Abstract
Conditional expression of estrogen receptor (ER)-α) was introduced into tetracycline-responsive MMTV-tTA/tetop-TAg mice to develop a mouse model of estrogen-responsive ER-α-positive mammary adenocarcinoma. Mammary adenocarcinomas developed in the mice with a mean latency of 11 months. Precursor lesions including ductal hyperplasia and hyperplastic alveolar nodules were present by the age of 4 months. The mammary adenocarcinomas exhibited histological features similar to human breast cancers. ER steroid-binding studies conducted on adenocarcinoma lysates demonstrated binding to estradiol. Tumor explant studies in the presence and absence of estradiol in ovariectomized athymic nude mice revealed that growth of mammary tumors was stimulated by estrogen. In addition, the presence of ER-α altered the tumor spectrum in other MMTV-targeted tissues in the tTA/TAg female mice. Lymphomas, which develop in 40% of tTA/TAg female mice, were found in only 4% of tTA/TAg/ER-α mice (P = 0.014, chi-square test). These experiments demonstrate that the introduction of an ER-α transgene targeted to mammary epithelial cells can be used to develop mouse models of ER-α-responsive mammary cancer.
Breast cancer is the most common malignancy in females and the second leading cause of cancer deaths in women in the United States. 1 Risk factors for this disorder, such as early menarche and late menopause, indicate an important etiological role for hormones, specifically estrogen. 2 Estrogen acts as a growth factor in normal development of the mammary gland, as well as in breast cancer, and may contribute to the molecular events leading to breast carcinogenesis. 3 Estrogen action on mammary epithelial cells (MECs) is mediated by estrogen receptors (ER-α and ER-β), members of the nuclear receptor superfamily and transcription factors that regulate expression of estrogen-responsive genes. Estrogen-dependent cancers account for ∼60% of all human breast cancer cases. 4 Approximately two-thirds of breast cancers express the ER gene and synthesize ER protein. 5 Expression of ER-α is a positive prognostic factor associated with more differentiated and less aggressive tumors that often respond to hormonal therapies. 4,6 However, increased expression of ER-α may stimulate the development of precancerous changes in the mammary gland that result in cancer initiation. 3 It is hypothesized that ER-α contributes to the carcinogenic process by increasing cell proliferation through ER-mediated signal transduction. This increase in proliferation leads to increased probability for mutation during DNA synthesis, accumulation of genetic damage, and stimulation of the synthesis of growth factors that act on the MECs. 7
The purpose of the current study was to develop a mouse model that can be used to examine the role ER-α signaling plays in mammary cancer initiation and progression. A conditional transgenic mouse model with dominant gain of ER-α (tetop-ER-α) was characterized previously. 8 To determine the effects of dominant gain of ER-α on cancer development, the tetop-ER-α transgene was introduced into the conditional Simian virus 40 T antigen (TAg) model (MMTV-tTA/tetop-TAg), 9 a well-established multistep mouse model of salivary carcinogenesis with time-dependent reversible and irreversible stages of dysplasia. Combining these two models resulted in the generation of a novel conditional mouse model of estrogen-responsive mammary cancer.
Materials and Methods
MMTV-tTA/tetop-TAg/tetop-ER-α Triple-Transgenic Mice
Conditional transgenic mice with mouse mammary tumor virus long-terminal repeat (MMTV)-driven expression of a tetracycline-responsive transactivator (tTA) (tet-off gene regulation) and tetracycline-responsive promoter (tetop)-Simian virus 40 large T antigen (TAg) double-transgenic mice (MMTV-tTA/tetop-TAg) 9 were mated to MMTV-tTA and tet-op-FLAG-tagged mouse estrogen receptor-α (tetop-ER-α) 8 double-transgenic mice to generate triple-transgenic MMTV-tTA/tetop-TAg/tetop-ER-α (tTA/TAg/ER-α) mice that demonstrated tet-off regulation of both the TAg and ER-α transgenes. Mice were identified using the polymerase chain reaction (PCR) from DNA extracted from either ear or tail tissue. 8-10 Double-transgenic tTA/TAg mice that did not carry the tetop-ER-α transgene were used as negative controls.
Observation of Tumor Formation, Development of Preneoplastic Lesions in the Mammary Gland, and Animal Care
Development of mammary, submandibular salivary, and lymphoid tumors was assessed by serial visual inspection and manual palpation in tTA/TAg/ER-α females (n = 27) and males (n = 40) and tTA/TAg females (n = 10) and males (n = 11). When palpable tumors reached 1 cm in diameter or the mice reached 12 months of age, the mice were euthanized and necropsied to confirm tumor presence, examine for the presence or absence of metastases, and obtain tissue for histological and biochemical studies. Mammary adenocarcinomas were harvested from all of the mice that developed mammary adenocarcinomas (10 tTA/TAg/ER-α females and 1 tTA/TAg/ER-α male). Salivary adenocarcinomas were harvested from all of the mice that developed salivary adenocarcinomas (20 tTA/TAg/ER-α females, 37 tTA/TAg/ER-α males, 4 tTA/TAg females, and 11 tTA/TAg males). Lymphomas were harvested from all of the mice that developed lymphomas (one tTA/TAg/ER-α female, one tTA/TAg/ER-α male, and four tTA/TAg females). Mammary glands were harvested from tTA/TAg/ER-α female mice (n = 10 at 4 months, n = 9 at 7 months; and n = 34 at 11 ± 0.5 months of age) and tTA/TAg-negative control female mice (n = 9 at 4 months, n = 9 at 7 months, and n = 18 at 11 ± 0.5 months of age). The presence of hyperplastic alveolar nodules (HANs) was determined by mammary gland whole mount analysis in tTA/TAg/ER-α female mice at 4 months of age (n = 9), 7 months of age (n = 9), and 11 ± 2 months of age (n = 28) and in tTA/TAg female mice at 4 months of age (n = 9), 7 months of age (n = 8), and 11 ± 2 months of age (n = 9). The presence of MEC hyperplasia and dysplasia were identified by examination of hematoxylin and eosin (H&E)-stained sections of mammary tissue from tTA/TAg/ER-α at 4 months of age (n = 8), 7 months of age (n = 8), and 11 ± 2 months of age (n = 25) and in tTA/TAg female mice at 4 months of age (n = 9), 7 months of age (n = 7), and 11 ± 2 months of age (n = 13). All procedures involving animals were performed in accordance with current federal (National Institutes of Health Guide for the Care and Use of Laboratory Animals) and university guidelines and were reviewed and approved by the Georgetown University Institutional Animal Use and Care Committee.
Histological Analyses, Immunohistochemistry, and Mammary Gland Whole Mounts
Mammary adenocarcinoma specimens were fixed in 10% buffered formalin overnight at 4°C and embedded in paraffin using standard techniques. Sections (5 μm) were cut and stained with H&E and immunohistochemical analyses. Detection of ER-α protein expression in mammary adenocarcinoma tissue was accomplished using the Mouse on Mouse (M.O.M) peroxidase kit (PK-2200; Vector Laboratories, Inc., Burlingame, CA). Tissue sections were deparaffinized, rehydrated, antigens exposed with high-pH target retrieval solution (S3307; DAKO, Carpinteria, CA) and high temperature, quenched with 3% hydrogen peroxide, blocked with mouse IgG-blocking reagent, incubated with M.O.M. diluent, exposed to a 1:25 dilution of the mouse monoclonal ER-α antibody (IM2133; Beckman Coulter Immunotech, Miami, FL) in M.O.M. diluent for 1 hour and to M.O.M. biotinylated anti-mouse IgG reagent for 10 minutes at room temperature. The tissues were exposed to M.O.M. elite stain for 30 minutes, then stained with diaminobenzidine peroxidase substrate kit (SK-4100, Vector) for 5 minutes, counterstained with hematoxylin, and mounted with GVA-mount. Immunohistochemical detection of proliferating cell nuclear antigen (PCNA) expression was performed as a relative measurement of cellular proliferation. Briefly, paraffin-embedded mammary adenocarcinoma tissue sections were deparaffinized, rehydrated, quenched with 3% hydrogen peroxide, exposed to two drops of the EPOS PCNA immunostaining system (U7032, DAKO) for 1 hour at room temperature, stained with the diaminobenzidine peroxidase substrate kit (Vector) for 5 minutes, counterstained with hematoxylin, and mounted with glycerol vinyl alcohol (GVA) mount. One whole no. 4 mammary gland from each animal was dissected and spread on a glass slide at the time of necropsy for whole mount analyses. The mammary glands were fixed in Carnoy’s solution (six parts ethanol, three parts chloroform, and one part glacial acetic acid) to defat the glands for at least 1 hour at room temperature, then rehydrated in successive washes of 70%, 50%, and 30% ethanol, rinsed in dH2O, and stained with carmine alum (carmine aluminum sulfate) for 1 to 3 days at 4°C. The stained mammary glands were then dehydrated in successive washes of 70%, 95%, and 100% ethanol, transferred to xylene, and coverslips were mounted with permount mounting media (Fisher Scientific, Pittsburgh, PA). Digital photographs of H&E-stained slides, immunohistochemistry, and whole mounts were taken using the Nikon Eclipse E800M microscope setup with Nikon DMX1200 camera and software (Nikon Instruments, Inc., Melville, NY).
RNA Isolation and Analysis of Transgene Tetop-ER-α Transgene by Reverse Transcriptase (RT)-PCR
Mammary adenocarcinoma specimens were snap-frozen in liquid nitrogen at the time of dissection. Total RNA was isolated from mammary tissue samples by Trizol extraction (Invitrogen, Carlsbad, CA), quantified on a spectrophotometer, and cDNA synthesis performed. 8 Total RNA from HC11 cells transiently transfected with both the MMTV-tTA and tetop-ER-α expression vectors was used as a positive control. Expression of endogenous β-actin mRNA was measured as a control for RNA integrity and cDNA synthesis. RT-PCR primers and conditions used to detect expression from the tetop-ER-α transgene and endogenous β-actin were published previously. 8
Protein Extraction, Immunoprecipitation, and Western Blot Analysis
Frozen mammary adenocarcinoma was homogenized in protein lysis buffer to extract whole proteins. Protein concentration was quantified by BCA protein assay (Pierce, Rockford, IL). Protein (1100 μg) was immunoprecipitated using Protein A/G Plus Agarose (sc-2003; Santa Cruz, Santa Cruz, CA) with 4.9 μg of the anti-FLAG M2 mouse monoclonal antibody (F3165; Sigma, St. Louis, MO) and was allowed to proceed overnight at 4°C. The agarose beads were washed with lysis buffer three times, resuspended in Laemmli sample buffer (161-0737; Bio-Rad Laboratories, Hercules, CA), boiled for 3 minutes, and spun down. The eluate was fractionated on precast 8% Tris-glycine gels (EC6015; Invitrogen Life Technologies, Carlsbad, CA) and transferred onto polyvinylidene difluoride membranes for Western blot analysis. Membranes were blocked in 4% nonfat dry milk in Tris-buffered saline and 1% Tween (TBS-T) overnight at 4°C, exposed to a 1:200 dilution of anti-ER-α (MC-20) rabbit polyclonal antibody (sc-542, Santa Cruz) for 1 hour, washed with TBS-T, and exposed to a 1:2000 dilution of anti-rabbit secondary antibody (sc-2004, Santa Cruz) for 1 hour at room temperature. Protein expression was visualized with the enhanced chemiluminescence plus Western blotting detection kit (RPN2133; Amersham Biosciences, Piscataway, NJ). The membrane was then stripped and reprobed with a 1:400 dilution of anti-FLAG antibody for 1.5 hours, washed with TBS-T, exposed to a 1:2000 dilution of mouse secondary antibody (sc-2005, Santa Cruz) for 1 hour at room temperature, and visualized as above. A salivary adenocarcinoma from a tTA/TAg mouse without the ER-α transgene was included as a negative control for the FLAG immunoprecipitation.
ER Steroid-Binding Assay
Lysates were prepared from fresh mammary adenocarcinoma (tTA/TAg/ER-α), salivary adenocarcinoma (tTA/TAg and tTA/TAg/ER-α), and uterus (nontransgenic) by homogenization in lysis buffer (10 mmol/L Tris, pH 7.4, 1 mmol/L ethylenediaminetetraacetic acid, 500 mmol/L NaCl, 1 mmol/L dithiothreitol, 10% glycerol) with protease inhibitors (1 μg/ml leupeptin, 7.7 μg/μl aprotinin, 25 μg/μl pefabloc, and 0.1 μg/μl pepstatin). The lysates were cleared by centrifugation at 100,000 × g for 30 minutes. Protein concentrations were determined by Bradford assay and samples of the lysate were incubated overnight at 4°C with 10 nmol/L of 3H-estradiol in the absence and presence of 100-fold molar excess unlabeled diethylstilbestrol. Binding was assayed using the dextran-coated charcoal assay as described previously. 11 Briefly, dextran-coated charcoal was added to adsorb free hormone and then pelleted by centrifugation. Aliquots of supernatant were removed and counted in 10 ml of liquid scintillation fluid in a liquid scintillation counter (Beckman Coulter, Inc., Fullerton, CA). Results are expressed as fmol/l/mg of protein. The mean and SD of duplicate assays are shown.
Mammary Adenocarcinoma Explants in Nude Mice
At the time of necropsy, a portion of the tumor, confirmed to be undifferentiated mammary adenocarcinoma on examination of histology on H&E sections, was cut into 1-mm3 pieces for explant into athymic ovariectomized nude mice. The nude mice (n = 10) were anesthetized by isofluorane-oxygen inhalation and placed on their backs. Their flanks were sanitized and a small incision was made between the nipples of the second and third mammary gland and a small pocket prepared into which the tumor pieces were inserted. Explants were placed on both the left and right sides of the mice (mean tumor burden: two tumor explants/mouse). After implanting the mammary adenocarcinoma pieces, half the mice (n = 5) were implanted with estradiol pellets (0.72 mg/pellet, 60-day release) (Innovative Research of America, Sarasota, FL) subcutaneously into the cervical interscapular space, the mice were removed from the anesthesia, and observed until they had recovered. Aseptic technique was used throughout and all procedures were approved by the Georgetown University Institutional Animal Use and Care Committee. Tumor volume was determined by daily measurement of the length, width, and height of the explanted tumor for 42 days to determine differences in growth, with (n = 10 tumors in five mice) and without exogenous estradiol (n = 9 tumors in five mice). Explant tumor measurements for one of the tumors implanted in a mouse without exogenous estrogen could not be determined because the explant did not grow and this site was excluded from the analysis of tumor growth rates. The experiment was terminated at day 42 because some of the explanted tumors began to display signs of necrosis, regardless of treatment condition, at this time point. All mice were necropsied between day 42 and day 60 after implantation and explant tumors examined for signs of necrosis. Tumors with significant degrees of necrosis demonstrated either a progressive decrease or a stable volume throughout time.
Statistical Analyses
Means and standard deviations were calculated using SPSS, version 11.0.0 (SPSS, Inc., Chicago, IL). Pearson chi-square analyses were performed to compare the prevalence of mammary hyperplasia, dysplasia, and HANs at 4, 7, and 11 months of age and the incidence of mammary adenocarcinomas, salivary adenocarcinomas, and lymphomas in triple-transgenic tTA/TAg/ER-α and double-transgenic tTA/TAg mice (SPSS, version 11.0.0). Student’s t-tests were used to compare tumor explant volumes in nude mice with and without estradiol treatment (SPSS, version 11.0.0).
Results
Development of Mammary Adenocarcinomas, Ductal Hyperplasia and Dysplasia, and HANs in tTA/TAg/ER-α Mice
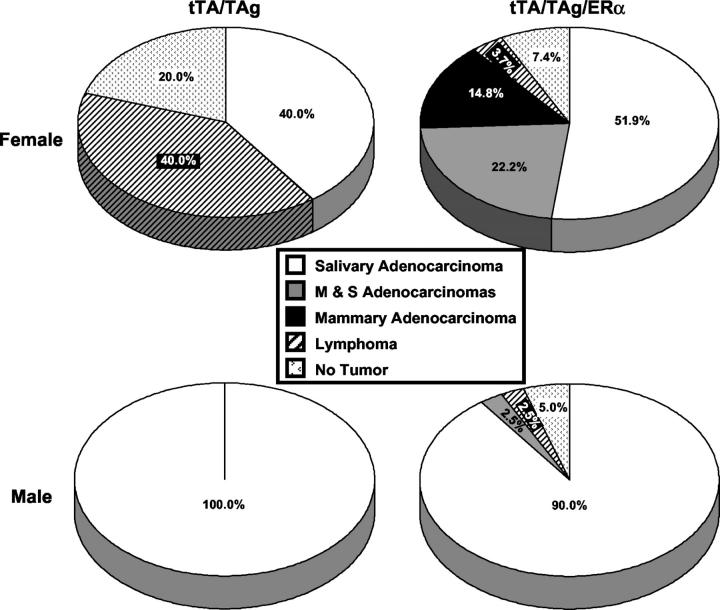
Undifferentiated mammary adenocarcinomas developed in 37.0% of tTA/TAg/ER-α female mice with a mean latency of 11 months of age (341 ± 16 days) and a mean tumor burden of one tumor per mouse (4 of 10 tTA/TAg/ER-α females developed two tumors) (Figure 1, A to D ▶ , and Figure 2 ▶ ). Of the 10 female tTA/TAg/ER-α mice that developed mammary tumors, 4 developed mammary adenocarcinomas only and 6 developed both mammary and salivary adenocarcinomas. Infiltration of nonencapsulated cancer cells into the surrounding stroma, as well as collections of small, darkly stained cells consistent with lymphocytes were found (Figure 1B) ▶ . Mammary adenocarcinomas also exhibited fibrous stroma (Figure 1C) ▶ and desmoplasia (Figure 1D) ▶ . All animals were euthanized when the tumor diameter reached 1 cm3. At the time the mice were euthanized no gross metastasis to the lung, liver, or other sites was found. No mammary tumors developed in negative control double-transgenic tTA/TAg mice (Figure 2) ▶ .
Figure 1.
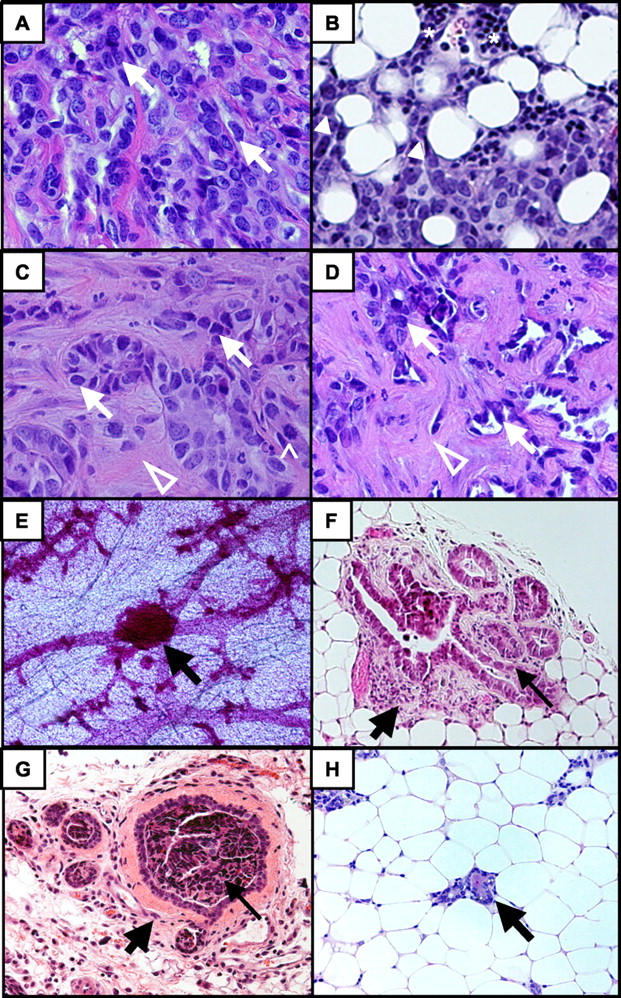
tTA/TAg/ER-α mice but not tTA/TAg mice developed mammary adenocarcinomas, HANs, and mammary hyperplasia. Representative examples of undifferentiated mammary adenocarcinoma (A–D), HAN (E), and mammary hyperplasia (F and G). A: Undifferentiated mammary cancer cells are indicated by white arrows. B: Infiltration of cancer cells (solid arrowheads) into adjacent adipose tissue. Asterisks indicate lymphocytes. C: Cancer cells (white arrows) embedded within a fibrous stroma (open arrowhead). ^, Mitotic figure. D: Cancer cells (white arrows) surrounded by desmoplasia (open white arrowhead). E: HAN (black arrow) in a mammary gland whole mount. F and G: Mammary hyperplasia (thin black arrows) with peri-ductal fibrosis (thick black arrows). H: Normal mammary ductal structure (black arrow) in a negative control tTA/TAg mouse for comparison to F and G. Original magnifications: ×40 (A–C); ×20 (D, F–H); ×4 (E).
Figure 2.
Tumor spectrum is changed in tTA/TAg/ER-α transgenic mice. Mammary adenocarcinomas developed only in triple-transgenic tTA/TAg/ER-α mice (females, n = 10; males, n = 1). Significantly fewer triple-transgenic females develop lymphomas than double-transgenic females (*, P = 0.014; chi-square test). M & S, development of both mammary and salivary adenocarcinomas.
Preneoplastic changes including HANs (Figure 1E) ▶ and mammary ductal hyperplasia (Figure 1, F and G) ▶ were present. Ductal hyperplasia and dysplasia, identified by examination of H&E-stained sections, appeared as early as 4 months of age. The percentage of mice demonstrating these lesions was significantly higher in older mice (11 ± 0.5 months) as compared to 4 and 7 months of age (4 months, 38%; 7 months, 62%; 11 ± 0.5 months, 84%; P = 0.036, chi-square). Similarly, HANs, identified on mammary gland whole mount analysis, were also present as early as 4 months of age in a percentage of the mice (4 months, 11%; 7 months, 22%; 11 ± 0.5 months, 32%; P = 0.438, chi-square). Both the histology and the timing of appearance of these abnormalities at earlier (4 and 7 months of age) time points than the adenocarcinomas (11 ± 0.5 months) were consistent with their representation as preneoplastic lesions. Mammary glands from double-transgenic-negative control tTA/TAg mice demonstrated normal histology (Figure 1H) ▶ and no HANs were found on mammary gland whole mount analysis.
Mammary adenocarcinomas and mammary ductal tissue from tTA/TAg/ER-α female mice expressed nuclear localized ER-α protein (Figure 3 ▶ ; A, C, E, and G). ER-α transgene-specific mRNA (Figure 3I) ▶ and protein (Figure 3J) ▶ expression in the adenocarcinomas was confirmed by RT-PCR and immunoprecipitation/Western blot assays. PCNA immunohistochemistry was consistent with a relative increase in proliferative rates in the adenocarcinomas (Figure 3 ▶ ; B, D, and F) as compared to the ductal tissue (Figure 3H) ▶ and mitotic figures were identified in the adenocarcinomas (Figure 1C) ▶ .
Figure 3.
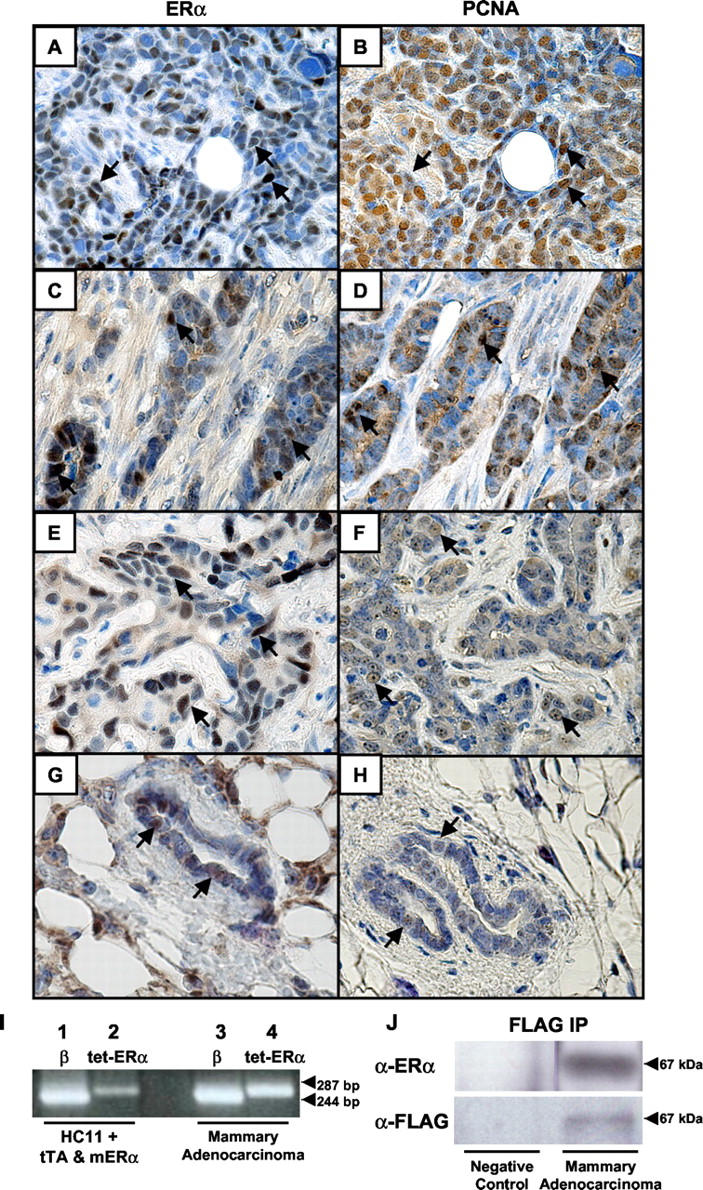
Expression of ER-α in tTA/TAg/ER-α mammary adenocarcinomas. ER-α immunohistochemistry was performed on undifferentiated mammary adenocarcinomas (A, C, and E) and mammary ductal tissue from tTA/TAg/ER-α mice (G). PCNA immunohistochemistry was performed on the same mammary adenocarcinoma (B, D, and F) and ductal tissue (H). Representative MECs with either nuclear-localized ER-α or PCNA are indicated by black arrows. I: Expression of the tetop-ER-α transgene (287 bp) in a mammary adenocarcinoma (lane 4) detected by RT-PCR. Positive control: HC11 cells transiently transfected with MMTV-tTA and tetop-ER-α expression vectors (lane 2). β-actin RT-PCR (lanes 1 and 3) was performed as a control for RNA integrity and cDNA synthesis. β, β-actin; tet-ER-α, tetop-ER-α transgene. J: Detection of FLAG-tagged ER-α protein by immunoprecipitation/Western blotting in the mammary adenocarcinoma shown in A. Anti-FLAG immunoprecipitation was followed by Western blotting with an anti-ER-α antibody (top right). The blot was stripped and reprobed with an anti-FLAG antibody (bottom right). Negative control: salivary adenocarcinoma tissue from a tTA/TAg double-transgenic mouse (left). Original magnifications, ×40.
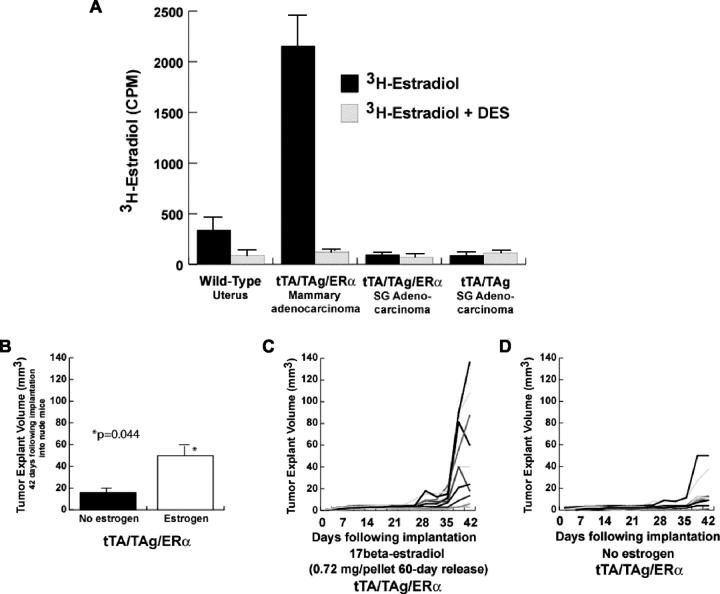
ER steroid-binding functional assays performed on the adenocarcinoma tissue demonstrated estradiol binding (Figure 4A) ▶ . To test if tumor growth was enhanced by estrogen exposure, explants from mammary adenocarcinomas were implanted into athymic ovariectomized nude mice. Mean tumor explant volume was significantly greater in the presence of estradiol as compared to explants without estradiol exposure at day 42 after implantation (P = 0.044, t-test) (Figure 4B) ▶ . The two explants exposed to estradiol that showed a decrease in tumor volume (Figure 4C) ▶ and the one nonexposed explant (Figure 4D) ▶ that showed a stable tumor volume at day 42 were found to be necrotic at the time of necropsy.
Figure 4.
Mammary adenocarcinomas in tTA/TAg/ER-α mice bind estrogen and are estrogen-responsive. A: Protein lysates from a nontransgenic uterus, a tTA/TAg/ER-α mammary adenocarcinoma, and salivary adenocarcinomas (tTA/TAg and tTA/TAg/ER-α) were subjected to ER steroid-binding assays. Uterus from a nontransgenic mouse was used as a positive control for binding. Data are represented as the mean of two replicates with SD error bars. B–D: Explants from a tTA/TAg/ER-α mammary adenocarcinoma were implanted into athymic ovariectomized nude mice in the presence (n = 10) and absence (n = 9) of exogenous estradiol. B: Explant tumor volume was increased significantly in mice exposed to estradiol at day 42 after implantation (Student’s t-test, P = 0.044). Bars indicate mean ± SEM. C: Individual explant volumes graphed throughout the time of observation in the presence of exogenous estradiol. D: Individual explant volumes graphed throughout the time of observation in the absence of exogenous estradiol.
Impact of ER-α-Dominant Gain on Tumor Spectrum
The tumor spectrum in the tTA/TAg model was altered significantly in female but not in male mice by the introduction of ER-α (Figure 2) ▶ . Only triple-transgenic tTA/TAg/ER-α mice developed mammary adenocarcinomas. Significantly fewer tTA/TAg/ER-α females developed lymphomas (4%) as compared to tTA/TAg female mice (40%) (P = 0.014, chi-square). Because the hallmark of the tTA/TAg mouse model is development of salivary adenocarcinomas, 9 tTA/TAg/ER-α mice were observed for growth of these tumors. ER-α expression had no effect on the incidence of salivary adenocarcinoma development because the percentages of salivary adenocarcinomas in the tTA/TAg/ER-α mice did not differ significantly from the percentage of salivary adenocarcinomas in the negative control (tTA/TAg) mice.
Discussion
The introduction of ER-α into the tTA/TAg mouse model exerted tissue-specific effects: the new appearance of mammary adenocarcinomas, a reduction in the incidence of lymphomas, and no change in either incidence or development of salivary adenocarcinomas. The development of an estrogen-responsive ER-α-positive conditional transgenic mouse model of breast cancer with histology that resembles human breast adenocarcinomas is novel. Although the adenocarcinomas that develop in some other transgenic mouse models do contain ER, none described so far have exhibited any responsiveness to estrogen stimulation. 12 However human breast cancers are predominantly ER-α-positive and estrogen responsive. In the future, the conditional tetop-ER-α transgene can be used in combination with other transgenic mouse models of mammary cancer to determine the impact of ER-α signaling pathways on mammary cancer initiated by specific oncogenes such as c-myc, 13 ErbB2, 14 and WNT. 15 Transgenic mice that express ER-α under control of the highly MEC-specific MMTV-rtTA transgene 16 and a promoter that targets to both epithelial and stromal cells 17 can be used to investigate the impact of ER-α-dominant gain in different tissue compartments. The combination of these models with mammary gland transplant techniques will enable investigators to isolate local and systemic effects. 18 Other tissue-specific tetracycline-responsive promoter constructs can be used to target conditional ER-α expression to other specific tissues.
The decreased incidence of lymphoma in the tTA/TAg/ER-α female mice is consistent with the role of estrogen and ER in modulating lymphocyte differentiation, maturation, and function. Estrogen can suppress lymphocyte mitogenesis through ER-α in B- and T-cell lymphopoiesis through an ER-α-specific mechanism in the marrow microenvironment. 19-21
In summary, we report the development of a novel conditional transgenic mouse model of estrogen-responsive mammary adenocarcinoma. Introduction of ER-α expression into specific tissues using the tetracycline-responsive system in transgenic mice can be used to study the effects of dominant gain of ER-α on carcinogenesis in a specifically targeted organ.
Acknowledgments
We thank the Animal Research and the Histopathology Shared Resources at the Lombardi Cancer Center for assistance [this resource is supported in part by a cancer center support grant from the National Cancer Institute (P30-CA51008)]; and Eric Osgood for his technical assistance.
Footnotes
Address reprint requests to Priscilla A. Furth, M.D., Lombardi Cancer Center, Department of Oncology, Georgetown University, Research Building, Room E518, 3970 Reservoir Rd., NW, Washington, DC 20057. E-mail: paf3@georgetown.edu.
Supported by the National Institutes of Health (RO1, HD38955 to J. A. F.); the National Cancer Institute (R01, CA89041 to P. A. F.); the Department of Defense (DAMD 17-01-1-0310 to P. A. F.); and the Women’s Health Research Group, University of Maryland, Baltimore (research project grant to M. T. T.).
Research was performed at the Lombardi Cancer Center, Department of Oncology, Georgetown University, Washington, DC.
References
- 1.Russo J, Hu HF, Yang X, Russo IH: Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr 2000, 27:17-37 [DOI] [PubMed] [Google Scholar]
- 2.Henderson BE, Feigelson HS: Hormonal carcinogenesis. Carcinogenesis 2000, 21:427-433 [DOI] [PubMed] [Google Scholar]
- 3.Shoker BS, Jarvis C, Sibson DR, Walker C, Sloane JP: Oestrogen receptor expression in the normal and pre-cancerous breast. J Pathol 1999, 188:237-244 [DOI] [PubMed] [Google Scholar]
- 4.Bundred NJ: Prognostic and predictive factors in breast cancer. Cancer Treat Rev 2001, 27:137-142 [DOI] [PubMed] [Google Scholar]
- 5.McGuire WL: Hormone receptors: their role in predicting prognosis and response to endocrine therapy. Semin Oncol 1978, 5:428-433 [PubMed] [Google Scholar]
- 6.Jordan VC: Molecular mechanisms of antiestrogen action in breast cancer. Breast Cancer Res Treat 1994, 31:41-52 [DOI] [PubMed] [Google Scholar]
- 7.Russo IH, Russo J: Role of hormones in mammary cancer initiation and progression. J Mammary Gland Biol Neoplasia 1998, 3:49-61 [DOI] [PubMed] [Google Scholar]
- 8.Hruska KS, Tilli MT, Ren S, Cotarla I, Kwong T, Li M, Fondell JD, Hewitt JA, Koos RD, Furth PA, Flaws JA: Conditional over-expression of estrogen receptor alpha in a transgenic mouse model. Transgenic Res 2002, 11:361-372 [DOI] [PubMed] [Google Scholar]
- 9.Ewald D, Li M, Efrat S, Auer G, Wall RJ, Furth PA, Hennighausen L: Time-sensitive reversal of hyperplasia in transgenic mice expressing SV40 T antigen. Science 1996, 273:1384-1386 [DOI] [PubMed] [Google Scholar]
- 10.Ren S, Li M, Cai H, Hudgins S, Furth PA: A simplified method to prepare PCR template DNA for screening of transgenic and knockout mice. Contemp Top Lab Anim Sci 2001, 40:27-30 [PubMed] [Google Scholar]
- 11.Edwards DP, McGuire WL: 17 Alpha-estradiol is a biologically active estrogen in human breast cancer cells in tissue culture. Endocrinology 1980, 107:884-891 [DOI] [PubMed] [Google Scholar]
- 12.Hennighausen L: Mouse models for breast cancer. Oncogene 2000, 19:966-967 [DOI] [PubMed] [Google Scholar]
- 13.D’Cruz CM, Gunther EJ, Boxer RB, Hartman JL, Sintasath L, Moody SE, Cox JD, Ha SI, Belka GK, Golant A, Cardiff RD, Chodosh LA: c-MYC induces mammary tumorigenesis by means of a preferred pathway involving spontaneous Kras2 mutations. Nat Med 2001, 7:235-239 [DOI] [PubMed] [Google Scholar]
- 14.Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, Innocent N, Cardiff RD, Schnall MD, Chodosh LA: Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell 2002, 2:451-461 [DOI] [PubMed] [Google Scholar]
- 15.Gunther EJ, Moody SE, Belka GK, Hahn KT, Innocent N, Dugan KD, Cardiff RD, Chodosh LA: Impact of p53 loss on reversal and recurrence of conditional Wnt-induced tumorigenesis. Genes Dev 2003, 17:488-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunther EJ, Belka GK, Wertheim GB, Wang J, Hartman JL, Boxer RB, Chodosh LA: A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. EMBO J 2002, 16:283-292 [DOI] [PubMed] [Google Scholar]
- 17.Shockett P, Difilippantonio M, Hellman N, Schatz DG: A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc Natl Acad Sci USA 1995, 92:6522-6526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furth PA: Conditional control of gene expression in the mammary gland. J Mammary Gland Biol Neoplasia 1997, 2:373-383 [DOI] [PubMed] [Google Scholar]
- 19.Smithson G, Medina K, Ponting I, Kincade PW: Estrogen suppresses stromal cell-dependent lymphopoiesis in culture. J Immunol 1995, 155:3409-3417 [PubMed] [Google Scholar]
- 20.Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW: The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J Immunol 1998, 161:27-34 [PubMed] [Google Scholar]
- 21.Sakazaki H, Ueno H, Nakamuro K: Estrogen receptor alpha in mouse splenic lymphocytes: possible involvement in immunity. Toxicol Lett 2002, 133:221-229 [DOI] [PubMed] [Google Scholar]