Abstract
Taste novelty can strongly modulate the speed and efficacy of taste aversion learning. Novel sweet tastes enhance c-Fos-like immunoreactivity (FLI) in the central amygdala and insular cortex. The present studies examined whether this neural correlate of novelty extends to different taste types by measuring FLI signals after exposure to novel and familiar polysaccharide (Polycose®) and salt (NaCl) tastes. Novel Polycose not only failed to elevate FLI expression in central amygdala and insular cortex, but also failed to induce stronger taste aversion learning than familiar Polycose. Novel NaCl, on the other hand, showed patterns of FLI activation and aversion learning similar to that of novel sweet tastes. Possible reasons for the resistance of Polycose to typical pre-exposure effects are discussed.
Keywords: learning, taste, neophobia, c-Fos, latent inhibition
Conditioned taste aversion (CTA) learning, in which an individual comes to reject a previously neutral or preferred taste because of a learned association between the taste and a subsequent period of illness, has been the subject of an extensive body of research (Bures, Bermúdez-Rattoni, & Yamamoto, 1998; Rozin & Kalat, 1971; Sander, 2004; Scalera, 2002; Welzl, D’Adamo, & Lipp, 2001). Indeed, interest in this learning model stems in part from the fact that CTAs differ from most forms of classical conditioning in the speed and efficacy with which they can be learned. Studies have shown that CTA learning is rapidly acquired, usually requiring only a single conditioning trial, can tolerate long delays on the order of minutes to hours between presentation of the taste (conditioned stimulus [CS]) and a treatment that induces illness (unconditioned stimulus [US]), and is very robust (Bernstein, 1991; Domjan, 1980; Garcia, Hankins, & Rusiniak, 1974). That it is possible to pinpoint the exact time when the animal learns the association (during the first CS–US pairing) and that the consequent learning is so strong, makes CTA learning a useful and intriguing model for understanding the molecular and neuronal changes that take place during learning (Chambers, 1990; Rosenblum, Meiri, & Dudai, 1993; Yamamoto & Fujimoto, 1991). To this end, several studies (Cubero, Thiele, & Bernstein, 1999; Houpt, Philopena, Joh, & Smith, 1996; Koh, Clarke, Spray, Thiele, & Bernstein, 2003a; Lamprecht & Dudai, 1996; Spray & Bernstein, 2004) have mapped pathways involved in the acquisition and recall of CTA learning by using the expression of activity-dependent immediate early genes such as c-fos—pathways that may represent taste memory–taste aversion processing.
Another area of interest in understanding the underlying circuitry of CTA learning stems from the fact that taste novelty dramatically impacts the speed and efficacy of the learning. Familiar tastes are quite resistant to formation of aversions and often require numerous CS–US pairings for learning to occur (Kalat & Rozin, 1973; Revusky & Bedarf, 1967). Using the decrease in CS efficacy that accompanies familiarity, Koh, Wilkins, and Bernstein (2003b) used c-Fos-like immunoreactivity (FLI) in response to a novel and familiar CS taste in order to identify changes in gene expression that may be necessary for learning while controlling for nonspecific gene expression associated with general exposure to sensory stimuli. When the taste was novel, significantly higher levels of FLI were seen in the insular cortex (IC) and central amygdala (CNA), two areas previously implicated in taste aversion learning. The results also suggest that synthesis and subsequent degradation of c-Fos protein within these cells may represent a biochemical “trace” of the novel taste that could have the potential to bridge some CS–US intervals and play a role in mediating long-delay learning.
The studies discussed above used a saccharin solution as the novel–familiar CS taste. Because taste aversions can be conditioned to virtually any novel tastant, it is important to establish that the novelty-based finding obtained with saccharin can generalize to other tastants. In this study we looked to see if patterns in neuronal activation and CTA learning with novel and familiar Polycose and salt (NaCl) tastes resembled those seen with saccharin.
Experiment 1
Our choice of testable taste qualities other than sweet tastes such as saccharin was constrained because we desired to use distinct and novel taste qualities while minimizing potential complications of odor cues. Primary tastes are limited to some five or six distinct types: salty, sour, sweet, bitter, and possibly umami (MSG) and polysaccharide (Brand, 2000; Sclafani, 2004). Sour and bitter stimuli, particularly in high concentrations, tend to be innately aversive, thus complicating the investigation of taste aversion learning as rats find the stimulus aversive even before conditioning. Even NaCl is aversive at high concentrations. We reasoned that Polycose®, a well-studied polysaccharide preparation, would be a good choice for the present studies because it is highly palatable to rats in a wide range of concentrations and does not appear to taste sweet, as aversions to Polycose fail to generalize to sweet stimuli (Nissenbaum & Sclafani, 1987; Sako et al., 1994). In this study we exposed rats to a familiar or novel aqueous solution of Polycose (30% wt/vol). This high, but palatable, concentration was chosen because prior studies in our lab indicated that high taste intensity as well as novelty might be necessary for detecting elevated fos gene expression.
For our first experiment, then, we compared FLI induced by novel and familiar Polycose in order to determine whether differences in patterns of neuronal activation would be evident with this nonsweet tastant.
Method
Subjects
Twenty-four experimentally naïve male Long–Evans rats (Charles River, Raleigh, NC) served as subjects for this study. The rats weighed between 300 and 410 g at the onset of the study and were individually housed in suspended stainless steel cages. Rats were maintained on a 12:12-hr light–dark cycle (lights on at 0700) with ad libitum access to food (LabDiet 5012, Brentwood, MO) and water except as noted below. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington.
Pre-exposure and testing
Three groups of rats (Polycose familiar, Polycose novel, and water control; n = 8 per group) were matched for body weight and fluid intake and maintained on a fluid restriction regimen in which access to liquids occurred twice a day from 10:00 a.m. to 10:30 a.m. and from 4:20 p.m. to 5 p.m. The Polycose familiar group was exposed to the Polycose solution during their morning drinking session and to water during their evening drinking session for 6 consecutive days, whereas the Polycose novel group received water for all drinking sessions. The water control group was composed of 4 rats that had and 4 that did not have prior experience with Polycose. Intakes were measured daily. On test day, the Polycose novel and Polycose familiar groups were given 5 ml of the Polycose solution from a graduated water bottle in their home cages. The water control group was given only water and served as a baseline for FLI in the absence of taste stimulation. The time needed to fully consume the solution or water was recorded for each rat.
Immunohistochemistry
Two hours after taste exposure, rats were deeply anesthetized with sodium pentobarbital and perfused initially with phosphate buffered saline (PBS) and then 4% paraformaldehyde. The rats’ brains were post-fixed with 4% paraformaldehyde for an additional 2–3 days. After post-fixation, the brains were blocked to include areas of interest and sectioned into 50 μm slices (in the transverse plane) using a vibratome. Slices were then rinsed (3× PBS), incubated for 20 min in 0.3% hydrogen peroxide in absolute methanol to quench endogenous peroxidase, and rinsed once again (3× PBS). This was followed by 1 hr incubation in a normal goat blocking agent composed of a 3% normal goat serum mixed with 0.1% bovine albumin in a PBS-0.4% Triton X 100 solution. Slices then were transferred, without rinsing, to the primary antibody solution, which consisted of 1:20k c-fos polyclonal rabbit IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and were incubated for 48 hr at approximately 4 °C. Following incubation, slices were rinsed (4× PBS, 30 min) and processed using the standard ABC method (Vector Laboratories, Burlingame, CA) and developed with 3,3′-diamino-benzidine and nickel chloride enhancement techniques to visualize the presence of FLI. After development, slices were rinsed (10× PB 1×, 30 min), mounted on gelatin coated glass slides, and counterstained with neutral red.
FLI counts were based on a single representative section per region for each rat. An investigator unaware of experimental conditions chose and plotted sections on the basis of anatomical landmarks. FLI positive neurons were defined as cells with nuclei that showed a solid reaction product that covered at least half of the nucleus.
Results and Discussion
FLI to novel and familiar Polycose do not differ
Mean latency to consume 5 ml of Polycose or water on test day is shown in Figure 1. Rats that were inexperienced with Polycose took no longer to fully consume the solution than rats that were previously familiarized with Polycose or just given water. This behavioral lack in neophobia was quite striking as it contrasted with previous work from this lab showing large differences in latency needed to drink a pre-exposed or novel saccharin solution.
Figure 1.

Latency needed to fully consume 5 ml of 30% Polycose (novel and familiar groups) or water (control group). No significant differences between groups were observed. Error bars represent the standard error of the mean.
Neural correlates of novelty, elevated FLI in the CNA and IC, were also absent in rats exposed to Polycose. Figure 2 displays average FLI expression in IC, CNA, and basolateral amygdala (BLA), and Figure 3 shows sample micrographs of these regions for representative rats across conditions. Unlike what was seen in previous experiments using novel and familiar saccharin, FLI levels were generally very low and no reliable differences were found as a function of taste novelty. In fact, neither group receiving Polycose differed from control rats that only received water. These findings imply that a behavioral lack of neophobia to Polycose is associated with a lack of differential FLI in areas previously found to be sensitive to taste novelty.
Figure 2.
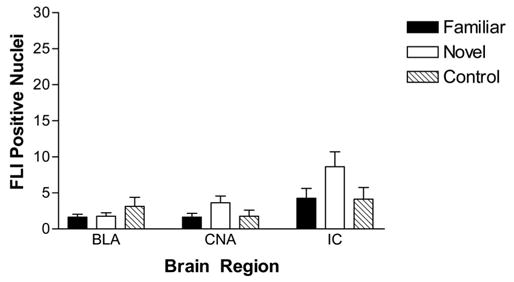
Mean number (±SEM) of nuclei positive for Fos-like immunoreactivity (FLI) in the basolateral (BLA) and central (CNA) subnuclei of the amygdala, and insular cortex (IC) following exposure to either a familiar Polycose solution, a novel Polycose solution, or water. Significant increases in FLI were not seen in any area for any group.
Figure 3.

Coronal sections stained for Fos-like immunoreactive (FLI) positive nuclei (shown as discrete, dark stained cells) in the basolateral amygdala (BLA), central amygdala (CNA), and insular cortex (IC) of rats receiving either a familiar (top) or novel (middle) Polycose taste, or water (bottom). FLI scoring was done at a 200× magnification. (CPu = caudate putamen).
Experiment 2
Unlike prior work with saccharin, pre-exposure to a Polycose taste failed to attenuate behaviors that are indicative of neophobia or produce differential expression of FLI. A more sensitive assessment of a pre-exposure effect, or latent inhibition, is the marked reduction of CTA learning after pre-exposure to a taste. To further assess whether rats respond differently to Polycose as a function of taste novelty, we evaluated the effect of Polycose taste pre-exposure on the strength of CTA learning.
Method
Subjects
Subjects for this experiment consisted of 28 male Long–Evans rats (315–390 g). Rats had not been used in any previous experiments and were housed exactly as in Experiment 1.
Pre-exposure, conditioning, and testing
Rats were maintained on the water restriction schedule as described in the previous experiment and received either six 30-min daily exposures to a 30% Polycose solution (familiar conditioned, n = 8, and familiar control, n = 6) or water (novel conditioned, n = 8, and novel control, n = 6). Rats were divided into groups matched on body weight and fluid intake during pre-exposure. On the conditioning day, all of the rats received 5 ml of Polycose in their home cages and were allowed to drink the entire amount. For novel rats, this was their first experience with Polycose. The time needed to fully consume the solution was recorded, and the rats were then given an immediate injection of either 0.15 M LiCl (1% body weight, ip) or 0.9% saline (1% body weight, ip). Conditioning day was followed by a recovery day when rats received only water in both the morning and afternoon.
Rats received a one-bottle taste test 2 days after conditioning in which they received Polycose in a graduated drinking tube. The amount each rat consumed after 1 hr was recorded and used as an index of CTA learning.
Analysis
A two-way analysis of variance (ANOVA) was used to find differences between groups. Because we were interested in only certain comparisons before hand, a priori planned contrasts were used whenever significant main effects were found.
Results and Discussion
CTAs to Polycose do not differ as a function of taste novelty
Figure 4 depicts Polycose intake on the test day. An ANOVA revealed a main effect for conditioning only, F(3, 24) = 11.579, p < .001; no differences were seen as a product of taste novelty. Novel rats that received LiCl on conditioning day drank significantly less than novel rats that received only an injection of saline (contrast value = −8.58 ± 1.65), t(11.78) = −4.99, p < .001. The same held true for familiar rats, with conditioned rats drinking significantly less than unconditioned control rats (contrast value = −4.58 ± 1.63), t(8.05) = −2.81, p = .023. Rats conditioned with LiCl showed significant CTAs, regardless of prior exposure to the taste and did not differ from each other in the amount consumed. Because we found no differences between familiar and novel conditioned rats, additional test trials were run to see if the two groups would differ in the time required to extinguish the learning. However, no significant differences emerged, and both groups showed extinction within 2 days (data not shown).
Figure 4.
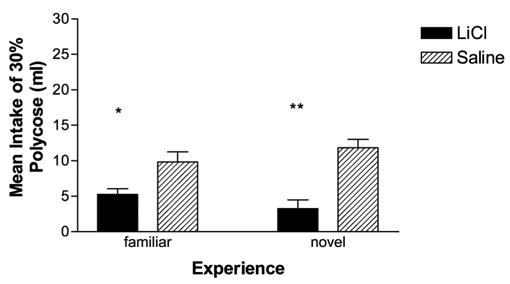
Average intake of Polycose (±SEM) after 1 hr of testing by rats previously conditioned (LiCl) to either a familiar or novel Polycose solution. Intakes were compared with unconditioned control rats (Saline) in order to evaluate if aversions were learned. Rats both familiar and unfamiliar with Polycose at the time of conditioning drank significantly less than unconditioned controls (* p < .05 between familiar LiCl and saline paired rats; ** p < .005 between novel LiCl and saline paired rats).
The fact that both familiar and novel conditioned groups showed comparable CTAs again contrasted with studies using saccharin, where familiarized rats showed greatly attenuated, or even no evidence of, learning (Koh & Bernstein, 2005). These findings further support the outcome of Experiment 1 and suggest that unlike saccharin, differential behavioral and neuronal responses to novelty were absent with Polycose.
Experiment 3
The previous experiments indicate that the effects of stimulus novelty commonly ascribed to taste stimuli may not apply to Polycose. Thus, Polycose may be unsuitable as a taste stimulus for assessing the generality of the effects reported by Koh et al. (2003b) with saccharin. The next experiment repeated Experiment 1 but used a NaCl solution as the novel and familiar taste stimulus. Because high NaCl solution concentrations are aversive to sodium replete rats, and because concentrated NaCl is far less hydrating to thirsty rats, we chose an isotonic (0.9%) concentration of NaCl. Studies have shown that most strains of rats find this concentration of NaCl to be palatable and prefer such salt solutions to water (Midkiff, Fitts, Simpson, & Bernstein, 1985). We examined whether a novel NaCl taste induces a pattern of neuronal activation that is more similar to novel saccharin.
Method
Eleven experimentally naïve Long–Evans rats (390–440 g) were used in this experiment. Methods were identical to those used in Experiment 1, except that rats received 0.9% NaCl (wt/vol) instead of Polycose. Familiar rats (n = 5) were pre-exposed to NaCl and novel rats (n = 6) were not; no water control rats were included. All other housing conditions were identical to previous experiments.
Results and Discussion
Induction of FLI by novel NaCl taste exposure
Evidence for neophobia to novel NaCl was evident on the test day, when all of the rats received 5 ml of the NaCl solution. Rats that were unfamiliar with the solution took significantly longer to consume 5 mls than those that were familiar with it, t(9) = 2.74, p = .028 (see Figure 5).
Figure 5.
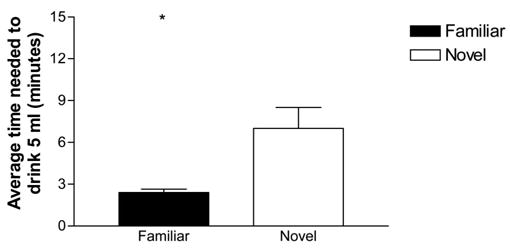
Latency (±SEM) needed to fully consume 5 ml of a 0.9% salt solution by rats that were or were not familiarized to the solution before hand. Familiar rats took significantly less time to drink all 5 ml (* p < .05).
In line with the behavioral evidence of neophobia, differential FLI induction as a function of taste novelty was evident. As depicted in Figures 6 and 7, NaCl taste induced significantly more FLI in IC and CNA when it was novel than when it was familiar, t(9) = 2.57, p = .03 and t(9) = 2.42, p = .038, respectively. Differences in FLI in the BLA as a function of taste novelty were not statistically significant (p > .05). These data closely replicate the results of Koh et al. (2003b) and tend to support the generality of those findings. Moreover, an association was demonstrated between behavioral evidence of neophobia and a difference in FLI as a function of taste novelty. Both saccharin and NaCl show a strong behavioral novelty effect as well as a neural correlate: increased FLI expression in IC and CNA.
Figure 6.

Mean number of Fos-like immunoreactive (FLI) positive nuclei (±SEM) in the basolateral (BLA) and central (CNA) nuclei of the amygdala and insular cortex (IC) after receiving a familiar or novel salt solution. Rats unfamiliar with the taste showed significantly higher FLI activity in the CNA and IC than did familiar rats (* p < .05).
Figure 7.
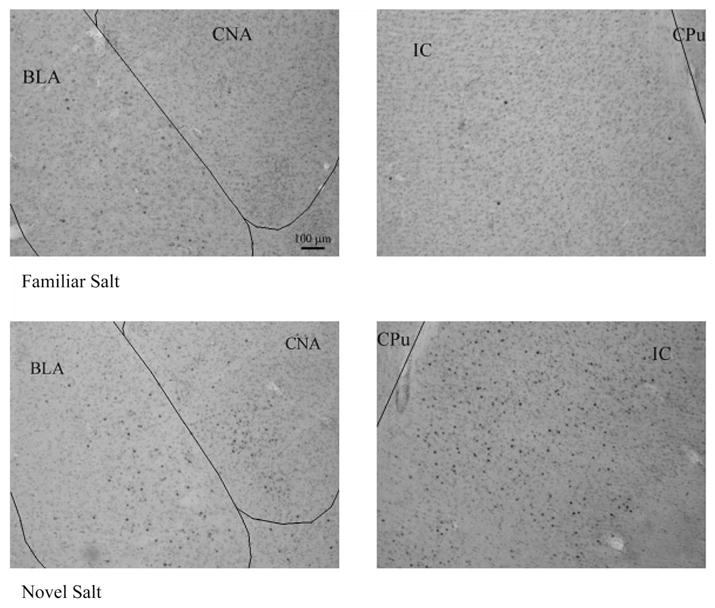
Fos-like immunoreactive (FLI) stained coronal sections of the basolateral amygdala (BLA), central amygdala (CNA), and insular cortex (IC) of rats given a familiar (top) or novel (bottom) 0.9% salt solution. Novel rats showed significantly higher levels of FLI activity in the CNA and IC than familiar rats. (CPu = caudate putamen).
Experiment 4
As a final measure of the generality of patterns observed with saccharin and taste novelty, we confirmed a differential pattern of CTA learning as a function of NaCl taste novelty. Thus, rats in this study were conditioned with either a novel or familiar 0.9% NaCl solution.
Method
Twenty-four male Long–Evans rats weighing between 340 and 420 g at the onset were used for this final experiment. Methods were identical to those in Experiment 2 except that rats received 0.9% NaCl solution instead of Polycose as the tastant (n = 6 per group). Once again, there were no other differences in experimental history, housing, and care of the rats from previous experiments in this study.
Results and Discussion
CTAs to NaCl differ dramatically as a function of taste novelty
Intake of NaCl on the test day (Figure 8) reveals a dramatic difference in CTA learning between novel and familiar conditioned rats. No aversions were seen in the familiar rats and aversions were so strong in the novel rats that they consumed virtually none of the solution. This was confirmed statistically, F(3, 20) = 14.93, p < .001, and contrast tests indicated that conditioned rats that were familiar with the CS taste drank significantly more on test day than rats that were not (contrast value = 18.17 ± 3.56), t(5.04) = 5.10, p < .005. In fact, familiar conditioned rats did not differ from unconditioned familiar control rats, indicating that they failed to learn the aversion. Novel conditioned rats, on the other hand, drank significantly less than their unconditioned control rats (contrast value = −19.33 ± 2.85), t(5.06) = −6.77, p < .005.
Figure 8.

Average intake (±SEM) after 1 hr of exposure to 0.9% salt by rats that previously had been conditioned (LiCl) to a familiar or novel 0.9% salt solution. Intakes were compared with unconditioned control rats (saline) in order to assess if aversions were learned. Novel conditioned (novel-LiCl) rats drank significantly less than their control rats (novel-Saline), whereas as familiar conditioned (familiar-LiCl) rats showed no evidence of learning and did not differ with their unconditioned control rats. Familiar-LiCl rats also drank significantly more than novel-LiCl rats. (* p < .005 for the difference between novel-LiCl rats and their control rats; and † p < .005 for the difference between novel-LiCl and familiar-LiCl rats).
This final study confirmed the well-established effect of taste pre-exposure on CTA learning using an isotonic NaCl taste. Again, results are very similar to those for saccharin (Koh & Bernstein, 2005; Koh et al., 2003b) but dissimilar to those seen in Experiments 1 and 2 with Polycose.
General Discussion
Two distinct tastants were tested for their sensitivity to taste exposure as a means of establishing the taste as familiar. Familiar tastes are resistant to the development of CTAs and are readily consumed with no evidence of hesitancy or neophobia. Novel tastes are potent targets for one-trial taste aversion conditioning, are consumed with some reluctance (neophobia), and are effective at increasing FLI in CNA and IC. A salt solution, as a taste CS, conformed to this pattern and was consistent with previous studies using saccharin and other tastants (Bennet, Wills, Wells, & Mackintosh, 1994; Franchina & Slank, 1988; Riley & Bornovalova, 2005). In contrast, Polycose was found to be quite dissimilar. No differences were observed between rats familiarized with Polycose and those for whom it was novel in amount consumed, latency to consume 5 ml, CTA acquisition, or FLI in the CNA and IC. If our findings with novel versus familiar Polycose were limited only to the FLI dependent measure, we would have questioned the generality of the FLI response as a correlate of taste novelty. However, the consistent absence of a pre-exposure effect across both behavioral and neural outcome measures led us to focus on this unusual property of Polycose as a tastant.
The surprising pattern observed with Polycose raises questions about why Polycose would be so unlike other tastants with regard to this taste novelty–familiarity distinction. Although novel and familiar Polycose are treated similarly by rats, it is not clear from these studies whether both are viewed as novel or both are viewed as familiar. The low level of FLI seen in the brains of rats given a novel Polycose solution would suggest that it is somehow familiar, inasmuch as FLI in CNA and IC is a correlate of taste novelty. Moreover, neither familiar nor novel rats differed in their latency to drink Polycose as compared with control rats receiving only water. On the other hand, the evidence of significant, albeit weak, CTAs after a single CS–US pairing in rats familiarized with Polycose would suggest that it is, at least, somewhat novel.
One possibility is that rats do not view Polycose as particularly novel even at first presentation because it is similar in taste to other starches or polysaccharides they have experienced in their lab life history. An argument against this explanation is that “learned safety” for tastes has been shown to be strikingly specific. Generalization of latent inhibition across different tastant concentrations is limited (Gilley & Franchina, 1985). Furthermore, one could clearly adopt a similar argument for NaCl, which is found in saliva, urine, and food, yet striking novelty effects were seen here with isotonic NaCl.
Another possibility is that the solution concentration used was somehow responsible for the anomalous findings. Koh et al. (2003b) found that 0.5% saccharin solution, but not a 0.15% saccharin solution, was associated with elevated FLI in CNA and IC as a correlate of taste novelty. However, both solutions were associated with strong novelty effects when CTA learning was examined. Thus, solution concentration may be a factor in the FLI response because it requires relatively high levels of neuronal activation for the detection of significant effects. However, this would not explain the failure to see behavioral evidence of novel–familiar taste differences, particularly in CTA acquisition. Moreover, the Polycose solution concentration was extremely high, and it would be hard to argue that lowering the concentration would change the pattern we saw.
On the other hand, one could argue that this high concentration could enhance characteristic differences between Polycose and saccharin–NaCl that are independent of taste quality. A 30% Polycose solution has a high caloric load—whereas saccharin and salt solutions are nonnutritive—and is rather viscous in texture. This lends to the possibility that differential texture and post-ingestive effects are responsible for our findings with Polycose. Although this possibility cannot be excluded on the basis of the studies presented here, we do not favor this interpretation for the following reasons. Texture and heavy caloric load may explain why familiar rats take longer to consume 5 ml of Polycose than NaCl, and why unconditioned animals drank less 30% Polycose than 0.9% NaCl on test day, but satiety, post-ingestive cues, and viscous texture cannot adequately explain the absence of behavioral and neuronal novelty effects in rats receiving Polycose. Sucrose, a highly palatable and nutritive tastant, elicits neophobic responses in the form of increased hesitancy and diminished consumption when it is novel and induces stronger CTA learning than familiar sucrose (Klein, Mikulka, & Hamel, 1976; Meinrath & Faherty, 1988; Nachman & Ashe, 1974). Moreover, one could argue that post-ingestive cues should not strongly affect a rat’s hesitancy or readiness to consume a novel tastant, as such cues would not be readily available for a tastant that has not been previously consumed. Rather, post-ingestive cues should shape behavioral responses to subsequent taste exposures and aid in familiarization. The highly reinforcing post-ingestive effects of Polycose (Sclafani & Nissenbaum, 1988) may explain why rats that are conditioned with a novel Polycose tastant learn only moderate aversions, but it does not explain why familiar rats also learn a comparable aversion. Finally, a viscous texture should, if anything, add an extra dimension of novelty to the tastant, as a highly viscous solution is unlike anything the rats have previously experienced.
The argument that the rats simply cannot taste Polycose is also not tenable for a number of reasons. Rats form significant aversions to it, and they prefer even low concentrations (0.0001 M) of Polycose to water (Sclafani & Nissenbaum, 1987). Moreover, electrophysiological recordings have shown activation of cells in the nucleus of the solitary tract in response to Polycose as well as to other nonsweet tastes (Giza, Scott, Sclafani, & Antonucci, 1991).
It has been suggested that the taste of polysaccharides such as Polycose may represent a fifth (or sixth) basic taste for rats, as its taste quality does not appear similar to sweet, salty, sour, bitter or umami stimuli (Ackroff, Manza, & Sclafani, 1993; Sclafani, 2004). However, the receptors and distinct coding circuits that underlie this taste are not yet well defined. Whether this “uniqueness” extends to a difference in the role of taste novelty remains to be determined.
Although the unusual property of Polycose identified here remains unexplained, the complementary studies with NaCl provide important confirmation of previous work defining the effect of taste novelty on FLI in CNA and IC. However, our findings with Polycose might require that we amend our definition of novel. Perhaps novelty should not be viewed solely as a characteristic of a tastant, but also as a characteristic of the rat’s behavioral response to the tastant. As such, FLI enhancement may be correlated to the rat’s behavioral response (i.e., presence or absence of neophobia) as much as to the novelty of the taste itself. Thus, when the rat displays a novelty response to a tastant, as in the case of novel saccharin and NaCl, an increase in FLI is observed, but when no novelty response is provoked (familiar saccharin and NaCl; familiar and novel Polycose), no enhancement in expression is detected. Why such dissociation was seen with Polycose between novelty of the taste and novelty response of the rats is an intriguing question worth further examination.
Footnotes
This research was funded by the National Institute of Health (NIH) NS37040 to Ilene L. Bernstein and by the National Science Foundation Graduate Research Fellowship Program to Sabiha K. Barot. We thank Ming Teng Koh and Benjamin Land for all of their help and support in conducting these experiments.
References
- Ackroff K, Manza L, Sclafani A. The rat’s preference for sucrose, Polycose and their mixtures. Appetite. 1993;21:69–80. doi: 10.1006/appe.1993.1037. [DOI] [PubMed] [Google Scholar]
- Bennet CH, Wills SJ, Wells JO, Mackintosh NJ. Reduced generalization following preexposure: Latent inhibition of common elements or a difference in familiarity? Journal of Experimental Psychology Animal Behavior Processes. 1994;20:232–239. doi: 10.1037//0097-7403.20.3.232. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nature Review Neuroscience. 2004;5:209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- Bernstein IL. Flavor aversion. In: Getchell TV, Doty RL, Bartoshuk LM, Snow JB, editors. Smell and taste in health and disease. New York: Raven Press; 1991. pp. 417–428. [Google Scholar]
- Brand JG. Receptor and transduction processes for umami taste. Journal of Nutrition. 2000;130:942–945. doi: 10.1093/jn/130.4.942S. [DOI] [PubMed] [Google Scholar]
- Bures J, Bermúdez-Rattoni F, Yamamoto T. Conditioned taste aversion: Memory of a special kind. New York: Oxford University Press; 1998. [Google Scholar]
- Chambers KC. A neural model for conditioned taste aversions. Annual Review of Neuroscience. 1990;13:373–385. doi: 10.1146/annurev.ne.13.030190.002105. [DOI] [PubMed] [Google Scholar]
- Cubero I, Thiele TE, Bernstein IL. Insular cortex lesions and taste aversion learning: Effects of conditioning method and timing of lesion. Brain Research. 1999;839:323–330. doi: 10.1016/s0006-8993(99)01745-x. [DOI] [PubMed] [Google Scholar]
- Domjan M. Ingestional aversion learning: Unique and general processes. In: Rosenblatt JS, Hinde RA, Beer C, Busnel M-C, editors. Advances in the study of behavior. Vol. 11. New York: Academic Press; 1980. pp. 275–336. [Google Scholar]
- Franchina JJ, Slank KL. Salience and the effects of CS preexposure on aversion conditioning. Behavioral and Neural Biology. 1988;50:367–373. doi: 10.1016/s0163-1047(88)91114-4. [DOI] [PubMed] [Google Scholar]
- Garcia J, Hankins WG, Rusiniak KW. Behavioral regulation of the milieu interne in man and rat. Science. 1974;185:824–831. doi: 10.1126/science.185.4154.824. [DOI] [PubMed] [Google Scholar]
- Gilley DW, Franchina JJ. Effects of preexposure flavor concentration on conditioned aversion and neophobia. Behavioral and Neural Biology. 1985;44:503–508. doi: 10.1016/s0163-1047(85)91000-3. [DOI] [PubMed] [Google Scholar]
- Giza BK, Scott TR, Sclafani A, Antonucci RF. Polysaccharides as taste stimuli: Their effect in the nucleus tractus solitarius of the rat. Brain Research. 1991;555:1–9. doi: 10.1016/0006-8993(91)90852-m. [DOI] [PubMed] [Google Scholar]
- Houpt TA, Philopena JM, Joh TH, Smith GP. c-Fos induction in the rat nucleus of the solitary tract correlates with the retention and forgetting of a conditioned taste aversion. Learning and Memory. 1996;3:25–30. doi: 10.1101/lm.3.1.25. [DOI] [PubMed] [Google Scholar]
- Kalat JW, Rozin P. “Learned safety” as a mechanism in long-delay taste-aversion learning in the rat. Journal of Comparative & Physiological Psychology. 1973;83:198–207. doi: 10.1037/h0034424. [DOI] [PubMed] [Google Scholar]
- Klein SB, Mikulka PJ, Hamel K. Influence of sucrose preexposure on acquisition of a conditioned aversion. Behavioral Biology. 1976;16:99–106. doi: 10.1016/s0091-6773(76)91186-x. [DOI] [PubMed] [Google Scholar]
- Koh MT, Bernstein IL. Mapping conditioned taste aversion associations using c-Fos reveals a dynamic role for insular cortex. Behavioral Neuroscience. 2005;119:388–398. doi: 10.1037/0735-7044.119.2.388. [DOI] [PubMed] [Google Scholar]
- Koh MT, Clarke SNDA, Spray KJ, Thiele TE, Bernstein IL. Conditioned taste aversion memory and c-Fos induction are disrupted in RIIβ-protein kinase A mutant mice. Behavioral Brain Research. 2003a;143:57–63. doi: 10.1016/s0166-4328(03)00024-x. [DOI] [PubMed] [Google Scholar]
- Koh MT, Wilkins EE, Bernstein IL. Novel tastes elevate c-Fos in the central amygdala and insular cortex: Implications for taste aversion learning. Behavioral Neuroscience. 2003b;117:1416–1422. doi: 10.1037/0735-7044.117.6.1416. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Dudai Y. Transient expression of c-fos in rat amygdala during training is required for encoding conditioned taste aversion memory. Learning & Memory. 1996;3:31–41. doi: 10.1101/lm.3.1.31. [DOI] [PubMed] [Google Scholar]
- Meinrath AB, Faherty CF. Effect of varied taste experience on negative contrast in consummatory behavior. American Journal of Psychology. 1988;101:87–96. [PubMed] [Google Scholar]
- Midkiff E, Fitts DA, Simpson JB, Bernstein IL. Absence of sodium chloride preference in Fischer-344 rats. American Journal of Physiology. 1985;249:R438–R442. doi: 10.1152/ajpregu.1985.249.4.R438. [DOI] [PubMed] [Google Scholar]
- Nachman M, Ashe JH. Effects of basolateral amygdala lesions on neophobia, learned taste aversions, and sodium appetite in rats. Journal of Comparative and Physiological Psychology. 1974;87:622–643. doi: 10.1037/h0036973. [DOI] [PubMed] [Google Scholar]
- Nissenbaum JW, Sclafani A. Qualitative differences in polysaccharide and sugar tastes in the rat: A two carbohydrate taste model. Neuroscience & Biobehavioral Reviews. 1987;11:187–196. doi: 10.1016/s0149-7634(87)80025-8. [DOI] [PubMed] [Google Scholar]
- Reilly S, Bornovalova MA. Conditioned taste aversion and amygdala lesions in the rat: A critical review. Neuroscience & Biobehavioral Reviews. 2005;29:1–22. doi: 10.1016/j.neubiorev.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Revusky SH, Bedarf EW. Association of illness with prior ingestion of novel foods. Science. 1967;155:219–220. doi: 10.1126/science.155.3759.219. [DOI] [PubMed] [Google Scholar]
- Rosenblum K, Meiri N, Dudai Y. Taste memory: The role of protein synthesis in gustatory cortex. Behavioral and Neural Biology. 1993;59:49–56. doi: 10.1016/0163-1047(93)91145-d. [DOI] [PubMed] [Google Scholar]
- Rozin P, Kalat JW. Specific hungers and poison avoidance as adaptive specializations of learning. Psychological Review. 1971;78:459–486. doi: 10.1037/h0031878. [DOI] [PubMed] [Google Scholar]
- Sako N, Shimura T, Komure M, Mochizuki R, Matsuko R, Yamamoto T. Differences in taste responses to Polycose and common sugars in the rat as revealed by behavioral and electrophysiological studies. Physiology & Behavior. 1994;56:741–745. doi: 10.1016/0031-9384(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Sander G. Lower animal conditioning studies help in the understanding of human memory and its disorders: The merits of conditioned taste, odor, and flavor aversion research. American Journal of Physiology—Regulatory, Integrative, and Comparative Physiology. 2004;286:251–253. doi: 10.1152/ajpregu.00596.2003. [DOI] [PubMed] [Google Scholar]
- Scalera G. Effects of conditioned food aversions on nutritional behavior in humans. Nutritional Neuroscience. 2002;5:159–188. doi: 10.1080/10284150290013059. [DOI] [PubMed] [Google Scholar]
- Sclafani A. The sixth taste? Appetite. 2004;43:1–3. doi: 10.1016/j.appet.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Nissenbaum JW. Taste preference thresholds for Polycose, maltose, and sucrose in rats. Neuroscience & Biobehavioral Reviews. 1987;11:181–185. doi: 10.1016/s0149-7634(87)80024-6. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Nissenbaum JW. Robust conditioned flavor preference produced by intragastric starch infusion in rats. American Journal of Physiology. 1988;255 (4 Pt 2):R672–R675. doi: 10.1152/ajpregu.1988.255.4.R672. [DOI] [PubMed] [Google Scholar]
- Spray KJ, Bernstein IL. Afferent and efferent connections of the parvicellular subdivisions of iNTS: Defining a circuit involved in taste aversion learning. Behavioural Brain Research. 2004;154:85–97. doi: 10.1016/j.bbr.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Welzl H, D’Adamo P, Lipp H. Conditioned taste aversion as a learning and memory paradigm. Behavioural Brain Research. 2001;125:205–213. doi: 10.1016/s0166-4328(01)00302-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Fujimoto Y. Brain mechanisms of taste aversion learning in the rat. Brain Research Bulletin. 1991;27:403–406. doi: 10.1016/0361-9230(91)90133-5. [DOI] [PubMed] [Google Scholar]


