Abstract
Conditioned fear (CF) is one of the most frequently used behavioral paradigms; however, little work has mapped changes in cerebral perfusion during CF in the rat—the species which has dominated CF research. Adult rats carrying an implanted minipump were exposed to a tone (controls, n = 8) or a tone conditioned in association with footshocks (CS group, n = 9). During reexposure to the tone 24 h later, animals were injected intravenously by remote activation with [14C]-iodoantipyrine using the pump. Significant group differences in regional CBF-related tissue radioactivity (CBF-TR) were determined by region-of-interest analysis of brain autoradiographs, as well as in the reconstructed, three-dimensional brain by statistical parametric mapping (SPM). CS animals demonstrated significantly greater, fear-enhanced increases in CBF-TR in auditory cortex than controls. The lateral amygdala was activated, whereas the basolateral/basomedial and central amygdala were deactivated. In the hippocampus and medial prefrontal cortex, CBF-TR increased significantly ventrally but not dorsally. Significant activations were noted in medial striatum and the thalamic midline and intralaminar nuclei. However, the ventrolateral/dorsolateral striatum and its afferents from motor and somatosensory cortex were deactivated, consistent with the behavioral immobility seen during CF. Significant activations were also noted in the lateral septum, periaqueductal gray, and deep mesencephalic nucleus/tegmental tract. Our results show that auditory stimuli endowed with aversive properties through conditioning result in significant redistribution of cerebral perfusion. SPM is a useful tool in the brain mapping of complex rodent behaviors, in particular the changes in activation patterns in limbic, thalamic, motor, and cortical circuits during CF.
Keywords: Brain mapping, Functional neuroimaging, Fear conditioning, Cerebral blood flow, Statistical parametric mapping, Medial prefrontal cortex, Amygdala, Hippocampus, Striatum, Septum, Periaqueductal gray
Introduction
Fear conditioning (CF) has been extensively used to explore the neural circuitry of fear and emotional learning (Fanselow and Tighe, 1988; Kim and Fanselow, 1992; Anagnostaras et al., 1999; LeDoux, 2000). Previous work in the rat relating changes in CF behavior to changes in brain function has been based to a large extent on lesion studies. While lesion methods have yielded substantial information, nonspecific damage to fibers of passage or overlying structures may complicate interpretation of results (Lomber, 1999). A question that has been repeatedly raised is whether freezing deficits observed after lesions truly represent a memory deficit or rather a deficit in performing the fear response (Gewirtz et al., 2000; Anagnostaras et al., 2001; Maren, 2003).
Experimental methods that do not require lesioning include electroencephalography (Romanski et al., 1993; Garcia et al., 1998; Tang et al., 2001), measurement of cytochrome oxidase (a rate-limiting mitochondrial enzyme for oxidative energy metabolism of ATP) (Poremba et al., 1998), measurement of the expression of early response genes such as c-fos (Beck and Fibiger, 1995), and measurement of glucose metabolism (Barrett et al., 2003). While brain electrical recordings offer the advantage of detailed information regarding the temporal and spatial synchronization of cognitive processes, only limited cortical and subcortical areas can be mapped in a single animal. Measurements of cytochrome oxidase and early gene responses offer spatial resolution at the cellular level, however, in order to elicit a robust signal, these methods require averaging of cerebral activation patterns over a prolonged period (days to weeks for cytochrome oxidase, minutes to hours for the early gene response). Likewise, mapping glucose metabolism using the deoxyglucose method signals is averaged over several minutes. Such averaging, when applied to a cognitive process such as CF, characterized by rapid formation and retrieval of a memory, may result in the inclusion of nonspecific mental processes in the overall picture of neural activation.
Regional cerebral perfusion has been extensively used as a surrogate marker of neural activation to obtain ‘snapshots’ of cognitive and sensory processes occurring over a brief time frame (seconds). Such methods have been used in human subjects exposed to the CF paradigm (Büchel and Dolan, 2000). However, little work has been done to map brain blood flow during CF in the rat—the species, which historically, has dominated CF research. A single prior study by Ledoux et al. measured local cerebral blood flow (CBF) in immobilized rats during auditory-cued CF and demonstrated an increase in blood flow in regions of interests corresponding to the hypothalamus and amygdala (LeDoux et al., 1983). In the present study, we examined local cerebral perfusion during auditory cued CF in nontethered, nonrestrained rats, free of the potential stress represented by immobilization (Hauger et al., 1990; Wan et al., 1992; Ma et al., 1997). In addition to a region-of-interest analysis, we applied statistical parametric mapping (SPM) (Friston et al., 1990, 1995), a comprehensive, voxel-by-voxel analysis of the functional brain activation patterns that can be applied to the reconstructed, three-dimensional rodent brain (Nguyen et al., 2004).
Methods
Animals
Experiments were carried out under a protocol approved by the Animal Research Committee of the University of Southern California. Adult, male Wistar rats (Harlan Sprague-Dawley, Indianapolis, IN) were randomized to one of 2 groups: conditioned stimulus (CS, body weight 462 ± 17 g, n = 9) or control (body weight 441 ± 15 g, n = 8). Prior to surgery, rats were housed in groups of 4 animals on a 12-h light:12-h dark cycle (7 p.m.-7 a.m. lights off) with free access to water and rodent chow. Following surgery, animals were individually housed.
Implantation of an infusion pump
We have developed a self-contained, fully implantable miniature infusion pump (MIP) that allows bolus administration of a radiotracer by remote activation (Holschneider et al., 2002). The MIP has been validated in freely moving rats for functional neuroimaging using injection of 14C-iodoantipyrine followed by autoradiographic analysis of the changes in cerebral blood flow tracer distribution (Holschneider et al., 2003, 2004). Both the design of the MIP and its surgical implantation have been described in detail elsewhere (Holschneider et al., 2002). In brief, the pump consists of an elastomeric reservoir, which creates a hydraulic pressure source to force liquid out of the MIP at a constant flow rate. Flow is controlled by a solenoid valve inside a separate silicone embedded electronics module, whose operation is determined by a phototransistor with peak sensitivity in the nearinfrared spectrum at 880 nm. Upon being transcutaneously illuminated by an infrared light source mounted above the cage, the phototransistor energizes the microvalve allowing bolus intravenous injection of the radiotracer, followed immediately by a separate bolus of a euthanasia solution.
To implant the MIP, the animals were anesthetized with isoflurane (2.5% induction, 1.5% maintenance). The ventral skin of the neck was aseptically prepared, and the right external jugular vein was catheterized with a 5 French silastic catheter, advanced 3.5 cm into the superior vena cava. The catheter was tunneled through the subcutaneous space to the back and connected to the MIP situated subcutaneously in the infrascapular region. The skin was sutured over the implant, except around the pump’s percutaneous access port (a 2-cm silastic tubing capped with a stainless steel plug). The percutaneous port allowed for flushes of the catheter every 2 days postoperatively to ensure patency (0.8 ml of 0.9% saline, followed by 0.1 ml of 20 U/ml heparin in 0.9% saline). It also allowed for loading of the radiotracer approximately 1 h prior to imaging.
Conditioned fear—training phase
Experiments were conducted at 1 week postsurgery using standard methods (LeDoux, 2000). Prior to the behavioral exposure, animals were habituated to the experimental room for 40 min in a stainless steel transport cage. Thereafter, rats were placed in the test chamber, which consisted of a Plexiglas/stainless steel box (30 cm × 30 cm × 30 cm) with a floor of stainless steel rods of 2-mm diameter and 8-mm separation. The chamber was illuminated with the indirect ambient fluorescent light from a ceiling panel and was subjected to background ambient sound level of 57 dB. After a 3-min baseline, the CS animals were subjected to the training phase in which they received 8 tone/footshock pairings, each separated by a 1-min silent interval. In each pairing, presentation of a tone (30 s, 72 dB, 1000 Hz/8000 Hz continuous, alternating sequence of 250-ms pulses) was followed immediately by a footshock (1 mA, 1 s). Control animals received identical exposure to the tone but without the footshock. One minute after the final footshock, rats were returned to their home cages. Rats were individually trained; animals awaiting their turn were placed in a separate room, separated by 3 doors, while in the presence of a low level white noise generator to minimize the possibility of detection of the ultrasonic vocalization, which may occur during CF training.
Functional neuroimaging during conditioned fear recall
Twenty-four hours after the training session, animals were immobilized for 5 min in a soft plastic rodent restrainer (Decapicone, Braintree Scientific, Braintree, MA) while the radiotracer ([14C]-iodoantipyrine, 100 μCi/kg in 300 μl of 0.9% saline, Amersham Biosciences, Piscataway, NJ) was loaded into the pump through the percutaneous port. At this time, a euthanasia agent was also loaded (1 ml of 100 mg/ml pentobarbital, 3 M KCl). Animals were returned to a transport cage, where they rested undisturbed for 40 min prior to exposure to the behavioral paradigm. Thereafter, recall of the fear conditioned response was tested in the absence of any footshock. CS and control animals received a 2-min exposure to a new context (a cylindrical, dimlylit, Plexiglas cage with a flat, paper floor), which was followed by a 2-min continuous exposure to the conditioned tone. Triggering of the pump at the end of the tone exposure resulted in bolus intravenous injection of the radiotracer, followed immediately by injection of the euthanasia solution. This resulted in cardiac arrest within ∼10 s, a precipitous fall of arterial blood pressure, termination of brain perfusion, and death. This 10-s time window provides the temporal resolution during which the distribution of CBF-TR is mapped (Holschneider et al., 2002). Brains were rapidly removed, flash frozen in dry ice/methylbutane (-55°C).
Behavioral analysis
Behaviors were recorded on tape by a camera mounted above the cage. The duration of the animal’s freezing response (in seconds) served as the behavioral measure of conditioned fear memory. Freezing was defined as the absence of all visible movements of the body and vibrissae aside from respiratory movement. Freezing was distinguished from resting responses by the presence of a tensed state, included horizontal positioning of the head, stretched and flattened position of the body, and stiffening of the tail (Tang et al., 2001). Behaviors were analyzed in a blinded fashion using the Observer, a software package for the analysis of animal behavior (Version 5.0, Noldus Information Technology, Leesburg, VA). The freezing data were transformed to a percentage of time spent freezing within each 30-s interval. Statistical comparison of group differences in the ‘percent freezing’ was performed with a univariate repeated measures analysis of variance (ANOVA), using “group” as a between-subject factor and “time” as a within-subjects factor. Analysis was performed separately for the following phases of the experiment: (a) day 1 baseline (minutes 1-3), (b) day 1 following delivery of the tone/shock (minutes 4-15), (c) day 2 baseline (minutes 1-2), (d) day 2 following exposure to the tone cue (minutes 3-4). Changes in freezing response in the baseline condition between days 1 and 2 were similarly evaluated for each group. Post hoc t tests (1-tailed, P < 0.05) were used to examine differences for each 30-s interval.
Autoradiography
Cerebral blood flow related tissue radioactivity (CBF-TR) was measured by the classic [14C]-iodoantipyrine method (Goldman and Sapirstein, 1973; Sakurada et al., 1978; Patlak et al., 1984) during the recall phase (day 2). In this method, there is a strict linear proportionality between tissue radioactivity and cerebral blood flow when the radioactivity data are captured within a brief interval (∼10 s) after the radiotracer injection (Van Uitert and Levy, 1978; Jones et al., 1991). Brains were sliced in a cryostat at –18°C in 20-μm sections, with an interslice spacing of 300 μm. Slices were heat dried on glass slides and exposed to Kodak Ektascan film for 15 days at room temperature with 12 [14C] standards (Amersham Biosciences). Images (autoradiographs) of brain sections were digitized on an 8-bit gray-scale with a ChromaPro 45 IAIS “Dumas” film illumination system and a Phillips charge-coupled device monochrome imaging module coupled to a Flashpoint 128 digitizing board on a microcomputer.
3D reconstruction of the digitized autoradiographs
In preparation for the statistical parametric mapping (SPM) analysis, a three dimensional (3D) reconstruction of each animal’s brain was computed using sixty-three serial coronal sections, selected starting at 4.3 mm anterior to bregma. Adjacent sections were aligned using TurboReg an automated pixel-based registration algorithm (Thevenaz et al., 1998). This algorithm registered each section sequentially to the previous section and used a nonwarping geometric model that included rotations and translations (rigid-body transformation) and used nearest-neighbor interpolation. Alignment was initiated beginning in the middle of the data set and proceeded separately to the rostral and caudal tip of the brain. Individual 3D images for each brain were spatially normalized into a standard space defined by a template of the rat brain. Creation of the rat brain template has been described in our previous work (Nguyen et al., 2004). Spatial normalization consisted in applying a 12-parameter affine transformation followed by a nonlinear spatial normalization using three-dimensional discrete cosine transforms (Ashburner and Friston, 1999). Normalized images were averaged to create a mean image. This image was smoothed with a Gaussian kernel (FWHM = 3 × voxel dimension, voxel size: 0.216 × 0.216 × 0.9 mm3) to create the final rat-brain template. Then each initial reconstructed brain was spatially normalized to this average template.
Statistical parametric mapping (SPM)
SPM (Friston et al., 1990, 1995), a system developed for analysis of imaging data in humans, has been recently adapted by us for use in rat brain autoradiographs (Nguyen et al., 2004). The nonbiased, voxel-by-voxel analysis of whole-brain activation using SPM was used for detection of significant changes in functional brain activation that would be difficult to predict a priori. Global differences in the absolute amount of radiotracer delivered to the brain were adjusted by the SPM software in each animal by scaling the voxel intensities so that the mean intensity for each brain was the same (proportional scaling). Using SPM, we implemented a Student t test (unpaired) at each voxel, testing the null hypothesis that there was no effect of group. Maps of positive and negative t were separately analyzed. We chose to set a significance threshold P < 0.05 for individual voxels within clusters of contiguous voxels and a minimum cluster size of 150 contiguous voxels (extent threshold). We then evaluated the significance of clusters of contiguous voxels exceeding the minimum cluster size, as well as the significance of individual voxels within clusters smaller than the threshold limit in the entire SPM. Regions determined to be significant at the voxel level were required to show significance in two or more autoradiographic slices. Brain regions were identified using an anatomical atlas of the rat brain (Paxinos and Watson, 1998).
Region-of-interest (ROI) analysis
Statistical significance of brain regions showing significant group differences in functional activation on the SPM analysis were reexamined using an ROI analysis, as previously reported (Holschneider et al., 2003). Brain regions were identified using the anatomic atlas of the rat brain (Paxinos and Watson, 1998) and transcribed to a visual template. Overlay of this template on to the digitized images allowed measurement of the optical density of locations in the cortical mantle in a manner invariant between animals. Optical density was measured with Image Pro-Plus software (Media Cybernetics, Silver Springs, MD). Twelve to 20 optical density measures, each spaced in 15° intervals along the cortical rim were taken per hemisphere for each slice. The optical density of symmetrical regions was averaged across the hemispheres. Structures sampled in the cortex included the regions outlined in Table 1, as well as the cingulate cortex (Cg), insular cortex (I), olfactory cortex (O), orbital cortex (OF), parietal cortex (PtA), visual cortex (V1), primary somatosensory cortex (S1BF, S1J, S1FL, S1HL, S1TR), and secondary somatosensory cortex (S2). Structures sampled in the subcortex included the regions outlined in Table 1, as well as inferior colliculus, superior colliculus, medial geniculate, median raphe (MnR), striatum (dorsolateral), substantia nigra (SN), thalamus (ventrolateral, ventromedial, VL, VM), lateral hypothalamus (LH), and posterior intralaminar nucleus (PIL). Cerebellar structures included the cerebellar lobules 1-4, the spinaltrigeminal, and lateral striatal tracts. A Z-score transformation was performed on the tissue radioactivity data to produce patterns of regional tracer concentrations for each animal (Hays, 1973). Differences in regional CBF Z-scores of animals exposed to the conditioned tone cue and controls were compared using t tests (unpaired, 2-tailed, P < 0.05).
Table 1.
Regions of statistically significant differences of functional brain activation in the cortex and subcortex between rats in response to a fear conditioned tone cue (CS) or a control condition (control)
| CS versus control | Sig. SPM | Sig. ROI | |
|---|---|---|---|
| Cortex | |||
| Isocortex | |||
| Auditory (Au) | ↑ | v, c, * | Y |
| Temporal association (TeA) | ↑ | v, c, * | Y |
| Visual, secondary lateral (V2) | ↑ | v | Y |
| Motor, primary, secondary (M1, M2) | ↓ | v, c | Y |
| Somatosensory, primary (S1, S1BF) | ↓ | v, c | Y |
| Cingulate area 2 (Cg2) | ↑ | v, c, * | N |
| Ectorhinal (Ect) | ↑ | v, c, * | Y |
| Entorhinal, medial posterior (MEnt) | ↑ | v, c, * | Y |
| Prelimbic (PrL), Infralimbic (IL) | ↑ | v, c, * | Y† |
| Piriform (Pir) | ↓ | v | Y |
| Subcortex | |||
| Amygdala | ↑ | ||
| Amygdala, lateral (LA) | ↓ | v, c, * | Y |
| Amygdala, anterior area, cortical anterior, cortical posterior, medial (AA, ACo, PLCo, Me) | ↓ | v, c | Y† |
| Amygdala, central (CE) | ↓ | v, c | Y |
| Amygdala, basomedial, basolateral (BMA, BLA) | ↓ | v, c | Y† |
| Hippocampal formation | |||
| Fimbria of hippocampus (fi) | ↑ | v | Y |
| Hippocampus ventral (CA1, CA2) | ↑ | v, c, * | Y |
| Subiculum, ventral posterior (S) | ↑ | v, c, * | Y |
| Hippocampus dorsal (CA1, CA2, CA3, DG) | ↓ | v, c, * | Y |
| Subiculum, dorsal (S) | ↓ | v, c, * | Y |
| Parasubiculum (PaS), presubiculum (PreS), postsubiculum (Post) | ↓ | v, c, * | Y |
| Hypothalamus | |||
| Hypothalamus, dorsomedial, ventromedial, posterior (DMH, VMH, PH) | ↑ | v | Y |
| Periaqueductal gray, dorsomedial, dorsolateral, lateral > ventrolateral (dmPAG, dlPAG, lPAG > PAG) | ↑ | v, c | Y |
| Striatum | |||
| Caudate-putamen, medial (mCPu) | ↑ | v, c, * | Y† |
| Caudate-Putamen, centrolateral (clCPu) | ↓ | v, c | Y |
| Thalamus | |||
| Thalamic midline nuclei: anteromedial, centromedial, paraventricular, paraventricular posterior, reunions, rhomboid (AM, CM, MD, PV, PVP, Re, Rh) | ↑ | v, c, * | Y† |
| Thalamus, posterior nucleus (Po) | ↓ | v, c, * | N |
| Thalamus, ventral posteromedial, ventral posterolateral nucleus (VPM, VPL) | ↓ | v, c, * | Y |
| Other | |||
| Deep mesencephalic nucleus (DpMe)/tegmental tract (tg) area | ↑ | v, c | N |
| Lateral septal nucleus, intermediate, ventral (LSI, LSV) | ↑ | v, c, * | Y† |
| Pons (Pn) | ↓ | v | Y |
| Pretectal nucleus, anterior (APT) | ↓ | v, c, * | N |
| Zona Inserta (ZI) | ↓ | v, c, * | N |
The first column lists the cortical and subcortical brain regions, with abbreviations taken from the Paxinos and Watson (1998) rat atlas.
The second column indicates whether such differences represent increases (i.e., CS minus control > 0) or decreases (i.e., CS minus control < 0).
The third column indicates for the SPM analysis whether presence of statistical significance was at the voxel level (v) and cluster level (c), as well as whether significance ( P < 0.05) was retained after correction for multiple comparisons.
The fourth column indicates whether the indicated region was significant (Yes/No) using the ROI method at the P < 0.05 level or P < 0.005 level.
Regions shown in Fig. 2 that were significant only at the voxel level, without significance at either the cluster level or the ROI level, are not shown.
Results
Behavioral response
Day 1
Baseline observation during the first 3 min after placement in the experimental cage showed animals to be engaged in active exploratory behavior, with little or no freezing activity (0-14%) and no significant group differences (P > 0.05) in this response. Delivery of the tone/footshock stimulus (minutes 4-15) resulted in a significant increase in the animal’s freezing response (42-89%) compared to controls (5-59%) (Group: F1,15 = 8.4, P < 0.01), with no significant group difference in this response over time. In the controls, delivery of the tone without a footshock increased freezing activity to a small extent above baseline, suggesting that the tone itself provided an unfamiliar, potentially fearful stimulus to the animal (Fig. 1).
Fig. 1.
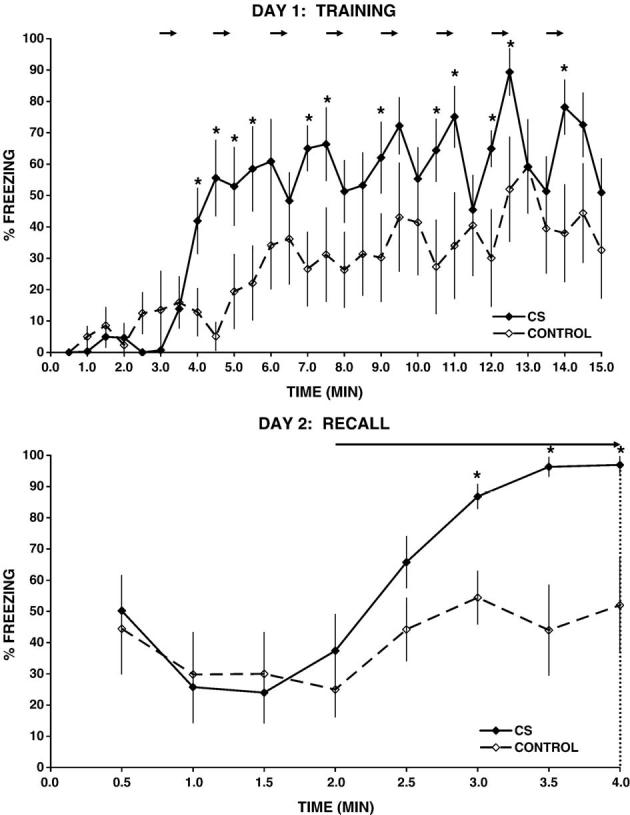
Freezing response during training (day 1) and during recall (day 2) for rats who have learned to associate a tone with an electric footshock (CS group, n = 9) or control animals (control, n = 8). Freezing scores are expressed as mean percentage of time (±SEM) spent immobile within each 30-s interval. Horizontal arrows on day 1 correspond to delivery of a tone stimulus (30-s duration) followed by delivery of a 1-s, 1-mA footshock (CS) or no footshock (control). On day 2, following a 2-min baseline, all animals were exposed to a 2-min continuous tone exposure (horizontal arrow), followed thereafter by triggering of the pump to inject the radiotracer (indicated by the vertical dotted line). *P < 0.05 for t test performed separately for each 30-s time interval.
Day 2
Behavioral freezing during the first 2 min following placement of the animal in the experimental cage was elevated in both groups compared to the baseline of the previous day (Time: F9,135 = 10.3, P < 0.0005), with no significant group difference in this response. This elevated freezing (CS 24-50%, control 25-44%) was likely due, in part, to the brief restraint stress, necessary for loading of the radiotracer, which preceded the exposure to the experimental cage by 40 min. Compared to the day 2 baseline without the tone(minutes 1-2), exposure to the tone stimulus (minutes 3-4) significantly increased immobility in CS animals (Tone exposure: F1,16 = 35.2, P < 0.0005) but not in controls. During the tone exposure, CS animals froze significantly more compared to the controls (Group: F1,15 = 12.4, P < 0.005; CS 66-97%, control 44-54%), a response which increased significantly during the continuous tone exposure (Group × Time, F3,45 = 3.0, P < 0.05). In fact, freezing for the CS group was near maximal (97%) during the final minute prior to triggering of the MIP.
Distribution of CBF-related tissue radioactivity
The significant positive and negative cerebral activations of rats exposed to the conditioned fear paradigm compared to control animals are displayed in several ways. First, group subcortical differences in the distribution of CBF-TR are shown in Fig. 2 as color-coded statistical parametric maps superimposed on the brain coronal, transverse, and sagittal slices. In Fig. 3, group differences in CBF-TR of the cerebral cortex obtained from the ROI analysis are shown in the form of group Z-score differences mapped onto the two-dimensional surface of the flattened cortex. Finally, Table 1 lists cortical and subcortical regions for which group differences were significant (P < 0.05) for the SPM and ROI analyses.
Fig. 2.
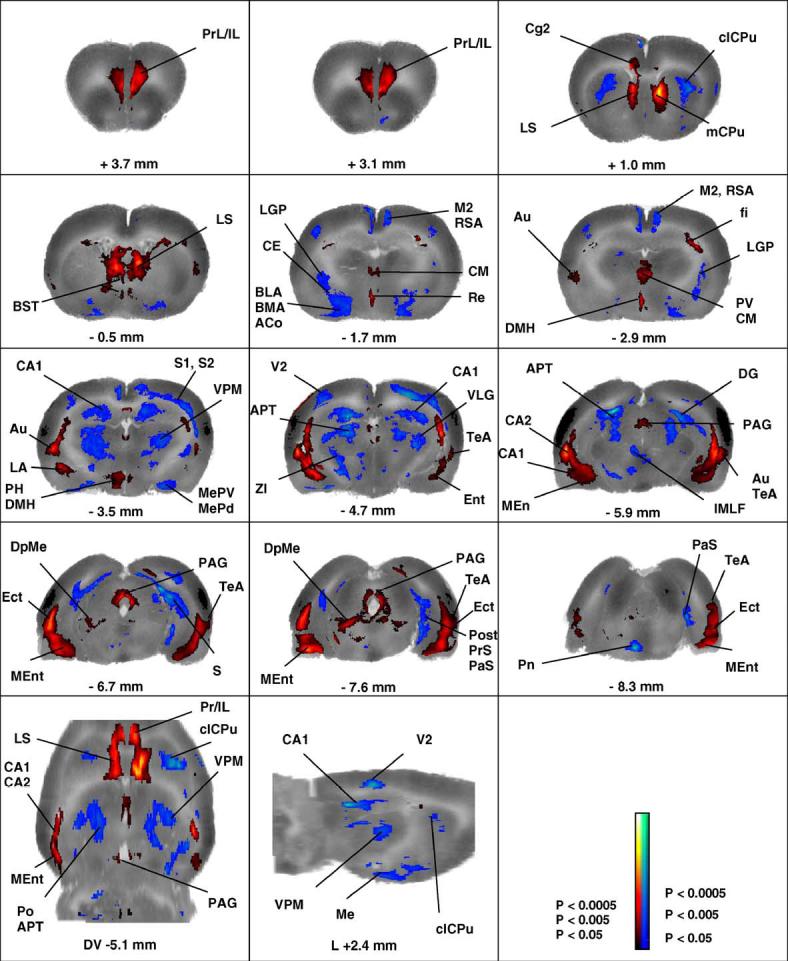
Changes in functional brain activity in rats in response to tone-conditioned fear (CS versus control). Depicted are a selection of representative coronal slices (anterior-posterior coordinates relative to bregma), a sagittal slice (+2.4 mm lateral to bregma) and a transverse brain slice (-5.1 mm dorsoventral to bregma). Colored overlays show statistically significant positive (red) and negative (blue) differences of CS animals (n = 9) compared to controls (n = 8). Significance is shown with a t statistic color scale, which corresponds to the level of the significance at the voxel level. Abbreviations are those from the Paxinos and Watson (1998) rat atlas: ACo (anterior cortical amygdaloid nucleus), APT (anterior pretectal nucleus), Au (auditory cortex), BLA/BMA (basolateral, basomedial amygdaloid nucleus), BST (bed nucleus of the stria terminalis), CA1/CA2 (hippocampus), CE (central amygdaloid nucleus), Cg2 (cingulated cortex, area 2), clCPU (centrolateral striatum), CM (central thalamic nucleus), DG (dentate gyrus), DMH (dorsomedial hypothalamic area), DpMe (deep mesencephalic nucleus), Ect (ectorhinal cortex), fi (fimbria of the hippocampus), IL (infralimbic cortex), IMLF (interstitial nucleus of the medial longitudinal fasciculus, LA (lateral amygdaloid nucleus), LGP (lateral globus pallidus), LS (lateral septum), M2 (secondary motor cortex), mCPU (medial striatum), Me (medial amygdaloid nucleus), MEnt (medial entorhinal cortex), PAG (periaqueductal gray), PCo (posterior amygdaloid nucleus), PH (posterior hypothalamus), Post/PrS/PaS (pre/post/parasubiculum), PrL (prelimbic cortex), PV (paraventricular thalamic nucleus), RSA (retrosplenial cortex), S1/S2 (primary and secondary somatosensory cortex), S (subiculum), TeA (temporal association cortex), VPM (ventral posteromedial thalamus).
Fig. 3.
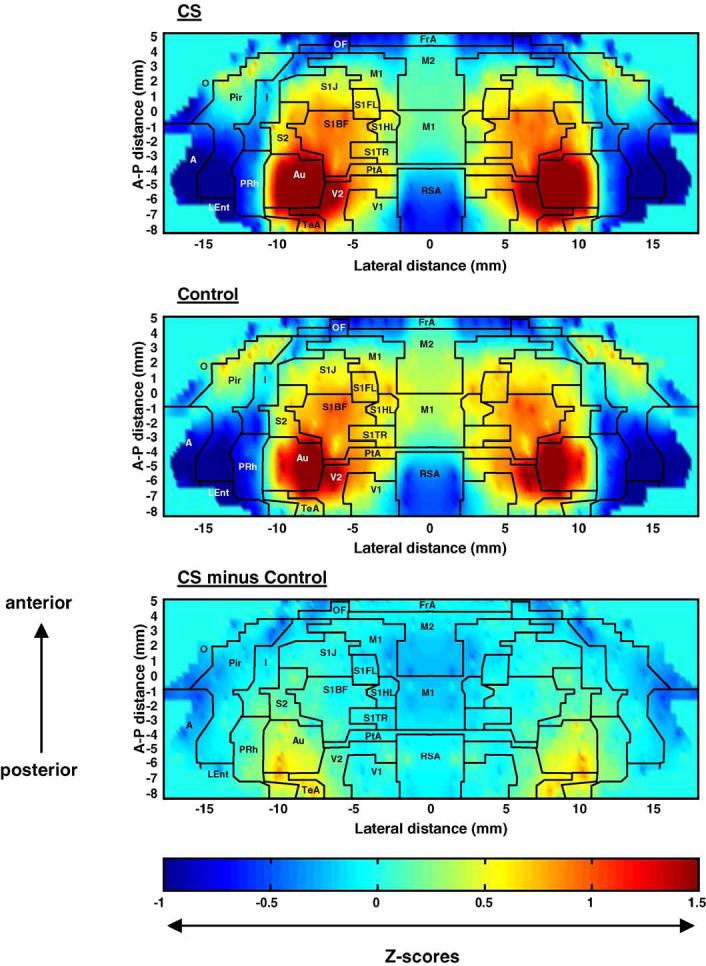
Maps of the color-coded average Z-scores. Depicted are Z-scores for rats exposed to the conditioned cue (CS) and control animals (control), as well as their differences, i.e., Z-scoreCS minus Z-scoreControl. In these two-dimensional maps of the flattened cortex, the x- and y-coordinates are obtained from measures of the anatomical distances within the autoradiographs. The x-axis (locations) represents lateral distance from the midline (in mm) along the cortical rim within a slice. The y-axis (slices) represents coronal slices, numbered from rostral to caudal, with distance relative to bregma in millimeters (positive values being rostral to this landmark). To avoid discontinuities in the graphic representation, the space between each coronal slice and the 12-20 locations within each slice, where there were no measurements, was filled with values calculated by linear interpolation (interpolation distance 300-1000 μm). Superimposed on the maps are the borders of the main cortical areas (Paxinos and Watson, 1998): A, cortical amygdaloid nucleus; Au, auditory; FrA, frontal association; I, insular; MEnt, medial entorhinal; M1, primary motor; M2, secondary motor; O, olfactory; OF, orbital frontal; PtA, parietal association; Pir, piriform; PRh, perirhinal; RSA, retrosplenial. Primary somatosensory mapping: S1FL, the forelimbs; S1HL, the hindlimbs; S1TR, the trunk; S1BF, the barrel fields; S1J, the jaw, lip, and oral region. S2, secondary somatosensory; TeA, temporal association; V1, primary visual; V2, secondary visual.
Cortex
Both CS and control animals exposed to the tone demonstrated increased CBF-TR in auditory cortex (Au) and temporal association cortex (TeA). Topographically, this replicated results from a previous study by our group that mapped the sensory response to an identical simple auditory challenge (Holschneider et al., 2004). Tone-conditioned animals demonstrated significantly greater increases in CBF-TR in the auditory area compared to control animals, despite the fact that both groups were exposed to the same sound (Fig. 2, Table 1).
CS animals compared to controls also showed a small region of significantly increased CBF-TR in secondary visual cortex (V2) at its lateral border with Au and TeA—an area spatially more restricted than that previously reported by our group during acute administration of a footshock in the absence of conditioning (Holschneider et al., 2004).
A low CBF-TR was noted in both groups in primary and secondary motor cortex (M1, M2), primary somatosensory cortex including the barrel fields (S1, S1BF), as well as piriform cortex (Pir). However, CS animals showed significantly lower values in these cortical regions compared to controls.
Within the medial prefrontal cortex (mPFC), significant increases in CBF-TR were noted primarily in ventral regions. This included infralimbic cortex (IL), prelimbic cortex (PrL), and to a small extent cingulate cortex area 2 (Cg2). CS animals compared to controls also showed increases in CBF-TR in the medial entorhinal cortex (MEnt) and ectorhinal cortex (Ect) but not the lateral entorhinal cortex (LEnt).
Subcortex
Within the amygdala, the fear response elicited by the tone cue resulted in a significant increase in CBF-TR in the lateral amygdala (LA). Significant decreases in CBF-TR were noted in anterior sites of the basolateral and basomedial amygdala (BLA, BMA) and the central nucleus of the amygdala (CE) (anterior to bregma AP - 2.6 mm), the cortical amygdala (Anterior ACo, Posteriolateral PLCo), the medial amygdala (Me), and the anterior amygdaloid area (AA). In the hippocampus (HPC), rats conditioned to the tone cue compared to controls showed significant increases in CBF-TR in the ventral HPC (CA1, CA2) and ventral subiculum, while a decrease was noted in the anterior, dorsal HPC (CA1-3, DG), the subiculum (ventral posterior, dorsal), and the pre, post and parasubiculum (PreS, Post, PaS). Significant increases in CBF-TR occurred in the intermediate and ventral parts of the lateral septal nucleus (LSI, LSV), as well as in the deep mesencephalic nucleus (DpMe)/tegmental tract (tg) area. Within the striatum, medial regions showed significant activations, while central and centrolateral regions showed significant deactivations. Within the thalamus, significant increases in CBF-TR in response to the conditioned tone were seen in midline and intralaminar nuclei (anteromedial, AM; centromedial, CM; paraventricular, PV; reuinens, Re; rhomboid, Rh), while significant decreases were noted in the ventral posteromedial and ventral posterolateral thalamic nuclei (VPM, VPL). Increases in CBF-TR were noted in the dorsomedial, ventromedial, and posterior hypothalamic area (DMH, VMH, PH), as well as in dorsomedial, dorsolateral, lateral periaqueductal gray (dmPAG, dlPAG, lPAG) at rostral and caudal sites, with increases in the ventrolateral subregions (vlPAG) being less extensive and seen only caudally.
Discussion
Isocortex
Tone-conditioned animals demonstrated significantly greater increases in CBF-TR in the auditory area (Au, TeA) compared to controls, though both groups were exposed to the same sound (Fig. 2, Table 1). This suggests that the emotional response elicited by CF enhanced the neural response to the auditory input. The greatest increase in CBF-TR was noted in the caudal portions of auditory cortex, an area thought to represent sound frequencies in the lower four octaves (from 0.5 to 8 kHz) (Doron et al., 2002), consistent with the frequencies used in the current paradigm. Others using tonotopic mapping of the Au have shown that CF indeed can result in a shift of tuning toward or to the frequency of the conditioned stimulus (Weinberger, 1998). The activation noted in visual cortex during recall of the CS was spatially more restricted than that previously reported by our group during acute administration of a footshock in the absence of conditioning (Holschneider et al., 2004), suggesting that during recall, attention was directed dominantly to the conditioned auditory cue, and less to the nonconditioned, visual aspect of the experimental cage. A low CBF-TR was noted in motor cortex (M1, M2) and primary somatosensory cortex (S1, S1BF) of both groups, with significantly lower values in the CS animals. Such deactivations were consistent with the increased motor immobility noted (and presumably decreased somatosensory input) in these animals compared to controls in response to the tone.
Amygdala
A central component in the acquisition and expression of CF is the amygdala. The afferent limb of the response is centered most importantly around the LA and also includes the BLA and BMA (Maren, 2000; Pare et al., 2004; Yaniv et al., 2004). Associative activity in the LA encodes fear memory and contributes to the expression of learned fear behaviors (Goosens et al., 2003). In our study, the fear response elicited by the tone cue resulted in a significant increase in CBF-TR in the LA, while a significant decrease in CBF-TR was noted in anterior portions of the BLA and BMA. Differences in the response of the LA to that of BLA/BMA are of interest in so far as a differential role for these regions in CF has been suggested (Amorapanth et al., 2000). Whereas the LA provides the primary sensory interface for unimodal, sensory processes associated with auditory stimuli, the BLA/BMA may serve as an amygdaloid sensory interface for multimodal, complex, configural, conditioned stimuli (Yaniv et al., 2001, 2004). The activation of the LA but not BLA/BMA seen in our study is consistent with the simple, conditioned auditory cue the animals were exposed to. Activation of the LA was larger in the right than left side, consistent with an increased role of the right LA proposed for anxiety (Andersen and Teicher, 1999) and CF memory (Baker and Kim, 2004).
Output from the LA (possibly via the intercalated cells) (Pare et al., 2004) is relayed to the CE, a critical component of the efferent limb of the CF response. The decrease in CBF-TR in the CE noted in our study was surprising, in so far as this structure is felt to control the animal’s motor and autonomic response via the midbrain, hypothalamus, medulla, and extended amygdala. A possible explanation may be found in the temporal changes in amygdala activity reported in human subjects during CF exposure. Here, it has been suggested that learning-related activation occurs only during early acquisition, whereas deactivation is seen during later stages of retention and extinction (Büchel et al., 1998; Büchel and Dolan, 2000; LaBar et al., 1998), possibly due to inhibitory modulation arising from the mPFC (Quirk et al., 2003). In our study, CE deactivations may have been the result of the fact that measurement of CBF-TR was performed during delayed recall, rather than during acquisition.
Medial prefrontal cortex
The mPFC has been proposed to be necessary for the normal expression and extinction of CF (Morgan and LeDoux, 1995; Vouimba et al., 2000; Milad and Quirk, 2002; Pezze et al., 2003). Specifically, activation of the ventral mPFC has been shown to enhance long-term extinction memory and activation of the dorsal mPFC to enhance acquisition. Our study showed a dominant importance of the ventral over the dorsal mPFC during recall in the absence of a footshock, with significant activations seen primarily in the PrL and IL. The ventral mPFC projects dominantly to limbic structures such as the amygdala, septum, medial parts of the preoptic and hypothalamic areas, the temporal and limbic association cortices, as well as the medial striatum (reviewed in (Heidbreder and Groenewegen, 2003))—regions that in our study demonstrated significant activation during CF recall. Decreases noted in our study in CBF-TR in the CE and BLA are consistent with the hypothesis that activation of IL neurons may serve to dampen the output of the CE and BLA, which over time results in a decrease of the immobility response associated with conditioned recall (Heidbreder and Groenewegen, 2003; Milad et al., 2004). Our study did not demonstrate significant activation of the dorsal mPFC (dorsal cingulate cortex, Cg1) or its projection areas (the dorsal and lateral parts of the preoptic and hypothalamic areas, the core of the nucleus accumbens, sensorimotor cortex, and the superior colliculus).
Our measurements of CBF-TR were taken following a 2-min exposure to the conditioned tone cue, 24 h after training. This time point likely precedes extinction, as reflected by the fact that freezing behavior remained maximal throughout the tone exposure. Electrophysiologic studies by Laviolette et al. (2005) support a role for both the PL and IL regions within minutes of the recall of a conditioned odor cue. However, single unit recordings by Milad and Quirk (2002) suggest that IL is not activated during recall delayed by 1 day, but only with the onset of extinction occurring on day 2 after repeated exposure to the conditioned cue. Differences between our results and those of Milad and Quirk may be methodologic (electrophysiology versus perfusion measurement) and possibly relate to issues of neurovascular coupling, further discussed below.
Hippocampus and parahippocampal region
CS rats compared to controls showed significantly increased CBF-TR in the ventral HPC (CA1, CA2) and the ventral subiculum, while a decrease was noted in the anterior, dorsal HPC (CA1-3, DG), and the dorsal subiculum. Afferents to the ventral two-thirds of the HPC arise from subcortical structures involved in defense and emotion, including the amygdala, hypothalamus, septum, and nucleus accumbens (Ottersen, 1982; Canteras and Swanson, 1992; Pitkanën et al., 2000; Petrovich et al., 2001). The ventral HPC may play a role in resolving conflicts, specifically as pertaining to “safety signaling” in situations involving anxiety and threat of punishment (Gray and McNaughton, 2000). Swanson et al. have emphasized a critical role for the ventral hippocampus (excluding the most ventral tip) in the expression of defensive, goal-oriented, motivated behavior such as freezing in response to external threat (Canteras and Swanson, 1992; Risold and Swanson, 1996; Petrovich et al., 2001). In particular, the CA1/subiculum domains 2 and 3 are described as projecting directly and indirectly (via a relay in the rostral LS) to the medial hypothalamus (Canteras, 2002; Sewards and Sewards, 2002). The CA1/subiculum domains 2 and 3 have also been identified as receiving the most dense terminal plexus from the LA (Petrovich et al., 2001). All these structures - LS, medial hypothalamus and LA - showed functional activation during tone-cued recall. In contrast, connections to the dorsal HPC may play a greater role in spatial navigation (Bannerman et al., 2004).
A differential role of the ventral HPC compared to the dorsal HPC in CF is consistent with work which has suggested that lesions of the ventral HPC result in a tone-conditioning deficit (Bast et al., 2001; Zhang et al., 2001), whereas lesions of the dorsal HPC do not (Phillips and LeDoux, 1992; Bast et al., 2003; Trivedi and Coover, 2004) or to a lesser extent (Bast et al., 2003) (but see also (Maren et al., 1997)). Our study supports these findings at the level of functional neural activation and corroborates earlier work that suggested increased metabolic activation in CA2 during tone cued recall (Barrett et al., 2003).
Past work has shown that manipulation of the neuronal activity in the Ent participates in the consolidation of emotional memories (Izquierdo and Medina, 1997), including learning during CF (Maren and Fanselow, 1997). Our study demonstrated activations in the MEnt but not the LEnt. This is consistent with the findings of Witter et al. (1989), who suggested that the LEnt is part of a mainly sensory circuitry that connects different cortical areas with the HPC, while the MEnt receives input from limbic areas and sends projections to the LEnt. Indeed, the LA provides a robust projection to the Ent in the rat, in particular the ventral and medial portions (Pikkarainen et al., 1999). Our results are consistent with recent work suggesting activation of the amygdalo-entorhinal pathway in fear conditioning (Majak and Pitkanen, 2003). In addition, efferent fibers from the Ent terminate both in PrL/IL cortex and the lateral septal complex (Witter and Amaral, 2004)— each of which in our study also showed significant increases in CBF-TR in cued animals compared to controls.
Lateral septum
Rats conditioned to the tone cue compared to controls showed broadly increased CBF-TR in the LS. Experimental data tend to implicate the LS in many behavioral and physiologic responses, including emotional behaviors (Risold and Swanson, 1997; Mizumori et al., 2000; Sheehan and Numan, 2000; Risold, 2004). Lesions of this nucleus result in what is thought to be exaggerated defensive behavior (Blanchard et al., 1979; Albert and Chew, 1980; Sparks and LeDoux, 1995; Sheehan and Numan, 2000). It has been suggested that LS synapses are part of a brain circuit that helps the animal predict the occurrence of an impending stressor (Garcia and Jaffard, 1996). Whereas some studies have documented a role for the LS in contextual CF, others have shown no effect on fear conditioning to a tone (Sparks and LeDoux, 1995; Garcia and Jaffard, 1996). Recent work suggests that alteration of neuronal excitability in the LS during tone-conditioned fear is dependent on the number of repetitions during training of the conditioned stimulus-unconditioned stimulus (Thomas et al., 1991; Hamamura et al., 1997; Desmedt et al., 2003), and that HPC-LS neurotransmission may modulate the strength of simple associations between the conditioned and unconditioned stimulus. Our animals were trained over 12 min with a series of 8 tone/footshock pairings. It appears that this was sufficient to yield a robust difference in functional brain activation in the LS during the recall of the conditioned cue.
Striatum
Tone cued animals compared to controls showed significant deactivation in the central and centrolateral striatum. These areas receive cortical projections, respectively, from motor cortex (M1, M2) and primary somatosensory cortex (S1)—both of which showed a pattern of decrease in CBF-TR or no change. In contrast, significant activations were seen in ventral mPFC and its efferent target, the medial striatum. The medial and ventral parts of the striatum are more densely innervated by limbic structures than the dorsal part (McDonald, 1991a,b; Berendse et al., 1992) and receive fibers originating not only from the PrL (McDonald, 1991a,b; Groenewegen and Berendse, 1994) but also from the amygdala (McDonald, 1991a,b; Pitkanen, 2000) and the ventral HPC (Groenewegen et al., 1987; Brog et al., 1993). Our results give a functional basis to both anatomic and behavioral observations suggesting a particular role of the medial striatum in the processing of emotional information (Cardinal et al., 2002).
The topographic organization seen in corticostriatal circuits becomes increasingly divergent in its downstream projections (Gerfen, 2004), with components of the motor/somatosensory projections intermingling with those of the limbic, midline cortex. Though efferent fibers remain segregated among themselves, a mosaic pattern is seen in the projections of both of these circuits to the lateral globus pallidus, the substantia nigra and the ventral and midline thalamic nuclei. This may explain the absence of significant changes in the substantia nigra (SN) and VM, where segregation of fibers from different cortical areas and subregions of the striatum is less distinct, and mixing of activated and deactivated circuits occurs over sufficiently close range to be outside of the estimated spatial resolution of our technique (∼0.1 mm).
One target downstream from the corticostriatal circuits that remains distinct in its afferent connections is the centromedial thalamus (CM). In our study, the CM showed a significant increase in CBF-TR, consistent with its projections from the prelimbic cortex→medial striatum→substantia nigra→CM.
Thalamus
A significant increase in CBF-TR in response to the conditioned tone was seen in midline and intralaminar nuclei (AM, CM, PV, PVP, Re, Rh). Much remains unknown about the functions of the thalamic midline and intralaminar complex (Groenewegen and Witter, 2004). The anterior portion of the CM, in addition to its role in the striatal circuitry, has been proposed to play a role in polymodal sensory awareness (Van der Werf et al., 2002). The PV has connections predominantly with the mPFC, the amygdala, the medial nucleus accumbens, and is part of the ‘viscerolimbic’ midline nuclei (Van der Werf et al., 2002), with a suggested role in stress and fear (Chastrette et al., 1991; Beck and Fibiger, 1995). The Re has been proposed as a critical relay in the transfer of emotional/cognitive information from limbic/limbic-associated structures to its major targets, the HPC, mPFC, and Ent (Dolleman-Van der Weel and Witter, 2000; McKenna and Vertes, 2004).
Cued animals compared to controls demonstrated a significant decrease in CBF-TR in the VPM and VPL. The VPM and VPL are a primary thalamic relay for nociceptive input from neurons in superficial laminae of the spinal cord (lamina I), as well as a tactile input from the dorsal column neurons, with projections to the primary somatosensory cortex. It has been proposed that this system is involved in the discriminative aspect of nociceptive processing (Gauriau and Bernard, 2002). A significant decrease in CBF-TR in cued animals compared to controls was also noted in the posterior thalamic nucleus (Po). The Po receives inputs from various different sensory modalities, including somatosensory, auditory, visual, and vestibular input. One of its proposed roles has been as a relay in the sensory-motor interface of the whisker system that an animal uses to explore its surroundings (Veinante et al., 2000). Since exploratory activity by the vibrissae is by definition absent during the immobility response, one can speculate that a lower CBF-TR in the Po is consistent with the presumably lower sensory-motor activity in the whisker system in the CS group compared to controls.
The absence of significant group differences in CBF-TR in the MG, a station of the auditory pathway that provides efferent input to the LA (LeDoux et al., 1990; Turner and Herkenham, 1991) and which shows associative plasticity during CF (Edeline and Weinberger, 1992), remains difficult to explain. Barrett et al., examining brain activity in mice using radiolabeled glucose metabolic mapping, noted increases in the MG, as well as Au during CF recall (Barrett et al., 2003). However, here, recall occurred 7 days after acquisition, and metabolic changes were averaged over several minutes of exposure to the conditioned cue, making direct comparison with our results difficult. Similar observations to ours have been made by LeDoux et al. (1983), who in their measurement of CBF in immobilized animals noted that while CBF increased in the MG and IC during tone exposure, this response did not significantly differ between CF animals and a control group that received simple acoustic stimulation. Our methods may not have been sufficiently sensitive to detect the rapid but relatively impoverished auditory input from the auditory thalamus to the LA in comparison to slower but richer input from the Au (Li et al., 1996). On the other hand, differences during recall may be related to those ascribed to the different roles played by the MG and Au, as has been shown in the acoustic startle response (Huang et al., 2005) or in the elicitation of long-term potentiation within the LA (Doyere et al., 2003).
Hypothalamus
Increases in CBF-TR were noted in the PH, DMH, and VMH. Past work has shown that stimulation or disinhibition of the PH and DMH increases blood pressure, heart, and respiratory rate and escape-defense behavior (Bunag et al., 1975; Bunag and Riley, 1979; Shekhar et al., 1990; Samuels et al., 2004). With projections to midline thalamic nuclei and brainstem cardiovascular centers (Vertes et al., 1995; Vertes and Crane, 1996), the PH has been proposed to be in a position to receive converging sensory and limbic input and, thus, alter the levels of cerebral activation, as well as cardiovascular tone. The DMH and VMH have been implicated in anxiety and defensive behaviors (Colpaert and Wiepkema, 1976; LeDoux et al., 1984; Inglefield et al., 1994; Beck and Fibiger, 1995; Sajdyk et al., 1997; Shekhar et al., 2002). These hypothalamic regions receive multimodal sensory input and substantial cortical and subcortical limbic input, including inputs from PrL, IL, the amygdala, the septum, and the HPC, as well as from the PAG and hypothalamus (for review, see (Simerly, 2004). Many of these connections appear to be bidirectional. It has been demonstrated that hypothalamic medial zone nuclei can influence the ventral hippocampal regions (including temporal Ammon’s horn/subiculum and MEnt) through dense projections onto midline nuclei of the thalamus, in particular the Re (Risold et al., 1994, 1997).
Periaqueductal gray
Past work has shown that the PAG is important in the immobility response (Amorapanth et al., 1999), and lesions attenuate CF freezing when made either before or after training. An inhibitory role has been proposed for both the dlPAG and vlPAG in CF acquisition, both of which derived strong afferent input from the mPFC. An additional role has been proposed for the vlPAG in recall (De Oca et al., 1998), which derives extensive afferent input from the CE (Rizvi et al., 1991). Efferents from the PAG project to specific hypothalamic and midline and intralaminar thalamic regions (CM, centrolateral, intermediodorsal, and PV nuclei). In particular, the dlPAG and lPAG then selectively target the dorsal and medial hypothalamic areas from which hypertension and tachycardia may be evoked, whereas the vlPAG targets the lateral hypothalamic area, a region associated with the elicitation of hypotension and bradycardia.
Our study demonstrated significant increases in CBF-TR in dmPAG, and dlPAG at rostral, as well as caudal sites. Increases in CBF-TR in the lPAG and vlPAG were less extensive and seen only caudally. This pattern of activation was equivalent to the pattern previously reported for Fos-like immunoreactivity in the PAG of rats exposed to a predator outside their cage (Canteras and Goto, 1999; Comoli et al., 2003). Our results showed a more prominent activation of the dmPAG and dlPAG than was noted in a study evaluating Fos expression during recall of contextual CF (Carrive et al., 1997), whose findings suggested a predominance of vlPAG activation. That such differences may be attributed to differences between recall of a context as compared to a tone cue is suggested by preliminary data from our laboratory, suggesting a dominant increase in CBF-TR in the vlPAG during CF to context (personal communication). The activation of both dorsal as well as ventral PAG sites in the current paradigm suggests that both passive coping, as well as active coping, may be elicited during CF recall (Keay and Bandler, 2004). Such global activation may represent a preparatory state in which the animal anticipates its repertoire of responses to the external stressor.
Technical considerations
Functional brain maps obtained represent a single ‘snapshot’ of patterns of perfusion that continuously change as a function of time. We chose to image the brain 24 h after acquisition and 2 min after reexposure to the conditioned cue, with CBF patterns integrated over a ∼10-s time window (Holschneider et al., 2002). Past imaging studies of the CF response in human subjects have underscored that brain activation during CF recall is a dynamic process (Büchel et al., 1998; Büchel and Dolan, 2000; LaBar et al., 1998). It is likely that functional brain maps obtained during acquisition or during late stages of extinction would differ from those obtained in the current study.
Although the existence of a flow/metabolism coupling at rest is well accepted, the exact relationship between neuronal activity, regional CBF (rCBF), and metabolism during cerebral activation remains a question of debate (Gsell et al., 2000; Mintun et al., 2001; Keri and Gulyas, 2003). Unresolved issues include (a) the assumption that behavioral performance shows correlations with rCBF equally in all brain regions, (b) the limits of proxy measures such as rCBF have in detecting changes in spatial and temporal neural processing, in which the overall energy demands may remain unchanged, and (c) the role of excitatory compared to inhibitory neurotransmitters have in altering brain perfusion and metabolism. In particular, hemodynamic changes evoked by activation can be driven by subthreshold synaptic activity, and the balance between synaptic excitation and inhibition, as well as the electroresponsive properties of the targeted nerve cells themselves, controls the amplitudes of the neuronal and vascular signals. Hence, it is not possible on the basis of an increase in regional CBF to definitively judge whether the output level of activity of that region is increased or not, since this will depend on the connections between the cells in that network. Of relevance here are findings that the development of extinction during repeated exposure of a conditioned cue appears in the mPFC to correspond with a shift in synaptic efficacy from depression to potentiation (Herry et al., 1999). While such shift may be detectable with electrophysiological methods (Milad and Quirk, 2002), they may not be detected by perfusion measures.
Limitations
A limitation of our methods is that we did not include a control group in whom the tone cue was presented in an unrelated fashion with the footshock. Such a group might have controlled for a number of possible nonspecific confounding effects such as differences in arousal or sensitization. Future work will need to evaluate this possibility.
The use of normalized data in the ROI and SPM analyses, necessary for addressing potential differences in the amount of radiotracer received by each animal, carries with it certain limitations for the interpretation of data. First, a change in the absolute amount of perfusion will not be detected if there is no change in the overall pattern. Second, a change in the mean flow may increase or decrease the normalized flow of an individual brain region, even if that region itself shows no change in absolute flow. Hence, the possibility exists for any individual activation or deactivation to be artifactually increased or decreased as a result of the normalization process.
Effectiveness of SPM analysis of whole brain data sets is limited by the accuracy of proper reconstruction prior to analysis of the brain’s coronal sections into a 3D volume (Nikou et al., 2003). These include potential errors introduced by artifacts in the coronal sections, as well as the propagation of errors due to misregistration of preceding slices. Incomplete alignment may be most apparent at border zones such as the interface of the cerebral ventricles with the brain tissue, the white-matter-gray matter transitions, or the external section contour. Misalignments are in part adjusted for by smoothing of the data within SPM. In an effort to further minimize such biases at border zones, we confirmed many of the findings in SPM by ROI analysis. Thus, prominent group differences adjacent to the cerebral ventricles such as, for instance, findings in the LS, the periventricular thalamic nucleus, the ventral CA1 region, PAG, and medial striatum were significant, both with the SPM and ROI analytic approaches. For the analysis of the cortical rim, which represents a continuous border, an analysis was used in which ROIs were manually placed on each slice by the investigator according to a set template. In addition, proper adjustment of the minimum cluster-size required for significance helped prevent false positives related to slice misalignment. For structures significant at the voxel level, significance was required on at least 2 autoradiographic planes. Furthermore, the frequent symmetrical appearance of significant changes across the hemispheres, though not accounted for statistically in the SPM methodology, reinforced the significance of these results, as they are unlikely to occur by chance.
SPM detected several regions of activation/deactivation that were not found by the ROI analysis. These included the cingulate (Cg2), anterior pretectal nucleus (APT), thalamic posterior nucleus (Po), and zona inserta (ZI). Differences between these methods may have been related to subjective placement of the subcortical ROIs and standardized warping inherent in spatial normalization in SPM. For brain structures that displayed irregular borders or a mosaic organization of their afferent and efferent projections, defining appropriate ROIs is typically difficult. This was the case for the abovementioned regions in which the patchy quality of significant changes made exact placement of the ROI challenging. Because significance in SPM was established at the voxel level, rather than with a user-defined ROI, it allowed improved detection of activation in geometrically complex regions. In this case, it provided a complementary method to the standard ROI analysis and a powerful tool for the exploration of brain function of complex behaviors.
Summary
Our study showed that auditory stimuli endowed with aversive properties through conditioning, resulted in significant alterations in brain perfusion patterns. These included activation of regions of the amygdala, HPC, LS, mPFC, PAG, and hypothalamus. Our results highlighted the dominant role of the ventral compared to the dorsal HPC during CF recall, as well as the ventral mPFC compared to the dorsal mPFC. They demonstrated that reexposure to the conditioned auditory cue resulted in activation of the LA, whereas deactivation was seen in anterior portions of the BLA/BME and CE B emphasizing the different functional roles for subnuclei of the amygdala during recall. A particular role during this memory facilitation by specific emotional cues was shown for the medial striatum, as well as its projections to midline and intralaminar nuclei, such as the centromedial nucleus of the thalamus. In contrast, the ventrolateral/dorsolateral striatum and its afferent input from motor and somatosensory cortex - all implicated in motor/somatosensory processing - were deactivated, consistent with the behavioral immobility characteristic of the CF response. In addition, the DpMe/tg was activated during CF recall, a finding not previously reported, and which will require future confirmation. Use of SPM for the analysis of rat brain autoradiographs is a novel application of this powerful, statistical tool routinely used in human functional neuroimaging. Previously, we showed the applicability of this analytic method to a standardized locomotor task (treadmill walking) (Nguyen et al., 2004); the current study shows its application to a more complex behavior.
Acknowledgments
We would like to thank Dr. Oscar U. Scremin for his helpful comments regarding the manuscript, and Dr. Mark A. Mandelkern for his expert assistance with the statistical parametric mapping. Supported by the NIBIB (8RO1 EB-00300, 1R01 NS050171), NCCAM (1R24AT002681), and the Whitaker Foundation (RG-99-0331).
References
- Albert DJ, Chew GL. The septal forebrain and the inhibitory modulation of attack and defense in the rat. A review. Behav. Neural Biol. 1980;30:357–388. doi: 10.1016/s0163-1047(80)91247-9. [DOI] [PubMed] [Google Scholar]
- Amorapanth P, Nader K, LeDoux JE. Lesions of periaqueductal gray dissociate-conditioned freezing from conditioned suppression behavior in rats. Learn. Mem. 1999;6:491–499. doi: 10.1101/lm.6.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat. Neurosci. 2000;3:74–79. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J. Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Serotonin laterality in amygdala predicts performance in the elevated plus maze in rats. NeuroReport. 1999;10:3497–3500. doi: 10.1097/00001756-199911260-00006. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum. Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Amygdalar lateralization in fear conditioning: evidence for greater involvement of the right amygdala. Behav. Neurosci. 2004;118:15–23. doi: 10.1037/0735-7044.118.1.15. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus-memory and anxiety. Neurosci. Biobehav. Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Barrett D, Shumake J, Jones D, Gonzalez-Lima F. Metabolic mapping of mouse brain activity after extinction of a conditioned emotional response. J. Neurosci. 2003;23:5740–5749. doi: 10.1523/JNEUROSCI.23-13-05740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. The ventral hippocampus and fear conditioning in rats. Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABA(A) agonist muscimol. Exp. Brain Res. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13:657–675. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J. Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ, Lee EM, Nakamura S. Defensive behaviors in rats following septal and septal-amygdala lesions. J. Comp. Physiol. Psychol. 1979;93:378–390. doi: 10.1037/h0077562. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp. Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Büchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Curr. Opin. Neurobiol. 2000;10:219–223. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Büchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Bunag RD, Riley E. Chronic hypothalamic stimulation in awake rats fails to induce hypertension. Hypertension. 1979;1:498–507. doi: 10.1161/01.hyp.1.5.498. [DOI] [PubMed] [Google Scholar]
- Bunag RD, Eferakeya AE, Langdon DS. Enhancement of hypothalamic pressor responses in spontaneously hypertensive rats. Am. J. Physiol. 1975;228:217–222. doi: 10.1152/ajplegacy.1975.228.1.217. [DOI] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol. Biochem. Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Goto M. Fos-like immunoreactivity in the periaqueductal gray of rats exposed to a natural predator. NeuroReport. 1999;10:413–418. doi: 10.1097/00001756-199902050-00037. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tracttracing study in the rat. J. Comp. Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carrive P, Leung P, Harris J, Paxinos G. Conditioned fear to context is associated with increased Fos expression in the caudal ventrolateral region of the midbrain periaqueductal gray. Neuroscience. 1997;78:165–177. doi: 10.1016/s0306-4522(97)83047-3. [DOI] [PubMed] [Google Scholar]
- Chastrette N, Pfaff DW, Gibbs RB. Effects of daytime and nighttime stress on Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus, the habenula, and the posterior paraventricular nucleus of the thalamus. Brain Res. 1991;563:339–344. doi: 10.1016/0006-8993(91)91559-j. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Wiepkema PR. Brief communication ventromedial hypothalamus: fear conditioning and passive avoidance in rats. Physiol. Behav. 1976;16:91–95. doi: 10.1016/0031-9384(76)90198-0. [DOI] [PubMed] [Google Scholar]
- Comoli E, Ribeiro-Barbosa ER, Canteras NS. Predatory hunting and exposure to a live predator induce opposite patterns of Fos immunoreactivity in the PAG. Behav. Brain Res. 2003;138:17–28. doi: 10.1016/s0166-4328(02)00197-3. [DOI] [PubMed] [Google Scholar]
- De Oca BM, DeCola JP, Maren S, Fanselow MS. Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses. J. Neurosci. 1998;18:3426–3432. doi: 10.1523/JNEUROSCI.18-09-03426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt A, Garcia R, Jaffard R. An 8-day extensive elemental, but not contextual, fear conditioning potentiates hippocampal-lateral septal synaptic efficacy in mice. Synapse. 2003;49:270–278. doi: 10.1002/syn.10243. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Witter MP. Nucleus reuniens thalami innervates gamma aminobutyric acid positive cells in hippocampal field CA1 of the rat. Neurosci. Lett. 2000;278:145–148. doi: 10.1016/s0304-3940(99)00935-0. [DOI] [PubMed] [Google Scholar]
- Doron NN, Ledoux JE, Semple MN. Redefining the tonotopic core of rat auditory cortex: physiological evidence for a posterior field. J. Comp. Neurol. 2002;453:345–360. doi: 10.1002/cne.10412. [DOI] [PubMed] [Google Scholar]
- Doyere V, Schafe GE, Sigurdsson T, LeDoux JE. Long-term potentiation in freely moving rats reveals asymmetries in thalamic and cortical inputs to the lateral amygdala. Eur. J. Neurosci. 2003;17:2703–2715. doi: 10.1046/j.1460-9568.2003.02707.x. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Associative retuning in the thalamic source of input to the amygdala and auditory cortex: receptive field plasticity in the medial division of the medial geniculate body. Behav. Neurosci. 1992;106:81–105. doi: 10.1037//0735-7044.106.1.81. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Tighe TJ. Contextual conditioning with massed versus distributed unconditional stimuli in the absence of explicit conditional stimuli. J. Exp. Psychol., Anim. Behav. Process. 1988;14:187–199. [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Dolan RJ, Lammertsma AA, Frackowiak RS. The relationship between global and local changes in PET scans. J. Cereb. Blood Flow Metab. 1990;10:458–466. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1995;2:189–210. [Google Scholar]
- Garcia R, Jaffard R. Changes in synaptic excitability in the lateral septum associated with contextual and auditory fear conditioning in mice. Eur. J. Neurosci. 1996;8:809–815. doi: 10.1111/j.1460-9568.1996.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Garcia R, Paquereau J, Vouimba RM, Jaffard R. Footshock stress but not contextual fear conditioning induces long-term enhancement of auditory-evoked potentials in the basolateral amygdala of the freely behaving rat. Eur. J. Neurosci. 1998;10:457–463. doi: 10.1046/j.1460-9568.1998.00027.x. [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp. Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Basal Ganglia G. Paxinos The Rat Nervous System. Elsevier Academic Press; San Diego: 2004. pp. 455–508. [Google Scholar]
- Gewirtz JC, McNish KA, Davis M. Is the hippocampus necessary for contextual fear conditioning. Behav. Brain Res. 2000;110:83–95. doi: 10.1016/s0166-4328(99)00187-4. [DOI] [PubMed] [Google Scholar]
- Goldman H, Sapirstein LA. Brain blood flow in the conscious and anesthetized rat. Am. J. Physiol. 1973;224:122–126. doi: 10.1152/ajplegacy.1973.224.1.122. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Hobin JA, Maren S. Auditory-evoked spike firing in the lateral amygdala and Pavlovian fear conditioning: mnemonic code or fear bias. Neuron. 2003;40:1013–1022. doi: 10.1016/s0896-6273(03)00728-1. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. Oxford Univ. Press; New York: 2000. [Google Scholar]
- Groenewegen HJ, Berendse HW. The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends Neurosci. 1994;17:52–57. doi: 10.1016/0166-2236(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Witter MP. Thalamus G. Paxinos The Rat Nervous System. Elsevier Academic Press; San Diego: 2004. pp. 407–453. [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Gsell W, De Sadeleer C, Marchalant Y, MacKenzie ET, Schumann P, Dauphin F. The use of cerebral blood flow as an index of neuronal activity in functional neuroimaging: experimental and pathophysiological considerations. J. Chem. Neuroanat. 2000 2000 Dec;20(34):215–24. doi: 10.1016/s0891-0618(00)00095-8. [DOI] [PubMed] [Google Scholar]
- Hamamura T, Ichimaru Y, Fibiger HC. Amphetamine sensitization enhances regional c-fos expression produced by conditioned fear. Neuroscience. 1997;76:1097–1103. doi: 10.1016/s0306-4522(96)00383-1. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Lorang M, Irwin M, Aguilera G. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Res. 1990;532:34–40. doi: 10.1016/0006-8993(90)91738-3. [DOI] [PubMed] [Google Scholar]
- Hays W. Statistics for the Social Sciences 2nd Holt. Rinehart and Winston; New York: 1973. [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci. Biobehav. Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Herry C, Vouimba RM, Garcia R. Plasticity in the mediodorsal thalamo-prefrontal cortical transmission in behaving mice. J. Neurophysiol. 1999;82:2827–2832. doi: 10.1152/jn.1999.82.5.2827. [DOI] [PubMed] [Google Scholar]
- Holschneider DP, Maarek J-M, Harimoto J, Yang J, Scremin OU. An implantable bolus infusion pump for use in freely moving, nontethered rats. Am. J. Physiol.: Heart Circ. Physiol. 2002;283:H1713–H1719. doi: 10.1152/ajpheart.00362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschneider DP, Maarek JM, Yang J, Harimoto J, Scremin OU. Functional brain mapping in freely moving rats during treadmill walking. J. Cereb. Blood Flow. Metab. 2003;23:925–932. doi: 10.1097/01.WCB.0000072797.66873.6A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschneider DP, Maarek JM, Yang J, Harimoto J, Scremin OU. Activation of cerebral cortex during acoustic challenge or acute foot-shock in freely moving, nontethered rats. Neurosci. Lett. 2004;354:74–78. doi: 10.1016/j.neulet.2003.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wu X, Yeomans J, Li L. Opposite effects of tetanic stimulation of the auditory thalamus or auditory cortex on the acoustic startle reflex in awake rats. Eur. J. Neurosci. 2005;21:1943–1956. doi: 10.1111/j.1460-9568.2005.04030.x. [DOI] [PubMed] [Google Scholar]
- Inglefield JR, Schwarzkopf SB, Kellogg CK. Alterations in behavioral responses to stressors following excitotoxin lesions of dorsomedial hypothalamic regions. Brain Res. 1994;633:151–161. doi: 10.1016/0006-8993(94)91534-2. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol. Learn. Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Jones SC, Korfali E, Marshall SA. Cerebral blood flow with the indicator fractionation of [14C]iodoantipyrine: effect of PaCO2 on cerebral venous appearance time. J. Cereb. Blood Flow Metab. 1991;11:236–241. doi: 10.1038/jcbfm.1991.55. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Periaqueductal Gray G. Paxinos The Rat Nervous System. Elsevier Academic Press; San Diego: 2004. pp. 243–257. [Google Scholar]
- Keri S, Gulyas B. Four facets of a single brain: behaviour, cerebral blood flow/metabolism, neuronal activity and neurotransmitter dynamics. NeuroReport. 2003;14:1097–1106. doi: 10.1097/00001756-200306110-00001. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J. Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Thompson ME, Iadecola C, Tucker LW, Reis DJ. Local cerebral blood flow increases during auditory and emotional processing in the conscious rat. Science. 1983;221:576–578. doi: 10.1126/science.6867731. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J. Neurosci. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J. Neurosci. 1990;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XF, Stutzmann GE, LeDoux JE. Convergent but temporally separated inputs to lateral amygdala neurons from the auditory thalamus and auditory cortex use different postsynaptic receptors: in vivo intracellular and extracellular recordings in fear conditioning pathways. Learn. Mem. 1996;3:229–242. doi: 10.1101/lm.3.2-3.229. [DOI] [PubMed] [Google Scholar]
- Lomber SG. The advantages and limitations of permanent or reversible deactivation techniques in the assessment of neural function. J. Neurosci. Methods. 1999;86:109–117. doi: 10.1016/s0165-0270(98)00160-5. [DOI] [PubMed] [Google Scholar]
- Ma XM, Levy A, Lightman SL. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: a study of both AVP and corticotropin-releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology. 1997;138:4351–4357. doi: 10.1210/endo.138.10.5446. [DOI] [PubMed] [Google Scholar]
- Majak K, Pitkanen A. Activation of the amygdalo-entorhinal pathway in fear-conditioning in rat. Eur. J. Neurosci. 2003;18:1652–1659. doi: 10.1046/j.1460-9568.2003.02854.x. [DOI] [PubMed] [Google Scholar]
- Maren S. Auditory fear conditioning increases CS-elicited spike firing in lateral amygdala neurons even after extensive overtraining. Eur. J. Neurosci. 2000;12:4047–4054. doi: 10.1046/j.1460-9568.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- Maren S. The amygdala, synaptic plasticity, and fear memory. Ann. N. Y. Acad. Sci. 2003;985:106–113. doi: 10.1111/j.1749-6632.2003.tb07075.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol. Learn. Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav. Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatallike areas of the rat brain. Neuroscience. 1991;44:15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Vertes RP. Afferent projections to nucleus reuniens of the thalamus. J. Comp. Neurol. 2004;480:115–142. doi: 10.1002/cne.20342. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav. Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Lundstrom BN, Snyder AZ, Vlassenko AG, Shulman GL, Raichle ME. Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6859–6864. doi: 10.1073/pnas.111164398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJ, Cooper BG, Leutgeb S, Pratt WE. A neural systems analysis of adaptive navigation. Mol. Neurobiol. 2000;21:57–82. doi: 10.1385/MN:21:1-2:057. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav. Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Nguyen PT, Holschneider DP, Maarek JM-I, Yang J, Mandelkern MA. Statistical parametric mapping applied to an autoradiographic study of cerebral activation during treadmill walking in rats. NeuroImage. 2004;23:252–259. doi: 10.1016/j.neuroimage.2004.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikou C, Heitz F, Nehlig A, Namer IJ, Armspach JP. A robust statistics-based global energy function for the alignment of serially acquired autoradiographic sections. J. Neurosci. Methods. 2003;124:93–102. doi: 10.1016/s0165-0270(02)00369-2. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Connections of the amygdala of the rat: IV. Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J. Comp. Neurol. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J. Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, Fenstermacher JD. An evaluation of errors in the determination of blood flow by the indicator fractionation and tissue equilibration (Kety) methods. J. Cereb. Blood Flow Metab. 1984;4:47–60. doi: 10.1038/jcbfm.1984.7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotactic Coordinates. 4th ed Academic Press; New York, NY.: 1998. [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res. Brain Res. Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Bast T, Feldon J. Significance of dopamine transmission in the rat medial prefrontal cortex for conditioned fear. Cereb. Cortex. 2003;13:371–380. doi: 10.1093/cercor/13.4.371. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J. Comp. Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Pitkanen A. Connectivity of the Rat Amygdaloid Complex J.P. Aggleton The Amygdala: A Functional Analysis. Oxford Univ. Press; New York: 2000. pp. 31–116. [Google Scholar]
- Pitkanën A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann. N. Y. Acad. Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Poremba A, Jones D, Gonzalez-Lima F. Classical conditioning modifies cytochrome oxidase activity in the auditory system. Eur. J. Neurosci. 1998;10:3035–3043. doi: 10.1046/j.1460-9568.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J. Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold PY. The Septal Region G. Paxinos The Rat Nervous System. Elsevier Academic Press; San Diego: 2004. pp. 605–632. [Google Scholar]
- Risold PY, Swanson LW. Structural evidence for functional domains in the rat hippocampus. Science. 1996;272:1484–1486. doi: 10.1126/science.272.5267.1484. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res. Brain Res. Rev. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Risold PY, Canteras NS, Swanson LW. Organization of projections from the anterior hypothalamic nucleus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J. Comp. Neurol. 1994;348:1–40. doi: 10.1002/cne.903480102. [DOI] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res. Brain Res. Rev. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J. Comp. Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav. Neurosci. 1993;107:444–450. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Katner JS, Shekhar A. Monoamines in the dorsomedial hypothalamus of rats following exposure to different tests of “anxiety”. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1997;21:193–209. doi: 10.1016/s0278-5846(96)00138-8. [DOI] [PubMed] [Google Scholar]
- Sakurada O, Kennedy C, Jehle J, Brown JD, Carbin GL, Sokoloff L. Measurement of local cerebral blood flow with iodo [14C] antipyrine. Am. J. Physiol. 1978;234:H59–H66. doi: 10.1152/ajpheart.1978.234.1.H59. [DOI] [PubMed] [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 2004;287:R472–R478. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. Fear and power-dominance drive motivation: neural representations and pathways mediating sensory and mnemonic inputs, and outputs to premotor structures. Neurosci. Biobehav. Rev. 2002;26:553–579. doi: 10.1016/s0149-7634(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Sheehan T, Numan M. The Septal Region and Social Behavior R. Numan The Behavioral Neuroscience of the Septal Region. Springer Verlag; New York: 2000. pp. 175–209. [Google Scholar]
- Shekhar A, Hingtgen JN, DiMicco JA. GABA receptors in the posterior hypothalamus regulate experimental anxiety in rats. Brain Res. 1990;512:81–88. doi: 10.1016/0006-8993(90)91173-e. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Katner JS, Sajdyk TJ, Kohl RR. Role of norepinephrine in the dorsomedial hypothalamic panic response: an in vivo microdialysis study. Pharmacol. Biochem. Behav. 2002;71:493–500. doi: 10.1016/s0091-3057(01)00688-8. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Anatomical Substrates of Hypothalamic Integration G. Paxinos The Rat Nervous System Elsevier. Academic Press; San Diego: 2004. pp. 335–368. [Google Scholar]
- Sparks PD, LeDoux JE. Septal lesions potentiate freezing behavior to contextual but not to phasic conditioned stimuli in rats. Behav. Neurosci. 1995;109:184–188. doi: 10.1037//0735-7044.109.1.184. [DOI] [PubMed] [Google Scholar]
- Tang J, Wotjak CT, Wagner S, Williams G, Schachner M, Dityatev A. Potentiated amygdaloid auditory-evoked potentials and freezing behavior after fear conditioning in mice. Brain Res. 2001;919:232–241. doi: 10.1016/s0006-8993(01)03020-7. [DOI] [PubMed] [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Thomas E, Yadin E, Strickland CE. Septal unit activity during classical conditioning: a regional comparison. Brain Res. 1991;547:303–308. doi: 10.1016/0006-8993(91)90975-2. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Coover GD. Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiol. Learn. Mem. 2004;81:172–184. doi: 10.1016/j.nlm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: a test of the amygdala’s role in sensory processing. J. Comp. Neurol. 1991;313:295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Brain Res. Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Van Uitert RL, Levy DE. Regional brain blood flow in the conscious gerbil. Stroke. 1978;9:67–72. doi: 10.1161/01.str.9.1.67. [DOI] [PubMed] [Google Scholar]
- Veinante P, Lavallee P, Deschenes M. Corticothalamic projections from layer 5 of the vibrissal barrel cortex in the rat. J. Comp. Neurol. 2000;424:197–204. doi: 10.1002/1096-9861(20000821)424:2<197::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Crane AM. Descending projections of the posterior nucleus of the hypothalamus: Phaseolus vulgaris leucoagglutinin analysis in the rat. J. Comp. Neurol. 1996;374:607–631. doi: 10.1002/(SICI)1096-9861(19961028)374:4<607::AID-CNE9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Crane AM, Colom LV, Bland BH. Ascending projections of the posterior nucleus of the hypothalamus: PHA-L analysis in the rat. J. Comp. Neurol. 1995;359:90–116. doi: 10.1002/cne.903590107. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Garcia R, Baudry M, Thompson RF. Potentiation of conditioned freezing following dorsomedial prefrontal cortex lesions does not interfere with fear reduction in mice. Behav. Neurosci. 2000;114:720–724. doi: 10.1037//0735-7044.114.4.720. [DOI] [PubMed] [Google Scholar]
- Wan XS, Liang F, Moret V, Wiesendanger M, Rouiller EM. Mapping of the motor pathways in rats: c-fos induction by intracortical microstimulation of the motor cortex correlated with efferent connectivity of the site of cortical stimulation. Neuroscience. 1992;49:749–761. doi: 10.1016/0306-4522(92)90353-4. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Physiological memory in primary auditory cortex: characteristics and mechanisms. Neurobiol. Learn. Mem. 1998;70:226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Hippocampal Formation G. Paxinos The Rat Nervous System. Elsevier Academic Press; San Diego: 2004. pp. 635–704. [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog. Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Yaniv D, Schafe GE, LeDoux JE, Richter-Levin G. A gradient of plasticity in the amygdala revealed by cortical and subcortical stimulation, in vivo. Neuroscience. 2001;106:613–620. doi: 10.1016/s0306-4522(01)00312-8. [DOI] [PubMed] [Google Scholar]
- Yaniv D, Desmedt A, Jaffard R, Richter-Levin G. The amygdala and appraisal processes: stimulus and response complexity as an organizing factor. Brain Res. Brain Res. Rev. 2004;44:179–186. doi: 10.1016/j.brainresrev.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-d-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behav. Brain Res. 2001;126:159–174. doi: 10.1016/s0166-4328(01)00256-x. [DOI] [PubMed] [Google Scholar]


