Summary
Chromatin-induced spindle assembly depends on regulation of microtubule depolymerizing proteins by the chromosomal passenger complex (CPC), consisting of Incenp, Survivin, Dasra (Borealin) and the kinase Aurora B, but the mechanism and significance of the spatial regulation of Aurora B activity remain unclear. Here we show that the Aurora B pathway is suppressed in the cytoplasm of Xenopus egg extract by phosphatases, but becomes activated by chromatin via a Ran-independent mechanism. While spindle microtubule assembly normally requires Dasra-dependent chromatin binding of the CPC, this function of Dasra can be bypassed by clustering Aurora B-Incenp using anti-Incenp antibodies, which stimulate autoactivation among bound complexes. However, such chromatin-independent Aurora B pathway activation promotes centrosomal microtubule assembly, and produces aberrant achromosomal spindle-like structures. We propose that chromosomal enrichment of the CPC results in local kinase autoactivation, a mechanism that contributes to the spatial regulation of spindle assembly and possibly to other mitotic processes.
Introduction
The microtubule-containing bipolar spindle drives eukaryotic chromosome segregation. Spindle microtubules are assembled from both chromosomes and centrosomes, and it is thought that local control of microtubule assembly by chromosomes is important for robust and accurate bipolar spindle formation [reviewed in (Bastiaens et al., 2006)].
At least three pathways contribute to chromatin-induced spindle assembly, the best characterized involving the small GTPase Ran. At M-phase, chromosomes organize the localized production of Ran-GTP through chromatin-associated RCC1, the Ran guanine nucleotide exchange factor [reviewed in (Harel and Forbes, 2004)]. Peri-chromosomal Ran-GTP then binds to importinβ, inducing the release of bound cargoes such as TPX2, NuMA, NuSAP and Rae1, which promote microtubule assembly (Blower et al., 2005; Gruss et al., 2001; Nachury et al., 2001; Ribbeck et al., 2006; Wiese et al., 2001).
A second pathway involves regulation of the small microtubule destabilizing protein Op18 (Stathmin), the activity of which has been associated with α/β-tubulin dimer sequestration and direct promotion of microtubule catastrophe [reviewed in (Cassimeris, 2002)]. Phosphorylation of Op18 is induced by chromatin at M phase, and at least three different phosphoacceptor sites are involved in inactivation of its microtubule destabilizing activity: serine 16 (S16), serine 25 and serine 39 (Andersen et al., 1997). While the latter two are potential Cdk1 target sites, the physiological mitotic kinase for S16 remains unclear. Recently, Gadea and Ruderman showed that Op18 hyperphosphorylation induced by sperm nuclei depends on Aurora B (Gadea and Ruderman, 2006).
We previously described a third pathway required for chromatin-dependent spindle formation (Sampath et al., 2004), which involves the ‘chromosomal passenger complex’ (CPC). The CPC, which in vertebrates consists of Aurora B, Incenp, Dasra A/B (Borealin), and Survivin (Figure 1A), shows a dynamic localization pattern throughout M-phase, playing important roles in promoting proper kinetochore-microtubule attachment, spindle formation, spindle checkpoint signaling, and cytokinesis (Gadea and Ruderman, 2005; Gadea and Ruderman, 2006; Sampath et al., 2004; Vagnarelli and Earnshaw, 2004). How Aurora B activity can be regulated spatially and otherwise to control such a diverse set of processes nevertheless remains an outstanding question. Such regulation might be achieved through the function of other CPC components; it has been reported that a C-terminal domain of Incenp can allosterically activate Aurora B kinase activity (Bishop and Schumacher, 2002; Honda et al., 2003; Kang et al., 2001; Sessa et al., 2005), while the Dasra proteins and Survivin can regulate Aurora B localization to centromeres (Carvalho et al., 2003; Gassmann et al., 2004; Lens et al., 2003; Romano et al., 2003; Sampath et al., 2004; Vader et al., 2006).
Figure 1. Dasra Proteins are Required for Efficient Binding of the CPC to Chromatin.
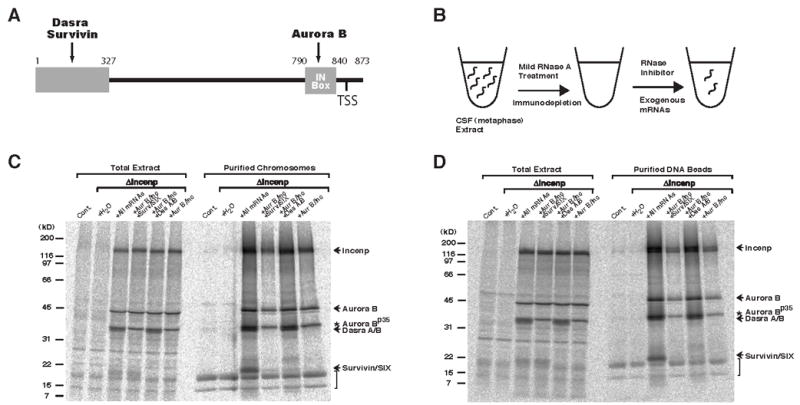
(A) Domain layout of Xenopus Incenp. Binding sites for Aurora B, Dasra and Survivin, as well as location of the TSS-motif are shown. (B) Schematic for the reconstitution of CPC-immunodepleted (ΔIncenp) egg extract with mRNA pools encoding CPC components. Cytostatic factor (CSF)-arrested metaphase extracts were treated with a low dose of RNase A, leading to destruction of endogenous mRNAs while sparing ribosomes. The CPC was then depleted using anti-Incenp antibodies coupled to protein A beads. RNasae inhibitor and pools of exogenous mRNAs were then added. Proteins encoded by exogenous mRNAs were translated during incubation at 20°C.
(C) Dasra proteins are required for efficient CPC binding to metaphase chromosomes. Control, ΔIncenp, or reconstituted ΔIncenp extracts containing sperm nuclei, 35S-methionine, and biotin-dUTP were cycled through interphase to metaphase, and biotinylated chromosomes were purified. The combination of mRNAs is indicated at the top of the lane, where “all mRNAs” represent in vitro transcribed mRNAs of Aurora B, Incenp, Dasra A, Dasra B, Survivin and SIX. Copurification of labeled proteins was analyzed by SDS-PAGE and quantified using a PhosphorImager. Asterisk indicates the Aurora Bp35 species, a product of internal translation initiation produced from exogenous Aurora B mRNA (T. A. M. and S. C. S., unpublished data). Arrow indicates Dasra proteins. Note that copurifed labeled histones (indicated by the bracket) are evident due to incomplete degradation of their abundant mRNAs during RNase treatment, and indicate equal chromosome recovery.
(D) Dasra proteins are required for efficient CPC binding to chromatin beads. Control, ΔIncenp, or reconstituted ΔIncenp extracts containing DNA-beads and 35S-methionine were cycled through interphase to metaphase, and the beads were isolated and analyzed as described above.
Downstream of Aurora B is the microtubule depolymerase MCAK, a kinesin 13 family member which catalytically depolymerizes microtubule ends (Desai et al., 1999b; Hunter et al., 2003). Phosphorylation of MCAK inhibits its microtubule depolymerizing activity, and non-phosphorylatable MCAK mutants are accordingly unable to support bipolar spindle assembly (Andrews et al., 2004; Lan et al., 2004; Ohi et al., 2004).
Here we demonstrate a critical role for chromatin-mediated activation of the Aurora B pathway during spindle assembly. We demonstrate that the Aurora B pathway is normally suppressed in the cytosol of Xenopus egg extract, but becomes activated by chromatin via a Ran-independent mechanism. Using an mRNA-dependent system for CPC reconstitution, we further show that Dasra A is required for loading of the CPC onto chromatin, and therefore chromatin-dependent activation of the Aurora B pathway. Surprisingly, we find that antibodies recognizing the C-terminus of Incenp cluster the CPC and can effectively bypass the requirement for Dasra proteins in spindle microtubule assembly. This activation of the CPC leads to the formation of abnormal spindle-like structures, including ‘achromosomal’ spindle formation by centrosomes. Our data demonstrate that clustering of the CPC by chromatin activates the Aurora B pathway, which plays a key role in confining spindle assembly to the vicinity of chromosomes.
Results
A System for Reconstitution of the CPC in Xenopus Egg Extracts
We previously described the identification of Dasra A and Dasra B, two new components of the vertebrate chromosomal passenger complex (CPC), and reported that immunodepletion of the CPC from Xenopus egg extract using anti-Incenp antibodies leads to failure of bipolar spindle formation around M-phase chromatin (Sampath et al., 2004). To test the function of individual CPC components, we adapted the previously described ‘mRNA-dependent’ extract system (Murray and Kirschner, 1989) (Figure 1B). For CPC reconstitution, pools of in vitro transcribed CPC mRNAs were added to RNase A treated, CPC-depleted (ΔIncenp) extracts. Labeling of the reconstituted extracts with 35S-methionine demonstrated efficient translation of all exogenous CPC mRNAs (Figures 1C and 1D, Total Extract), including Aurora B, Incenp, Dasra A, Dasra B, Survivin, and SIX, a second Survivin-related protein in Xenopus [Figure S1; (Song et al., 2003)]. Immunoblots with antibodies recognizing Incenp, Aurora B, Dasra A, and Survivin indicated that all of these proteins were reconstituted to approximately endogenous levels (data not shown, but see Figures 2C and 5B).
Figure 2. Dasra A Is Required for Spindle Assembly.
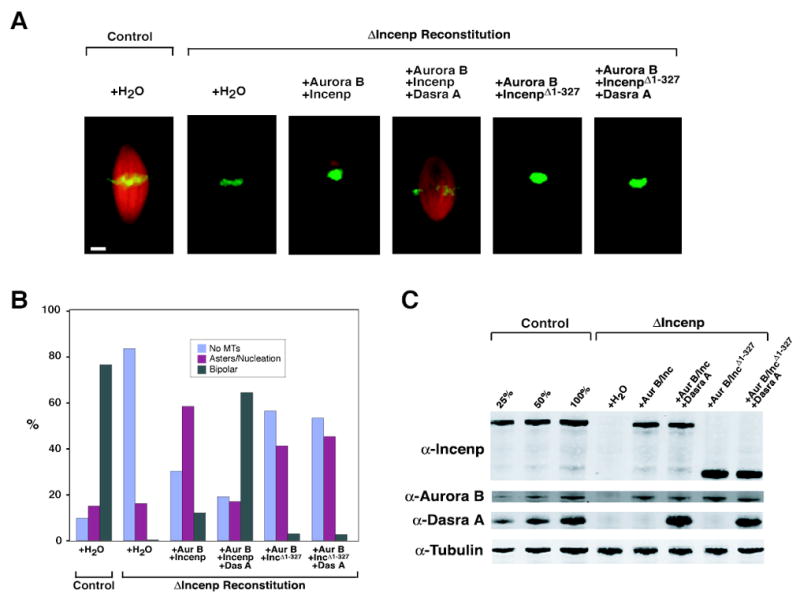
(A) Spindle assembly in reconstituted egg extracts. Control or ΔIncenp extracts were reconstituted as indicated, cycled through interphase to metaphase with sperm nuclei, and rhodamine-tubulin (red) was added to visualize microtubules; extracts were fixed 60 min after entry into metaphase. Hoechst 33258 (green) was used to visualize DNA. Bar, 10 μm.
(B) Quantitation of structures formed in the extracts described in (A). At least 200 chromosome-containing samples were analyzed per sample.
(C) Western blot of samples from (A).
Figure 5. Anti-Incenp Antibodies Bypass the Requirement of Dasra A in Spindle Microtubule Assembly and Induce Aberrant Achromosomal Microtubule Assembly.
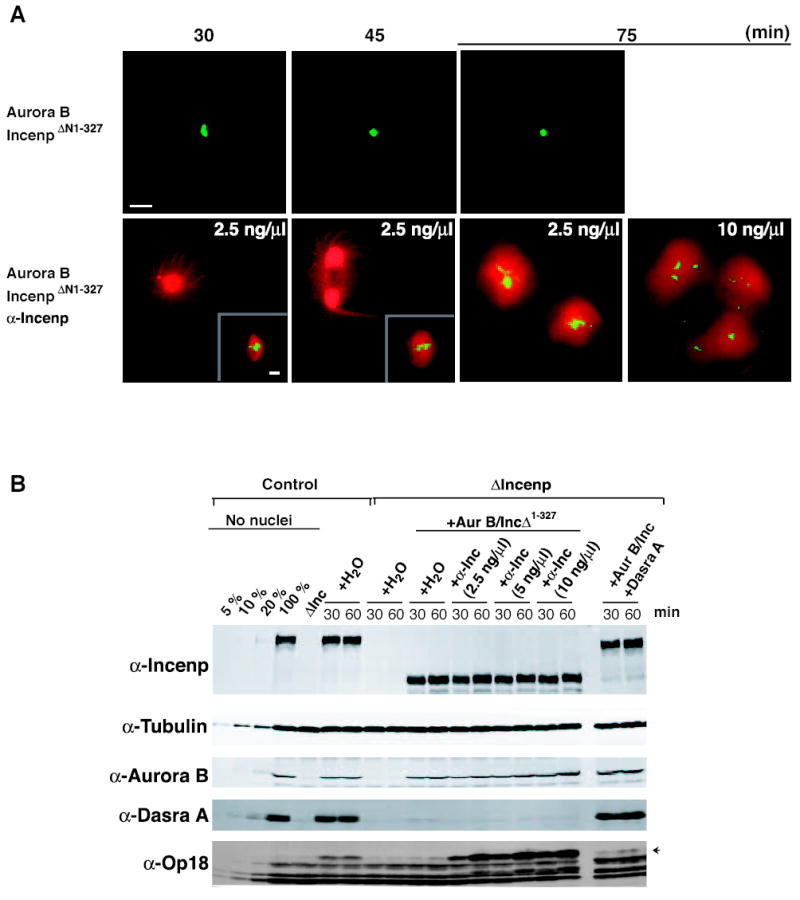
Spindle formation around sperm chromosomes was monitored in control extract or in ΔIncenp extract complemented with either Aurora B/IncenpΔ1-327 in the presence or absence of anti-Incenp antibody (2.5, 5, or 10 ng/μl), or Aurora B/Incenp/Dasra A. Extracts were prepared as described (Figure 2), except that 24 μg/ml cyclin BΔ90 was added. A full analysis of each condition is described in Supplemental Figure S8. (A) Representative images of structures formed at 30, 45 and 75 minutes after entry into M phase for ΔIncenp extracts containing Aurora B/IncenpΔ1-327, Aurora B/IncenpΔ1-327 + 2.5 ng/μl anti-Incenp, or Aurora B/IncenpΔ1-327 + 10 ng/μl anti-Incenp. Representative bipolar spindle structures formed (inset) are shown for the 30 and 45-minute time points. Rhodamine-tubulin (red) and Hoechst 33258 (green) were used to visualize microtubules and DNA, respectively. Bars, 20 μm. (B) All samples were analyzed by quantitative Western blot at 30 and 60 minutes after entry into M phase. Left-most lanes represent indicated amounts of control extracts.
Dasra Proteins Facilitate Loading of the CPC onto Chromatin
To investigate how Dasra proteins contribute to CPC function, we analyzed the ability of the CPC to co-purify with chromosomes in the presence or absence of the Dasra proteins. Metaphase chromosomes were purified from reconstituted ΔIncenp extract that had been metabolically labeled with 35S-methionine, and the extent of copurification of the labeled CPC members was examined (Figure 1C). We observed that ~9-fold less Incenp and Aurora B, and ~25-fold less Survivin copurified with chromosomes when Dasra proteins were absent than when present. Essentially the same effect was seen on purified chromatin beads lacking centromeric sequences (Figure 1D), indicating that centromeres are not required for Dasra-dependent loading of the CPC onto chromosomes. Consistent with this conclusion, immunofluorescence analyses revealed reduction of Incenp staining on both centromeres and chromosome arms in the absence of Dasra proteins (Figure S2B).
Dasra A, together with Aurora B and Incenp, Is Required for Spindle Assembly in Xenopus Egg Extracts
We previously proposed that the CPC on chromosomes locally controls phosphorylation of MCAK to promote chromatin-induced microtubule assembly (Sampath et al., 2004), suggesting that Dasra proteins, which facilitate CPC-binding to chromatin, would be important for spindle assembly. Indeed, we found that CPC depletion led to the expected absence of chromatin-associated microtubules, whereas addition of a pool containing Aurora B, Incenp and Dasra A was sufficient for rescuing bipolar spindle assembly (Figure 2). Furthermore, Aurora B, Incenp (data not shown) and Dasra A (Figure 2) are all necessary for the rescue.
A small population (~11%) of DNA-based structures seen in the Aurora B and Incenp condition had “mini-spindles” (Figures S2 and S3) with average lengths of 13 ± 4 μm (vs. 38 ± 6 μm for control spindle length). We believe these mini-spindles reflect the recruitment of a small amount of residual undepleted Dasra proteins onto the chromosomes (Figure S3). Indeed, when a deletion mutant of Incenp (IncenpΔ1-327), which retains binding to Aurora B, but which cannot bind to Dasra proteins or Survivin/SIX [Figures 1A and S1; (Bolton et al., 2002)], was used, an even smaller population of spindles exists in the Aurora B and IncenpΔ1-327 condition (3%; Figure 2B) in the presence or absence of Dasra A. This result reflects the importance of the Incenp-Dasra A interaction for CPC binding to chromosomes and spindle assembly (Figures S3 and S4).
Hyperphosphorylation of Op18 Is Dependent on the CPC
The results described so far suggest the close link between chromosomal localization of the CPC and spindle assembly. We therefore asked if chromosomes activate the Aurora B pathway, and sought useful markers that indicate the level of pathway activation. We considered that Op18 hyperphosphorylation might be a good candidate, because it is induced by chromatin (Andersen et al., 1997), and because Ser16, a critical site of Op18 phosphorylation, resides within an Aurora target consensus site (Ferrari et al., 2005).
To determine whether Aurora B can phosphorylate Op18, the kinase activity of immunoprecipitated CPC was examined towards recombinant Op18 and Op18-S16A mutant proteins. Plx1 and control IgG immunoprecipitations were used as controls for specificity, and myelin basic protein (MBP) was used as a positive control for kinase activity. Immunoprecipitated CPC robustly phosphorylated both MBP and wild-type Op18 whereas phosphorylation of Op18-S16A was ~14 fold less than that of wild-type Op18, demonstrating that S16 is the major site of Aurora B-dependent phosphorylation in vitro (Figure 3A). In contrast, Plx1 had only relatively weak activity against both wild-type and S16A Op18 (Figures 3A).
Figure 3. Chromatin- and Centrosome-Mediated Op18 Hyperphosphorylation Depend on the CPC.
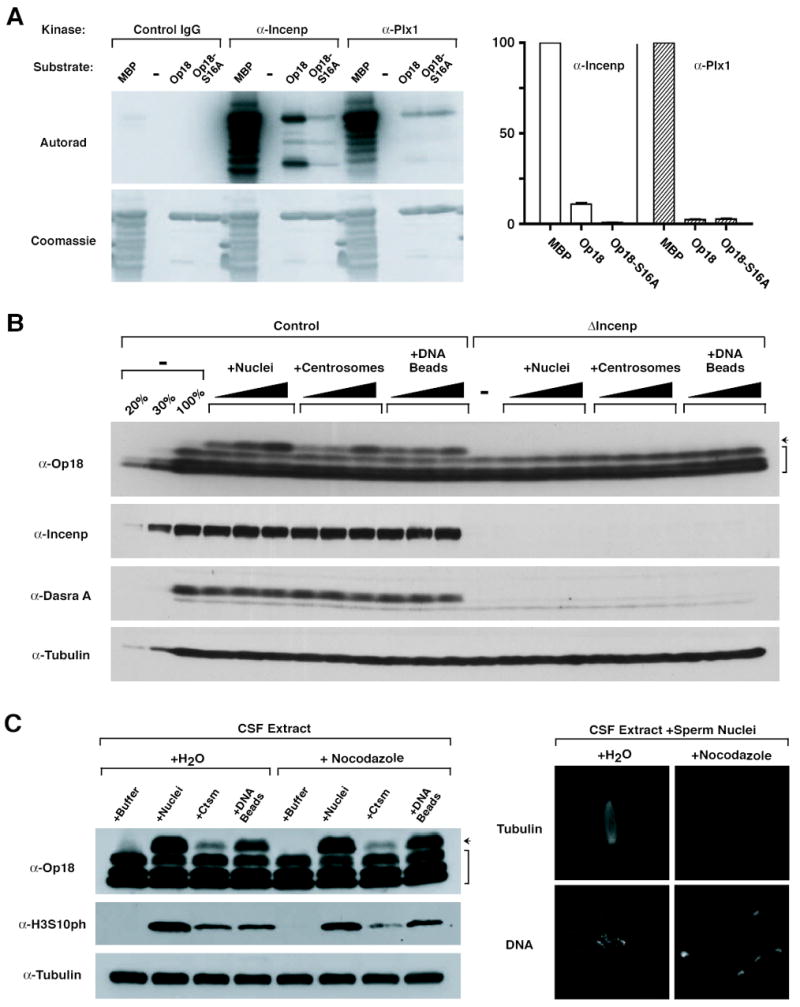
(A) In vitro kinase assay against recombinant Op18. The CPC and Plx1 were immunoprecipitated from CSF extract with anti-Incenp and anti-Plx1 antibodies, respectively, and tested for kinase activity using recombinant 6xHis-Op18 or 6xHis-Op18-S16A as a substrate. Control IgG precipitation was used as a negative control, and Myelin Basic Protein (MBP) was used as a positive control for the kinase assays. Quantitation (right) was performed following SDS-PAGE and exposure to a PhosphorImager; all values are normalized against activity towards MBP. Mean and range of two experiments are shown.
(B) CPC-dependent Op18 phosphorylation is induced by chromatin and centrosomes. Increasing amounts of sperm nuclei (500, 2000, or 10000/μl), purified centrosomes (250, 1000, or 10000/μl), or DNA beads (1000, 2000, 5000 beads/μl extract, respectively) were added to control or ΔIncenp extract, and were cycled through interphase to metaphase. Samples were taken 45 min after entry into metaphase and analyzed by Western blot. Arrow indicates the slowest migrating, hyperphosphorylated form of Op18, and bracket indicates faster migrating Op18 species having various states of phosphorylation.
(C) Centrosomes and chromatin induce CPC-dependent but microtubule-independent phosphorylation of both Op18 and H3S10. Sperm nuclei (Nuclei; 10000/μl), centrosomes (Ctsm; 3000/μl), or DNA beads (5000/μl) were added to CSF extracts containing rhodamine-tubulin in the presence or absence of 33 μM nocodazole. Samples were taken after 100 min and analyzed by Western blot. Representative images of structures assembled in the presence or absence of nocodazole are shown at right for tubulin (top) and Hoechst 33258 (bottom).
Previous studies have shown that addition of sperm nuclei, purified centrosomes, or DNA-beads to metaphase egg extracts induces hyperphosphorylation of Op18 (Andersen et al., 1997; Kuntziger et al., 2001). We similarly observed that the most hyperphosphorylated (slowest migrating) form of Op18 accumulated in a dose-dependent manner under all three conditions, but the formation of this hyperphosphorylated species was completely blocked in ΔIncenp extract (Figure 3B). Although it has been suggested that Plx1 mediates Op18 phosphorylation in Xenopus egg extract (Budde et al., 2001), depletion of Plx1 showed relatively a minor effect (Figure S5). Altogether, these data demonstrate that Aurora B leads to chromatin- and centrosome-induced hyperphosphorylation of Op18.
Op18 and Histone H3 Phosphorylation Are Reporters of Aurora B Pathway Activation by Chromatin, Centrosomes, and Stabilized Microtubules
As Op18 hyperphosphorylation stimulated by taxol-induced microtubule stabilization (Kuntziger et al., 2001) also requires the CPC (Figure S6), we investigated whether hyperphosphorylation induced by chromatin and centrosomes is caused by microtubules assembled by these structures. We observed no effect of nocodazole on Op18 phosphorylation (Figure 3C, left), even though microtubule polymerization was clearly inhibited (Figure 3C, right). In addition, phosphorylation of histone H3 serine 10 (H3S10), a canonical chromosomal substrate of Aurora B (Murnion et al., 2001), closely mirrored the pattern of Op18 hyperphosphorylation (Figures 3C and S6). Thus, Op18 and H3S10 phosphorylation can be thought of as markers of Aurora B activity, isolated from extracts preincubated with anti-Incenp beads at 4°C and 20°C represent the pre-and post-activated status of the CPC, respectively. The phosphorylation of the Incenp TSS motif isolated from beads preincubated at 20°C was increased by 2 fold when compared to the 4°C sample, as analyzed by quantitative mass-spectroscopy (Figures 4B and S7). Moreover, addition of 0.5 μM okadaic acid to metaphase extracts, previously shown to inhibit PP2A acitivity by 50% (Félix et al., 1990), increased Incenp TSS phosphorylation by 2.9 fold. Recently, cytoplasmic suppression of TSS phosphorylation was also shown using an anti-phospho-S850 Incenp antibody (Knowlton et al., 2006). These data suggest that the TSS motif is underphosphorylated in the cytoplasm due to phosphatase activity, but that clustering of the CPC increases the strength and lifetime of this signal, in turn increasing the net Aurora B kinase activity in the system.
Figure 4. Incubation of Anti-Incenp Antibodies with Egg Extracts Stimulate Op18 Phosphorylation.
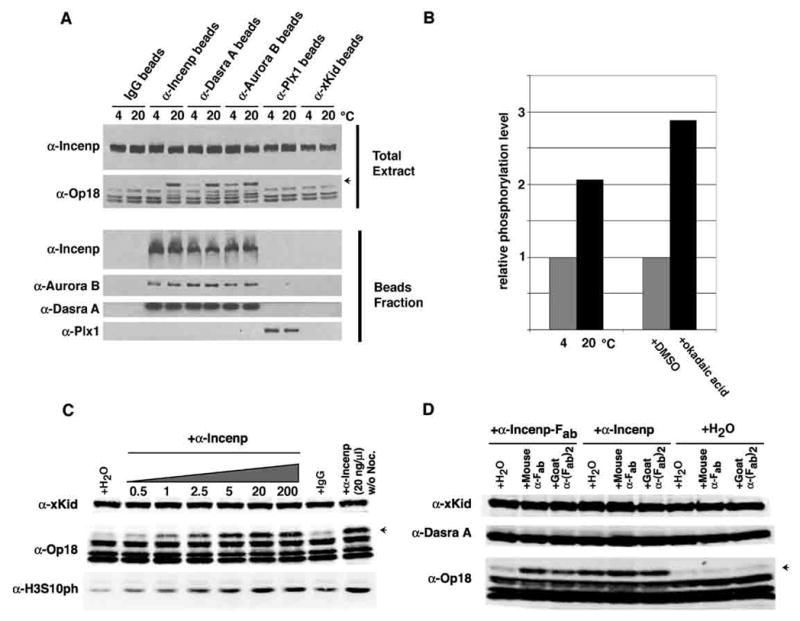
(A) Indicated antibodies (2 μg) were crosslinked to protein A-beads and incubated with 20 μl of CSF extract containing nocodazole at 4 or 20°C for 85 min. Total input (extracts and beads) and isolated beads fraction were analyzed by Western blotting. Antibodies against CPC components induced Op18 hyperphosphorylation (indicated by an arrow) when incubated at 20°C.
(B) Relative phosphorylation of Incenp tryptic peptide (847-862) TSSAVWHSPPLSSNR isolated from extracts incubated at 4°C and 20°C with anti-Incenp antibodies (left) analyzed by mass spectrometry. Relative phosphorylation from extracts treated with 0.5 μM okadaic acid or DMSO control is also shown (right).
(C) Indicated amount (ng/μl) of anti-Incenp antibodies or 200 ng/μl of control IgG were incubated with 30 μl CSF extract at 20°C for 60 minutes in the presence of nocodazole, except for the last lane. Op18 and H3S10 phosphorylation level was measured by quantitative Western blotting analysis. An arrow indicates the hyperphosphorylated form of Op18. xKid was used as a loading control.
(D) Fab fragment of anti-Incenp antibody (20.8 ng/μl ≅ 400 nM), native anti-Incenp antibody (70 ng/μl ≅ 400 nM) or water was incubated with CSF extracts together with water, mouse anti-light-chain antibody (30 ng/μl ≅ 200 nM), or goat-anti-(Fab)2 antibody (30 ng/μl ≅ 200 nM) at 20°C for 60 minutes in the presence of nocodazole. Op18 hyperphosphorylation was analyzed by quantitative Western blot.
To our surprise, addition of soluble anti-Incenp antibody to control extract induced Op18 hyperphosphorylation and H3S10 phosphorylation in a dose-dependent manner in both the presence and absence of nocodazole (Figures 4C and 7A). To examine if this anti-Incenp antibody induces Aurora B pathway activation by increasing the local concentration of the CPC, we tested if the Fab fragment of the anti-Incenp antibody (which contains one binding site per molecule instead of two) could increase Op18 hyperphosphorylation (Figure 4D). The Fab fragment was not able to induce hyperphosphorylation of Op18, but this activity was restored when the Fab fragment was supplemented with secondary antibodies recognizing the Fab fragment (mouse anti-light chain and goat anti-(Fab)2), demonstrating that the Fab fragment can still recognize Incenp, but such binding was not sufficient for activation.
Figure 7. The Aurora B Pathway Is Activated and Can Promote Microtubule Assembly by a Ran-Independent Mechanism.
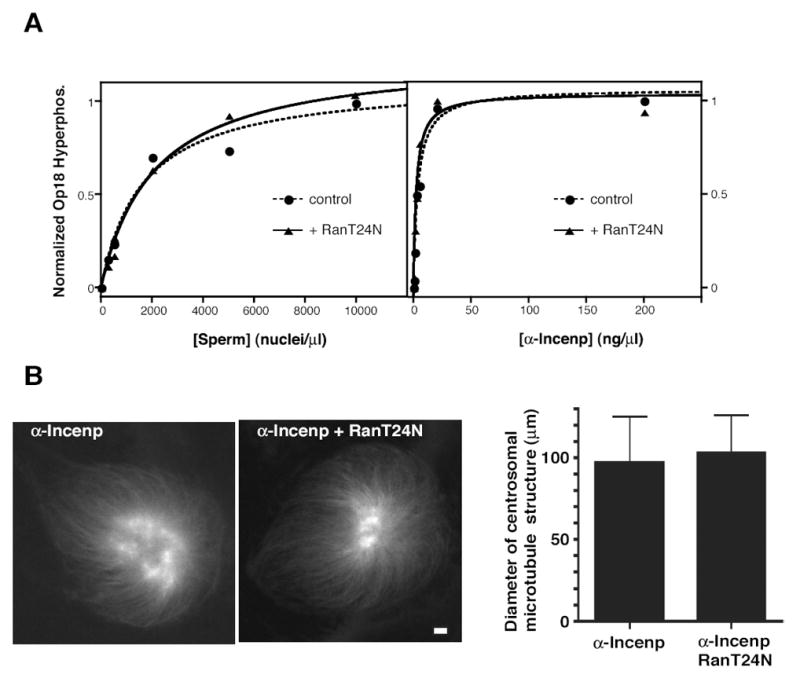
(A) Nocodazole-treated CSF extract was incubated with the indicated concentrations of either sperm nuclei (left) or anti-Incenp antibody (right) at 20°C for 60 minutes, in the presence (triangle) or absence (circle) of 15 μM RanT24N (a gift of E. Arias). The degree of hyperphosphorylation of Op18 was determined by quantitative Western blotting. Data points were fit to a hyperbolic dose response curve.
(B) Centrosomes (500/μl) were added to CSF extract which had been pre-incubated with 10 ng/μl of anti-Incenp antibodies at 4°C for 70 minutes and incubated at 20°C for 70 minutes in the presence or absence of 15 μM RanT24N. Representative images of rhodamine-tubulin are shown. Bar, 10 μm. Average diameter with standard deviation of microtubule structures associated with mono-asters in each sample is shown. N=134 and 98, respectively
We conclude that Aurora B-dependent phosphorylation is stimulated by the anti-Incenp antibody by the forced proximity of Aurora B-containing complexes caused by the existence of which is suppressed in the cytoplasm of Xenopus egg extracts, but can be induced by chromatin in a microtubule-independent manner.
Anti-Incenp Antibodies Activate the Aurora B Pathway By Increasing the Local Concentration of the CPC
We next sought to identify a simple manipulation that activates the CPC in the cytoplasm independent of chromatin, centrosomes or microtubules. We considered that cytoplasmic phophatase activity may dampen the kinase activity of Aurora B but the high local concentration of the CPC on chromatin facilitates autostimulation of the CPC. Indeed, incubation of beads coated with anti-Incenp, anti-Dasra A, or anti-Aurora B with metaphase extract, which should increase the local concentration of the CPC, induced Op18 hyperphosphorylation (Figure 4A). This effect was specific to antibodies against components of the CPC, as anti-Plx1 or anti-xKid antibodies did not induce Op18 hyperphosphorylation.
The simplest explanation of the result above is that antibody-mediated clustering facilitates phosphorylation among the CPCs, which stimulates the kinase activity. It has been shown that phosphorylation of the C-terminal TSS motif in Incenp is carried out by Aurora B, and is important for full activation for the kinase activity (Bishop and Schumacher, 2002; Honda et al., 2003; Kang et al., 2001; Sessa et al., 2005; Yasui et al., 2004). To test if antibody-mediated clustering induces phosphorylation of these sites, anti-Incenp-coated beads were incubated with metaphase extracts at 4°C or 20°C, and then the CPC was purified with beads. At 4°C, a condition predicted to diminish enzymatic activity, the CPC can still associate with antibody-beads, but a smaller degree of Op18 hyperphosphorylation was observed when compared to samples incubated at 20°C (Figure 4A). Therefore, we speculated that complex two antigen binding sites per antibody molecule, and/or by further clustering of antibodies mediated by the Fc fragment. Addition of this antibody can therefore circumvent the need for physiological Aurora B pathway activators in Xenopus egg extracts.
Effects of Anti-Incenp Antibodies in Spindle Assembly
Having established that the anti-Incenp antibody can autonomously activate the Aurora B pathway in the cytoplasm, we then investigated if the Aurora B pathway requires local activation by chromosomes to spatially restrict microtubule assembly.
Spindle morphologies were monitored in control extracts, ΔIncenp extracts supplemented with Aurora B, Incenp and Dasra A, or ΔIncenp extracts with Aurora B, IncenpΔ1-327 in the presence or absence of 2.5, 5 or 10 ng/μl anti-Incenp antibody at various time points after entry into M phase (Figures 5 and S8). IncenpΔ1-327 was employed in place of full-length Incenp to avoid any interaction with undepleted Dasra A. In control extract and ΔIncenp extract complemented with Aurora B, Incenp and Dasra A, visible microtubule structures were predominantly associated with chromosomes throughout the time course, and bipolar spindle was the major form at 60 minutes after entry into M phase (Figure S8). At this point, hyperphoshorylation of Op18 was also observed in these extracts (Figure 5B). In ΔIncenp extracts with no complementation or with Aurora B, IncenpΔ1-327, more than 50% of chromosomes did not associate with any visible microtubules as previously shown (Figures 2, 5A, and S8). Strikingly, in ΔIncenp extract containing Aurora B, IncenpΔ1-327 and anti-Incenp antibody (2.5 ng/μl), the majority (> 90%) of chromosomes were associated with microtubules, and about 50 % of chromosome-containing structures were bipolar spindles at 45 minutes (Figures 5A and S8). Furthermore, robust Op18 hyperphosphorylation was observed even in the absence of Dasra A. At later time points (60 and 75 minutes) in extracts containing anti-Incenp antibody, the bipolarity of spindles was greatly diminished, and spindles were often clustered together. These abnormal structures correlate with the hyperactivation (Figure 5B) and mislocalization of the CPC (Figure S9; the majority of Incenp is localized to microtubules instead of chromosomes), which may affect the spatial regulation of microtubule assembly. This unusual Incenp-microtubule association does not seem to be mediated by anti-Incenp antibodies, since Incenp is preferentially localized to microtubules when its chromosome-binding is abrogated upon depletion of Dasra A (Figure S2B).
Another apparent phenotype caused by anti-Incenp antibodies was the presence of achromosomal microtubule structures, including bipolar, spindle-like structures. At 15 minutes, 13, 56 and 56% of achromosomal microtubule structures are bipolar in the presence of 2.5, 5 and 10 ng/μl anti-Incenp, respectively. After 45 minutes, more than 70% of achromosomal structures are bipolar in all cases with anti-Incenp. Anti-Incenp antibodies were colocalized to these achromosomal spindle microtubules, indicating that the CPC may promote microtubule assembly in part through interaction with them (Figure S9). We assumed that these structures are formed by the microtubules nucleated from centrosomes derived from sperm nuclei, as they were not formed by when DNA beads were used for spindle assembly instead of sperm nuclei (data not shown). These results indicate that anti-Incenp antibody is able to activate the Aurora B pathway independent of Dasra A and to assemble microtubules that are nucleated from either chromosomes or centrosomes.
Microtubule Assembly by Aurora B Pathway Activation
To avoid the pleiotropic phenotype seen on sperm chromosomes, we quantified the effect of the anti-Incenp antibody using purified centrosomes as the sole source of microtubule nucleation (Figure 6). Microtubules were highly unstable in control M phase extracts or ΔIncenp extracts complemented with water or Aurora B and Incenp, and the average diameter size of centrosomal microtubule structures was about 10 μm in all of these extracts (Figures 6A and B). In control extract containing the anti-Incenp antibody or in ΔIncenp extract complemented with Aurora B and Incenp together with the anti-Incenp antibody, however, the average diameter of centrosomal microtubule structures was 70–90 μm, and centrosomal interactions were frequently observed (Figure 6C).
Figure 6. Antibody-Induced Aurora B Pathway Activation Leads to Achromosomal Spindle Formation by Centrosomes.
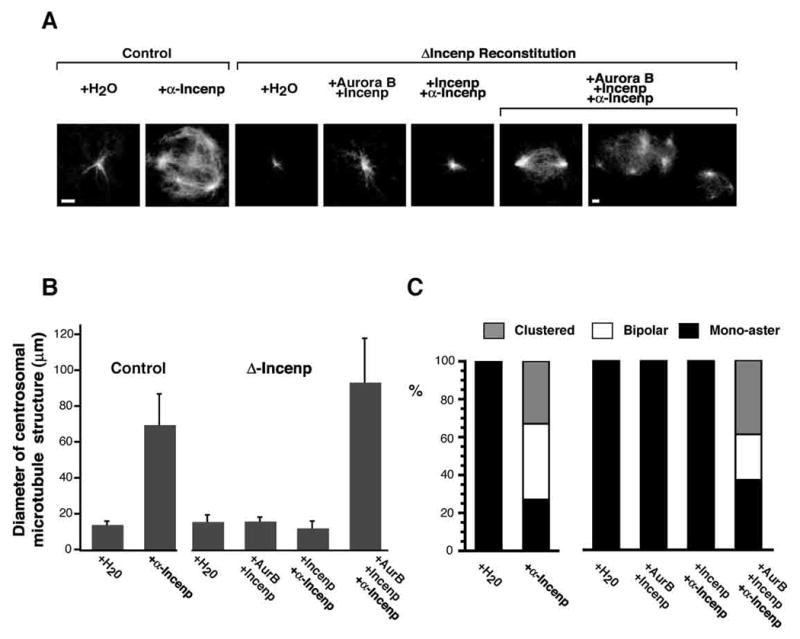
Representative fields of microtubules formed in cycled control or ΔIncenp extract containing human centrosomes (400/μl), and reconstituted with either water or the indicated CPC components. Where indicated, anti-Incenp antibody (5 ng/μl) was added to extracts. (A) All images are shown at equal magnification, except for the last image. Rhodamine-tubulin was added to visualize microtubules. Bars, 10 μm. (B) Average diameter with standard deviation of microtubule structures associated with mono-asters in each sample is shown. (C) Aster structures were categorized into “mono-aster”, “bipolar” and “clustered”, and frequency of each category is presented. At least 110 microtubule-containing structures were counted for each sample.
To rule out the possibility that the antibody exerted its effects on microtubules independently of Aurora B by clustering the microtubule binding domain of Incenp (Wheatley et al., 2001), the anti-Incenp antibody was added to ΔIncenp extract complemented with only Incenp. No centrosomal interactions were seen and there was no increase in the appearance of astral microtubules around centrosomes (Figures 6A and B). Together, these data demonstrate that promotion of microtubule assembly and centrosomal interaction are caused by Aurora B kinase activated by anti-Incenp antibodies.
The Aurora B Pathway Is Activated by a Ran-Independent Mechanism
One prevailing model implies that Ran-GTP locally generated by chromatin is critical for chromatin-induced spindle formation (Caudron et al., 2005; Kalab et al., 2006). To determine if chromatin activates the Aurora B pathway through the Ran-GTP pathway, the Op18 hyperphosphorylation response induced by a broad range of sperm chromosome concentrations was monitored in the presence or absence of 15 μM RanT24N, which prevents production of Ran-GTP. As previously shown (Carazo-Salas et al., 1999), RanT24N effectively blocked microtubule assembly from DNA beads (data not shown). However, RanT24N did not reduce the capacity of sperm chromosomes to induce hyperphosphorylation of Op18 (Figure 7A, left panel). Similarly, RanT24N did not show any effect on Op18 phosphorylation induced by anti-Incenp antibody (Figure 7A, right panel). These data demonstrate that the Aurora B pathway can be activated by chromosomes via a Ran-GTP independent mechanism.
Finally, the effect of RanT24N on microtubule assembly induced by anti-Incenp antibodies was examined by monitoring microtubules nucleated from centrosomes, since RanT24N inhibits microtubule nucleation from chromatin. The positive effect of the anti-Incenp antibody on microtubule assembly was not affected by RanT24N (Figure 7B). This result strongly suggests that once microtubules are nucleated, activation of the anti-Incenp-mediated Aurora B pathway can promote their assembly even in the absence of an active Ran pathway.
Discussion
We previously reported that the CPC is required for chromatin-induced spindle assembly, and proposed that this regulation occurs in part through phosphorylation of the microtubule depolymerase MCAK by Aurora B (Sampath et al., 2004). Here we show that Aurora B can regulate another microtubule depolymerizing protein, Op18. During the course of revising this manuscript, similar results were reported (Gadea and Ruderman, 2006). Using Op18 hyperphosphorylation as an indicator for the Aurora B pathway activation, we demonstrate that the pathway is suppressed in cytoplasm, but is activated by chromatin through a Ran-independent mechanism. Below we discuss the mechanism and significance of chromatin-induced activation of the Aurora B pathway.
Mechanisms of Aurora B Activation by Chromatin
How does chromatin activate Aurora B-dependent phosphorylation? Four lines of evidence support a model in which Aurora B is activated by increasing the local concentration of CPC molecules on chromatin: 1) Chromatin can bind to multiple molecules of the CPC and induce Aurora B pathway activation (Figures 1 and 3); 2) Antibody alone can activate Aurora B kinase activity and this activity is dependent on having multiple binding sites (Figures 4C and D); 3) The responses of Op18 hyperphosphorylation induced by sperm nuclei and antibodies are similar and Ran-independent (Figure 7A); 4) Op18 hyperphosphorylation induced by antibody clustering is insensitive to the geometry of attachment (Figure 4A).
Full activation of Aurora B requires Aurora B-mediated phosphorylation of the C-terminal TSS motif of Incenp, and structural analysis suggests that this phosphorylation must occur in trans (Sessa et al., 2005). Thus, the simplest model is that the Incenp TSS motif is actively dephosphorylated in the cytoplasm, but chromatin increases the local concentration of the CPC, resulting in initiation of a positive feedback loop among bound CPC holocomplexes. It is worth noting other possible mechanisms: clustering may also activate Aurora B independent of phophorylation, as is the case for kinases such as Raf and EGFR (Goetz et al., 2003; Zhang et al., 2006), or chromatin or its associated molecules might directly induce a non-clustering-mediated structural change in Aurora B.
It is also possible that chromatin exerts its effect on the Aurora B pathway by inhibiting protein phosphatase activities. However, our data indicate that chromatin directly stimulates the kinase activity of Aurora B, since we demonstrate that Dasra proteins (which are required for loading of the CPC onto chromatin) are needed for spindle assembly. Importantly, more than 90% of Dasra A is associated with Incenp and Aurora B in the cytoplasm of Xenopus egg extracts (Sampath et al., 2004). In addition, it has been reported that recombinant human Dasra B/Borealin does not affect the in vitro kinase activity of Aurora B (Gassmann et al., 2004). Thus, it is unlikely that Dasra proteins stimulate the enzymatic activity of Aurora B simply by virtue of their interactions.
The spatial distribution of phosphorylated substrates around chromatin can be finely regulated by the level of phosphatase activity, and subtrate diffusibility and stability, whereas the amplitude of the gradient is most sensitive to kinase activity (Kholodenko et al., 2000). For example, the freely diffusible Op18-tubulin interaction is abrogated in the vicinity of chromosomes (4 to 8 μm) by a gradient of Op18 phosphorylation, the extent of which is mainly determined by phosphatase activity/concentration and Op18 diffusion rate (Niethammer et al., 2004). Alternatively, if the substrate is immobilized on chromosomes, kinase activity dictates the behavior of the phospho-substrate. MCAK, a protein that is bound to centromeric chromatin, is more efficiently phosphorylated at Ser196 by Aurora B on centromeres of unaligned chromosomes than on aligned chromosomes (Lan et al., 2004). This raises the question of whether a change in chromatin status between sister kinetochores can effectively regulate Aurora B activity by modulating its local concentration. In summary, our results illustrating that Aurora B is activated by increased local concentration have important implications for the several roles of this complex throughout mitosis.
Importance of Chromatin-Dependent Activation of the Aurora B Pathway in Spindle Assembly
Integrating centrosomal and chromosomal microtubules into one spindle is a particularly important task for the egg, since the diameter of the cell at the first mitotic division in Xenopus laevis is ~1 mm, as compared to the ~30 μm spindle. Centrosomal microtubules are highly unstable in metaphase egg extracts, and are not capable of forming bipolar spindles in the absence of associated chromosomes (Sawin and Mitchison, 1991). Using an antibody against Incenp, however, we were able to stimulate Aurora B-mediated phosphorylation in a chromatin-independent manner, resulting in the formation of chromosome-free bipolar spindle-like structures by centrosomes. From these observations, we speculate that local activation of Aurora B-dependent phosphorylation around chromosomes acts to couple spindle assembly initiated by chromatin and centrosomes. This function of Aurora B might be related to the “long-range communication” previously demonstrated to occur between centrosomes and chromatin (Carazo-Salas and Karsenti, 2003), as localized Aurora B activation by chromatin could promote the polarizing effect of chromatin on centrosome growth.
We have shown that the functions of the Dasra proteins in spindle microtubule assembly can be bypassed by the addition of anti-Incenp antibodies. Dasra-dependent recruitment of the CPC to chromatin appears to make the Aurora B pathway responsive to chromatin, but alternative mechanisms, such as the Ran-GTP pathway, can be engaged, permitting microtubule assembly on chromosomes if the Aurora B pathway is activated in a chromatin-independent manner. Indeed, we have previously shown that the Ran-GTP pathway remains active even in the absence of the CPC (Sampath et al., 2004). Here we showed that chromosomes were able to activate the Aurora B pathway in the presence of dominant negative Ran. These results altogether indicate that the Ran-GTP pathway and the Aurora B pathway are both activated by chromatin, and they cooperate to assemble bipolar spindles.
We currently do not understand the biological significance of centrosomal activation of the Aurora B pathway, since centrosomal microtubule size was not largely affected in ΔIncenp extracts (Figure 6). However, if the Aurora B pathway is artificially activated in the cytoplasm, spindle assembly is not restricted to chromosomes and can be elicited by centrosomes alone. Intriguingly, achromosomal spindles are also assembled by beads coated with anti-Aurora A antibody in the presence of Ran-GTP (Tsai and Zheng, 2005). These results suggest that the Aurora pathways do not merely promote microtubule assembly, but may contribute to establishing microtubule bipolarity.
In conclusion, we have demonstrated the importance of spatial regulation of the Aurora B pathway during spindle assembly. Previous studies have shown that chromatin creates a chromosome-centered Ran-GTP gradient responsible for the localized activation of microtubule-assembly factors around chromosomes (Caudron et al., 2005). While the catalytic activity of Rcc1 for Ran-GTP generation is directly induced by H2A/H2B interaction (Nemergut et al., 2001), we suggest that local enrichment of the CPC on chromatin drives Aurora B pathway activation. In light of the data presented here, it is interesting to consider why two pathways must be regulated by chromatin in order to generate a spindle. Of note, the two pathways differ in the effects of their downstream targets; while two major microtubule-catastrophe regulators, MCAK and Op18, are inhibited by Aurora B, microtubule assembly factors such as TPX2, NuMA and NuSAP are activated by Ran-GTP. Each downstream protein may have structural preferences or limitations for its regulation by phosphorylation or Ran-GTP/Importin β. In addition, having two independent mechanisms may contribute to the stability and dynamicity of the system. Understanding the integration between the Ran-GTP and Aurora B pathways will be crucial to elucidating the principles governing microtubule assembly around chromatin.
Experimental Procedures
See Supplemental Data for a detailed description of the methods.
Immunodepletion From RNase-treated Xenopus Egg Extracts
Control rabbit IgG or anti-xIncenp (Sampath et al., 2004) were crosslinked to protein-A Dynabeads (Invitrogen) by DMP (dimethyl pimelimidate dihydrochloride, Pierce). To prepare RNase-treated Xenopus laevis egg extracts, a method described by Murray was applied (Murray, 1991). Extracts were depleted twice using anti-xIncenp-beads. In the second round of depletion, protein-A Dynabeads were added to remove any leached antibody that remained in the extract.
We found that chemical crosslinking of the antibodies to beads by DMP was critical for mRNA-dependent rescue experiments, as antibodies that were passively conjugated to beads without crosslinking leached into extracts during immunodepletion, and caused activation of the Aurora B pathway in an Incenp-dependent manner. Although our previous report (Sampath et al., 2004) used a non-crosslinked method for immunodepletion, the conclusion was not affected by leached antibodies, as little Incenp remained in depleted extracts and no activation of the Aurora B pathway was seen (A.E.K, S.C.S, unpublished results).
Spindle Assembly in RNase-treated, Immunodepleted Egg Extracts
All incubations for spindle assembly using immunodepleted egg extracts were carried out at 20ºC. For a typical spindle assembly on replicated chromosomes, 20 μl of RNase-treated control or immunodepleted egg extract containing sperm nuclei (final concentration 500/μl) or purified centrosomes (a gift of K. Kinoshita), 2 μl mRNA, and 0.3 mM calcium chloride were incubated for 80 min at 20ºC to prepare interphase extracts. Metaphase spindles were assembled by the addition of 40 μl of fresh RNase-treated control or immunodepleted extract, followed by incubation at 20ºC for 60 min. To score phenotypes, 3 μl of Fix (Murray, 1991) were added to 1 μl of extract on a slide, and repeated three times.
For spindle assembly and Western blot analysis using chromatin beads, 5 μl of DNA-coated beads per sample, prepared as previously described (Heald et al., 1998), were washed once with 20 μl of RNase-treated control or immunodepleted extract, added to 20 μl of RNase-treated control or immunodepleted extract containing rhodamine-tubulin, and incubated for 80 min at 20°C after addition of 0.3 mM calcium chloride. M phase entry was induced as described above.
Microscopy
Spindles assembled in Xenopus egg extract were processed for immunofluorescence as described (Desai et al., 1999a). Affinity purified anti-Incenp antibodies, anti-Dasra A antibodies and control IgG were used at 2 μg/ml in AbDil (TBS, 0.1% Triton X100, 2% BSA). Alexa488-conjugated goat anti-rabbit antibody (Molecular Probes) was used for detection. Hoechst 33258-stained chromosomes, rhodamine-tubulin labeled microtubules, and fluorescent antibodies were imaged using a Carl Zeiss Axioplan 2 microscope equipped with a Photometrics CoolSnap HQ cooled CCD camera, and controlled by MetaMorph software (Universal Imaging). Images were processed with MetaMorph and Adobe Photoshop. To obtain the size of centrosomal microtubule structures, images were acquired with a Plan Neofluar 20X Objective, and the diameter of the major and minor axes of each microtubule-occupied region was measured using MetaMorph.
Conventional Western Blots
Primary and secondary antibodies were diluted in PBS/4% nonfat milk at the following concentrations: 5 μg/ml anti-xDasra A (Sampath et al., 2004), 5 μg/ml anti-xIncenp, 5 μg/ml anti-xAurora B (raised against a C-terminal peptide CRRVLPPVYQSTQSK), 5 μg/ml anti-Plx1 (raised against a C-terminal peptide CQSSKSAVAHVKASA), 4 μg/ml anti-histone phospho-H3S10 (Abcam (ab14955) or Millipore (06-570)), 0.2 μg/ml anti-Op18 (a gift of R. Heald), 1:5000 anti-α-Tubulin (DM1, Sigma), 3 μg/ml anti-xKid antibody (Funabiki and Murray, 2000). Antibodies were detected using either ECL (Amersham; for anti-xDasra A, anti-xIncenp, anti-Op18, anti-Plx1, anti-Tubulin, anti-xKid) or Visualizer (Upstate; for anti-xAurora B).
Quantitative Western Blots
Procedures are similar to the conventional Western Blot, except that 0.1% Tween 20 was added to primary and secondary antibodies diluted in PBS/4 % nonfat milk. IRDye 680 goat anti-rabbit IgG (Li-Cor) and IR Dye 800CW goat anti-mouse IgG (Li-Cor) were used as secondary antibodies. The Odyssey Infrared Imaging System (Li-Cor) was used for detection and quantitation. Op18 hyperphosphorylation was measured by calculating the total fluorescence of the slowest-migrating band for each sample using xKid (Funabiki and Murray, 2000) or α-Tubulin as a loading normalization.
Quantitative Mass Spectrometry
To purify the pre-activated and post-activated form of CPCs from metaphase egg extracts, CSF extracts were incubated with anti-Incenp antibodies crosslinked to Dynabeads at 4°C and 20°C for 75 minutes. In addition, CSF extracts to which okadaic acid (0.5 μM) or DMSO (0.5% v/v) was added, were incubated at 20°C for 30 minutes, cooled to 4°C for ten minutes, mixed with anti-Incenp beads and incubated further for 70 minutes at 4°C. Immunoprecipitated complexes were separated by SDS-PAGE and visualized by GelCode blue staining (Pierce). The band corresponding to xIncenp was excised, destained, alkylated, and digested with trypsin using published methods (Chang et al., 2004). xIncenp tryptic peptides were then simultaneously extracted from the gel slice and propionylated for the purpose of quantifying phosphorylation by mass spectrometry, as described previously (Jin et al., 2005). Briefly, the 4°C and DMSO samples were treated with a sequence of solvents containing 1% d10-propionic anhydride, while the 20°C and okadaic acid samples were treated with solvents containing 1% d0-propionic anhydride. The resulting differentially labeled peptides were dried and crystallized with 2,5-dihydroxybenzoic acid (DHB) MALDI matrix and subjected to single-stage and tandem mass spectrometry for relative quantification of peptide phosphorylation (Chang et al., 2004).
Supplementary Material
Acknowledgments
We thank E. Arias, R. Heald, T. Hirano, K. Kinoshita and B. S. Tseng for reagents, and S. Biggins, T. Kapoor, K. Kinoshita, R. Ohi, L. Postow, S. Sampath, B. Tseng and K. Yap for comments on the manuscript. A.E.K. was supported by NRSA Training Grant CA09673. S.C.S. was supported by NIH MSTP grant GM07739 to the Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program. E.M.W. was supported by an HHMI Predoctoral Fellowship. B.T.C. was supported by the NIH (RR00862), H.F. was supported by the Rockefeller University, the NIH (GM075249), a Searle Scholarship, the Alexandrine and Alexander Sinsheimer Fund, and the Irma T. Hirschl/Monique Weill-Caulier Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SS, Ashford AJ, Tournebize R, Gavet O, Sobel A, Hyman AA, Karsenti E. Mitotic chromatin regulates phosphorylation of Stathmin/Op18. Nature. 1997;389:640–643. doi: 10.1038/39382. [DOI] [PubMed] [Google Scholar]
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6:253–268. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- Bastiaens P, Caudron M, Niethammer P, Karsenti E. Gradients in the self-organization of the mitotic spindle. Trends Cell Biol. 2006;16:125–134. doi: 10.1016/j.tcb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Bishop JD, Schumacher JM. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J Biol Chem. 2002;277:27577–27580. doi: 10.1074/jbc.C200307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blower MD, Nachury M, Heald R, Weis K. A Rae1-containing ribonucleoprotein complex is required for mitotic spindle assembly. Cell. 2005;121:223–234. doi: 10.1016/j.cell.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Bolton MA, Lan W, Powers SE, McCleland ML, Kuang J, Stukenberg PT. Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol Biol Cell. 2002;13:3064–3077. doi: 10.1091/mbc.E02-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde PP, Kumagai A, Dunphy WG, Heald R. Regulation of Op18 during spindle assembly in Xenopus egg extracts. J Cell Biol. 2001;153:149–158. doi: 10.1083/jcb.153.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carazo-Salas RE, Guarguaglini G, Gruss OJ, Segref A, Karsenti E, Mattaj IW. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature. 1999;400:178–181. doi: 10.1038/22133. [DOI] [PubMed] [Google Scholar]
- Carazo-Salas RE, Karsenti E. Long-range communication between chromatin and microtubules in Xenopus egg extracts. Curr Biol. 2003;13:1728–1733. doi: 10.1016/j.cub.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Carvalho A, Carmena M, Sambade C, Earnshaw WC, Wheatley SP. Survivin is required for stable checkpoint activation in taxol-treated HeLa cells. J Cell Sci. 2003;116:2987–2998. doi: 10.1242/jcs.00612. [DOI] [PubMed] [Google Scholar]
- Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol. 2002;14:18–24. doi: 10.1016/s0955-0674(01)00289-7. [DOI] [PubMed] [Google Scholar]
- Caudron M, Bunt G, Bastiaens P, Karsenti E. Spatial coordination of spindle assembly by chromosome-mediated signaling gradients. Science. 2005;309:1373–1376. doi: 10.1126/science.1115964. [DOI] [PubMed] [Google Scholar]
- Chang EJ, Archambault V, McLachlin DT, Krutchinsky AN, Chait BT. Analysis of protein phosphorylation by hypothesis-driven multiple-stage mass spectrometry. Anal Chem. 2004;76:4472–4483. doi: 10.1021/ac049637h. [DOI] [PubMed] [Google Scholar]
- Desai A, Murray A, Mitchison TJ, Walczak CE. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 1999a;61:385–412. doi: 10.1016/s0091-679x(08)61991-3. [DOI] [PubMed] [Google Scholar]
- Desai A, Verma S, Mitchison TJ, Walczak CE. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 1999b;96:69–78. doi: 10.1016/s0092-8674(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Félix MA, Cohen P, Karsenti E. Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: evidence from the effects of okadaic acid. EMBO J. 1990;9:675–683. doi: 10.1002/j.1460-2075.1990.tb08159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Marin O, Pagano MA, Meggio F, Hess D, El-Shemerly M, Krystyniak A, Pinna LA. Aurora-A site specificity: a study with synthetic peptide substrates. Biochem J. 2005;390:293–302. doi: 10.1042/BJ20050343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki H, Murray AW. The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell. 2000;102:411–424. doi: 10.1016/s0092-8674(00)00047-7. [DOI] [PubMed] [Google Scholar]
- Gadea BB, Ruderman JV. Aurora kinase inhibitor ZM447439 blocks chromosome-induced spindle assembly, the completion of chromosome condensation, and the establishment of the spindle integrity checkpoint in Xenopus egg extracts. Mol Biol Cell. 2005;16:1305–1318. doi: 10.1091/mbc.E04-10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea BB, Ruderman JV. Aurora B is required for mitotic chromatin-induced phosphorylation of Op18/Stathmin. Proc Natl Acad Sci U S A. 2006;103:4493–4498. doi: 10.1073/pnas.0600702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R, Nigg EA, Gerloff DL, Earnshaw WC. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol. 2004;166:179–191. doi: 10.1083/jcb.200404001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CA, O’Neil JJ, Farrar MA. Membrane localization, oligomerization, and phosphorylation are required for optimal raf activation. J Biol Chem. 2003;278:51184–51189. doi: 10.1074/jbc.M309183200. [DOI] [PubMed] [Google Scholar]
- Gruss OJ, Carazo-Salas RE, Schatz CA, Guarguaglini G, Kast J, Wilm M, Le Bot N, Vernos I, Karsenti E, Mattaj IW. Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell. 2001;104:83–93. doi: 10.1016/s0092-8674(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Harel A, Forbes DJ. Importin beta: conducting a much larger cellular symphony. Mol Cell. 2004;16:319–330. doi: 10.1016/j.molcel.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Vernos I, Murray A, Hyman T, Karsenti E. In vitro assays for mitotic spindle assembly and function. In: Celis J, editor. Cell Biology: A laboratory handbook. Academic Press; 1998. pp. 326–336. [Google Scholar]
- Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14:3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter AW, Caplow M, Coy DL, Hancock WO, Diez S, Wordeman L, Howard J. The kinesin-related protein MCAK is a microtubule depolymerase that forms an ATP-hydrolyzing complex at microtubule ends. Mol Cell. 2003;11:445–457. doi: 10.1016/s1097-2765(03)00049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Bateup H, Padovan JC, Greengard P, Nairn AC, Chait BT. Quantitative analysis of protein phosphorylation in mouse brain by hypothesis-driven multistage mass spectrometry. Anal Chem. 2005;77:7845–7851. doi: 10.1021/ac051519m. [DOI] [PubMed] [Google Scholar]
- Kalab P, Pralle A, Isacoff EY, Heald R, Weis K. Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature. 2006;440:697–701. doi: 10.1038/nature04589. [DOI] [PubMed] [Google Scholar]
- Kang J, Cheeseman IM, Kallstrom G, Velmurugan S, Barnes G, Chan CS. Functional cooperation of Dam1, Ipl1, and the inner centromere protein (INCENP)-related protein Sli15 during chromosome segregation. J Cell Biol. 2001;155:763–774. doi: 10.1083/jcb.200105029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodenko BN, Brown GC, Hoek JB. Diffusion control of protein phosphorylation in signal transduction pathways. Biochem J . 2000;350(Pt 3):901–907. [PMC free article] [PubMed] [Google Scholar]
- Knowlton AL, Lan W, Stukenberg PT. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr Biol. 2006;16:1705–1710. doi: 10.1016/j.cub.2006.07.057. [DOI] [PubMed] [Google Scholar]
- Kuntziger T, Gavet O, Manceau V, Sobel A, Bornens M. Stathmin/Op18 phosphorylation is regulated by microtubule assembly. Mol Biol Cell. 2001;12:437–448. doi: 10.1091/mbc.12.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT. Aurora B Phosphorylates Centromeric MCAK and Regulates Its Localization and Microtubule Depolymerization Activity. Curr Biol. 2004;14:273–286. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- Lens SM, Wolthuis RM, Klompmaker R, Kauw J, Agami R, Brummelkamp T, Kops G, Medema RH. Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. Embo J. 2003;22:2934–2947. doi: 10.1093/emboj/cdg307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnion ME, Adams RR, Callister DM, Allis CD, Earnshaw WC, Swedlow JR. Chromatin-associated protein phosphatase 1 regulates aurora-B and histone H3 phosphorylation. J Biol Chem. 2001;276:26656–26665. doi: 10.1074/jbc.M102288200. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell Cycle Extracts. Meth Cell Biol. 1991;36:573–597. [PubMed] [Google Scholar]
- Murray AW, Kirschner MW. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Maresca TJ, Salmon WC, Waterman-Storer CM, Heald R, Weis K. Importin beta is a mitotic target of the small GTPase Ran in spindle assembly. Cell. 2001;104:95–106. doi: 10.1016/s0092-8674(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Nemergut ME, Mizzen CA, Stukenberg T, Allis CD, Macara IG. Chromatin docking and exchange activity enhancement of RCC1 by histones H2A and H2B. Science. 2001;292:1540–1543. doi: 10.1126/science.292.5521.1540. [DOI] [PubMed] [Google Scholar]
- Niethammer P, Bastiaens P, Karsenti E. Stathmin-tubulin interaction gradients in motile and mitotic cells. Science. 2004;303:1862–1866. doi: 10.1126/science.1094108. [DOI] [PubMed] [Google Scholar]
- Ohi R, Sapra T, Howard J, Mitchison TJ. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol Biol Cell. 2004;15:2895–2906. doi: 10.1091/mbc.E04-02-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbeck K, Groen AC, Santarella R, Bohnsack MT, Raemaekers T, Kocher T, Gentzel M, Gorlich D, Wilm M, Carmeliet G, et al. NuSAP, a mitotic RanGTP target that stabilizes and cross-links microtubules. Mol Biol Cell. 2006;17:2646–2660. doi: 10.1091/mbc.E05-12-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A, Guse A, Krascenicova I, Schnabel H, Schnabel R, Glotzer M. CSC-1: a subunit of the Aurora B kinase complex that binds to the survivin-like protein BIR-1 and the incenp-like protein ICP-1. J Cell Biol. 2003;161:229–236. doi: 10.1083/jcb.200207117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Sawin KE, Mitchison TJ. Mitotic spindle assembly by two different pathways in vitro. J Cell Biol. 1991;112:925–940. doi: 10.1083/jcb.112.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa F, Mapelli M, Ciferri C, Tarricone C, Areces LB, Schneider TR, Stukenberg PT, Musacchio A. Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol Cell. 2005;18:379–391. doi: 10.1016/j.molcel.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Song K, Kim TM, Kim HJ, Kim JW, Kim HH, Kwon HB, Kim WS, Choi HS. Molecular cloning and characterization of a novel inhibitor of apoptosis protein from Xenopus laevis. Biochem Biophys Res Commun. 2003;301:236–242. doi: 10.1016/s0006-291x(02)03013-9. [DOI] [PubMed] [Google Scholar]
- Tsai MY, Zheng Y. Aurora A kinase-coated beads function as microtubule-organizing centers and enhance RanGTP-induced spindle assembly. Curr Biol. 2005;15:2156–2163. doi: 10.1016/j.cub.2005.10.054. [DOI] [PubMed] [Google Scholar]
- Vader G, Kauw JJ, Medema RH, Lens SM. Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO Rep. 2006;7:85–92. doi: 10.1038/sj.embor.7400562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnarelli P, Earnshaw WC. Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma. 2004;113:211–222. doi: 10.1007/s00412-004-0307-3. [DOI] [PubMed] [Google Scholar]
- Wheatley SP, Kandels-Lewis SE, Adams RR, Ainsztein AM, Earnshaw WC. INCENP binds directly to tubulin and requires dynamic microtubules to target to the cleavage furrow. Exp Cell Res. 2001;262:122–127. doi: 10.1006/excr.2000.5088. [DOI] [PubMed] [Google Scholar]
- Wiese C, Wilde A, Moore MS, Adam SA, Merdes A, Zheng Y. Role of importin-beta in coupling Ran to downstream targets in microtubule assembly. Science. 2001;291:653–656. doi: 10.1126/science.1057661. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Urano T, Kawajiri A, Nagata K, Tatsuka M, Saya H, Furukawa K, Takahashi T, Izawa I, Inagaki M. Autophosphorylation of a newly identified site of Aurora-B is indispensable for cytokinesis. J Biol Chem. 2004;279:12997–13003. doi: 10.1074/jbc.M311128200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


