Abstract
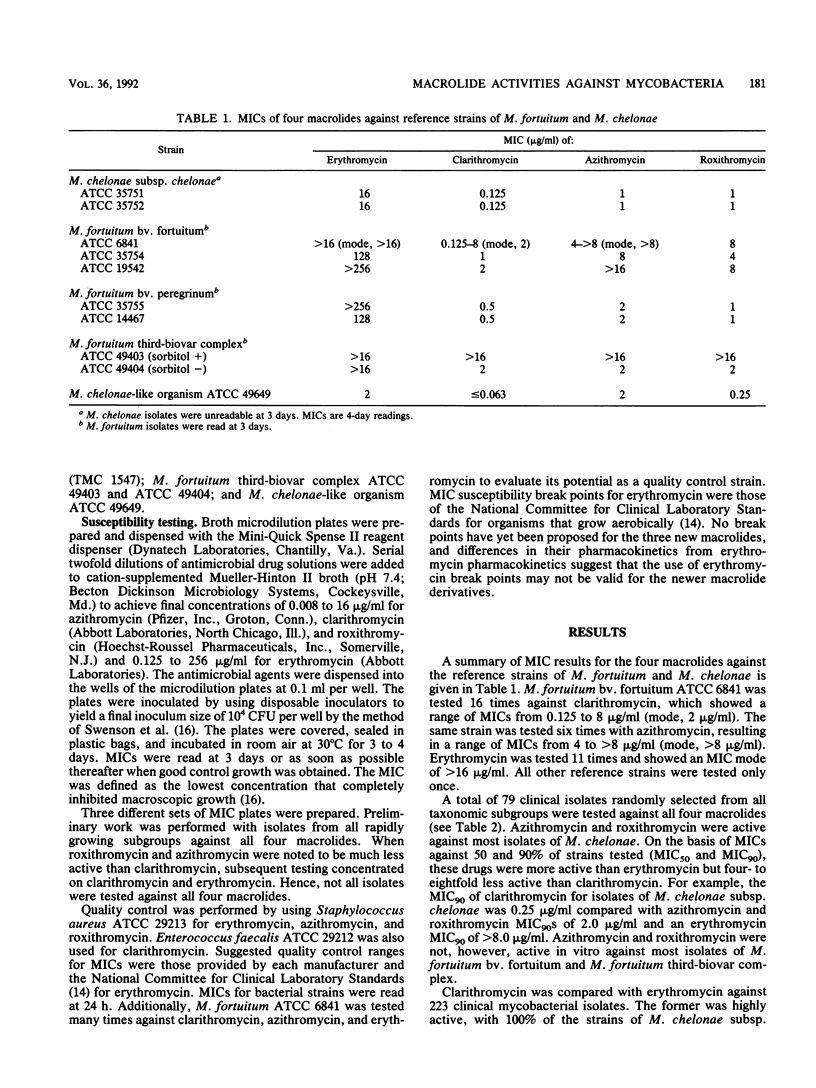
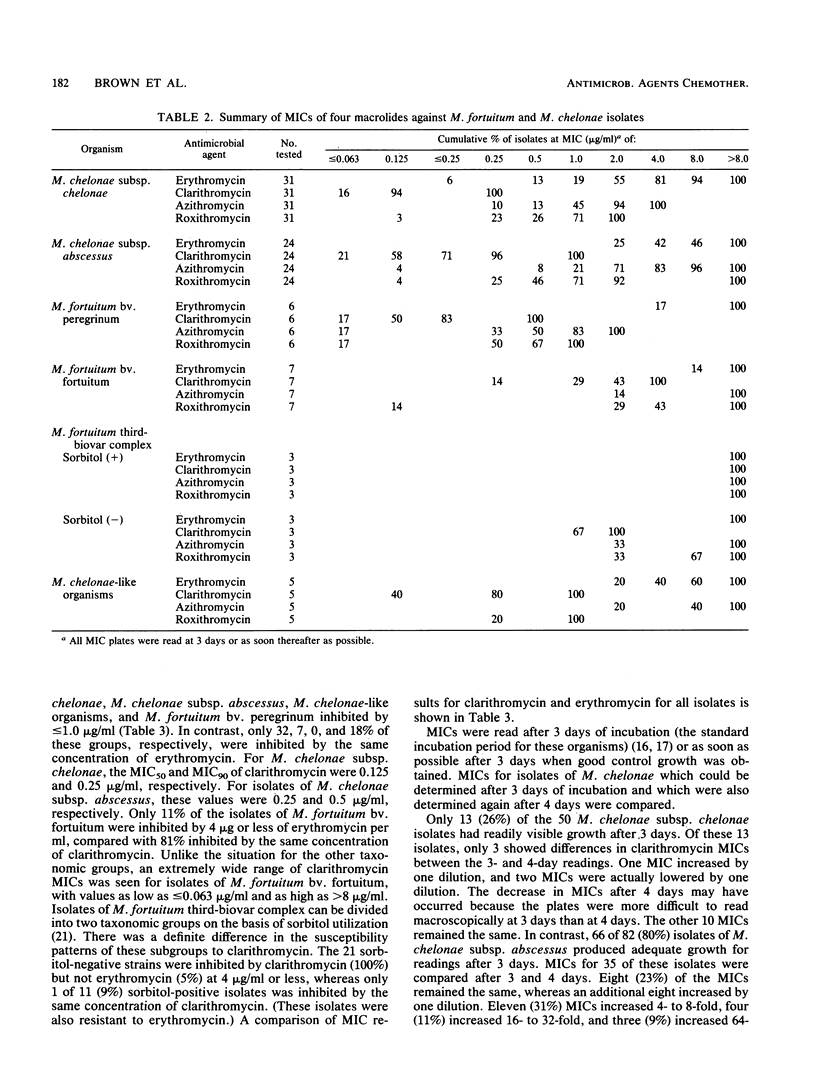
Susceptibilities to erythromycin by broth microdilution were compared with those to the newer macrolide clarithromycin for 223 isolates of rapidly growing mycobacteria belonging to seven taxonomic groups. Seventy-nine random isolates were also tested against azithromycin and roxithromycin. The MIC of clarithromycin for 90% of strains tested (MIC90) was 0.25 microgram/ml for isolates of Mycobacterium chelonae subsp. chelonae and 0.5 microgram/ml for M. chelonae subsp. abscessus, with 100% of strains inhibited by less than or equal to 1 microgram/ml. Clarithromycin was 10 to 50 times more active than erythromycin and four- to eightfold more active than the other newer macrolides against M. chelonae. MICs of clarithromycin frequently increased with prolonged incubation with isolates of M. chelonae subsp. abscessus but not M. chelonae subsp. chelonae. MICs of clarithromycin were much higher for M. fortuitum bv. fortuitum (MIC50, 2.0 microgram/ml; MIC90, greater than 8.0 microgram/ml). The three newer macrolides had comparable activity against M. fortuitum bv. peregrinum (MIC90s of 0.5 to 2.0 microgram/ml compared with erythromycin MIC90s of greater than 8.0 microgram/ml). Overall, clarithromycin was the most active agent, inhibiting all isolates of M. chelonae subsp. chelonae, M. chelonae subsp. abscessus, M. fortuitum bv. peregrinum, and the M. chelonae-like organisms and 35% of M. fortuitum bv. fortuitum at less than or equal to 1 microgram/ml. Clinical trials of the newer macrolides, especially clarithromycin, against these environmental mycobacterial species appear to be warranted.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Jones R. N., Thornsberry C. In vitro activities of azithromycin (CP 62,993), clarithromycin (A-56268; TE-031), erythromycin, roxithromycin, and clindamycin. Antimicrob Agents Chemother. 1988 May;32(5):752–754. doi: 10.1128/aac.32.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin O. G., Young L. S., Floyd-Reising S. A., Bruckner D. A. Comparative in vitro activity of the new macrolide A-56268 against mycobacteria. Eur J Clin Microbiol. 1987 Aug;6(4):486–487. doi: 10.1007/BF02013117. [DOI] [PubMed] [Google Scholar]
- Dalovisio J. R., Pankey G. A., Wallace R. J., Jones D. B. Clinical usefulness of amikacin and doxycycline in the treatment of infection due to Mycobacterium fortuitum and Mycobacterium chelonei. Rev Infect Dis. 1981 Sep-Oct;3(5):1068–1074. doi: 10.1093/clinids/3.5.1068. [DOI] [PubMed] [Google Scholar]
- Dautzenberg B., Truffot C., Legris S., Meyohas M. C., Berlie H. C., Mercat A., Chevret S., Grosset J. Activity of clarithromycin against Mycobacterium avium infection in patients with the acquired immune deficiency syndrome. A controlled clinical trial. Am Rev Respir Dis. 1991 Sep;144(3 Pt 1):564–569. doi: 10.1164/ajrccm/144.3_Pt_1.564. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Bailer R., Swanson R., Hanson C. W., McDonald E., Ramer N., Hardy D., Shipkowitz N., Bower R. R., Gade E. In vitro and in vivo evaluation of A-56268 (TE-031), a new macrolide. Antimicrob Agents Chemother. 1986 Dec;30(6):865–873. doi: 10.1128/aac.30.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P. B., Hardy D. J., McDaniel D., Hanson C. W., Swanson R. N. In vitro and in vivo activities of clarithromycin against Mycobacterium avium. Antimicrob Agents Chemother. 1989 Sep;33(9):1531–1534. doi: 10.1128/aac.33.9.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraschini F., Scaglione F., Pintucci G., Maccarinelli G., Dugnani S., Demartini G. The diffusion of clarithromycin and roxithromycin into nasal mucosa, tonsil and lung in humans. J Antimicrob Chemother. 1991 Feb;27 (Suppl A):61–65. doi: 10.1093/jac/27.suppl_a.61. [DOI] [PubMed] [Google Scholar]
- Lowry P. W., Jarvis W. R., Oberle A. D., Bland L. A., Silberman R., Bocchini J. A., Jr, Dean H. D., Swenson J. M., Wallace R. J., Jr Mycobacterium chelonae causing otitis media in an ear-nose-and-throat practice. N Engl J Med. 1988 Oct 13;319(15):978–982. doi: 10.1056/NEJM198810133191504. [DOI] [PubMed] [Google Scholar]
- Meisler D. M., Friedlaender M. H., Okumoto M. Mycobacterium chelonei keratitis. Am J Ophthalmol. 1982 Sep;94(3):398–401. doi: 10.1016/0002-9394(82)90368-3. [DOI] [PubMed] [Google Scholar]
- Naik S., Ruck R. In vitro activities of several new macrolide antibiotics against Mycobacterium avium complex. Antimicrob Agents Chemother. 1989 Sep;33(9):1614–1616. doi: 10.1128/aac.33.9.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silcox V. A., Good R. C., Floyd M. M. Identification of clinically significant Mycobacterium fortuitum complex isolates. J Clin Microbiol. 1981 Dec;14(6):686–691. doi: 10.1128/jcm.14.6.686-691.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Thornsberry C., Silcox V. A. Rapidly growing mycobacteria: testing of susceptibility to 34 antimicrobial agents by broth microdilution. Antimicrob Agents Chemother. 1982 Aug;22(2):186–192. doi: 10.1128/aac.22.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Wallace R. J., Jr, Silcox V. A., Thornsberry C. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985 Dec;28(6):807–811. doi: 10.1128/aac.28.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Brown B. A., Onyi G. O. Susceptibilities of Mycobacterium fortuitum biovar. fortuitum and the two subgroups of Mycobacterium chelonae to imipenem, cefmetazole, cefoxitin, and amoxicillin-clavulanic acid. Antimicrob Agents Chemother. 1991 Apr;35(4):773–775. doi: 10.1128/aac.35.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Brown B. A., Silcox V. A., Tsukamura M., Nash D. R., Steele L. C., Steingrube V. A., Smith J., Sumter G., Zhang Y. S. Clinical disease, drug susceptibility, and biochemical patterns of the unnamed third biovariant complex of Mycobacterium fortuitum. J Infect Dis. 1991 Mar;163(3):598–603. doi: 10.1093/infdis/163.3.598. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr Diagnostic and therapeutic consideration in patients with pulmonary disease due to the rapidly growing mycobacteria. Semin Respir Infect. 1986 Dec;1(4):230–233. [PubMed] [Google Scholar]
- Wallace R. J., Jr, Steele L. C., Labidi A., Silcox V. A. Heterogeneity among isolates of rapidly growing mycobacteria responsible for infections following augmentation mammaplasty despite case clustering in Texas and other southern coastal states. J Infect Dis. 1989 Aug;160(2):281–288. doi: 10.1093/infdis/160.2.281. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Swenson J. M., Silcox V. A., Bullen M. G. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J Infect Dis. 1985 Sep;152(3):500–514. doi: 10.1093/infdis/152.3.500. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Swenson J. M., Silcox V. A., Good R. C., Tschen J. A., Stone M. S. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis. 1983 Jul-Aug;5(4):657–679. doi: 10.1093/clinids/5.4.657. [DOI] [PubMed] [Google Scholar]



