages of tumors indicated that SPF45 is highly expressed in numerous carcinomas including bladder, breast, colon, lung, ovarian, pancreatic, and prostate. Interestingly, this study revealed that overexpression of SPF45 in HeLa, a cervical carcinoma cell line, resulted in drug resistance to doxorubicin and vincristine, two chemotherapeutic drugs commonly used in cancer. To our knowledge, this is the first study showing tumor overexpression of an alternate splicing factor resulting in drug resistance.
The development of multidrug resistance is a major hurdle in the treatment of cancer. Although some genes that confer multidrug resistance are known, it is clear that many other genes remain to be identified. The characterization of novel mechanisms that lead to drug resistance would enable the development of a targeted new generation of anti-cancer drugs. To identify novel genes involved in drug resistance we performed suppressive-subtractive polymerase chain reaction analyses of the cyclophosphamide-resistant EMT6 mouse mammary tumor cells and the parental EMT6 tumors. This led to the identification of a gene that was highly homologous to a DNA damage response protein, DRT111 of Arabidopsis (unpublished observations). 1 While this work was in progress, Neubauer and colleagues, 1 identified a novel protein from the spliceosome. An analysis of all proteins present in the spliceosomal complex led to the identification of 25 previously identified proteins and 20 novel proteins. One of the novel proteins thus identified was named SPF45 (splicing factor 45 kd). This protein was identical to the one that was identified by our suppressive-subtractive polymerase chain reaction analysis. Neubauer and colleagues, 1 further showed that SPF45 was a nuclear protein that co-localizes with U1A snRNP in nuclear speckles, a known reservoir of splicing factors. 2-4 Recently Rappsilber and colleagues, 5 have described the identification of 311 proteins in the human spliceosomal complex of which 96 were novel proteins. Again, in this study, SPF45 was identified as a component of the spliceosome.
Lallena and colleagues, 6 demonstrated that SPF45 regulates the alternate splicing of the Drosophila sex-lethal gene (sxl). SXL protein is expressed only in female flies, but not in males. 7 Exon3 of Sxl plays a very important role in determining the expression of SXL because its inclusion results in a nonfunctional protein (male flies) whereas its exclusion produces a functional protein. 8,9 Intron 2 of sxl has an unusual sequence in that it has two different AG dinucleotides [proximal and distal AGs that can both serve as 3′ splice sites (ss)]. 10 Lallena and colleagues, 6 showed that in males, SPF45 binds specifically to the proximal AG and activates this 3′ ss resulting in the inclusion of exon 3. However, in female flies, SXL protein interacts with the amino-terminus of SPF45 and blocks its ability to activate this splice site for splicing, resulting in the exclusion of exon 3 through exon skipping. 6 Alternate splicing that is dependant on SPF45 occurs not only in Drosophila but also in humans. In a subgroup of patients that have β-thalassemia, a single nucleotide mutation in intron I generates a new proximal splice site (3′ ss) AG 11 that is activated by SPF45 for splicing. 6 This results in the translation of a nonfunctional β-globin protein that is characteristic of β-thalassemia. Thus, SPF45, a component of the spliceosome, is a novel splicing factor acting at the second catalytic step of intron removal. It is involved in alternative splicing through the recognition of proximal AGs within introns. It may also recognize distal AGs and may have other unknown roles in splicing.
The primary objective of this study was to analyze the expression patterns of SPF45 in normal tissues and in carcinomas and to identify a phenotype associated with tumor overexpression. Our study demonstrates that SPF45 is expressed in most normal ductal epithelium in the body. In addition, overexpression of SPF45 is seen in numerous carcinomas including bladder, breast, colon, lung, pancreatic, and prostate carcinomas. Using HeLa cells that stably overexpress SPF45, we demonstrate that SPF45 overexpression is sufficient to confer drug resistance to doxorubicin and vincristine, two drugs from two different classes of chemotherapeutic agents 12,13 commonly used in cancer treatment. To our knowledge, this is the first study that demonstrates that overexpression of a splicing factor is sufficient to confer a multidrug-resistant phenotype to transfected cells. Thus, SPF45 potentially plays a role in multidrug resistance to anti-cancer agents in a wide range of tumor types in which SPF45 overexpression has been detected.
Materials and Methods
Cell Culture
HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (Invitrogen), 2 mmol/L glutamine, 0.1 mmol/L nonessential amino acids, 10 mmol/L Hepes buffer (pH 7.5), and 1 mmol/L sodium pyruvate. The cells were maintained at 37°C under 5% CO2. Transient transfections using SPF45 expression vectors or empty vectors were performed using the standard calcium phosphate co-precipitation protocol.
Cloning of SPF45 cDNA
To identify genes overexpressed in cyclophosphamide-resistant EMT6/CTX mouse mammary tumor cells relative to sensitive parental EMT6 cells, 14 suppression-subtractive hybridization was performed using a PCR-Select kit (Clontech, Palo Alto, CA) and products were cloned and sequenced. BLAST analysis 15 (web address: http://www.ncbi.nlm.nih.gov/BLAST) of one of the sequences revealed that it was identical to mouse SPF45 1 (GenBank AF083384). The predicted protein translation of this sequence was used to identify an EST clone (GenBank AA644366) representing the human SPF45 cDNA (NM_032905) in the human EST database at National Center for Biotechnology Information using the TBLASTN server. The human SPF45 cDNA was cloned into the pMiNeo vector resulting in the plasmid pMiNeo-hSPF45. The vector plasmid, pMiNeo contains a CMV SR-α promoter and cloning sites from the vector pMet7 plus the IRES element and neomycin resistance genes from pIRES-Neo (BD Biosciences–Clontech). A modified form of the human SPF45 cDNA containing 6-His and Xpress tags (Xpress tag amino acid sequence DLDDDDK) fused upstream of the third amino acid residue of SPF45 was cloned into the EBNA-based episomal vector EW1969 described previously 16 to generate the EW1969-hisB-hSPF45 that was used in transient transfection experiments.
SPF45 Recombinant Protein Purification
For production of SPF45 protein in baculovirus, wild-type human SPF45 cDNA was cloned into the pFASTBAC vector (Invitrogen). Generation of recombinant bacmids, transfection into Sf9 cells, determining viral titers by plaque formation assay, and infection of Sf9 cells for protein production were performed using reagents and protocols provided by the manufacturer (Invitrogen). SPF45 protein was purified using metal chelate affinity chromatography by binding to 5-ml HiTrap Ni-columns (Amersham Biosciences, Piscataway, NJ).
Antibody Production
Purified SPF45 protein produced in baculovirus as described above was used to generate rabbit polyclonal antisera by Covance Research Products (Denver, PA).
Generation of Stable Transfectants
The pMiNeo-hSPF45 plasmid or the empty vector was transfected into HeLa cells (200,000cells/p100 plate) plated the day before, using lipofectamine (Invitrogen) according to the supplied protocol. The day after transfection, the media was changed and the cells incubated overnight at 37°C with 5% CO2. The following day, the cells from each p100 plate were trypsinized and replated at low density into 10 p100 dishes and allowed to attach for 4 hours. Then 600 μg/ml of G418 was added to the media and the cells were allowed to grow until visible colonies could be seen. During this time, the media was changed every other day. Individual colonies were picked and expanded and used for further analyses.
Preparation of Protein Lysates
Adherent cells were scraped off the p100 plates and transferred to 50-ml Falcon tubes (Fisher Scientific, Chicago, IL) and washed once with 1× phosphate-buffered saline (PBS) (137 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, 2 mmol/L KH2PO4). The cells were then centrifuged at 1000 × g for 5 minutes and the pellet was resuspended in an appropriate amount of RIPA (50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L sodium chloride, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 1% sodium deoxycholate) buffer containing protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN) and incubated on ice for 10 minutes. The lysate was then sonicated for 1 minute in 20-second pulses on ice and centrifuged. An aliquot of the supernatant was then used to quantitate the protein by the Bradford method (Bio-Rad, Hercules, CA).
To prepare protein lysates from normal tissues; tissue samples were placed in lysing matrix D columns (Q.Biogene, Carlsbad, CA) with high-salt RIPA buffer (composition as above except that instead of 150 mmol/L sodium chloride, 420 mmol/L sodium chloride was used) containing protease inhibitor cocktail (Boehringer Mannheim). The tissue was homogenized in the FastPrep instrument (Q.Biogene) according to the manufacturer’s recommendation at 4°C. The total lysate obtained was centrifuged at 16,000 × g for 1 minute at 4°C in an Eppendorf centrifuge (Brinkman, Westbury, NY) and the supernatant was used for Western blots.
Western Blots
Total protein preparations isolated from either tissues or cell lines were run on Novex 4 to 20% gradient polyacrylamide sodium dodecyl sulfate-Tris-glycine gels (Invitrogen) and proteins were transferred to nitrocellulose membranes. The membranes were then used for Western blots with either the polyclonal rabbit anti-SPF45 or a mouse monoclonal anti-Xpress tag antibody (Invitrogen) or a β-actin antibody (Clontech) along with appropriate secondary antibodies. The blots were blocked with PBST (1× PBS, 0.1% Tween 20) containing 5% nonfat dry milk. The washes were performed with PBST and the antibodies were diluted in PBST-5% milk. The enhanced chemiluminescent system (Amersham, Chicago, IL) was used to visualize the protein bands. Further details are provided in the figure legends.
Tissue Specimens
All tissue specimens were retrieved from the tissue bank of Lilly Research Laboratories. Surgical specimens were obtained during the period extending from 1997 to 1999. None of the patients had been treated with chemotherapy before surgery.
Tissue Microarray Slide Production
The hematoxylin and eosin section was used as a guide to select the most representative area of the tumor from which the individual core was taken. Tissue cores with a diameter of 0.6 mm were punched from tumor areas of each donor block and brought into the recipient paraffin block using a custom-made precision instrument (Beecher Instruments, Silver Springs, MA). Samples for all tumors were distributed in four regular-sized paraffin blocks each containing 40 to 60 individual tumor samples. Six-μm sections of the resultant tissue microarray blocks were transferred to positive-charged glass slides. At one end of the array, a core of kidney and spleen was inserted to enable correct identification of all tumor core samples.
Sausage Slide Production
Carcinomas in individual paraffin-embedded blocks were used to create a tumor sausage containing multiple tumor samples. A 4-mm skin punch biopsy device was used to remove the tumor sample from the original block and six samples were placed in a new recipient block. This created a new paraffin block with six different individual cases.
Slide Preparation
Tissues (both normal as well as tumor tissues) were fixed overnight in zinc-buffered formalin and then transferred to 70% ethanol before processing through paraffin. Five-μm sections were microtomed and the sections were placed on positive-charged slides. The slides were then baked overnight at 60°C in an oven.
Immunohistochemistry
Ten percent zinc formalin-fixed, paraffin-treated tissue sections (either individual sections or sausages or tissue arrays) were first deparaffinized with xylene, and rehydrated through incubations with graded alcohol to water. The tissue sections on slides were then treated for 20 minutes (and in some cases 40 minutes) with target-retrieval solution (DAKO, Carpinteria, CA) at 85°C and at room temperature for 10 minutes. Excess buffer was removed and the tissue sections were marked with a wax pen. Then the slides were placed in an autostainer (DAKO) and treated with 3% hydrogen peroxide for 5 minutes and washed with TBST (50 mmol/L Tris-HCl, pH7.6, 150 mmol/L NaCl, and 0.1% Tween 20) twice. Nonserum protein block solution (DAKO) was added and the slides were incubated for 45 minutes at room temperature. After the incubation, the solution was dabbed off, the primary antibody (α-SPF45) at a dilution of 1:200 was added and the slides were incubated for an hour at room temperature. The slides were then washed twice with TBST and incubated with biotinylated anti-rabbit secondary antibody (DAKO) for 10 minutes at room temperature. As before, the slides were washed twice and horseradish peroxidase conjugated to streptavidin (DAKO) was added and incubated for 10 minutes. After washing twice with the buffer (TBST), the slides were incubated for 2 minutes with diaminobenzidine substrate (DAKO) and washed twice. The slides were then counterstained with hematoxylin and dehydrated through graded alcohols and xylene. Coverslips were placed on the tissue sections and the slides were visualized under a bright-field microscope.
Confocal Microscopy
HeLa cells stably transfected with either vector control (pMiNeo) or with pMiNeo-hSPF45 expression plasmid were plated at a density of 10,000/ml on chamber slides (Nalgene, Naperville, IL) and incubated overnight at 37°C with 5% CO2. The following day the media was discarded and the cells were fixed in 1% formaldehyde in PBS for 10 minutes at room temperature. After fixing, the cells were washed in 1× PBS three times. After the wash, the cells were permeabilized with 1× PBS containing 1% bovine serum albumin and 0.025% Nonidet P-40 for 15 minutes and then washed twice with 1× PBS containing 1% bovine serum albumin. The cells were then blocked with Protein Block (DAKO) for 30 minutes at room temperature. Primary antibodies [rabbit polyclonal SPF45 antibody (1:100 dilution) and a 1:250 dilution of a mouse monoclonal anti-SR antibody (Zymed, South San Francisco, CA)] were added, and the cells incubated for 1 hour at room temperature. The cells were washed with 1× PBS containing 1% bovine serum albumin three times. An anti-rabbit IgG conjugated with Alexa 488 and anti-mouse IgG conjugated with Alexa 568 (1:500 dilutions) were added, and the cells incubated for 1 hour at room temperature. The cells were washed three times as before with 1× PBS containing 1% bovine serum albumin. The cells were then observed under a confocal microscope. As controls, the secondary antibodies were tested separately.
Antibody Blocking
The α-SPF45 polyclonal antibody was added to a fivefold higher concentration of Baculovirus-purified SPF45 protein and the mix was diluted 1:2 in DAKO antibody diluent buffer (DAKO) and incubated overnight at 4°C with gentle mixing. After incubation, the Eppendorf tube was centrifuged at 16,000 × g in a tabletop centrifuge for 5 minutes and the supernatant was transferred to a fresh tube and used either for immunohistochemistry or Western blots as described previously. The immunohistochemistry was performed with a couple of modifications. The primary antibody concentration used for immunohistochemistry was a 1:400 dilution rather than a 1:200 dilution as described earlier. The primary antibody incubation time was increased from 60 minutes to 90 minutes. As a positive control, immunohistochemistry using a 1:400 dilution of the antibody (unblocked) was performed along side the ones with the blocked antibody.
Cytotoxicity Assays
To test the sensitivity of HeLa vector and SPF45-overexpressing stable cells to doxorubicin and vincristine (Sigma, St. Louis, MO), 10,000 cells of each clone were plated/well on a 96-well plate and incubated overnight. The next day different concentrations of the drugs (in triplicate) were added to the wells and the cells incubated for 72 hours and MTT (Promega, Madison, WI) reagent was added to all of the wells and incubated for 1 to 2 hours and then the absorbance at OD492 nm was measured. All incubations were performed at 37°C with 5% CO2.
Statistical Methods
The IC50 values (concentration of the drug at which 50% of cells die) after drug treatment of HeLa cells stably transfected with pMiNeo vector or pMiNeo-hSPF45 cells were estimated after fitting the dose-response curves using the nonlinear model, 1 − 1/[1 + (IC50/X)slope]. IC50 and slope are the parameters to be estimated and X refers to the drug concentration. Estimates of other parameters, IC25 and IC75 (concentrations of the drug at which 25% and 75% of the cells die, respectively), were obtained by fitting this nonlinear model again, in which IC50 is replaced by IC25*(1/3)(1/slope) and IC75*3(1/slope), respectively. Because the variability of the response is not constant across the range of the response, these nonlinear models were fit using appropriate weights for the response. The weights were estimated by the power-of-the-mean function using the pseudo-likelihood method. 17 In addition, when fitting these nonlinear models, the parameters were represented as a factorial function of the treatment groups (HeLa vector and HeLa SPF45) and studies (number of times the experiment was repeated). This enabled the estimates of IC25, IC50, and IC75 to be statistically compared between the treatment groups for each experiment and between experiments. Any comparisons that resulted in a P value of <0.05 were considered as statistically significant. These analyses were performed using the gnls routine in S-PLUS software/language, version 2000, in a Windows 2000 workstation.
Results
Characterization of Anti-SPF45 Polyclonal Antibody
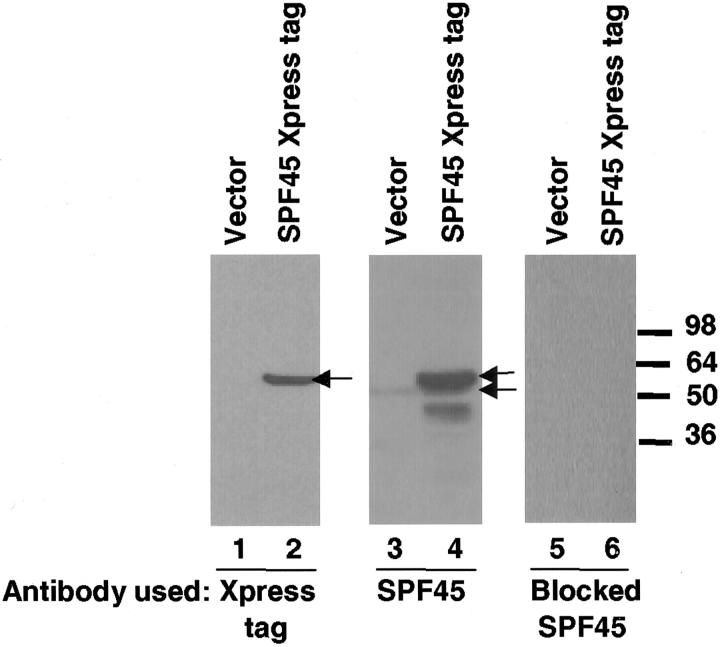
To verify that the polyclonal rabbit antibody that has been developed specifically detected SPF45, we transfected either the vector plasmid (EW1969) or EW1969-hisB-hSPF45 (containing an Xpress tag and His tag) transiently into HeLa cells. The lysate was subjected to Western blot analysis using either the SPF45 rabbit polyclonal antibody or a mouse monoclonal antibody directed toward the Xpress tag. As shown in Figure 1 ▶ , lanes 1 and 2, after a 30-second exposure the Xpress antibody detected a protein of ∼54 kd only in the HeLa cells transfected with SPF45 but not in the empty vector-transfected cells. Again with a 30-second exposure, the SPF45 antibody detected this same protein as well as a smaller protein present in all of the samples (Figure 1 ▶ , lanes 3 and 4). The smaller band (52 kd) detected in all lanes is the endogenous SPF45 present in HeLa cells. The endogenous protein is smaller as it lacks the Xpress and His tags. We also performed Western blots using SPF45 antibody that had been blocked with purified SPF45 protein (at a ratio of antibody to SPF45 of 1:5). SPF45 was not detected by Western blot after a 30-second exposure (Figure 1 ▶ , lanes 5 and 6). In fact only a very faint band was detected even after overnight exposure (data not shown). This further confirms the fact that the antibody generated is indeed specific for SPF45.
Figure 1.
Anti-SPF45 rabbit polyclonal antibody is specific for SPF45. HeLa cells were transiently transfected either with empty vector EW1969 (lanes 1, 3, and 5) or with EW1969-hisB-hSPF45 expression vector containing an Xpress tag (lanes 2, 4, and 6). Total cell lysates were prepared and 100 μg of total protein from each was run on 4 to 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the proteins transferred to a nitrocellulose membrane. Western blotting was performed using an antibody to the Xpress tag (lanes 1 and 2) or a SPF45-specific rabbit polyclonal antibody (lanes 3 and 4) or SPF45 antibody blocked with purified SPF45 protein (antibody to pure SPF45 protein ratio of 1:5) (lanes 5 and 6). The Xpress antibody detected a protein of 54 kd (top arrow), SPF45-specific antibody detected both the Xpress-tagged SPF45 (54 kd) (top arrow) and endogenous SPF45 (52 kd) (bottom arrow). The blocked antibody failed to recognize SPF45 under the same exposure conditions (lanes 5 and 6) but a barely visible band corresponding to SPF45 was visible in lanes 5 and 6 after an overnight exposure (not shown).
SPF45 Co-Localizes with SR Proteins to Nuclear Speckles
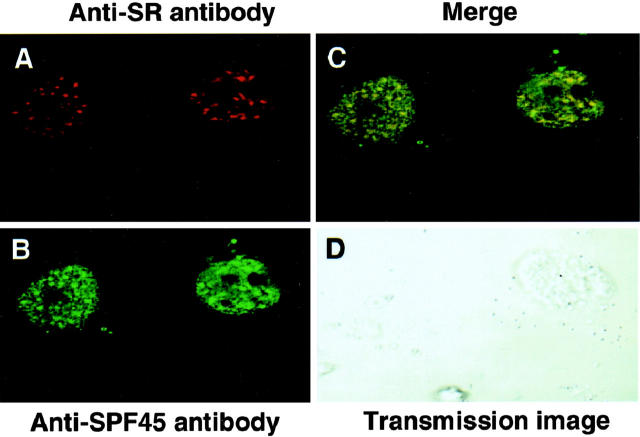
To analyze the subcellular localization of SPF45, we performed confocal microscopic analysis on HeLa cells stably transfected with pMiNeo-hSPF45, using the anti-SPF45 polyclonal antibody. Our analysis revealed that it was mostly confined to the nucleus in a speckled pattern as was reported previously for GFP-tagged SPF45 in transient transfections. 1 Because SPF45 has been previously identified to be a spliceosomal protein, 1,5 we performed co-localization studies using an antibody for SR (serine-arginine rich) proteins, a group of proteins known to localize to nuclear speckles. 18 Our analysis revealed that there is at least 89% co-localization of SPF45 with SR proteins (Figure 2) ▶ in HeLa cells stably overexpressing SPF45. HeLa cells stably transfected with the empty vector had a very low staining for SPF45 hence they were not used for analysis.
Figure 2.
SPF45 co-localizes with SR proteins in nuclear speckles. HeLa cells stably transfected with pMiNeo-hSPF45 were used for confocal microscopy using a α-SPF45 antibody and αSR antibody as described in Materials and Methods. A: Red spots indicate the location of SR proteins. B: Green spots indicate the location of SPF45. C: Yellow color resulting from merge red (SR) and green (SPF45) indicates co-localization (∼89% co-localization was observed). D: Transmission image of the two healthy cells, used for analysis.
Western Blot Analysis Reveals that SPF45 Is a Ubiquitously Expressed Protein in Normal Tissues
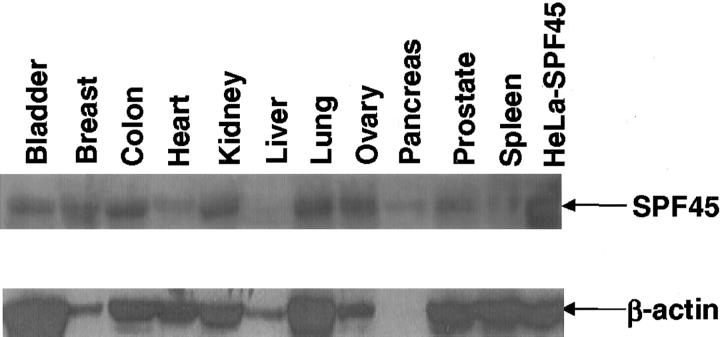
To analyze the normal tissue distribution of SPF45, we prepared protein lysates from various normal tissues and performed Western blot analyses using the polyclonal antibody described above. A unique band corresponding to SPF45 was seen in all of the tissues tested although the levels of SPF45 varied (Figure 3 ▶ , top). The highest expression was observed in breast, bladder, colon, kidney, lung, and ovary. Intermediate levels of expression were detected in the prostate and spleen. Low levels of expression were detected in heart, liver, and pancreas. The expression of β-actin in these tissues was also determined (Figure 3 ▶ , bottom). Because equal amounts of protein were loaded in each well [as confirmed by Ponceau S staining of the membrane (data not shown)], the expression of SPF45 could be compared between tissues. As we observed the β-actin expression level to be lower in the breast, liver, and pancreatic tissues, we did not normalize SPF45 expression level to that of β-actin but used it just as an indicator to show that intact proteins were isolated.
Figure 3.
SPF45 is ubiquitously expressed in normal tissues as assessed by Western blot. Total protein from bladder tissue, breast, colon, heart, kidney, liver, lung, ovary, pancreas, prostate, and spleen was isolated as described in Material and Methods and 50 μg of the total protein was used for each analysis. Total protein from HeLa cells stably transfected with pMiNeo-hSPF45 (lane 12) was used as a control. Two identical gels were run and the proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Because Ponceau S staining of the membrane showed equal protein loading in each well (not shown), the expression of SPF45 could be compared between tissues. Because we observed the β-actin expression level to be lower in the breast, liver, and pancreatic tissues, we did not normalize SPF45 expression level to that of β-actin but used it just as an indicator to show that intact proteins were isolated. One of the blots was used for Western blot analysis with α-SPF45 rabbit polyclonal antibody and the other blot was probed with anti-β-actin antibody. Western blots were performed at least twice and representative blots with the same patterns of expression are shown.
SPF45 Is Expressed in the Epithelium of Normal Tissues
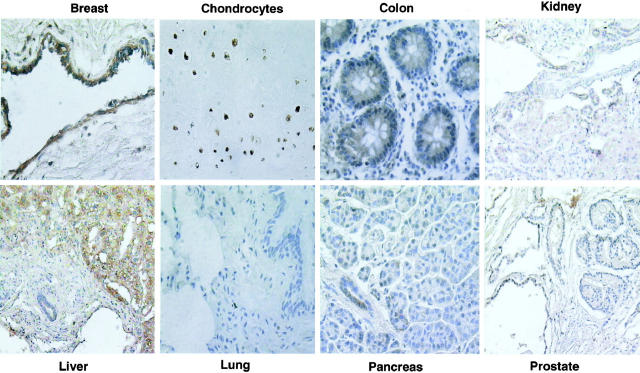
To analyze the specific localization of SPF45 in normal tissues, paraffin sections of the same normal tissues that were used for the Western blot analyses were embedded in slides and used for immunohistochemistry studies. The expression of SPF45 in various normal tissues is tabulated using an expression scale of 1 to 4, with 4 being maximal expression (Table 1) ▶ . SPF45 was present in the ductal epithelium of breast, liver, pancreas, and prostate (Figure 4) ▶ . In the liver, expression of SPF45 was also observed in hepatocytes. In the prostate, a lower intensity of staining was observed in the glandular epithelium. In the kidney, staining was present in the distal tubules. SPF45 was highly expressed in the nucleus of chondrocytes in the cartilage in the pharynx and trachea. In virtually all cases, Western blot results and the immunohistochemistry results agreed. The only exception was the lung tissue where the Western blot analysis indicated the presence of a high level of SPF45, whereas, it was not detected by immunohistochemistry. Further analysis indicated that this was because of the presence of inflammatory cells, which showed a high level of SPF45 expression (data not shown), contributing to the Western blot signal. These same inflammatory cells in the lung tissue were not scored in the immunohistochemistry analysis resulting in a negative signal. Overall, the immunohistochemistry analysis complemented the Western blot analysis and also revealed that SPF45 is not expressed in all of the cell types but is limited to specific cell types in any given tissue.
Table 1.
Expression of SPF45 in Normal Tissues Determined by Immunohistochemistry
| Tissue | SPF45 |
|---|---|
| Urinary system | |
| Kidney | − |
| Bladder | − |
| Bladder, lumen | +/− (V) |
| Respiratory system | |
| Trachea, upper brochium | +4 |
| Lung, lower brochium | − |
| Tonsil | − |
| Larynx/pharynx | − |
| Throat chondrocytes | +4 |
| Trachea chondrocytes | +4 |
| Alveolus | − |
| Digestive system | |
| Esophagus | − |
| Colon | − |
| Liver | +1 (V) |
| Gallbladder, transitional epithelium | + |
| Pancreas | − |
| Appendix | − |
| Cecum crypts | +1 |
| Bile ducts | − |
| Small intestine, crypts | − |
| Small intestine, vagal nerves | + |
| Stomach | − |
| Duodenum | − |
| Ileum | − |
| Reproductive system | |
| Testis | +/− (V) |
| Prostate | − |
| Breast, ductal epithelium | +3 |
| Uterus | − |
| Ovary | +2 |
| Endometrium | − |
| Immune system | |
| Spleen | − |
| Thymus | − |
| Lymph node | − |
| Endocrine system | |
| Adrenal | − |
| Islets | − |
| Neural tissues | |
| Spinal cord, white matter | +2 |
| Cerebellum | − |
| Brain | +1 |
| Other | |
| Placenta | − |
| Adipose | − |
| Smooth muscle | − |
Expression of SPF45 in various normal tissues was tested using the polyclonal SPF45 antibody and the results are tabulated. The expression level was graded with a 1 to 4 scale with 4 indicating the highest level of expression. Lack of expression is indicated by −. V is used to indicate variable expression in tissues where the expression was not found uniformly in all the cells.
Figure 4.
Immunohistochemical analysis reveals that SPF45 protein expression is localized to epithelial cells of normal tissues. Zinc formalin-fixed, paraffin-treated tissue sections were analyzed by immunohistochemistry using the rabbit polyclonal SPF45 antibody as described in Material and Methods. Brown color indicates the presence/abundance of SPF45 in each tissue. One representative slide is shown for each tissue.
SPF45 Is Overexpressed in Numerous Cancers
Because our analyses revealed that SPF45 was expressed predominantly in epithelial tissues and because most of the tumors have an epithelial origin, we extended our studies of SPF45 expression to human tumor specimens. One example of each type of tumor is shown in Figure 5 ▶ and the results of the analyses are tabulated (Table 2) ▶ . Our results clearly revealed that SPF45 was overexpressed in a majority of the bladder, breast, colon, lung, ovarian, pancreatic, and prostate carcinomas examined. The immunolocalization of the antibody was confined mainly to tumor cells from epithelial origin. There were minimal differences in staining between the breast, colon, lung, ovarian, pancreatic, and prostate carcinomas. The cell nucleus was distinctly stained with the antibody. In some tumors, in ∼35 to 40% of the cells that were positive, the cell cytoplasm was also stained. There was some staining variation between tumor cells within a neoplasm. Some cells had minimal staining whereas other tumor cells had moderate to very strong staining. For the most part, the surrounding connective tissue stroma, blood vessel smooth muscle, and endothelial cells, in and around the neoplastic foci were not stained with the antibody. Generally, the associated inflammatory cells in and around the tumor were stained with the antibody. In comparison to tumor tissue, there was less intensity in staining in the normal tissue in most cases.
Figure 5.
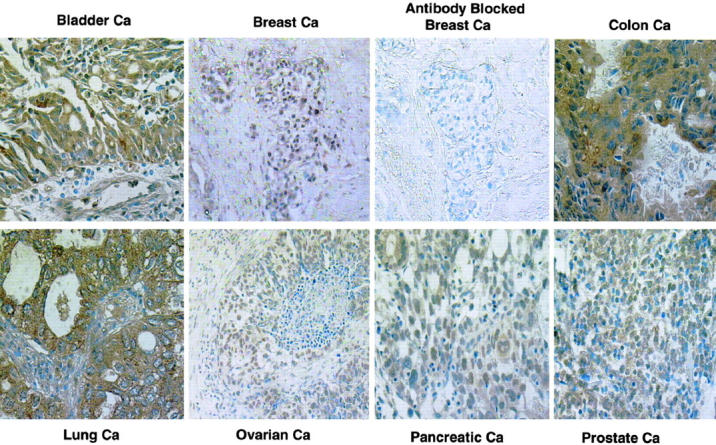
SPF45 is overexpressed in numerous cancers as measured by immunohistochemistry. Paraffin-embedded tumor tissue specimens were analyzed by immunohistochemistry for SPF45 expression. Cases from bladder, breast, colon, lung, ovarian, pancreatic, and prostate carcinoma are shown. As before, brown color indicates the presence of SPF45. Nuclear staining was observed in most of the tumors with only a few showing nuclear and cytoplasmic staining.
Table 2.
Overexpression of SPF45 in Tumors
| Tumor type | SPF45-positive tumors/total number of tumors (percentage positives) |
|---|---|
| Bladder tumor | 10/13 (77) |
| Breast carcinoma | 50/58 (86) |
| Colon cancer | 31/34 (91) |
| Lung cancer | 33/35 (94) |
| Ovarian carcinoma | 5/6 (83) |
| Pancreatic cancer | 6/7 (86) |
| Prostate carcinoma | 13/18 (72) |
A number of various types of tumors were analyzed by immunohistochemistry using anti-SPF45 polyclonal antibody. The total number of tumors analyzed and those that showed the presence of SPF45 are tabulated. Percentage positives are indicated within parentheses.
We also performed immunohistochemical analysis using SPF45 antibody that was blocked with purified SPF45 protein. A representative example of a breast cancer shown in Figure 5 ▶ clearly indicates that the antibody was efficiently blocked by the purified protein. This further confirms that the antibody generated is indeed specific for SPF45.
SPF45 Overexpression Results in Resistance to Chemotherapeutic Drugs
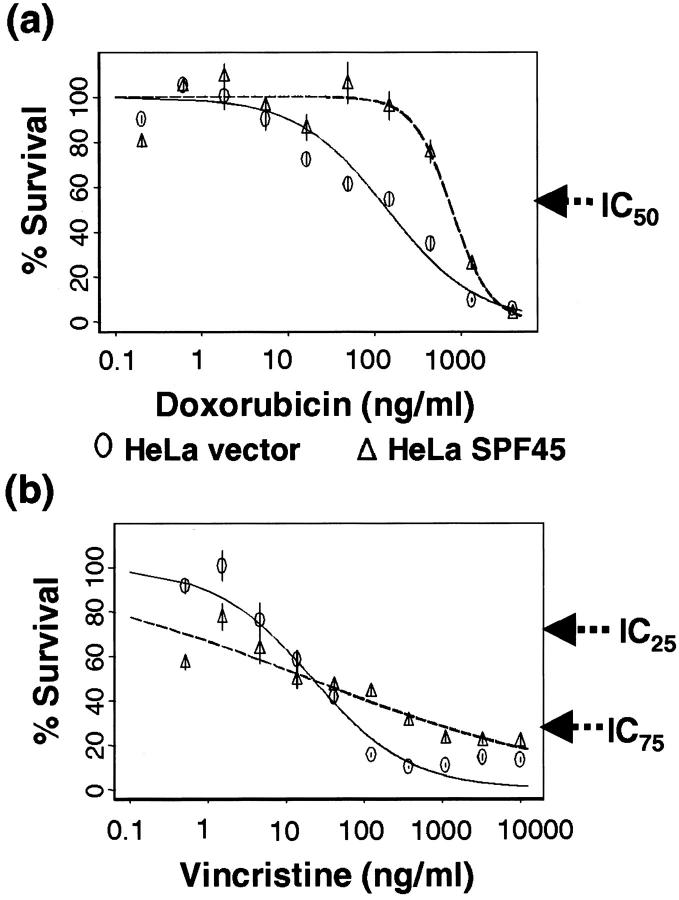
Because subtractive suppression polymerase chain reaction has identified SP45 as a candidate gene associated with the development of cyclophosphamide resistance in EMT6/CTX mammary tumor cells (unpublished data), we tested whether transfection of SPF45 could confer drug resistance to sensitive cells in culture. Our analysis using HeLa cells, stably transfected with either vector plasmid (pMiNeo) or the pMiNeo-hSPF45 expression plasmid, clearly showed that SPF45 overexpression results in drug resistance (Figure 6, a and b ▶ , shows a representative graph for doxorubicin and vincristine, respectively). Comparison of the IC50 values for doxorubicin between HeLa cells stably transfected with empty vector plasmid and SPF45-overexpressing cells revealed that the SPF45-overexpressing HeLa cells were fourfold to sevenfold resistant relative to vector control cells. The absolute IC50 values (concentration of the drug at which 50% of cells die) for both cell lines varied between experiments, however, the fold resistance remained the same. As evident from the graph (Figure 6a) ▶ , SPF45 overexpression resulted in resistance to doxorubicin at all concentrations. However, the graph for vincristine (Figure 6b) ▶ showed an unusual pattern in that at low doses of the drug, the SPF45-overexpressing cell line was more sensitive to the drug, whereas at high doses, it was more resistant. Comparison of the IC75 values (concentration of the drug at which 75% of cells die) of the HeLa cells that overexpress SPF45 with that of the vector control cells show that SPF45 overexpression results in 9- to 35-fold resistance to vincristine.
Figure 6.
SPF45 overexpression results in altered drug sensitivity. HeLa cells stably transfected with either the empty vector (pMiNeo) or pMiNeo-hSPF45 expression vector were used in cytotoxicity assays as described in Materials and Methods and the data obtained using doxorubicin (a) and vincristine (b) are plotted. Statistical analyses were performed as described in Material and Methods and the IC25, IC50, and IC75 values (concentrations of the drug at which 25%, 50%, or 75% of the cells die) were estimated. The IC50 values for HeLa vector and HeLa SPF45 cells for doxorubicin were 140 ± 17.4 ng/ml and 784.4 ± 58.4 ng/ml, respectively, with a P value <0.0001. The IC25 values for HeLa vector and HeLa SPF45 cells for vincristine were 4.36 ± 1.3 ng/ml and 0.17 ± 0.12 ng/ml with a P value of 0.0024 and the IC75 values for HeLa vector and HeLa SPF45 cells for vincristine were 103.3 ± 19.9 ng/ml and 2064.1 ± 912.11 ng/ml with a P value of 0.0355.
Conclusions
This study characterized the normal human tissue distribution of SPF45 expression in the most common epithelial carcinomas and the phenotype conferred by its overexpression. Immunohistochemical analysis revealed that SPF45 has limited expression in most normal tissues and is predominantly expressed in epithelial cells (Figure 4 ▶ and Table 1 ▶ ). Because SPF45 is a known spliceosomal protein, 1,5 this specific expression pattern suggests that SPF45 most likely participates in the splicing of selected mRNAs that are expressed in only a subset of cells (such as the epithelial cells) within a given tissue. This idea is supported by the observations of Lallena and colleagues, 6 that SPF45 is involved in alternate splicing of a subset of mRNAs that possess proximal AGs within the intron.
Our analysis has revealed that overexpression of SPF45 is a common event in a variety of different tumors including those derived from breast, colon, lung, ovary, pancreas, and prostate carcinomas (compare Table 1 ▶ and Figure 4 ▶ with Figure 5 ▶ ). SPF45 expression was seen in many of the common carcinomas that are clinically relevant in humans. In most cases, SPF45 was observed to be in the nucleus whereas in other cases, it was present both in the nucleus as well as the cytoplasm. Because SPF45 has been shown to be a splicing factor localized in the nucleus of HeLa cells (this study), 1,5 our analysis of SPF45 localization predominantly in the nucleus in tumors suggests that SPF45 is not frequently mislocalized in these tumors because of its overexpression. Neubauer and colleagues, 1 demonstrated that SPF45-GFP (green fluorescent protein) fusion protein construct co-localized with U1snRNP in nuclear speckles of HeLa cells. This predominant subcellular localization of SPF45 is further confirmed by our confocal analysis using specific SPF45 and SR antibodies using HeLa cells stably transfected with SPF45 (Figure 2) ▶ . This shows that overexpression of SPF45 still results in nuclear speckle localization.
Other splicing factors have also been shown to be overexpressed in cancer cells. A recent report showed that the p32 subunit of the splicing factor SF2, was differentially expressed in metastasis-associated transformation in colon cancer; 19 similarly hnRNP A2/B1 has been found to be overexpressed in lung cancer 20 and is in fact an early marker for lung cancer. U2AF65 was found to be overexpressed whereas SF2 was found to be down-regulated during transformation induced by chemical carcinogens in BALB/3T3 cells. 21 However, the results of the alterations in the expression of these splicing factors during cancer progression and/or metastasis are not known. Because a number of genes have been characterized to be alternately spliced in cancer, 22 it is very likely that alterations in the splicing of key genes is the result of alterations in splicing factors.
Finally, we have demonstrated that overexpression of SPF45 was sufficient to confer drug resistance to doxorubicin and high-dose vincristine, drugs that belong to two different classes of commonly used chemotherapeutic agents. 12,13 The dose-response curve for vincristine looks unusual in that at low doses the HeLa vector transfectants are more resistant than the SPF45-overexpressing cells, whereas at high doses of the drug the SPF45-overexpressing cells are more resistant that the vector control. This pattern was consistently seen in repeat experiments. This probably indicates that, at least for vincristine, an endogenous gene or genes have to be altered (either activated or repressed) for SPF45-mediated drug resistance to occur and that this occurs only in high concentrations of the drug. SPF45-mediated resistance was also observed against a number of chemotherapeutic drugs that have different mechanisms of action in A2780, an ovarian carcinoma cell line (unpublished observations). 23 Thus, SPF45 plays an important role in multidrug resistance.
It is currently unknown how overexpression of SPF45 leads to drug resistance and this is the subject of intense investigation. However, unlike the ATP-binding cassette transporters, 24 and the anti-apoptotic proteins 25 that have been shown to contribute to drug resistance, SPF45 is neither a plasma membrane protein effluxing drugs out of the cell, nor a mitochondrial protein affecting apoptosis, but a nuclear protein involved in splicing. Thus SPF45 overexpression results in a novel form of multidrug resistance. To our knowledge, this is the first demonstration that overexpression of a splicing factor is sufficient to confer a multidrug-resistant phenotype to cancer cells.
Acknowledgments
We thank Kovacevic Steven, Yeh Wu-Kuang, Bright Stuart, and Kushstoss Stuart, for their help in development of the antibody and its initial characterization.
Footnotes
Address reprint requests to Dr. Robert E. Moore, Research Scientist, Cancer Research Division, Lilly Research Laboratories, Eli Lilly & Company, Drop Code 0424, Indianapolis, IN 46285. E-mail: remoore@lilly.com.
References
- 1.Neubauer G, King A, Rappsilber J, Calvio C, Watson M, Ajuh P, Sleeman J, Lamond A, Mann M: Mass spectrometry and EST-database searching allows characterization of the multi-protein spliceosome complex. Nat Genet 1998, 20:46-50 [DOI] [PubMed] [Google Scholar]
- 2.Carmo-Fonseca M, Tollervey D, Pepperkok R, Barabino SM, Merdes A, Brunner C, Zamore PD, Green MR, Hurt E, Lamond AI: Mammalian nuclei contain foci which are highly enriched in components of the pre-mRNA splicing machinery. EMBO J 1991, 10:195-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmo-Fonseca M, Pepperkok R, Sproat BS, Ansorge W, Swanson MS, Lamond AI: In vivo detection of snRNP-rich organelles in the nuclei of mammalian cells. EMBO J 1991, 10:1863-1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang S, Spector DL: U1 and U2 small nuclear RNAs are present in nuclear speckles. Proc Natl Acad Sci USA 1992, 89:305-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rappsilber J, Ryder U, Lamond AI, Mann M: Large-scale proteomic analysis of the human spliceosome. Genome Res 2002, 12:1231-1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lallena MJ, Chalmers KJ, Llamazares S, Lamond AI, Valcarcel J: Splicing regulation at the second catalytic step by sex-lethal involves 3′ splice site recognition by SPF45. Cell 2002, 109:285-296 [DOI] [PubMed] [Google Scholar]
- 7.Sanchez L, Granadino B, Torres M: Sex determination in Drosophila melanogaster: x-linked genes involved in the initial step of sex-lethal activation. Dev Genet 1994, 15:251-264 [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto H, Inoue K, Higuchi I, Ono Y, Shimura Y: Control of Drosophila sex-lethal pre-mRNA splicing by its own female-specific product. Nucleic Acids Res 1992, 20:5533-5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell LR, Maine EM, Schedl P, Cline TW: Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell 1988, 55:1037-1046 [DOI] [PubMed] [Google Scholar]
- 10.Penalva LO, Lallena MJ, Valcarcel J: Switch in 3′ splice site recognition between exon definition and splicing catalysis is important for sex-lethal autoregulation. Mol Cell Biol 2001, 21:1986-1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young K, Margulies L, Donovan-Peluso M, Clyne J, Driscoll MC, Dobkin C, Leibowitz D, Russo G, Schiliro G, Bank A: Structure and expression of two beta genes in a beta thalassemia homozygote. J Mol Appl Genet 1985, 3:1-6 [PubMed] [Google Scholar]
- 12.Cummings J, Smyth JF: DNA topoisomerase I and II as targets for rational design of new anticancer drugs. Ann Oncol 1993, 4:533-543 [DOI] [PubMed] [Google Scholar]
- 13.Rowinsky EK, Donehower RC: The clinical pharmacology and use of antimicrotubule agents in cancer chemotherapeutics. Pharmacol Ther 1991, 52:35-84 [DOI] [PubMed] [Google Scholar]
- 14.Teicher BA, Herman TS, Holden SA, Wang YY, Pfeffer MR, Crawford JW, Frei E, III: Tumor resistance to alkylating agents conferred by mechanisms operative only in vivo. Science 1990, 247:1457-1461 [DOI] [PubMed] [Google Scholar]
- 15.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ: Basic local alignment search tool. J Mol Biol 1990, 215:403-410 [DOI] [PubMed] [Google Scholar]
- 16.Godinot N, Iversen PW, Tabas L, Xia X, Williams DC, Dantzig AH, Perry WL, III: Cloning and functional characterization of the multidrug resistance associated protein (MRP1/ABCC1) from the cynomolgus monkey. Mol Cancer Ther 2003, 2:307-316 [PubMed] [Google Scholar]
- 17.Carroll RJ, Ruppert D: Transformation and Weighting in Regression, ch 2. Carroll RJ Ruppert D eds. 1988. Chapman & Hall New York
- 18.Chaudhary N, McMahon C, Blobel G: Primary structure of a human arginine-rich nuclear protein that colocalizes with spliceosome components. Proc Natl Acad Sci USA 1991, 88:8189-8193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parle-McDermott A, McWilliam P, Toghe O, Dunican D, Croke DT: Serial analysis of gene expression identifies putative metastasis-associated transcripts in colon tumor cell lines. Br J Cancer 2000, 83:725-728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou J, Mulshine JL, Unsworth EJ, Scott FM, Avis IM, Vos MD, Treston AM: Purification and characterization of a protein that permits early detection of lung cancer. Identification of heterogeneous nuclear ribonucleoprotein-A2/B1 as the antigen for monoclonal antibody 703D4. J Biol Chem 1996, 271:10760-10766 [DOI] [PubMed] [Google Scholar]
- 21.Maeda T, Hiranuma H, Jikko A: Differential expression of the splicing regulatory factor genes during two-step chemical transformation in a BALB/3T3-derived cell line, MT-5. Carcinogenesis 1999, 20:2341-2344 [DOI] [PubMed] [Google Scholar]
- 22.Mercatante D, Kole R: Modification of alternative splicing pathways as a potential approach to chemotherapy. Pharmacol Ther 2000, 85:237-243 [DOI] [PubMed] [Google Scholar]
- 23.Perry III WL, Shepard RL, Pratt S, Godinot N, Williamson M, Tighe M, Lesoon A, Jin S, Shyjan A, Rolfe M, Moore RE, Dantzig AH: Overexpression of the Human Splicing Factor SPF45 Results in Multidrug Resistance. American Association for Cancer Research, annual meeting 2003, Abstract no. 5540
- 24.Borst P, Elferink RO: Mammalian ABC transporters in health and disease. Annu Rev Biochem 2002, 71:537-592 [DOI] [PubMed] [Google Scholar]
- 25.Reed JC, Miyashita T, Takayama S, Wang HG, Sato T, Krajewski S, Aime-Sempe C, Bodrug S, Kitada S, Hanada M: BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem 1996, 60:23-32 [DOI] [PubMed] [Google Scholar]