Abstract
The biosynthesis of hormones and neuropeptides involves post-translational cleavage of precursors at basic amino acids by prohormone convertases (PCs) predominantly in secretory granules that bud from the trans-Golgi Network. This study reports that the amino acid sequence of PC3 (aa617–638), previously identified as a novel transmembrane (TM) domain, confers lipid raft association and facilitates sorting of the enzyme to the secretory granules of Neuro2A cells for prohormone cleavage. Floatation analysis on sucrose density gradients showed that a proportion of full length (PC3-FL) and carboxyl terminus-truncated PC31–638 (PC3-638) containing the TM domain were associated with lipid rafts in Neuro2A cells, while PC31–616 (PC3-616) and PC3-ΔTM lacking the TM domain were not. Secondly, PC3-FL and PC3-638 underwent stimulated secretion and were shown to be colocalized with a secretory granule marker, chromogranin A, by immunocytochemistry. In contrast, PC3-616 and PC3-ΔTM were constitutively secreted and primarily localized in the Golgi. These data indicate that the transmembrane domain of PC3 plays a key role in sorting the enzyme to the regulated secretory pathway.
Keywords: prohormone convertase, enzyme, sorting, lipid raft, transmembrane protein
1. Introduction
In eukaryotic cells, biologically active peptides are synthesized in the endoplasmic reticulum (ER) as larger, inactive precursors, or preproproteins. Processing of these multivalent precursors to release the biologically active molecules is mediated by proprotein/prohormone convertases and the active molecules are secreted from the cell following specific stimuli (Burgess and Kelly, 1987; Seidah and Chretien, 1997; Steiner, 1998). In neuroendocrine cells, peptide hormones and peptide neurotransmitters are sorted away from lysosomal and constitutively secreted proteins into secretory granules for secretion by the regulated secretory pathway (RSP). Successful biosynthesis and regulated secretion of these molecules is dependent on a range of factors including correct targeting of the precursor and the processing enzymes to the RSP. Several different mechanisms have been proposed for sorting proteins to the RSP, encapsulated within two non-mutually exclusive targeting models; the “sorting for entry” and the “sorting by retention” models (Arvan and Castle, 1998). Targeting is likely to involve specific sorting motifs, interaction of proteins with the immature granule membrane or membrane components and aggregation/condensation of proteins (Rindler, 1992; Zhang et al., 1999; Lou et al., 2005).
Prohormone convertase 3 (PC3) is a member of the mammalian Kex2/subtilisin-like endoprotease family, capable of cleaving prohormones into smaller biologically active peptides (Hakes et al., 1991; Seidah et al., 1991; Steiner et al., 1992). PC3 is synthesized as an inactive precursor that undergoes autocatalytic truncation of its pro-region (Zhou, Y. and Lindberg, 1994; Shennan et al., 1995), generating a full length 86 kDa PC3 (Christie et al., 1991; Benjannet et al., 1992; Jean et al., 1993). Further carboxy-terminal truncation occurs in a post-TGN compartment (Zhou, Y. and Lindberg, 1993; Milgram and Mains, 1994; Coates and Birch, 1997), yielding the major PC3-ΔC, a 64kDa~66kDa form that is found in neuroendocrine secretory granules (Hill et al., 1995).
The sorting of PC3 to the RSP has been studied by several laboratories. Initial observations by Zhou et al (Zhou, A. et al., 1995) indicated the involvement of the C-terminus for efficient trafficking and/or storage in the RSP in AtT20 cells, a finding that was supported by another study in AtT20 cells (Jutras et al., 1997). Studies in PC12 cells, initially appeared to conflict with this (Zhou, Y. et al., 1995), however, recent work by Bernard et al (Bernard et al., 2003) showed that PC3 lacking its C-terminus (PC3-ΔC (PC31–616)) is reduced in its regulated secretion and increased in its constititutive secretion in PC12 cells demonstrating a reliance of PC3 on its C-terminus for correct trafficking to the RSP. A more definitive study using fusion proteins of Fc fragments and C-terminal domains of PC3 demonstrated that there were two amphipathic alpha helical stretches within the C-terminus that were sufficient for sorting these chimeras to the RSP (Jutras et al., 2000). In addition, these authors confirmed that the C-terminus was necessary for sorting PC3 to the RSP in GH4 cells since a construct expressing PC3-ΔC (PC31–616) was secreted constitutively. Recently, this group has further characterized one of these alpha helical domains and showed again with Fc-fusion proteins a sufficiency for this domain to sort this reporter protein to the RSP and indeed enhance its sorting when functioning in combination with the dibasic residue sorting signal found within the proregion of prorenin (Lacombe et al., 2005). Other domains or sequences within PC3 have also been shown to be important in its sorting to the RSP. These include the cleavage site, R616–R617, in combination with other dibasic sequences within the C-terminus (Bernard et al., 2003) and the highly conserved integrin binding sequence, RGD, adjacent to the P domain, which when mutated, resulted in the constitutive secretion of PC3 and a reduction in the generation of the 64KDa PC3 form in GH4C1, PC12 and AtT20 cells (Lusson et al., 1997; Rovere et al., 1999).
Recently, we detected the C-terminus of PC3 on the outside of purified granules from bovine adrenal medulla (Arnaoutova et al., 2003), implicating that at least some PC3 was in a transmembrane (TM) orientation and that in situ; this C-terminus was cytosolic. We further identified immunologically that amino acids 619–638 were responsible for the TM orientation of PC3 in secretory granules based on its resistance to carbonate extraction and 2D-gel electrophoresis. However, this is not a conventional TM domain and its insertion into the membrane may not occur until PC3 arrives at the TGN. Indeed this is consistent with the recent work by Stettler et al. which showed that transfected and overexpressed PC3 is not synthesized as a TM protein at the ER in the non-endocrine COS 1 cells (Stettler et al., 2005). Insertion through the membrane of the Golgi, although anti-dogmatic is possible, given the precedent of the diphtheria toxin (Ren et al., 1999) and the cytosolic TA-proteins (Borgese et al., 2003). For example, diphtheria toxin by itself fails to insert into model membranes as a TM protein but in the presence of molten globule-like proteins it does (Ren et al., 1999). Furthermore, we showed that amino acids 619–638 were sufficient to direct the sorting of a reporter protein to the granules of the RSP as assayed by immunofluorescence microscopy (Arnaoutova et al., 2003). These data demonstrated that amino acids 619–638 contained information sufficient for sorting to the RSP and that the mechanism possibly involved insertion of PC3 through the membrane.
The goal of the current set of experiments was to investigate the importance of aa617–638 of PC3 in the trafficking of PC3 itself to the RSP. To do this we analyzed the secretory behavior, cellular localization and lipid-raft association of full length PC3 (PC3-FL), PC3 with intact C-terminus but without the TM domain (PC3-ΔTM), PC3 with the C-terminus deleted but containing the TM domain (PC3-638), and PC3 with both the C-terminus and the TM domain deleted (PC3-616). We found that the sequence aa617–638 in the context of PC3-638 conferred association of PC3-638 with lipid rafts, targeting of PC3-638 to the RSP and co-localization with a secretory granule marker of the RSP. In the absence of aa617–638, PC3-616 was not associated with lipid rafts, did not appreciably co-localize with a secretory granule marker, and was not targeted to the RSP as evidenced by increased constitutive secretion and the absence of regulated secretion. Furthermore, PC3 missing only the TM domain (PC3-ΔTM) was secreted via the constitutive pathway.
2. Materials and Methods
2.1. Generation of PC3 constructs
The full length rat PC3 expression construct was prepared by cloning a ~2.9 kb fragment containing the complete coding sequence of rat preproPC3 into pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA) at EcoRI and DraI restriction sites. To generate carboxyl terminus-truncated PC3 constructs, an XhoI site was introduced at base pairs 1673–1678 by silent mutagenesis using Pwo Polymerase (Roche Molecular Biochemicals, Mannheim, Germany), a heat stable polymerase with 3′ to 5′ exonuclease activity to increase the fidelity of amplification. We used a PCR-based strategy with the internal overlapping primers containing an XhoI site: 5′-CGCGCTCGAGCATGTGCAATTTGAA-3′ (sense) and 5′-GCGCCTCGAGGGAATTGATAGCATT-3′ (antisense) and primers to amplify the full length PC3 coding sequence: 5′-CCGGAATTCGTGCGAGCCATGAAGC-3′ (sense primer with EcoR1 site), 5′-GCTCTAGACAACAACTCCAGACCCAGG-3′ (antisense primer with XbaI site). The 5′ amplicon contained EcoRI and XhoI restriction sites at the 5′ and 3′ termini respectively. The 3′ amplicon contained XhoI and XbaI restriction sites at the 5′ and 3′ termini respectively.
All carboxyl terminus truncation mutants collectively referred as PC3-ΔC were prepared by PCR. rPC3-616 was prepared using the sense primer: 5′-CCGCTCGAGCATGTGCAATTTGAAGCAACAATCGC-3′ (XhoI site) and antisense primer: 5′GGGGAAGCTTCTAGTCATTCTGGACAGTATTGTAGG-3′ (HindIII site). PC3-638 and r/hPC3-638 were amplified using the same sense primer and either antisense primer: 5′-GGGCTCTAGACTAGCCATTCAGGCTG-3′ (XbaI site, PC3-638) or 5′-CCTCTAGACTACTCCTTCGGGTTCTCTTGTGTGGGCTGCTCCTCCCCAGGATCCACC-3′ (r/hPC3-638).
PC3-ΔTM was prepared by PCR using pcDNA3.1-PC3 as template with two pairs of primers (PC3-pcrXI and SPC3delC, and SPC3del and BGH reverse). The sequences of the primers were as follow: PC3-pcrXI: 5′-CGCGCTCGAGCATGTGCAATTTGAA-3′; SPC3delC (XhoI site, antisense): 5′-GGGTACCAGGTCATTCTGGACAGTATTG-3′; SPC3del (sense): 5′-CCAGAATGACCTGGTACCCAAAAACTCC-3′; and BGH reverse: 5′-TAGAAGGCACAGTCGAGG-3′. The products of the two reactions were purified, combined and re-amplified with PC3-pcrXI and BGH reverse primers. The final PCR product was digested with Xho I and Xba I. All the fragments were directionally ligated into pcDNA3.1 vector and the final constructs confirmed by DNA sequence analysis.
2.2. Transfection
Neuro2A cells, (N2A) a clone of the mouse neuroblastoma cell line expressing very low levels of endogenous PC3 (Zhang et al., 2003), were cultured in Dulbecco’s modified Eagles medium (DMEM) supplemented with 10% fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 4 mM glutamine at 37°C under an atmosphere of 5% CO2. The constructs for various molecular forms of PC3 and vector control were transiently transfected for 24 hours using Lipofectamine™2000 (Invitrogen) according to the manufacturer’s protocol. For analysis of floatation experiments, cells were grown to approximately 70% confluence in a 10 cm dish and transfected with a DNA concentration of 10 μg/dish. For Western blot analysis of PC3 secretion experiments, cells were plated into 6 cm dishes and transfected with cDNA constructs at 4 μg/dish. For enzymatic analysis of stimulated secretion experiments, 2 × 105 cells were plated into each well of a 6 well plate and transfected with cDNA (1 μg/well) using Fugene 6 (Roche Molecular Biochemicals) according to the manufacturer’s recommendations. Following transfection, cells were incubated for 2 days prior to measurements of secreted PC3 activity.
2.3. Sucrose density gradient centrifugation
Cells from over-night transfections were lysed with 1% Triton X-100 (TX-100) in TNE buffer (50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 4°C) supplemented with 1x complete inhibitor cocktail (Roche Molecular Biochemicals) for 30 min on ice. The cells were collected into eppendorf tubes on ice and centrifuged at 3000 × g for 10 min at 4°C to make a post nuclear fraction. The supernatant was mixed in an ultracentrifuge tube with 1.875 M sucrose to adjust to 1.2 M sucrose (1.2 ml total). This was overlaid with 1.6 ml of 1.1 M sucrose and 0.8 ml of 0.15 M sucrose before centrifugation for 15 h at 140,000 × g at 4°C. Ten fractions (360 μl each) were collected from the top of the gradient and proteins precipitated in 20% (v/v) trichloroacetic acid on ice. The pellets were washed once with cold acetone, suspended and denatured with 1x sample buffer, followed by SDS-PAGE using 4–20% gel and Western blotting. Detection of PC3 was performed with a rabbit PC3 fusion antibody (PC3-fus, at dilution of 1:5000) (Hill et al., 1995) and ECL plus detection system (Amersham Biosciences Inc, Piscataway NJ, USA). The blot of PC3-ΔTM and PC3-616 were stripped and reprobed with antibody against the raft protein caveolin-1 (Affinity Bioreagents, Golden, CO, USA) or the non-raft protein transferrin receptor (TfR, Zymed Laboratories, San Francisco, CA, USA) as controls.
2.4. Fluorescence Immunocytochemistry
N2A cells, grown on 15 mm diameter glass slides until ~70% confluent, were transfected with PC3 cDNA of PC3-FL, PC3-ΔTM, PC3-638 and PC3-616 and co-transfected with either secretory granule marker, chromogranin A (bovine CGA, inserted in pcDNA 3.1- vector) (Kim et al., 2001). After overnight transfection, cells were rinsed with PBS, fixed in 4% formaldehyde and permeabilized with 0.1% TX-100 in PBS. Primary and secondary antibodies were diluted in PBS containing 1% BSA as follows: rabbit anti-PC3-fus (1:600); guinea pig anti-bovine CGA (bCGA) (1:2000); mouse anti-p115 (Transduction Laboratories) as a marker for the Golgi apparatus (1:5000). Goat anti-rabbit IgG Alexa 488 (green), goat anti-guinea pig IgG Alexa 568 (red) and goat anti-mouse IgG Alexa 568 (red) (Molecular Probes, Eugene, OR, USA) were used at 1:1000. Double labeling of PC3 with bCGA or p115 was carried out for all four constructs in transfected cells. The fluorescent images were collected and processed as described previously (Lou et al., 2005). To evaluate targeting of PC3 to the regulated secretory pathway, cells with processes were selected. The number of cells containing PC3 punctate staining that overlapped with CGA punctate staining in the processes was recorded in a blind manner. The data were expressed as the mean ± SEM of the percentage of cells showing co-localization of punctate staining in the processes from three separate experiments. The difference between PC3-FL and the other groups (PC3ΔTM, PC3-638 or PC3-616) was compared by two way Student t-test.
2.5. Basal Secretion of PC3
After overnight transfection with the PC3 constructs, the cells were washed with DMEM. The basal release of the PC3 was performed by incubation of the cells with DMEM supplemented with 0.02 % ovalbumin (Amersham Biosciences Inc.) for three one-hour incubation periods. The media were collected from 3 consecutive 1 h incubations and 1x complete inhibitor cocktail was immediately added to the collected media to prevent protein degradation. The media were then centrifuged to remove detached cells and cell debris, and concentrated 5-fold with Amicom Ultra-4 Centrifugal Filter Devices (10,000 MWCO, Millipore Corp, Bedford, MA USA) for 30 min at 4°C. The cells were lysed with 1x TX-100/TNE buffer supplemented with the inhibitor cocktail. The cell lysate was collected and centrifuged at 15,000 × g for 10 min at 4°C. The supernatant of the cell lysate and 20 μl of concentrated media were denatured, run on 4–20% SDS-PAGE gels and transferred onto nitrocellulose membrane. The PC3 was detected using PC3-fus antibody (1:5000) followed by IRDye conjugated secondary antibodies (Rockland Immunochemicals, Gilbertsville, PA, USA) and analyzed with an Odyssey Infrared Imager System and application software (LI-COR Biosciences, Lincoln, NE USA). The intensities of the fluorescence from corresponding bands were recorded as arbitrary units. The units for PC3-FL include both 86 kDa and PC3ΔC bands. To assess protein loadings, the cell lysate blots were double labeled with PC3-fus antibody and a mouse antibody against endogenous tubulin (Transduction laboratories, KY, USA). The expression of intracellular PC3 was normalized to tubulin expression in each sample. The secretion level of PC3 for each construct was corrected for the PC3 expression level in the matched cell lysate and expressed as the mean ±SEM from four separate experiments. The secretion level of PC3-638 and PC3-616 in the basal medium was then expressed as a percent of PC3-FL (made equal to 100%). The difference between the constructs was compared by Student t-test.
2.6. Stimulated Secretion Experiment
Neuro2A cells, transfected with PC3-FL, PC3-ΔTM, PC3-638 and PC3-616, were rinsed and equilibrated in DMEM/0.02% ovalbumin for 2 h at 37°C. The cells were then subjected to a 15 min incubation with DMEM/0.02% ovalbumin (Basal medium) followed by a 15 min incubation with DMEM containing 50 mM KCl/2mM BaCl2/0.02% ovalbumin (Stimulation medium). The cells were then harvested in lysis buffer. The basal and stimulation media were concentrated 10-fold by centrifugation filtration and PC3 levels in the samples were detected by Western blot analysis as described above for the basal secretion study. The level of PC3 secretion was expressed as the mean ±SEM from five separate experiments. The difference between the basal and stimulated medium was assessed statistically using the Student t-test.
The stimulated secretion study was also carried out using an enzymatic assay for the detection of PC3 in the medium. N2A cells transiently transfected with pcDNA3.1 (vector only) or PC3/pcDNA3.1 constructs were washed with PBS and replicate wells incubated for 2 h with basal release medium or with stimulated release medium. Basal release medium contained 25 mM Hepes, 125 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 5.6 mM glucose and 0.5 mM MgCl2 (pH 7.4). Stimulated release medium contained 25 mM Hepes, 80 mM NaCl, 51 mM KCl, 1.2 mM KH2PO4, 5.6 mM glucose, 0.5 mM MgCl2 and 5.2 mM CaCl2 (pH 7.4). Bovine serum albumin was added to a final concentration of 0.2 mg/ml. Medium samples were centrifuged at 2000 × g for 5 min to pellet any detached cells and supernatants were assayed for enzymatic activity immediately. PC3 enzymatic activity was determined using the fluorescent substrate Boc-Arg-Val-Arg-Arg-MCA (Peninsula Laboratories, CA, USA). Media were assayed in quadruplicate in assay buffer containing 100 mM 2-[N-Morpholino]ethane-sulfonic acid-HCl (pH 6.0), 50 μM Boc-Arg-Val-Arg-Arg-MCA, 5 mM CaCl2, and 0.05% CHAPS in a final volume of 200 μl. Samples were incubated at 37°C for 5 h in 96 well plates and fluorescence values measured directly using a SpectraMAX Gemini X5 fluorescent plate reader (Molecular Devices Corp. Sunnyvale, CA, USA) with excitation at 360 nm and emission at 480 nm. Levels of enzymatic activity were calculated as total pmol AMC after subtracting of AMC from the basal or stimulated secretion media from vector controls. The significance of differences between unstimulated and stimulated samples and between different constructs was determined using the Welch t-test.
3. Results
3.1. Expression of exogenous PC3 constructs
In this study we have investigated a role for the carboxy-terminal transmembrane domain of PC3 (PC3619–638) for sorting PC3 to the regulated secretory pathway. Constructs for the various molecular forms of PC3 were prepared (Fig. 1A) and transfected into N2A cells. Control N2A cells transfected with vector only (Fig. 1B, V) showed very low levels of immunoreactive PC3. N2A cells transfected with PC3-FL (Fig. 1B, FL) express the full length 752 amino acid protein which is cleaved to generate carboxy-terminal (C-terminal) truncated forms, 64–66 kDa PC3ΔC. The molecular forms seen in N2A cells following transfection with the full length PC3 construct are similar to those seen in the anterior pituitary AtT20 cell line, which expresses PC3 endogenously (Fig. 1B, lane AtT). In contrast, expression of the PC3-638 and PC3-616 constructs resulted in detection of single immunoreactive forms with molecular masses of ~66 kDa and ~64 kDa, respectively. The PC3-616 construct, terminating at aspartic acid 616 is considered to be the mature form of PC3 found in secretory granules produced by autocatalytic cleavage at the dibasic Arg617–Arg618 cleavage site (Zhou, Y. and Lindberg, 1994; Zhou, A. et al., 1995; Coates and Birch, 1997; Bernard et al., 2003). Transfection of N2A cells with the PC3-ΔTM construct (ΔTM) resulted in expression of an 83 kDa immunoreactive band which represents the unprocessed form of PC3 (Fig. 1B, ΔTM). The expression level for each construct was quantified relative to expression levels for full length PC3 (FL = 100%) with correction for slight differences in protein load, determined by quantification of the endogenous protein tubulin (Fig. 1C). The expression level of PC3-638 (97±8%) and PC3-ΔTM (94±13%) were not significantly different from PC3-FL. The expression of PC3-616 was slightly higher than PC3-FL (113±13%, p>0.05) but the difference was not statistically significant.
Fig. 1.
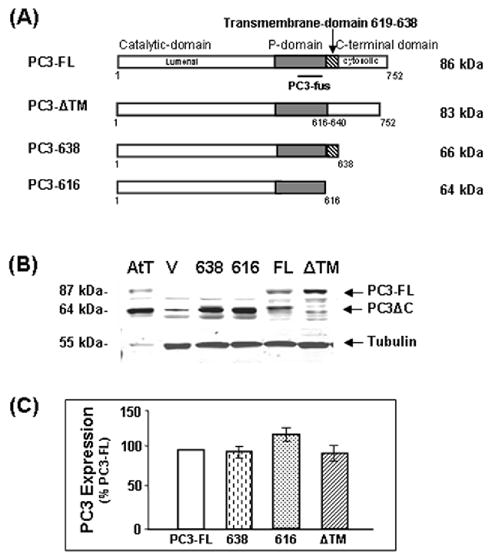
Analysis of PC3 expression in transfected Neuro2A cells. (A) Schematic representation of the PC3 constructs: PC3-FL, full length PC3; PC3-ΔTM, PC3 without the transmembrane domain; PC3-638, C-terminally truncated PC3 with the transmembrane domain; PC3-616, C-terminally truncated PC3 without the transmembrane domain. (B) Western blot of PC3 expression in AtT-20 cell lysate (AtT) as a positive control (5 μg), cell lysates (30 μg) of Neuro-2A cells transfected with vector only (V), PC3-638 (638), PC3-616 (616), full-length PC3 (FL), or PC3-ΔTM (−ΔTM). (C) Quantification of expression levels of intracellular PC3 in the cells transfected with truncated PC3 enzymes relative to PC3-FL (open bar) designated as 100%, after normalizing to expression levels of endogenous protein, tubulin (55 kDa), (mean ±SEM, n=4). There was no significant difference between the four constructs as assessed by the Student t test.
3.2. Lipid raft association of PC3 TM domain
To assess whether PC3 was associated with lipid rafts, transfected N2A were extracted with TX-100/TNE buffer and analyzed by floatation through sucrose density gradients followed by Western Blotting (Fig. 2). A band of immunoreactive PC3 was found in the low density fraction (fraction 1) in cells transfected with PC3-FL and PC3-638 constructs. In contrast, neither PC3-616 nor PC3-ΔTM floated to this fraction at the top of the sucrose gradient. Caveolin-1, a known lipid raft protein (Kurzchalia and Parton, 1999), was detected in the low density fraction (fraction 1) in PC3-616 or PC3-ΔTM gradients, while the non raft-associated transferrin receptor (Chamberlain and Gould, 2002), did not float (fraction 9), demonstrating the integrity of the gradients. These data indicate that a population of PC3-FL and PC3-638, but not PC3-ΔTM or PC3-616, exhibit lipid-raft membrane association properties in transfected N2A cells. The results for PC3-FL and PC3-616 were similar to previous studies investigating the corresponding endogenous forms of PC3 in isolated chromaffin granules (Blazquez et al., 2001; Arnaoutova et al., 2003). A proportion of PC3-FL and PC-638 was found in the high density fractions which is likely to represent non-raft associated, ER and Golgi localized PC3 enzymes, since total cell lysate was loaded on the gradient and only granule/TGN PC3 was expected to be raft-associated, as previously reported for the prohormone processing enzyme carboxypeptidase E (CPE) (Dhanvantari and Loh 2000).
Fig. 2.
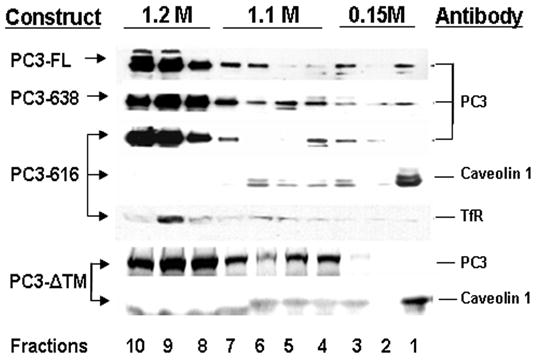
Sucrose density gradient analysis of raft association of PC3. N2A cells transfected with PC3 constructs were extracted with 1% TX-100/TNE and fractionated on a sucrose gradient. Fractions were collected from the top of the gradient (1–10) and precipitated proteins analyzed by Western blotting using various antibodies. Left column: PC3 constructs used in the transfection. Right column: antibodies used for Western blots. The blots of the gradient for PC3-616 and PC3-ΔTM were stripped and reprobed with antibodies to caveolin-1 or the transferrin receptor (TfR) as controls.
3.3. Subcellular co-localization of PC3 and CGA
The localization of PC3 was investigated by immunocytochemical analysis of transfected N2A cells (Fig. 3). PC3-FL and PC3-638 immunoreactivities were colocalized with the secretory granule marker, CGA, in processes of N2A cells, whereas PC3-616 or PC3-ΔTM immunoreactivity was mainly restricted to the perinuclear region of the cell body. To assess the sorting of PC3 mutants to the regulated secretory pathway, the number of cells showing co-localization of PC3 and CGA immunoreactivity in the processes were quantified from three experiments (Table 1). The data clearly indicate that the PC3-FL and PC3-638 showed a high level of co-localization with CGA (92.7±2.3% and 81.7±2.2%, respectively). However, both PC3-616 and PC3-ΔTM constructs exhibited significantly reduced levels of co-localization with CGA (20.3±2.9%, p<0.002; 25.0±1.9%, p<0.002, respectively). The images from double labeling of PC3 and p115 (right panel) indicated that in the majority of the cells, PC3-616 or PC3-ΔTM staining overlapped with the Golgi marker, p115 (Fig. 3).
Fig. 3.
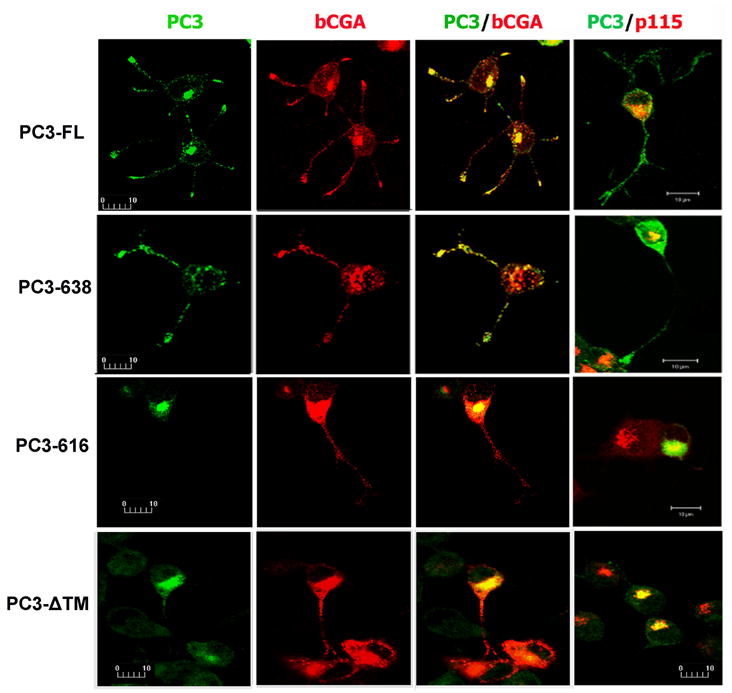
Subcellular distribution of PC3 in transfected N2A cells by fluorescence immunocytochemistry and confocal microscopy. N2A cells were co-transfected with four PC3 constructs and an expression construct encoding bovine chromogranin A (bCGA). The green signal indicates immunoreactive PC3 (1st column) and the red signal indicates bCGA, the secretory granule marker, in transfected cells (2nd column). The merged confocal images show the co-localization of PC3 and bCGA (yellow, in 3rd column) in the cell processes of full length PC3 (PC3-FL) and carboxyl terminus truncated PC-638 transfected cells. The 4th column shows co-localization of PC3 (green) and p115 (red) in transfected cells. The PC3 staining in PC3-616 or PC3-ΔTM transfected cells was primarily present in the cell body and colocalized with p115, a Golgi marker. The bar represents 10 μm.
Table 1.
Quantification of PC3 constructs colocalized with the secretory granule marker chromogranin A in transfected Neuro-2A cells.
| Construct | Exp. 1 | Exp.2 | Exp. 3 | %Mean ± SEM | P value |
|---|---|---|---|---|---|
| PC3-FL | 79/86 (91.9%) | 32/33 (97.0%) | 68/76 (89.5%) | 92.7% ± 2.3 | |
| PC3-ΔTM | 11/45 (24.4%) | 12/42 (28.7%) | 19/86 (22.1%) | 25.0% ± 1.9 | P<0.002 |
| PC3-638 | 66/84 (78.6%) | 42/49 (85.7%) | 48/60 (80.0%) | 81.7% ± 2.2 | P< 0.05 |
| PC3-616 | 11/52 (21.2%) | 8/52 (15.4%) | 39/155(25.2%) | 20.3% ± 2.9 | P< 0.002 |
Fractions (e.g. 79/86) represent the number of cells showing punctate staining for PC3 that co-localized with CGA in cell processes over the total number of cells with processes. Means and standard error of the mean (SEM) were calculated from three separate experiments.
3.4. High basal secretion of PC3ΔC
To evaluate the role of the transmembrane domain of PC3 in the secretory pathway, basal release of PC3 at steady state was examined in N2A cells transfected with PC3-FL or C-terminal truncated forms of PC3 (PC3-638 and PC3-616). The secretion levels of the different forms of PC3 were analyzed by Western blot after various incubation times. Truncated forms of PC3 (638 and 616) detected in the media were compared with full-length PC3 (Fig. 4A). After normalization of expression levels in the cell lysates, PC3-616 showed significantly higher basal release in the first (205±27%, p<0.05), second (207±39%, p<0.02) and third hour (203±46%, p<0.05) compared to the PC3-FL (100%). The secretion of PC3-638 was similar to the full-length form (105±9%, 108±13% and 110±10% in the first, second and third hour, respectively, p>0.05) (Fig. 4B). Higher basal secretion of PC3-616 indicated missorting of this enzyme to the constitutive secretory pathway and is consistent with previous work by others (Zhou, A. et al., 1995; Jutras et al., 1997; Jutras et al., 2000; Bernard et al., 2003).
Fig. 4.
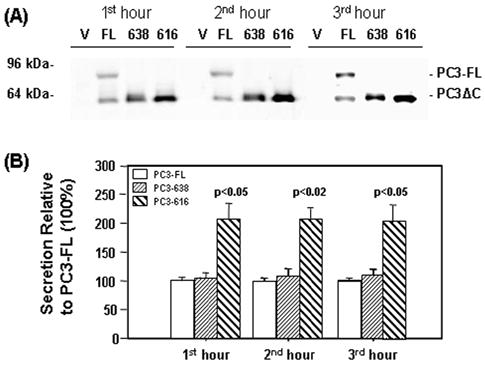
Steady state secretion of full length and truncated PC3 from transfected N2A cells.
(A) Representative Western blot of PC3 in concentrated basal medium (20 μl) collected from 3 consecutive 1 hour incubations (1st hour, 2nd hour and 3rd hour) using the PC3-fus antibody and analyzed with the Odyssey Infrared Imager. Basal medium was collected from cells transfected with empty vector (V), or vector encoding full-length PC3 (FL), C-terminus truncated PC3-638 (638) and PC3-616 (616). (B) Quantification of PC3 levels detected on Western blots from 4 separate experiments. PC3 levels for PC3-638 and PC3-616 constructs are shown as a percentage of full-length PC3 (PC3-FL) at each time point. Note that higher levels of PC3-616 in the medium compared to full-length PC3 and PC3-638.
3.5. Stimulated Secretion of PC3ΔC with TM domain
To assess targeting of the PC3 proteins to the regulated secretory pathway, high KCl depolarization was used to stimulate secretion in transfected N2A cells. Analysis by Western blots (N=5) demonstrated a 2.45±0.30-fold increase in secretion upon stimulation for PC3-FL transfected cells [(basal) 3.06±0.74 vs. (stim.) 6.26±1.36 arbitrary units, p<0.02] as shown in Figs. 5A and B. PC3-638 transfected cells showed 1.75±0.21-fold increase in stimulation [(basal) 3.90±0.92 vs. (stim.) 6.90±0.92, p<0.05). In contrast, PC3-616 showed no increased secretion (0.8 fold) after stimulation (5.78±0.80) compared to its unstimulated control (7.73±1.48) (Figs. 5A and B).
Fig. 5.
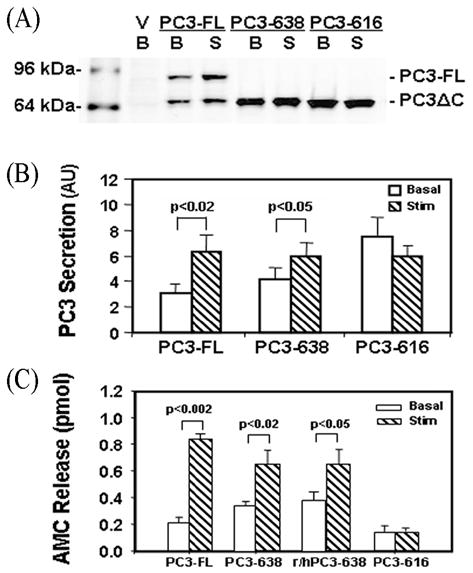
Stimulated secretion of PC3 from transfected N2A cells.
(A) Representative PC3 Western blot of concentrated basal (B) or stimulated (S) media collected from three PC3 constructs. Medium was collected from cells transfected with empty vector (V), or vectors encoding full-length PC3 (FL), and C-terminally truncated PC3-638 (638) and PC3-616 (616). There were significant increases in the stimulated secretion of PC3 in FL and 638 transfected cells, but not for PC3-616.
(B) Quantification of PC3 secretion levels expressed as mean ±SEM from stimulated media, (hashed bars) compared to basal media (open bars) from Western blots (N=5). AU: arbitrary units; (C) Analysis of PC3 activity using the fluorescent substrate Boc-Arg-Val-Arg-Arg-MCA for the enzymatic activity in basal release (clear bars) or stimulated release medium (hatched bars). Levels of enzymatic activity were calculated as total pmol AMC released. Data are presented as mean release ±SEM from two experiments in quadruplicates after subtraction of AMC from the vector-only controls. The enzymatic activity in vector-only transfected cells in basal medium was 1.03 ± 0.02 pmol AMC and in stimulation medium 1.16± 0.03 pmol AMC.
The secretion of the various forms of PC3 was also assayed enzymatically (Fig. 5C). PC3-FL showed a 4-fold increase of enzymatic activity upon stimulation [(Basal) 0.21±0.04 vs. (Stimulated) 0.84±0.03, p<0.002] and PC3-638 showed a ~2-fold increase upon stimulation [(Basal) 0.34±0.03 vs. (Stimulated) 0.65±0.01, p<0.02]. The activity level of a second construct expressing the human 619–638 sequence fused to rat PC3-618 (r/hPC3-638) also increased 1.6-fold relative to basal control [(Basal) 0.40±0.02 vs. (Stimulated) 0.65±0.1, p<0.05]. In contrast, PC3-616 showed no increase in activity after stimulation compared to its unstimulated control [(Basal) 0.14±0.01 vs. (Stimulated) 0.14±0.05, p>0.05]. Interestingly, the enzymatic activity level in the basal medium from cells transfected with the PC3-616 construct was lower than that of the PC3-FL and PC3-638 constructs (0.14±0.05 vs. 0.21±0.04 and 0.34±0.03, respectively) unlike the Western blot analysis (Fig. 4 and Fig. 5B). This apparent lower level of specific enzymatic activity of PC3-616 may be due to partial misfolding of PC3-616 protein, as a result of deletion of the transmembrane domain.
3.6. Constitutive secretion of PC3 without TM domain (PC3-ΔTM)
To assess the importance of transmembrane domain and the carboxyl terminus sequences beyond the transmembrane domain (amino acids 617–639) we compared the secretion of a construct lacking the residues 617–639, PC3-ΔTM with PC3-FL constructs in N2A cells by Western blotting (Fig. 6A). PC3-ΔTM was secreted mainly as an unprocessed 83 kDa form (Fig 6A); while the exogenous full length PC3 (FL) was present in both 87 kDa and 64–66 kDa PC3-ΔC forms, similar to the endogenous PC3 found in AtT-20 cell lysate (Fig. 6A, lane AtT). However, PC3-ΔTM showed higher basal release than PC3-FL [3.07±0.69 vs. 1.69±0.27 arbitrary units (AU), respectively, p<0.05] and no stimulated secretion [(basal) 3.07±0.69 vs. (stim.) 2.55±0.58, p>0.05]. The positive control PC3-FL demonstrated 2.6 fold stimulated secretion (4.32±0.76 vs. basal 1.69±0.27, p<0.02) (Fig. 6B).
Fig. 6.
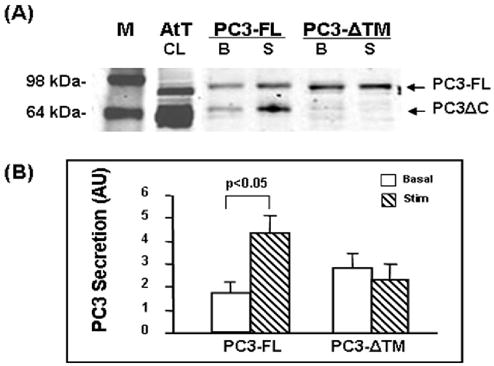
Analysis of PC3-ΔTM secretion in transfected Neuro2A cells.
(A) Representative Western blot showing PC3 secretion levels from concentrated medium. M: molecular weight marker; AtT20 cell lysate (AtT, CL, 5 μg); 20 μl of basal medium (B) and stimulated medium (S) from PC3-FL (FL) or PC3-ΔTM (ΔTM) transfected N2A cells.
(B) Quantification of PC3 secretion levels expressed as mean ±SEM from stimulated medium (hashed bars) versus basal medium (open bars) from Western blots from four separate experiments (AU: arbitrary units).
4. Discussion
The present study identifies an amino acid sequence (617–639) in the carboxyl terminus of PC3 as a domain that is necessary for targeting PC3 to the regulated secretory pathway (RSP). Analysis of sorting domains within the native molecule is critical given discrepancies that may arise between this approach and construction of chimeras linking sorting domains to constitutively sorted proteins. Previous studies demonstrated that expression of the 64 kDa form of PC3 (PC3(1–616)) yielded a protein that was secreted through the constitutive secretory pathway (Zhou, A. et al., 1995; Jutras et al., 2000; Bernard et al., 2003). This result has been repeated in the present study confirming that the carboxyl terminus of PC3 is necessary for targeting PC3 to the RSP. In an effort to define the domains within the C-terminus of PC3 that conferred this sorting, Jutras et al (Jutras et al., 2000) demonstrated a sufficiency for two C-terminal amphipathic alpha-helices (667–713 and 711–753) of PC3 to target a reporter protein. However, until now, the only deletion mutation experiment that has been performed, save for the PC3(1–616) studies described above, in an effort to demonstrate necessity, was by Bernard at al (Bernard et al., 2003). That study clearly showed that PC3 sequence beyond K687 did not contain primary sorting signals for efficient sorting of PC3 to the RSP but suggested that aa617–687 contained the necessary information. In our present study we analyzed a further deletion mutant, PC3-638, and demonstrated that this construct was sorted to the RSP, although at reduced efficiency compared to WT PC3, whereas a construct lacking residues 617–639 was not sorted. These results indicated that residues beyond aa638 were also not primary sorting signals for PC3 sorting. The implication, therefore is that aa617–638 contains primary information for sorting of PC3 and supports our previous study showing that this sequence was sufficient to target the extracellular domain of the soluble IL2 receptor α-subunit to secretory granules (Arnaoutova et al., 2003). Our current study showing that the construct (PC3-ΔTM) with a deletion of the 617–639 sequence, but including the two carboxyl terminus sorting domains was not trafficked to the RSP, supports the key role of this domain for sorting into the RSP.
The R617–R618 cleavage site has been reported to contribute to efficient sorting to the RSP (Bernard et al., 2003). Investigated as part of a larger study probing the role of paired basic amino acids in the carboxyl terminus of mouse PC3 for processing and targeting, mutation of R617–R618 with more than one of the remaining three other dibasics (R629–R630; K654–R655 and K686–K687) in the carboxyl-terminal domain, blocked sorting to the RSP. However, mutation of R617–618 alone had no effect (Bernard et al., 2003). It has been proposed that the concerted binding of multiple basic residues to negatively charged molecules (proteins or phospholipids) within the RSP facilitates the sorting of PC3 (Bernard et al., 2003). This suggestion is weakened somewhat by the lack of evolutionary conservation between rat and mouse of R630 and K687 since this would argue for a less significant role for these residues in this process. However, in the context of our current data, interaction of positively charged residues with the membrane or membrane protein(s) may facilitate the initial binding and/or insertion of the C-terminal tail through the Golgi/TGN membrane by an as yet unknown mechanism.
Other reports, as mentioned above, have identified amino acid sequences located within the carboxyl terminus of PC3 that act as sorting domains for PC3. A peptide region spanning mouse PC3 residues 627–670 was shown to be less efficient in sorting an Fc fusion protein to secretory granules in transfected GH4 cells compared to the complete C-terminal sequence (Jutras et al., 2000). In light of our current study, the poor targeting efficacy of this Fc-PC3(627–670) fusion protein may be due to the absence of ~50% of the sorting domain described herein (aa617–638), although further analysis of the 627–670 sequence within the framework of the PC3 molecule is still needed. Additionally, two carboxyl terminus sorting domains encompassing residues 667–713 and 711–753 which are closer to the carboxyl terminus than the domain (aa617–638) identified in our present study, have been reported to target Fc-PC3 fusion proteins to the RSP in transfected GH4 cells (Jutras et al., 2000). Since we previously showed that residues 617–638 constitute a novel transmembrane domain, the two regions (667–713 and 711–753) would be predicted to have a cytoplasmic localization once in a TM orientation. Given that the full or partial removal of these two domains did not eliminate sorting, as shown by this study (PC3-638, Figs. 3 and 5) and Bernard et al (construct ST687) (Bernard et al., 2003), the hierarchy and participation of these domains in the sorting of PC3 in vivo within the framework of full length PC3 remains to be determined.
These three carboxyl terminal sequences have some intriguing similarities. Both peptide domains (667–713 and 711–753) in the Jutras et al study are predicted to contain α-helices and can associate with GH4 cell membranes, leading the authors to suggest that these sequences mediated the binding of fusion proteins to membranes within the sorting compartments of the secretory pathway. The 617–638 sorting domain identified in the present study is also predicted to form an amphipathic α-helix (Snell C.R., personal communication) similar to that of the transmembrane domain of CPE (Dhanvantari et al., 2002). Additionally, the r/hPC3-638 chimera, expressing the human PC3(619–638) sequence which differs from the rat sequence at 9 out of the 20 residues, was also sorted to the RSP, supporting a conformational role for this domain. Thus, all three domains are suggested to interact with membranes. This may increase the efficiency of sorting of PC3 since the stimulated secretion of PC3-638, lacking these domains, is 30-40% less than PC3-FL. These C-terminal α-helical domains may initially bind on the inner face of the TGN membrane to facilitate flipping and transmembrane orientation of the PC3 to effect more efficient sorting, However, evidence reported here suggests that aa617–638 are critical for this sorting to occur.
In conclusion, our present study has demonstrated that aa617–638 of PC3 plays a critical role in sorting PC3 to the RSP. We propose that interaction of this domain with lipid rafts at the trans-Golgi network may be the mechanism by which PC3 is sorted into the budding granules of the RSP, analogous to another prohormone processing enzyme, carboxypeptidase E (Dhanvantari et al., 2002; Lou et al., 2005). We speculate that the transmembrane insertion of a proportion of PC3 molecules into lipid rafts at the TGN may be enough to carry other PC3 molecules into the RSP to enhance sorting efficiency since PC3 can form self-aggregates at an acidic pH (unpublished data), similar to that of CPE (Rindler, 1998) and PC2 (Jan et al., 1998).
Acknowledgments
We thank John Pearson for advice on the statistical analysis. This research was supported by a Marsden Grant from the Royal Society of New Zealand to N.P.B. and Y.P.L. and by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnaoutova I, Smith AM, Coates LC, Sharpe JC, Dhanvantari S, Snell CR, Birch NP, Loh YP. The prohormone processing enzyme PC3 is a lipid raft-associated transmembrane protein. Biochemistry. 2003;42:10445–55. doi: 10.1021/bi034277y. [DOI] [PubMed] [Google Scholar]
- Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjannet S, Reudelhuber T, Mercure C, Rondeau N, Chretien M, Seidah NG. Proprotein conversion is determined by a multiplicity of factors including convertase processing, substrate specificity, and intracellular environment. Cell type-specific processing of human prorenin by the convertase PC1. J Biol Chem. 1992;267:11417–23. [PubMed] [Google Scholar]
- Bernard N, Kitabgi P, Rovere-Jovene C. The Arg617-Arg618 cleavage site in the C-terminal domain of PC1 plays a major role in the processing and targeting of the enzyme within the regulated secretory pathway. J Neurochem. 2003;85:1592–603. doi: 10.1046/j.1471-4159.2003.01823.x. [DOI] [PubMed] [Google Scholar]
- Blazquez M, Docherty K, Shennan KI. Association of prohormone convertase 3 with membrane lipid rafts. J Mol Endocrinol. 2001;27:107–16. doi: 10.1677/jme.0.0270107. [DOI] [PubMed] [Google Scholar]
- Borgese N, Brambillasca S, Soffientini P, Yabal M, Makarow M. Biogenesis of tail-anchored proteins. Biochem Soc Trans. 2003;31:1238–42. doi: 10.1042/bst0311238. [DOI] [PubMed] [Google Scholar]
- Burgess TL, Kelly RB. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–93. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Chamberlain LH, Gould GW. The vesicle- and target-SNARE proteins that mediate Glut4 vesicle fusion are localized in detergent-insoluble lipid rafts present on distinct intracellular membranes. J Biol Chem. 2002;277:49750–4. doi: 10.1074/jbc.M206936200. [DOI] [PubMed] [Google Scholar]
- Christie DL, Batchelor DC, Palmer DJ. Identification of kex2-related proteases in chromaffin granules by partial amino acid sequence analysis. J Biol Chem. 1991;266:15679–83. [PubMed] [Google Scholar]
- Coates LC, Birch NP. Posttranslational maturation of the prohormone convertase SPC3 in vitro. J Neurochem. 1997;68:828–36. doi: 10.1046/j.1471-4159.1997.68020828.x. [DOI] [PubMed] [Google Scholar]
- Dhanvantari S, Arnaoutova I, Snell CR, Steinbach PJ, Hammond K, Caputo GA, London E, Loh YP. Carboxypeptidase E, a prohormone sorting receptor, is anchored to secretory granules via a C-terminal transmembrane insertion. Biochemistry. 2002;41:52–60. doi: 10.1021/bi015698n. [DOI] [PubMed] [Google Scholar]
- Hakes DJ, Birch NP, Mezey A, Dixon JE. Isolation of two complementary deoxyribonucleic acid clones from a rat insulinoma cell line based on similarities to Kex2 and furin sequences and the specific localization of each transcript to endocrine and neuroendocrine tissues in rats. Endocrinology. 1991;129:3053–63. doi: 10.1210/endo-129-6-3053. [DOI] [PubMed] [Google Scholar]
- Hill RM, Ledgerwood EC, Brennan SO, Pu LP, Loh YP, Christie DL, Birch NP. Comparison of the molecular forms of the Kex2/subtilisin-like serine proteases SPC2, SPC3, and furin in neuroendocrine secretory vesicles reveals differences in carboxyl-terminus truncation and membrane association. J Neurochem. 1995;65:2318–26. doi: 10.1046/j.1471-4159.1995.65052318.x. [DOI] [PubMed] [Google Scholar]
- Jan G, Taylor NA, Scougall KT, Docherty K, Shennan KI. The propeptide of prohormone convertase PC2 acts as a transferable aggregation and membrane-association signal. Eur J Biochem. 1998;257:41–6. doi: 10.1046/j.1432-1327.1998.2570041.x. [DOI] [PubMed] [Google Scholar]
- Jean F, Basak A, Rondeau N, Benjannet S, Hendy GN, Seidah NG, Chretien M, Lazure C. Enzymic characterization of murine and human prohormone convertase-1 (mPC1 and hPC1) expressed in mammalian GH4C1 cells. Biochem J. 1993;292:891–900. doi: 10.1042/bj2920891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras I, Seidah NG, Reudelhuber TL. A predicted alpha -helix mediates targeting of the proprotein convertase PC1 to the regulated secretory pathway. J Biol Chem. 2000;275:40337–43. doi: 10.1074/jbc.M004757200. [DOI] [PubMed] [Google Scholar]
- Jutras I, Seidah NG, Reudelhuber TL, Brechler V. Two activation states of the prohormone convertase PC1 in the secretory pathway. J Biol Chem. 1997;272:15184–8. doi: 10.1074/jbc.272.24.15184. [DOI] [PubMed] [Google Scholar]
- Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell. 2001;106:499–509. doi: 10.1016/s0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- Kurzchalia TV, Parton RG. Membrane microdomains and caveolae. Curr Opin Cell Biol. 1999;11:424–31. doi: 10.1016/s0955-0674(99)80061-1. [DOI] [PubMed] [Google Scholar]
- Lacombe MJ, Mercure C, Dikeakos JD, Reudelhuber TL. Modulation of secretory granule-targeting efficiency by cis and trans compounding of sorting signals. J Biol Chem. 2005;280:4803–7. doi: 10.1074/jbc.M408658200. [DOI] [PubMed] [Google Scholar]
- Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron. 2005;45:245–55. doi: 10.1016/j.neuron.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Lusson J, Benjannet S, Hamelin J, Savaria D, Chretien M, Seidah NG. The integrity of the RRGDL sequence of the proprotein convertase PC1 is critical for its zymogen and C-terminal processing and for its cellular trafficking. Biochem J. 1997;326 (Pt 3):737–44. doi: 10.1042/bj3260737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram SL, Mains RE. Differential effects of temperature blockade on the proteolytic processing of three secretory granule-associated proteins. J Cell Sci. 1994;107 (Pt 3):737–45. doi: 10.1242/jcs.107.3.737. [DOI] [PubMed] [Google Scholar]
- Ren J, Kachel K, Kim H, Malenbaum SE, Collier RJ, London E. Interaction of diphtheria toxin T domain with molten globule-like proteins and its implications for translocation. Science. 1999;284:955–7. doi: 10.1126/science.284.5416.955. [DOI] [PubMed] [Google Scholar]
- Rindler MJ. Biogenesis of storage granules and vesicles. Curr Opin Cell Biol. 1992;4:616–22. doi: 10.1016/0955-0674(92)90080-v. [DOI] [PubMed] [Google Scholar]
- Rindler MJ. Carboxypeptidase E, a peripheral membrane protein implicated in the targeting of hormones to secretory granules, co-aggregates with granule content proteins at acidic pH. J Biol Chem. 1998;273:31180–5. doi: 10.1074/jbc.273.47.31180. [DOI] [PubMed] [Google Scholar]
- Rovere C, Luis J, Lissitzky JC, Basak A, Marvaldi J, Chretien M, Seidah NG. The RGD motif and the C-terminal segment of proprotein convertase 1 are critical for its cellular trafficking but not for its intracellular binding to integrin alpha5beta1. J Biol Chem. 1999;274:12461–7. doi: 10.1074/jbc.274.18.12461. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Chretien M. Eukaryotic protein processing: endoproteolysis of precursor proteins. Curr Opin Biotechnol. 1997;8:602–7. doi: 10.1016/s0958-1669(97)80036-5. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Marcinkiewicz M, Benjannet S, Gaspar L, Beaubien G, Mattei MG, Lazure C, Mbikay M, Chretien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol. 1991;5:111–22. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- Shennan KI, Taylor NA, Jermany JL, Matthews G, Docherty K. Differences in pH optima and calcium requirements for maturation of the prohormone convertases PC2 and PC3 indicates different intracellular locations for these events. J Biol Chem. 1995;270:1402–7. doi: 10.1074/jbc.270.3.1402. [DOI] [PubMed] [Google Scholar]
- Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–9. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- Steiner DF, Smeekens SP, Ohagi S, Chan SJ. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992;267:23435–8. [PubMed] [Google Scholar]
- Stettler H, Suri G, Spiess M. Proprotein convertase PC3 is not a transmembrane protein. Biochemistry. 2005;44:5339–45. doi: 10.1021/bi047430c. [DOI] [PubMed] [Google Scholar]
- Zhang CF, Dhanvantari S, Lou H, Loh YP. Sorting of carboxypeptidase E to the regulated secretory pathway requires interaction of its transmembrane domain with lipid rafts. Biochem J. 2003;369:453–60. doi: 10.1042/BJ20020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CF, Snell CR, Loh YP. Identification of a novel prohormone sorting signal-binding site on carboxypeptidase E, a regulated secretory pathway-sorting receptor. Mol Endocrinol. 1999;13:527–36. doi: 10.1210/mend.13.4.0267. [DOI] [PubMed] [Google Scholar]
- Zhou A, Paquet L, Mains RE. Structural elements that direct specific processing of different mammalian subtilisin-like prohormone convertases. J Biol Chem. 1995;270:21509–16. doi: 10.1074/jbc.270.37.21509. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Lindberg I. Purification and characterization of the prohormone convertase PC1(PC3) J Biol Chem. 1993;268:5615–23. [PubMed] [Google Scholar]
- Zhou Y, Lindberg I. Enzymatic properties of carboxyl-terminally truncated prohormone convertase 1 (PC1/SPC3) and evidence for autocatalytic conversion. J Biol Chem. 1994;269:18408–13. [PubMed] [Google Scholar]
- Zhou Y, Rovere C, Kitabgi P, Lindberg I. Mutational analysis of PC1 (SPC3) in PC12 cells. 66-kDa PC1 is fully functional. J Biol Chem. 1995;270:24702–6. doi: 10.1074/jbc.270.42.24702. [DOI] [PubMed] [Google Scholar]


