Abstract
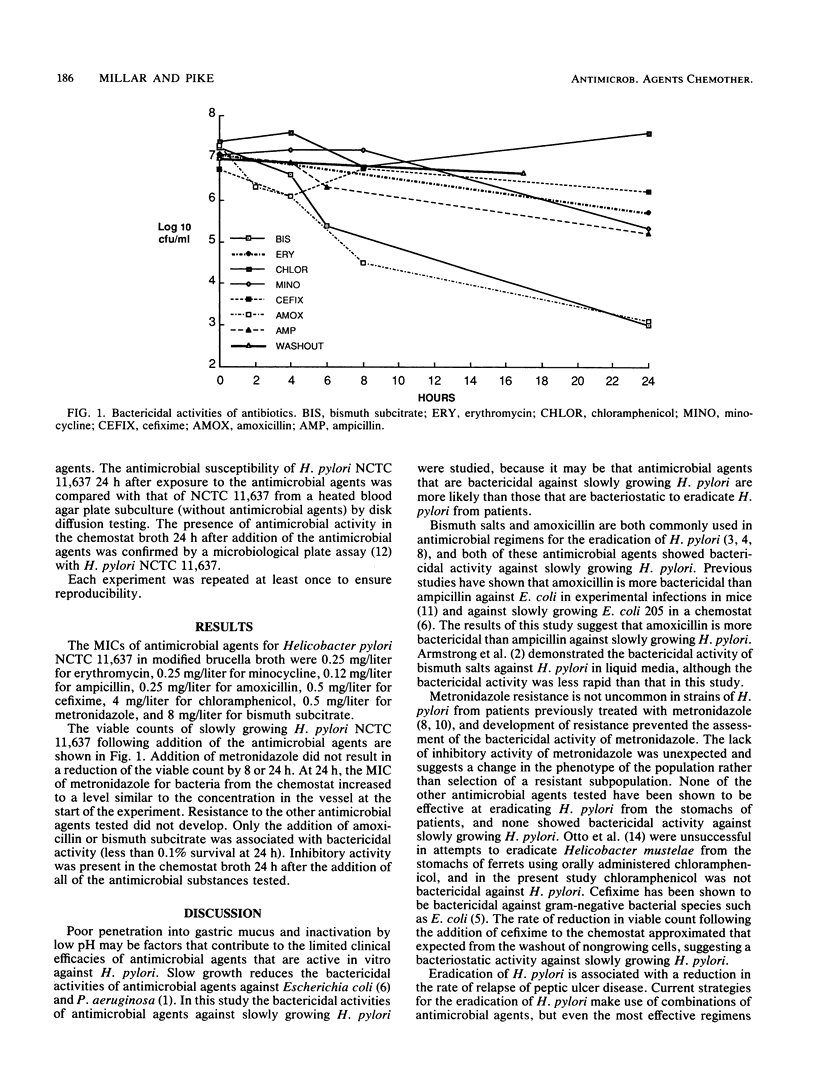
The doubling times of bacteria at sites of colonization or infection are considerably longer than those in laboratory culture media, and slow growth reduces the susceptibility of bacteria to antimicrobial agents. Helicobacter pylori is susceptible to a wide range of antimicrobial agents in vitro; however, tests for inhibitory activity do not adequately predict which antimicrobial agents will eradicate slowly growing H. pylori from the stomachs of patients. The chemostat can be used to compare the bactericidal activities of antimicrobial substances against slowly growing bacteria. In this study we compared the bactericidal activities of antimicrobial agents against slowly growing H. pylori. The bactericidal activities of erythromycin, minocycline, ampicillin, amoxicillin, cefixime, metronidazole, and bismuth subcitrate against slowly growing H. pylori NCTC 11,637 in a chemostat were compared. Antimicrobial agents were added to the system at four to eight times the MIC. Exposure of H. pylori to metronidazole was associated with the rapid development of metronidazole resistance, preventing assessment of the bactericidal activity of metronidazole. Resistance to the other antimicrobial agents tested did not develop. The poor bactericidal activities of the antimicrobial agents against slowly growing H. pylori may be a contributory factor in limiting their clinical efficacies. Of the agents tested, only amoxicillin and bismuth subcitrate showed bactericidal activity against slowly growing H. pylori. The chemostat allows comparison of the bactericidal activities of antimicrobial agents against slowly growing H. pylori and may therefore provide results which more accurately identify those agents or combinations of agents that will eradicate H. pylori from patients.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar H., van Biesen T., Dasgupta M., Lam K., Costerton J. W. Interaction of biofilm bacteria with antibiotics in a novel in vitro chemostat system. Antimicrob Agents Chemother. 1989 Oct;33(10):1824–1826. doi: 10.1128/aac.33.10.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Wee S. H., Goodwin C. S., Wilson D. H. Response of Campylobacter pyloridis to antibiotics, bismuth and an acid-reducing agent in vitro--an ultrastructural study. J Med Microbiol. 1987 Dec;24(4):343–350. doi: 10.1099/00222615-24-4-343. [DOI] [PubMed] [Google Scholar]
- Axon A. R. Duodenal ulcer: the villain unmasked? BMJ. 1991 Apr 20;302(6782):919–921. doi: 10.1136/bmj.302.6782.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain D. C., Scully B. E., Hirose T., Neu H. C. The pharmacokinetic and bactericidal characteristics of oral cefixime. Clin Pharmacol Ther. 1985 Nov;38(5):590–594. doi: 10.1038/clpt.1985.229. [DOI] [PubMed] [Google Scholar]
- Börsch G., Mai U., Müller K. M. Monotherapy or polychemotherapy in the treatment of Campylobacter pylori-related gastroduodenal disease. Scand J Gastroenterol Suppl. 1988;142:101–106. doi: 10.3109/00365528809091722. [DOI] [PubMed] [Google Scholar]
- Cozens R. M., Tuomanen E., Tosch W., Zak O., Suter J., Tomasz A. Evaluation of the bactericidal activity of beta-lactam antibiotics on slowly growing bacteria cultured in the chemostat. Antimicrob Agents Chemother. 1986 May;29(5):797–802. doi: 10.1128/aac.29.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eudy W. W., Burrous S. E. Generation times of Proteus mirabilis and Escherichia coli in experimental infections. Chemotherapy. 1973;19(3):161–170. doi: 10.1159/000221451. [DOI] [PubMed] [Google Scholar]
- Goodwin C. S., Blake P., Blincow E. The minimum inhibitory and bactericidal concentrations of antibiotics and anti-ulcer agents against Campylobacter pyloridis. J Antimicrob Chemother. 1986 Mar;17(3):309–314. doi: 10.1093/jac/17.3.309. [DOI] [PubMed] [Google Scholar]
- Goodwin C. S., Marshall B. J., Blincow E. D., Wilson D. H., Blackbourn S., Phillips M. Prevention of nitroimidazole resistance in Campylobacter pylori by coadministration of colloidal bismuth subcitrate: clinical and in vitro studies. J Clin Pathol. 1988 Feb;41(2):207–210. doi: 10.1136/jcp.41.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P. A., Rolinson G. N., Witting D. A. Comparative activity of amoxycillin and ampicillin in an experimental bacterial infection in mice. Antimicrob Agents Chemother. 1973 Sep;4(3):285–293. doi: 10.1128/aac.4.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maw J., Meynell G. G. The true division and death rates of Salmonella typhimurium in the mouse spleen determined with superinfecting phage P22. Br J Exp Pathol. 1968 Dec;49(6):597–613. [PMC free article] [PubMed] [Google Scholar]
- Otto G., Fox J. G., Wu P. Y., Taylor N. S. Eradication of Helicobacter mustelae from the ferret stomach: an animal model of Helicobacter (Campylobacter) pylori chemotherapy. Antimicrob Agents Chemother. 1990 Jun;34(6):1232–1236. doi: 10.1128/aac.34.6.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauws E. A., Tytgat G. N. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990 May 26;335(8700):1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- Rees W. D., Turnberg L. A. Mechanisms of gastric mucosal protection: a role for the 'mucus-bicarbonate' barrier. Clin Sci (Lond) 1982 Apr;62(4):343–348. doi: 10.1042/cs0620343. [DOI] [PubMed] [Google Scholar]



