Abstract
Purpose
To quantify the impact of radiotherapy (RT) dose escalation on the timing of biochemical failure (BF) and distant metastasis (DM) for prostate cancer treated with RT alone.
Methods and Materials
A total of 667 men with clinically localized intermediate and high risk prostate cancer treated with 3D-conformal RT alone were retrospectively analyzed. The interval hazard rates of DM and biochemical failure (BF), using ASTRO and Phoenix (Nadir+2) definitions, were determined. Median follow-up was 77 months.
Results
Multivariate analysis showed that increasing RT dose was independently associated with decreased ASTRO BF (P<.0001), Nadir+2 BF (P=.001), and DM (P=.006). The preponderance (85%) of ASTRO BF occurred at ≤4 years after RT, while Nadir+2 BF was more evenly spread over years 1-10, with 55% BF in ≤4 years. RT dose escalation caused a shift in the BF from earlier to later years. The interval hazard function for DM appeared to be biphasic (early and late peaks) overall and for the <74 Gy group. In patients receiving ≥74 Gy, there was a reduction in the risk of DM in both the early and late waves, although the late wave appears reduced to a greater degree.
Conclusions
The ASTRO definition of BF systematically underestimates late BF due to backdating. RT dose escalation diminished and delayed BF; the delay suggests that there may still be local persistence in some patients. For DM, higher RT dose reduced both the early and late waves, suggesting that persistence of local disease contributes to both.
Keywords: Prostate cancer, Radiotherapy dose, Hazard function, Biochemical failure, Distant metastasis
Introduction
Local failure after radiotherapy for prostate cancer predisposes to distant metastasis (1-3). Some have argued that local failure is a surrogate for tumor aggressiveness and that patients who fail locally would have failed distantly anyway (4). More recently, Coen et al (5) provided evidence supporting the alternate view, that local persistence contributes to late distant metastasis (DM). A late wave of distant metastasis was seen in patients who experienced local failure. To our knowledge, their observation has not been substantiated in another patient group.
In the PSA era, local persistence of disease is poorly documented. Many patients are not considered good candidates for further local therapy when biochemical failure (BF) is recognized after radiotherapy. Even if predictive factors for DM, such as the PSA doubling time, are considered favorable, prostate biopsies are often not performed. Likewise, there is no point in obtaining a prostate biopsy clinically if predictive factors suggest rapid progression to distant metastasis. Thus, the relationship between local persistence of disease and distant metastasis in the PSA era is poorly characterized.
Radiation dose escalation for prostate cancer has been shown to reduce biochemical (6-11) and distant (6-8) failure. The main question asked here was whether RT dose escalation caused complete tumor eradication or just a reduction in tumor clonogens and a delay in failure. We hypothesized that by looking at the hazard functions for BF and DM over discrete time intervals after RT it would be possible to 1) illustrate the problematic effect of backdating on the changes in BF risk over time, 2) demonstrate a greater reduction in late BFs with RT dose escalation using the Nadir+2 definition because of a reduction in local persistence of disease and 3) also show a reduction with RT dose in late DMs for the same reason. To better understand the relationship between RT dose escalation and local persistence of disease, the interval hazard rates for BF and DM in men with intermediate and high risk prostate cancer were retrospectively examined.
Methods and Materials
Patient Selection and Treatment
From April 1989 to November 1999, a total of 667 men with intermediate and high risk prostate cancer were treated consecutively with 3D-conformal radiotherapy at Fox Chase Cancer Center (Philadelphia, PA); they comprise the study group. All patients had disease that was clinically localized to the prostate, stage N0/Nx M0 according to the 2002 AJCC staging guidelines (12). Only intermediate and high risk prognostic risk groups, as defined by the single-factor method of Chism et al (13), were included. Low-risk group patients who presented with palpation stage T1-2, initial pretreatment prostate-specific antigen (iPSA) ≤10 ng/mL, and Gleason score of ≤6 disease were excluded. All patients had a PSA available prior to treatment and had serial PSA values post-treatment. No patient received neoadjuvant or adjuvant androgen deprivation (AD). Those who received salvage AD initiated ≥6 months after RT were included.
All patients were treated using a 3-D conformal technique, as previously described (14). In general they underwent a treatment planning computed tomography scan in the treatment position (supine on a custom-made alpha cradle for immobilization) and were treated with daily fractions of 2 Gray (Gy). Patients with clinical stage T1/T2a-b and Gleason score 2-6 typically received treatment to the prostate only. Patients with more advanced prostate cancer, T2c/T3/T4 or Gleason score 7-10, received 46-50 Gy to a small pelvis field, followed by a conformal boost to the prostate and seminal vesicles. Typical small pelvis field borders were the middle of the sacroiliac joints superiorly, the bottom on the ischial tuberosities inferiorly, the symphysis pubis anteriorly, the S2/S3 interspace posteriorly, and 1.5 centimeters beyond the pelvic brim laterally. These pelvic fields were shaped only by corner blocks and were delivered with 2-field, 3-field, or 4-field beam arrangements. The planning target volume (PTV) for conformal radiotherapy included the prostate with or without the seminal vesicles, with a margin of 1-1.5 centimeters to the block edge. All conformal treatments utilized 10 to 18 MV photons with a 4-field or 5-field beam arrangement. The radiation dose was prescribed to the 95% isodose line of the beam arrangements. The mean dose to the PTV was typically between -5% and +7% of the prescribed dose, and 99% of the PTV received at least 95% of the prescribed dose. As recommended by the International Commission on Radiation Units and Measurements, radiation dose is reported here as the dose delivered to isocenter (the ICRU reference point) (15). The range of radiotherapy doses was 51-82 Gy.
Typical follow-up consisted of a serum PSA level evaluation and a digital rectal exam every 3-6 months after RT for 2 years, then every 6-12 months thereafter. Two definitions of biochemical failure (BF) were applied to the study cohort: the American Society of Therapeutic Radiation Oncology (ASTRO) consensus definition (three consecutive rises in PSA with backdating to between the nadir and the first rise)(16) and the Phoenix (Nadir+2) definition (rise of at least 2 ng/mL greater than the PSA nadir after RT) (17-22). The classification of distant metastasis (DM) was determined based on radiological evidence of hematogenous spread. Outcomes for men receiving <74, 74-76, and >76 Gy to the prostate were retrospectively compared.
Estimates of BF and DM were calculated using the Kaplan-Meier product-limit method (23). Univariate comparisons of outcome were performed using the log-rank test (24). For univariate analysis RT dose and iPSA were treated as categorical variables, with groupings of <74, 74-76, and >76 Gy and ≤10, >10-20, and >20 ng/mL, respectively. The 74 Gy and 76 Gy cut-points were chosen because multiple studies have identified the greatest dose-response in this range (7-9, 14, 25, 26). Multivariate analysis (MVA) utilized stepwise Cox proportional hazards regression models. Radiotherapy dose and iPSA were treated as increasing continuous variables in the MVA. Categorical covariates for MVA included T stage (T1-2 vs. T3-4) and Gleason score (2-6 vs. 7-10). Hazard functions were estimated using life-table methodology, with comparisons assessed using the log-rank test for overall differences and the Wilcoxon statistic for early differences.
Results
The clinical characteristics of the 667 patients are summarized in Table 1. Patient age and clinical T-stage were well matched among the three RT dose groups. Initial pretreatment PSA and Gleason score distributions were similar in the >74 and 74-76 Gy groups, however the >76 Gy group had more favorable iPSA (P<.0001) and more adverse Gleason score (P<.0001). Median follow-up was also shorter in the >76 Gy group (65 months), compared with 85-87 months in the lower dose groups (P<.0001).
Table 1.
Patient Characteristics
| <74 Gy (n = 224) | 74 - 76 Gy (n = 270) | >76 Gy (n = 173) | |
|---|---|---|---|
| Age (y) | |||
| Mean | 69 | 69 | 68 |
| Median | 70 | 70 | 69 |
| Range | 55-86 | 45-84 | 49-83 |
| T-stage | |||
| T1-T2 | 202 (90%) | 245 (91%) | 159 (92%) |
| T3-T4 | 22 (10%) | 25 (9%) | 14 (8%) |
| iPSA, ng/mL | |||
| 0-10 | 52 (23%) | 57 (21%) | 73 (42%)* |
| >10-20 | 117 (52%) | 146 (54%) | 74 (43%)* |
| >20 | 55 (25%) | 67 (25%) | 26 (15%)* |
| Gleason Score | |||
| 2-6 | 137 (61%) | 153 (57%) | 67 (39%)* |
| 7 | 77 (34%) | 96 (36%) | 91 (53%)* |
| 8-10 | 10 (4%) | 21 (8%)* | 15 (9%)* |
| RT Dose, Gray | |||
| Mean | 71.4 | 75.6* | 80.5* |
| Median | 71.6 | 75.8* | 80.0* |
| Range | 67.4-73.9 | 74.3-75.9 | 76.2-82.1 |
| Follow-up, months | |||
| Mean | 87 | 84 | 61* |
| Median | 85 | 87 | 65* |
| Range | 11-185 | 4-152 | 5-161 |
Abbreviations: Gy = Gray; RT = radiotherapy; iPSA = initial preradiotherapy prostate specific antigen level.
P-value <0.05 when compared to <74 Gy group.
Biochemical failure was examined using two definitions, one encouraged by a consensus conference sponsored by ASTRO (16) and the other from a joint ASTRO-RTOG conference in Phoenix (22). The Phoenix definition, the PSA nadir + 2 ng/mL after RT, is a better approximation of eventual clinical failure (17-22). A primary reason for making the comparison here is that, because the ASTRO definition incorporates backdating, the interval hazard function for ASTRO BF will not be an accurate reflection of the risk of BF at a given interval of time, while the Nadir+2 definition will.
Biochemical failure using the ASTRO definition was documented in 227 (34%) of the 667 study patients. The majority of these failures occurred early, with 194/277 (85%) in years 0-4 post-RT. The 5-year actuarial ASTRO BF rates decreased from 50% to 30% with increasing RT dose (P<.0001, Table 2). Univariate analysis revealed that higher T-stage and iPSA were significantly associated with an increased ASTRO BF rate (P≤.0001, Table 3). Conversely, higher RT doses were significantly associated with lower ASTRO BF rates (P<.0001). Multivariate analysis confirmed that higher RT dose as a continuous variable was independently associated with decreased ASTRO BF (hazard ratio [HR] 0.92; 95% confidence interval [CI], 0.88 to 0.95; P<.0001, Table 4). Thus the risk of ASTRO BF was reduced by 8% for each additional Gray of radiation administered, within the dose range of the study (67-82 Gy). Gleason score 7-10, stage T3-T4 and higher iPSA were also significantly associated with increased ASTRO BF in the MVA.
Table 2.
Biochemical Failure, Distant Metastasis, and Salvage Androgen Deprivation
| Event Type | <74 Gy (n = 224) | 74 - 76 Gy (n = 270) | >76 Gy (n = 173) |
|---|---|---|---|
| ASTRO Biochemical Failure* | |||
| No. (5-year actuarial) | 96 (50%) | 91 (36%) | 40 (30%) |
| Nadir+2 Biochemical Failure† | |||
| No. (5-year actuarial) | 86 (32%) | 85 (29%) | 37 (18%) |
| Distant Metastasis | |||
| No. (5-year actuarial) | 27 (8%) | 15 (4%) | 5 (2%) |
| Salvage Androgen Deprivation, Total No. (%) | 47 (21%) | 44 (16%) | 17 (10%) |
| Initiated for BF only, no subsequent DM | 23 (10%) | 29 (11%) | 12 (7%) |
| Initiated for BF only, develop subsequent DM | 6 (3%) | 7 (3%) | 3 (2%) |
| Initiated after previously diagnosed DM | 18 (8%) | 8 (3%) | 2 (1%) |
Abbreviations: Gy = Gray; ASTRO = American Society of Therapeutic Radiation Oncology; BF = Biochemical Failure; DM = distant metastasis.
ASTRO definition of biochemical failure: 3 consecutive rises in PSA, with backdating to midpoint between nadir and the first rise (16).
Table 3.
Univariate Analysis of Potential Predictors of Biochemical Failure and Distant Metastasis
| Biochemical Failure |
Distant Metastasis |
||||||
|---|---|---|---|---|---|---|---|
| No. of Patients (5-year) | ASTRO* |
Nadir+2† |
|||||
| Predictor | (5-Year) | P | (5-Year) | P | (5-Year) | P | |
| T-stage | |||||||
| T1-T2 | 606 | 37% | 26% | 4% | |||
| T3-T4 | 61 | 59% | .0001 | 45% | .002 | 12% | .016 |
| Gleason score | |||||||
| 2-6 | 357 | 48% | 25% | 3% | |||
| 7-10 | 310 | 42% | .559 | 31% | .042 | 8% | .002 |
| iPSA (ng/mL) | |||||||
| ≤10 | 182 | 37% | 22% | 5% | |||
| >10-20 | 337 | 30% | 23% | 4% | |||
| >20 | 148 | 66% | <.0001 | 47% | <.0001 | 8% | .043 |
| RT Dose (Gy) | |||||||
| <74 | 224 | 50% | 32% | 8% | |||
| 74-76 | 270 | 36% | 29% | 4% | |||
| >76 | 173 | 30% | <.0001 | 18% | .040 | 2% | .010 |
Abbreviations: ASTRO = American Society of Therapeutic Radiation Oncology; iPSA = initial pretreatment prostate-specific antigen level; RT = radiotherapy; Gy = Gray.
ASTRO definition of biochemical failure: 3 consecutive rises in PSA, with BF backdated to midpoint between nadir and the first rise (16).
Table 4.
Multivariate Analysis of Potential Predictors of Biochemical Failure and Distant Metastasis
| Biochemical Failure |
Distant Metastasis |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ASTRO* |
Nadir+2† |
||||||||
| Predictor | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P |
| T-stage | |||||||||
| T3-4 (vs T1-2) | 1.94 | 1.34 to 2.82 | .0005 | 1.76 | 1.19 to 2.60 | .004 | 2.49 | 1.20 to 5.16 | .014 |
| Gleason score | |||||||||
| 7-10 (vs 2-6) | 1.48 | 1.13 to 1.95 | .005 | 1.80 | 1.34 to 2.41 | <.0001 | 3.56 | 1.90 to 6.67 | <.0001 |
| iPSA (continuous) | |||||||||
| 1 ng/mL increase | 1.02 | 1.01 to 1.02 | <.0001 | 1.02 | 1.01 to 1.02 | <.0001 | 1.02 | 1.01 to 1.03 | .0001 |
| RT Dose (continuous) | |||||||||
| 1 Gy increase | 0.92 | 0.88 to 0.95 | <.0001 | 0.93 | 0.90 to 0.97 | .001 | 0.88 | 0.80 to 0.96 | .006 |
Abbreviations: ASTRO = American Society of Therapeutic Radiation Oncology; iPSA = initial pretreatment prostate-specific antigen level; RT = radiotherapy; Gy = Gray; HR = hazard rate; CI = confidence interval.
ASTRO definition of biochemical failure: 3 consecutive rises in PSA, with BF backdated to midpoint between nadir and the first rise (16).
Biochemical failure using the Nadir+2 definition was documented in 208 (31%) of the 667 study patients. These failures were not restricted to the early years following RT, with 94/208 (45%) occurring at year 5 or later. The 5-year actuarial Nadir+2 BF rates decreased from 32% to 18% with increasing RT dose (P=.040, Table 2). Univariate analysis revealed that higher T-stage, Gleason score, and iPSA, as well as lower RT dose were all significantly associated with increased Nadir+2 BF (Table 3). These variables remained significant on MVA (Table 4).
The interval hazard rates of BF using the ASTRO and Nadir+2 definitions are shown in Figure 1 for each 2-year time interval of follow-up after RT. In general, ASTRO BF was documented at earlier times than Nadir+2 BF because of backdating in the former. There were similar changes in the interval hazard functions with increasing RT dose. First, the risk of BF was reduced in magnitude overall. Second, higher RT doses were associated with a shift in the risk of BF to the later time intervals. This effect is particularly evident using the Nadir+2 definition, where the peak hazard rate for BF was at 4-6 years in the <74 Gy group and was at 8-10 years in the 74-76 and >76 Gy groups. It should be noted, however, that in the >76 Gy group, the large spike in the Nadir+2 BF hazard function observed in the 8-10 year interval was based on only 3 patients at risk. The difference in the BF hazard function between RT dose <74, 74-76, and >76 was statistically significant using the log-rank test for overall differences (ASTRO BF, P<.0001; Nadir+2 BF, P=.040) and the Wilcoxon statistic for early differences (ASTRO BF, P<.0001; Nadir+2 BF, P=.018). When the BF hazard functions for men with intermediate and high risk disease were analyzed separately, the overall patterns of failure and response to RT dose escalation described above persisted in each risk group.
Fig. 1.
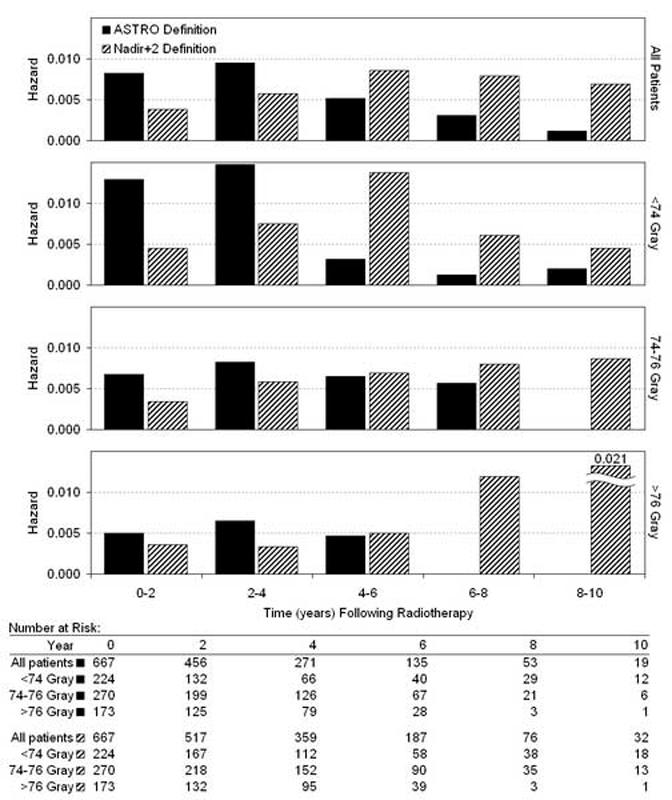
Interval hazard rates for biochemical failure during each 2-year time interval after radiotherapy, according to the ASTRO definition (3 consecutive rises in PSA, backdated) (16) and the Nadir+2 definition (PSA ≥ nadir + 2 ng/mL) (17-22).
Distant metastasis was documented in 47 (7%) of the 667 study patients. The 5-year actuarial DM rates decreased from 8% to 2% with increasing RT dose (P=.010, Table 2). The 10-year actuarial DM rates were 16%, 7%, and 3% for RT dose <74, 74-76, and >76 Gy groups, respectively. Univariate analysis revealed that higher T-stage, Gleason score, and iPSA, as well as lower RT dose were all significantly associated with increased DM (Table 3). These significant associations were confirmed on MVA (Table 4). The hazard ratio was .88 (95% CI, .80 to .96; P=.006) for RT dose as an increasing continuous variable. Thus the risk of DM was decreased by 12% with each additional Gy of radiation administered, within the dose range of the study (67-82 Gy).
The interval hazard rates for DM are shown in Figure 2. Because only 5 DM events occurred in patients receiving >76 Gy, hazard analysis of this subgroup would be based on only 1-2 events in each time interval. We therefore compared all patients who received ≥74 Gy (DM events = 20) to those who received <74 Gy (DM events = 27). For the entire study cohort, the distribution of DM over time was biphasic, with an early wave peaking at 0-4 years and a late wave at 8-10 years. This biphasic pattern was driven primarily by the <74 Gy group. In patients receiving ≥74 Gy, the incidence of both early and late DM was reduced. The difference in the DM hazard function between RT dose <74 and ≥74 Gy was statistically significant using the log-rank test for overall differences (P=.003) and the Wilcoxon statistic for early differences (P=.0027). The percentage men with intermediate risk disease (67%) and high risk disease (33%) was identical in the <74 and ≥74 Gy groups. When the DM hazard functions for intermediate and high risk patients were analyzed separately, the biphasic pattern of DM persisted in each risk group and RT dose escalation reduced both the early wave (0-4 years) and the late wave of DM (8-10 years), as observed in the entire study cohort in Figure 2.
Fig. 2.
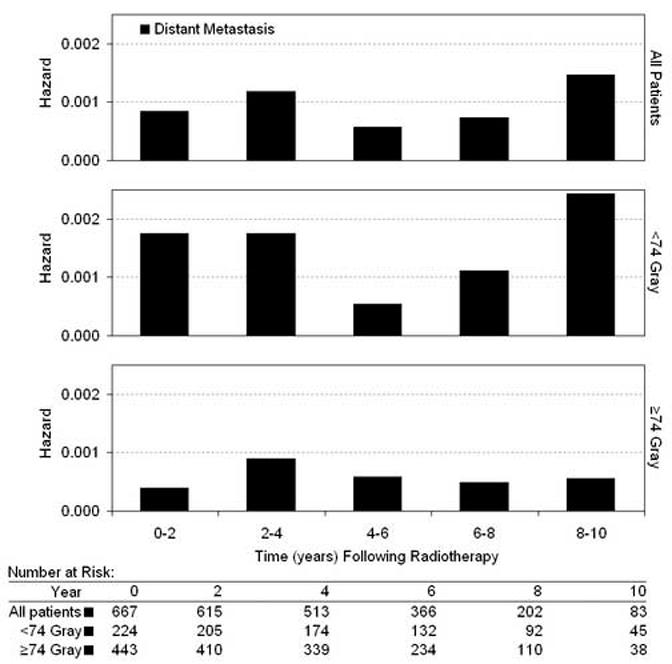
Interval hazard rates for distant metastasis during each 2-year time interval after radiotherapy.
One potentially confounding factor in an analysis of DM is the use of salvage androgen therapy. Androgen deprivation given to patients for a rising PSA could postpone or eliminate the subsequent appearance of DM, thus altering the DM interval hazard function. Salvage AD was administered to 108 (16%) of the 667 study patients (Table 2). Salvage AD was initiated after a diagnosis of DM in 28 men (4%). This would not affect the DM hazard function. The remaining 80 patients (12%) had hormone therapy initiated for BF, at a time when no DM had been identified. However, these patients were distributed fairly evenly among the RT dose groups (range 9-14%) and a similar percentage went on to develop subsequent DM in each dose group (range 2-3%, Table 2).
Discussion
The impact of local therapy for prostate cancer on distant metastasis and survival has been difficult to establish. A trial randomizing patients between radical prostatectomy and observation documented a cause specific survival advantage for prostatectomy (27). For men treated with radiotherapy, there are no published randomized trials with an observation arm. An analogous trial structure for examining the effect of prostate radiotherapy on patient outcome is dose escalation.
There are now three published PSA-era Phase III prostate cancer radiation dose escalation series (8, 28, 29). All have shown an improvement in freedom from biochemical failure with higher radiation doses. None have reported a reduction in distant metastasis or mortality from prostate cancer, although a trend toward lower DM (p=0.056) was seen in the men treated with the higher RT dose in the M.D. Anderson Cancer Center trial (8). Since biochemical failure has been correlated with distant metastasis (30), the likely explanation for the lack of effect of RT dose on DM is that these studies were underpowered. Along these lines, a prior retrospective analysis from our group (6) showed that RT dose was associated with reduced DM and death. The results described herein provide additional evidence that RT dose escalation has a significant impact on DM, altering the risk of both early (0-4 years) and late (8-10 years) metastasis.
We observed that RT dose was independently associated with reduced DM in multivariate analysis (Table 4) and the interval hazard function for DM was biphasic. The initial wave peaked at about 0-4 years and the second wave at 8-10 years following completion of RT. Coen et al (5) showed that early and late DM are both increased in prostate cancer patients with local failure after RT, with a more dramatic increase in the late wave. The data presented here substantiate and extend those of Coen et al (5), showing that the DM interval hazard function is biphasic and is altered by increasing RT dose. When RT doses of ≥74 Gy were used, both the early and late waves were reduced (Figure 2). These data suggest that local persistence is an important mechanism contributing to the progression to DM, even when DM occurs within four years of RT.
We also examined the relationship of RT dose to BF using the ASTRO and Nadir+2 definitions. Figure 1 clearly illustrates the problem of the ASTRO definition, with the backdating of BF to the midpoint between the nadir and first PSA rise. Under these conditions, late biochemical failures are nearly absent, suggesting that almost all of the failures occurred early. This artifact of the ASTRO definition has previously been interpreted as suggesting prostate cancer cure (31). In contrast, the interval hazard function for BF using the Nadir+2 definition is spread more evenly between years 0-10. The Nadir+2 events are recorded at the time observed, without backdating. In comparison to the Nadir+2 definition, the ASTRO definition systematically underestimates late BF.
In distinction to the interval hazard function for DM, the Nadir+2 BF hazard function was not biphasic. At RT doses of <74 Gy, Nadir+2 BF peaked at 4-6 years and began to decline. At RT doses ≥74 Gy, Nadir+2 BF was lower in the 0-6 year range, but continued to rise over time, suggesting that there may still be local persistence of disease. The late rise in the Nadir+2 BF hazard function further suggests that the higher RT doses may be postponing, rather than truly eliminating, the risk of late DM beyond the follow-up period of this cohort.
Several potentially confounding factors exist due to the retrospective, non-randomized design of this study. It is well established that androgen suppression therapy delays the time to DM. Twelve percent of the study patients received salvage AD for a rising PSA, at a time when they had no evidence of DM. These patients were relatively well balanced among the three RT dose groups (13%, 14%, and 9%, Table 2) and a similar percentage of them in each dose group went on to develop subsequent DM after AD (3%, 3%, and 2%, Table 2). Secondly, the pretreatment PSA and Gleason scores were imperfectly balanced among the RT dose groups. Twenty-five percent of patients in the <74 and 74-76 Gy groups had iPSA>20, versus only 15% in the >76 Gy group (Table 1). This introduces a bias toward less late failures in the high dose group. Conversely, a higher percentage of Gleason score 8-10 tumors at RT doses ≥74 Gy (8-9%), versus 4% in the <74Gy group, introduces a bias toward more late failures in the higher dose groups. Adjusting for these imbalances in MVAs demonstrated that increasing RT dose is a statistically significant and independent predictor of reduced BF and DM. Finally, the median follow-up in the >76 Gray group (65 months) is 20 months shorter than the lower dose groups (85-87 months), which introduces a potential follow-up bias toward less late failures, which is partially mitigated by the use of actuarial statistical methods.
In conclusion, RT dose escalation reduced both early and late DM. The impact of RT dose is most likely explained by direct local effects. The late rise in Nadir+2 BF at the higher RT doses suggests that local disease continues to persist in a significant proportion. Further RT dose escalation, the addition of neoadjuvant/adjuvant AD, or the use of other treatment approaches (e.g., targeted therapy) should improve cure rates in men with intermediate to high risk clinically localized prostate cancer. Cure would be implied by a gradual reduction in the late interval hazard functions for both BF and DM. The data presented here also suggest that the early identification of local persistence and consequent salvage therapy before the manifestation of BF should be a goal of future research. These findings support the more routine use of postradiotherapy biopsies at 2-3 years, since positive biopsies strongly predict for eventual BF (32, 33).
Acknowledgments
This research was supported in part by grants CA-006927 and CA101984-01 from the National Cancer Institute, and a grant from Varian Medical Systems, Palo Alto, CA. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or Varian Medical Systems. The authors thank Dr. Gerald Hanks for his leadership in the establishment of the Fox Chase Cancer Center database for the treatment of prostate cancer reported herein and Ruth Peter for her dedication to its maintenance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous Presentations of these Data: None
Conflicts of Interest Notification: The authors have no actual or potential conflicts of interest to disclose.
References
- 1.Kuban DA, el-Mahdi AM, Schellhammer PF. Effect of Local Tumor Control on Distant Metastasis and Survival in Prostatic Adenocarcinoma. Urology. 1987;30:420–426. doi: 10.1016/0090-4295(87)90372-4. [DOI] [PubMed] [Google Scholar]
- 2.Zagars GK, von Eschenbach AC, Ayala AG, et al. The influence of local control on metastatic dissemination of prostate cancer treated by external beam megavoltage radiation therapy. Cancer. 1991;68:2370–2377. doi: 10.1002/1097-0142(19911201)68:11<2370::aid-cncr2820681107>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 3.Fuks Z, Leibel SA, Wallner KE, et al. The effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with 125I implantation. Int J Radiat Oncol Biol Phys. 1991;21:537–547. doi: 10.1016/0360-3016(91)90668-t. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald I. Biological predeterminism in human cancer. Surg Gynecol Obstet. 1951;92:443–452. [PubMed] [Google Scholar]
- 5.Coen JJ, Zietman AL, Thakral H, et al. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. 2002;20:3199–3205. doi: 10.1200/JCO.2002.01.086. [DOI] [PubMed] [Google Scholar]
- 6.Jacob R, Hanlon AL, Horwitz EM, et al. The relationship of increasing radiotherapy dose to reduced distant metastases and mortality in men with prostate cancer. Cancer. 2004;100:538–543. doi: 10.1002/cncr.11927. [DOI] [PubMed] [Google Scholar]
- 7.Hanks GE, Hanlon AL, Epstein B, et al. Dose response in prostate cancer with 8-12 years’ follow-up. Int J Radiat Oncol Biol Phys. 2002;54:427–435. doi: 10.1016/s0360-3016(02)02954-1. [DOI] [PubMed] [Google Scholar]
- 8.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 9.Zelefsky MJ, Fuks Z, Hunt M, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166:876–881. [PubMed] [Google Scholar]
- 10.Kupelian PA, Mohan DS, Lyons J, et al. Higher than standard radiation doses (> or =72 Gy) with or without androgen deprivation in the treatment of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2000;46:567–574. doi: 10.1016/s0360-3016(99)00455-1. [DOI] [PubMed] [Google Scholar]
- 11.Zelefsky M, Leibel S, Gaudin P, et al. Dose Escalation with Three-Dimensional Conformal Radiation Therapy Affects the Outcome in Prostate Cancer. Int J Radiat Oncol Biol Phys. 1998;41:491–500. doi: 10.1016/s0360-3016(98)00091-1. [DOI] [PubMed] [Google Scholar]
- 12.American Joint Committee on Cancer . Prostate. In: Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. Springer; New York: 2002. pp. 337–345. [Google Scholar]
- 13.Chism DB, Hanlon AL, Horwitz EM, et al. A comparison of the single and double factor high-risk models for risk assignment of prostate cancer treated with 3D conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:380–385. doi: 10.1016/j.ijrobp.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz EM, Hanlon AL, Pinover WH, et al. Defining the optimal radiation dose with three-dimensional conformal radiation therapy for patients with nonmetastatic prostate carcinoma by using recursive partitioning techniques. Cancer. 2001;92:1281–1287. doi: 10.1002/1097-0142(20010901)92:5<1281::aid-cncr1449>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Monti AF, Ostinelli A, Frigerio M, et al. An ICRU 50 radiotherapy treatment chart. Radiother Oncol. 1995;35:145–150. doi: 10.1016/0167-8140(95)01541-n. [DOI] [PubMed] [Google Scholar]
- 16.Cox J, Grignon D, Kaplan R, et al. Consensus Statement: Guidelines for PSA Following Radiation Therapy. Int J Radiat Oncol Biol Phys. 1997;37:1035–1041. [PubMed] [Google Scholar]
- 17.Horwitz EM, Thames HD, Kuban DA, et al. Definitions of biochemical failure that best predict clinical failure in patients with prostate cancer treated with external beam radiation alone: a multi-institutional pooled analysis. J Urol. 2005;173:797–802. doi: 10.1097/01.ju.0000152556.53602.64. [DOI] [PubMed] [Google Scholar]
- 18.Kestin LL, Vicini FA, Martinez AA. Practical application of biochemical failure definitions: what to do and when to do it. Int J Radiat Oncol Biol Phys. 2002;53:304–315. doi: 10.1016/s0360-3016(02)02707-4. [DOI] [PubMed] [Google Scholar]
- 19.Pickles T, Kim-Sing C, Morris WJ, et al. Evaluation of the Houston biochemical relapse definition in men treated with prolonged neoadjuvant and adjuvant androgen ablation and assessment of follow-up lead-time bias. Int J Radiat Oncol Biol Phys. 2003;57:11–18. doi: 10.1016/s0360-3016(03)00439-5. [DOI] [PubMed] [Google Scholar]
- 20.Vicini FA, Kestin LL, Martinez AA. The correlation of serial prostate specific antigen measurements with clinical outcome after external beam radiation therapy of patients for prostate carcinoma. Cancer. 2000;88:2305–2318. doi: 10.1002/(sici)1097-0142(20000515)88:10<2305::aid-cncr15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Thames H, Kuban D, Levy L, et al. Comparison of alternative biochemical failure definitions based on clinical outcome in 4839 prostate cancer patients treated by external beam radiotherapy between 1986 and 1995. Int J Radiat Oncol Biol Phys. 2003;57:929–943. doi: 10.1016/s0360-3016(03)00631-x. [DOI] [PubMed] [Google Scholar]
- 22.Roach M, 3rd, Hanks G, Thames H, Jr., et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:447–457. [Google Scholar]
- 24.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;50:163–170. [PubMed] [Google Scholar]
- 25.Pollack A, Smith LG, von Eschenbach AC. External beam radiotherapy dose response characteristics of 1127 men with prostate cancer treated in the PSA era. Int J Radiat Oncol Biol Phys. 2000;48:507–512. doi: 10.1016/s0360-3016(00)00620-9. [DOI] [PubMed] [Google Scholar]
- 26.Pollack A, Hanlon AL, Horwitz EM, et al. Prostate cancer radiotherapy dose response: an update of the fox chase experience. J Urol. 2004;171:1132–1136. doi: 10.1097/01.ju.0000111844.95024.74. [DOI] [PubMed] [Google Scholar]
- 27.Holmberg L, Bill-Axelson A, Helgesen F, et al. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med. 2002;347:781–789. doi: 10.1056/NEJMoa012794. [DOI] [PubMed] [Google Scholar]
- 28.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. Jama. 2005;294:1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 29.Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 30.Pollack A, Hanlon AL, Movsas B, et al. Biochemical failure as a determinant of distant metastasis and death in prostate cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:19–23. doi: 10.1016/s0360-3016(03)00538-8. [DOI] [PubMed] [Google Scholar]
- 31.Hanlon AL, Hanks GE. Failure patterns and hazard rates for failure suggest the cure of prostate cancer by external beam radiation. Urology. 2000;55:725–729. doi: 10.1016/s0090-4295(99)00605-6. [DOI] [PubMed] [Google Scholar]
- 32.Pollack A, Zagars GK, Antolak JA, et al. Prostate biopsy status and PSA nadir level as early surrogates for treatment failure: analysis of a prostate cancer randomized radiation dose escalation trial. Int J Radiat Oncol Biol Phys. 2002;54:677–685. doi: 10.1016/s0360-3016(02)02977-2. [DOI] [PubMed] [Google Scholar]
- 33.Crook J, Malone S, Perry G, et al. Postradiotherapy prostate biopsies: what do they really mean? Results for 498 patients. Int J Radiat Oncol Biol Phys. 2000;48:355–367. doi: 10.1016/s0360-3016(00)00637-4. [DOI] [PubMed] [Google Scholar]


