Abstract
Lipids extracted from the skin of C57BL/6J mice injected subcutaneously with α-(4-pyridyl-1-oxide)-N-tert-butylnitrone (POBN) and exposed to topical protoporphyrin IX (PPIX) and visible light had significantly higher levels of POBN spin adducts as compared to dark PPIX exposed or vehicle treated controls. Computer analysis of the POBN adduct EPR spectra indicated that two radical species were present in each extract, one of which was a lipid derived carbon-centered adduct (1, aN=14.8 G and aH =2.6 G) while the other (2, aN=13.8 G and aH=1.8 G) was probably oxygen-centered. Adduct 2 was present in greater proportion in lipids extracted from PPIX/light exposed mice as compared to dark or vehicle treated controls. findings suggest that PPIX/light generates free radicals in mouse skin, thus providing a radical mechanism for PPIX induced photosensitivity. Our approach may be useful for the detection of free radicals generated by other skin photosensitizers and may also provide a means for testing putative skin protecting agents.
Keywords: EPR, spin trapping, protoporphyrin IX, visible light, free radicals, photosensitization, skin
Abbreviations: EPR, electron paramagnetic (spin) resonance; PBN, α-4-pyridyl-N-tert-butylnitrone; POBN, α-(4-pyridyl-1-oxide)-N-tert-butylnitrone; PPIX, protoporphyrin IX
Introduction
Skin photosensitivity is a universal feature of erythropoietic protoporphyria, a disease that results from mutations in the ferrochelatase gene and the concomitant overproduction of protoporphyrin IX (1). PPIX is also used in the photodynamic therapy of skin tumors (2). The mechanism of action of PPIX, both phototoxic and phototherapeutic, may involve the photogeneration of reactive oxygen species, such as hydrogen peroxide and singlet oxygen (3), although it is probable that free radicals are the ultimate damaging agents (4,5). To date there are no reports of free radical generation in skin caused by PPIX and light
The direct detection of free radicals in a tissue such as skin is difficult due to their high reactivity and consequently their ephemeral nature (6–10). While it is possible to infer the generation of free radicals from an analysis of products or inhibition by scavengers, detection by EPR remains the gold standard. There have been several successful reports of free radical generation in skin using EPR alone or with the aid of spin traps (7–10). For example, we have successfully used EPR to detect an increase in the ascorbyl radical in mouse skin exposed to chlorpromazine and UVA, however, the initiating radical could not be identified (9). Miller and coworkers (11) have detected free radicals in the perfused dog skin flap using the nitrone spin trap PBN. PBN has also been successfully used to detect free radicals in skin lipids from mice exposed to cumene hydroperoxide (10), while POBN trapped radicals in lung lipids from mice exposed to lipopolysaccharide (12). In this study we have employed POBN to provide evidence for the in vivo generation of free radicals in the skin of mice treated with PPIX and irradiated with visible light.
Materials and Methods
Reagents
2,2'-Dipyridyl and PPIX were purchased from Sigma Chemical Co. (St. Louis, MO). α-(4-Pyridyl-1-oxide)-N-tert-butylnitrone (POBN) was obtained from Alexis (San Diego, CA). All other chemicals were reagent grade or better.
Animals and Treatments
Male C57BL/6J strain mice (20-25g) were group-housed in a temperature-controlled room at 23-24 °C with a 12/12 h. light/dark cycle and allowed free access to food and water. The studies adhered to the National Institutes of Health guidelines for the care and handling of experimental animals and were approved by the NIEHS Institutional Review Board. Protoporphyrin IX (1 mg) was dissolved in 1.0 ml of 50 % acetone water, and 100μl topically applied to the shaved back of each mouse anesthetized with pentobarbital. Controls were treated with 50 % acetone water. Thirty min later, 100μl of a POBN solution (200mg/ml) was injected subcutaneously. After exposure to a 100W halogen flood lamp, filtered through a 4 cm water filter to remove IR (fluence rate 1 kW/m2 measured with a YSI Model 65A Radiometer, Yellow Springs Instrument Co., OH) for 10 min, the mice were sacrificed, and the lipid phase of the dorsal skin extracted and the radical adducts detected by EPR.
EPR Measurements
Extraction of the lipid components of the skin was carried out as previously described by Sato et al. (12). Briefly, dorsal skin tissue was homogenized with a Polytron (Brinkman Instruments, Westbury, NY) in a mixture containing 2.5 ml 2:1 chloroform: methanol, 0.5 ml of 30 mM 2,2'-dipyridyl and 4 ml of deionized water, cooled in ice water. The 2,2'-dipyridyl, a ferrous chelator, was used to inhibit ex vivo ferrous-dependent free radical generation. To the extract, 16 ml 2:1 chloroform: methanol were added, and the resultant mixture was shaken and then centrifuged at 2,000 rpm for 10 min. The chloroform layer was isolated and dried by passing through an anhydrous sodium sulfate column. The sample was evaporated to a volume of 1ml under a stream of nitrogen gas, and EPR spectra were acquired immediately at room temperature using a quartz flat cell in a Bruker EMX EPR spectrometer equipped with a super high-Q cavity. Spectra were recorded on an IBM-compatible computer interfaced to the spectrometer using instrument settings of 9.79 GHz, 20.2 mW microwave power, 100 kHz modulation frequency, 1300 ms conversion time, and 655 ms time constant. Simulations of EPR spectra were performed using a computer program, WINSIM, developed in this laboratory (13). Assignment of the POBN adducts based on their EPR parameters was achieved using the STDBII database (14).
Results and Discussion
The total integrated EPR intensities of skin lipids extracted from mice treated with PPIX and visible light irradiated is significantly higher than those of the controls (Figure 1). This suggests that the radical yield is higher in the PPIX/light treated skins. Typical EPR spectra are shown in Figure 2. We simulated the EPR spectra (13) in Figure 2 assuming two radical species as this gave the best fit to the experimental data; the resultant parameters are given in Table 1. While POBN is a good trap for in vivo studies the EPR spectra of POBN adducts are often very similar and so it is difficult to identify the trapped radicals. We have previously proposed the use of the NoH parameter (defined as the ratio aN/aH) as an aid to adduct identification (15). The NoH ratio removes instrumental calibration differences and is relatively insensitive to solvent polarity (14,15). An examination of the NoH parameters in Table 1 confirms that in each case the two POBN adducts are indeed different. We therefore searched the STDII database (14) for POBN adducts with splitting constants and NoH values close to the observed adducts. Our findings suggest (Table 2) that radical 1 is a lipid derived carbon-centered adduct. In contrast radical 2 is probably oxygen-centered possibly superoxide or the hydroxyl radical adduct. The observation that radical 2 is present in the highest amount in lipids extracted from PPIX/light exposed mice (Table 1) is consistent with the known photochemistry of PPIX which can generate superoxide via a Type I mechanism or singlet oxygen via a Type II pathway (5,16,17). Dismutation of superoxide to hydrogen peroxide is a possible route to the hydroxyl radical via the Fenton reaction.
Figure 1.
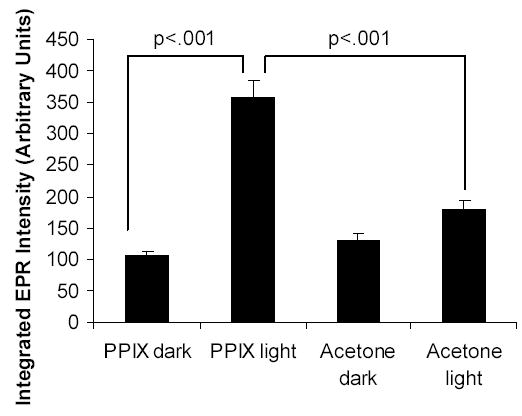
The integrated EPR intensities of skin lipids extracted from mice injected subcutaneously with POBN and treated topically with PPIX dissolved in 50% aqueous acetone or vehicle alone, followed by exposure to visible light (see Methods). Results are the mean ± standard error of data from six mice.
Figure 2.
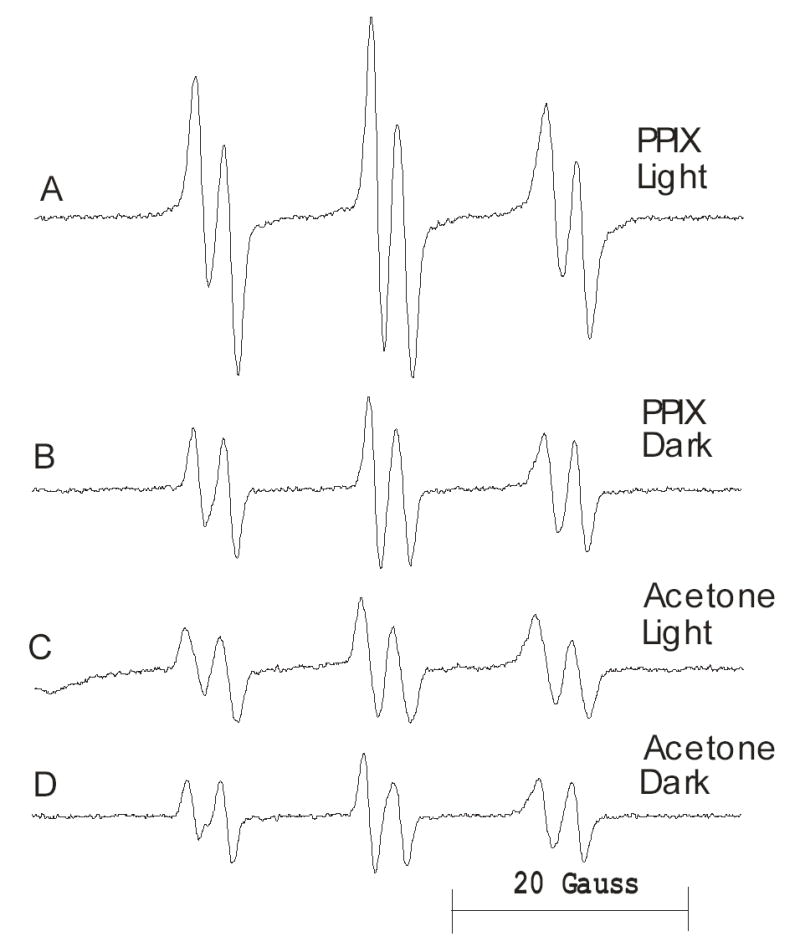
Typical EPR spectra of skin lipids extracted from mice injected subcutaneously with POBN and treated topically with PPIX dissolved in 50% aqueous acetone or vehicle alone, followed by exposure to visible light (see Methods).
Table 1.
EPR Parameters of Simulations of EPR Spectra in Figure 2
| Treatment | Species | % | aN(G) | aH(G) | aN/aH |
|---|---|---|---|---|---|
| Acetone dark | 1 | 58 | 14.9 | 2.6 | 5.7 |
| 2 | 42 | 13.8 | 2.0 | 6.9 | |
| Acetone light | 1 | 67 | 14.9 | 2.8 | 5.3 |
| 2 | 33 | 13.9 | 1.9 | 7.3 | |
| PPIX dark | 1 | 73 | 14.9 | 2.8 | 6.0 |
| 2 | 27 | 13.8 | 1.9 | 7.3 | |
| PPIX light | 1 | 49 | 14.8 | 2.6 | 5.7 |
| 2 | 51 | 13.8 | 1.8 | 7.7 |
Table 2.
Possible Assignments of POBN Adducts Detected in Skin Lipids from PPIX/light Exposed Mice
Conclusions
These in vivo studies have shown that PPIX in combination with visible light generates free radicals in the skin of mice, thus providing a mechanism for PPIX induced photosensitivity. Our approach may be useful for the in vivo detection of free radicals generated by other skin photosensitizers and may also provide a means for testing putative skin protecting agents.
Acknowledgments
Supported by the Intramural Program of the NIH, NIEHS.
References
- 1.Baart de la Faille H, Bijlmer-Iest JC, van Hattum J, Koningsberger J, Rademakers LK, van Weelden H. Erythropoietic protoporphyria: clinical aspects with emphasis on the skin. Curr Probl Dermatol. 1991;20:123–134. doi: 10.1159/000420016. [DOI] [PubMed] [Google Scholar]
- 2.Radakovic-Fijan S, Blecha-Thalhammer U, Kittler H, Honigsmann H, Tanew A. Efficacy of 3 different light doses in the treatment of actinic keratosis with 5-aminolevulinic acid photodynamic therapy: A randomized, observer-blinded, intrapatient, comparison study. J Am Acad Dermatol. 2005;53:823–827. doi: 10.1016/j.jaad.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Niedre MJ, Patterson MS, Giles A, Wilson BC. Imaging of photodynamically generated singlet oxygen luminescence in vivo. Photochem Photobiol. 2005;81:941–943. doi: 10.1562/2005-03-15-TSN-462. [DOI] [PubMed] [Google Scholar]
- 4.Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention and management. Drug Saf. 2002;25:345–372. doi: 10.2165/00002018-200225050-00004. [DOI] [PubMed] [Google Scholar]
- 5.Niziolek M, Korytowski W, Girotti AW. Self-sensitized photodegradation of membrane-bound protoporphyrin mediated by chain lipid peroxidation: inhibition by nitric oxide with sustained singlet oxygen damage. Photochem Photobiol. 2005;81:299–305. doi: 10.1562/2004-10-25-RA-351. [DOI] [PubMed] [Google Scholar]
- 6.Sakurai H, Yasui H, Yamada Y, Nishimura H, Shigemoto M. Detection of reactive oxygen species in the skin of live mice and rats exposed to UVA light: a research review on chemiluminescence and trials for UVA protection. Photochem Photobiol Sci. 2005;4:715–720. doi: 10.1039/b417319h. [DOI] [PubMed] [Google Scholar]
- 7.Mader K, Bacic G, Swartz HM. In vivo detection of anthralin-derived free radicals in the skin of hairless mice by low-frequency electron paramagnetic resonance spectroscopy. J Invest Dermatol. 1995;104:514–517. doi: 10.1111/1523-1747.ep12605998. [DOI] [PubMed] [Google Scholar]
- 8.Herrling T, Zastrow L, Fuchs J, Groth N. Electron spin resonance detection of UVA-induced free radicals. Skin Pharmacol Appl Skin Physiol. 2002;15:381–383. doi: 10.1159/000064545. [DOI] [PubMed] [Google Scholar]
- 9.Buettner GR, Motten AG, Hall RD, Chignell CF. ESR detection of endogenous ascorbate free radical in mouse skin: enhancement of radical production during UV irradiation following application of chlorpromazine. Photochem Photobiol. 1987;46:161–164. doi: 10.1111/j.1751-1097.1987.tb04751.x. [DOI] [PubMed] [Google Scholar]
- 10.Shvedova AA, Kisin ER, Murray AR, Kommineni C, Castranova V, Mason RP, Kadiiska MB, Gunther MR. Antioxidant balance and free radical generation in vitamin E-deficient mice after dermal exposure to cumene hydroperoxide. Chem Res Toxicol. 2002;15:1451–1459. doi: 10.1021/tx0200313. [DOI] [PubMed] [Google Scholar]
- 11.Miller C, Chen G, Janzen E. Detection of free radicals in reperfused dog skin flaps using electron paramagnetic resonance spectroscopy: A pilot study. Microsurgery. 1999;19:171–175. doi: 10.1002/(sici)1098-2752(1999)19:4<171::aid-micr2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Sato K, Kadiiska MB, Ghio AJ, Corbett J, Fann YC, Holland SM, Thurman RG, Mason RP. In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. FASEB J. 2002;16:1713–1720. doi: 10.1096/fj.02-0331com. [DOI] [PubMed] [Google Scholar]
- 13.Duling DR. Simulation of multiple isotropic spin-trap EPR spectra. J Magn Reson B. 1994;104:105–110. doi: 10.1006/jmrb.1994.1062. [DOI] [PubMed] [Google Scholar]
- 14.Li ASW, Chignell CF. STDBII, a database for storing, retrieving and analyzing spin trapping data on an IBM or Macintosh personal computer. Res Chem Intermediates. 1990;14:235–257. [Google Scholar]
- 15.Li AS, Chignell CF. The NoH value in EPR spin trapping: a new parameter for the identification of 5,5-dimethyl-1-pyrroline-N-oxide spin adducts. J Biochem Biophys Methods. 1991;22:83–87. doi: 10.1016/0165-022x(91)90084-a. [DOI] [PubMed] [Google Scholar]
- 16.Molnár A, Dedic R, Korinek M, Svoboda A, Hála J. Protoporphyrin IX and hematoporphyrin derivatives interactions with oxygen studied by time and spectral resolved phosphorescence. Journal of Molecular Structure. 2005;744–747:723–726. [Google Scholar]
- 17.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: Part one - Photosensitizers, photochemistry and cellular localization. Photodiagnosis and Photodynamic Therapy. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mottley C, Connor HD, Mason RP. [17O]oxygen hyperfine structure for the hydroxyl and superoxide radical adducts of the spin traps DMPO, PBN and 4-POBN. Biochem Biophys Res Commun. 1986;141:622–628. doi: 10.1016/s0006-291x(86)80218-2. [DOI] [PubMed] [Google Scholar]
- 19.Leaustic A, Babonneau F, Livage J. Photoreactivity of tungsten trioxide dispersions: spin trapping and electron spin resonance detection of radical intermediates. J Phys Chem. 1986;90:4193–4198. [Google Scholar]


