Abstract
The amygdala is critically important for fear learning, and specific kinases have been implicated as contributors to the mechanisms that underlie learning. We examined levels of protein kinase C βII (PKC βII) in the left and right lateral and basolateral nuclei (LA/BLA) of the amygdala from animals that were classically fear conditioned with tones as cues and footshocks. Groups consisted of animals that received neither tones nor shocks, paired tones and shocks, or unpaired tones and shocks. At 1 hour after conditioning, some animals from each group were used for biochemical measurements of PKC βII levels and other animals were given probe trials to assess freezing behavior to cue and context. The levels of PKC βII were greater in the left hemisphere in animals receiving neither tones nor shocks and animals receiving paired tones and shocks. PKC βII levels were greater in the right hemisphere of animals receiving randomly presented tones and shocks. Freezing times to cue were long (>80% of probe trial time) in both the paired tone/shock and randomly unpaired tone/shock groups. Freezing times to context were long in the unpaired tone/shock group, but not the paired tone/shock group. Correlational analyses showed that freezing times to context, but not cue, precisely predicted the right/left relation of PKC βII levels in the LA/BLA: the greater the time spent freezing to context, the greater the increase in right hemisphere PKC βII levels. We conclude that fear conditioning causes hemisphere and input specific increases in PKC βII in the rat LA/BLA.
INTRODUCTION
Classical fear conditioning requires long-lasting changes in neocortical, thalamic, and hippocampal inputs to the lateral and basolateral nuclei (LA/BLA) of the amygdala carrying information about conditioned stimuli (CS, tones) (Bordi and LeDoux, 1994b, Bordi and LeDoux, 1994a, Doron and LeDoux, 1999), unconditioned stimuli (US, foot shocks) (Shi and Davis, 1999, LeDoux, 2000), and context (Fanselow et al., 1994, Maren and Fanselow, 1995, Maren and Fanselow, 1997, McEchron et al., 1998).
The CS – US association is not the only kind of learning possible in such a paradigm. If tones (cues) occur during the same conditioning session as shocks, but the tones do not reliably predict the shocks (i.e., tones and shocks are “unpaired”), animals exhibit fear responses to both the tones (cue) and the chamber itself (context) (Anagnostaras et al., 2001). Depending upon the details of how tones and shocks occur in relation to one another, animals may learn that the tones never coincide with shocks and therefore, tones actually indicate a safety period (Walasek et al., 1995). The distinction between fearing the cue and fearing the context also depends on the animal’s total experience with the chamber. A näive animal that is given paired tones and shocks will more likely show cue and context conditioning after CS – US pairing, whereas animals that were repeatedly exposed to the chamber without any tones or shocks before CS – US pairing show little or no context conditioning.
Some kinds of memory formation are believed to depend on the serine-threonine kinases, referred to as “cognitive kinases,” because these kinases are present in nervous tissue and because they can remain active in the absence of transmitter or second messengers (Murray et al., 1987, Schonwasser et al., 1998, Newton, 2002, Hirai and Chida, 2003). The protein kinase C family is involved in a wide variety signaling cascades and consists of at least 10 isoforms (Ohno and Nishizuka, 2002). The isoforms of PKC designated as α, βI, βII, γ, ε, and ζ are expressed in many tissues, but are all highly expressed in the brain (Nishizuka, 1988, Hosoda et al., 1989, Saito et al., 1989). The subcellular localization of PKC isoforms appears to be isozyme-specific, cell-specific, and species-specific (Angenstein et al., 1999, Yang et al., 2003, Libien et al., 2005)(see also this paper). While different isoforms show some tissue specificity, often more than one isoform is present in a single cell (Kose et al., 1988).
The PKC’s have been implicated by various methods in the formation of memories in the amygdala. Intra-amygdalar (LA/BLA) infusions of H7, an inhibitor of PKCs and PKA, selectively inhibited the formation of long-term fear memories (Maren et al., 2003). Intraventricular injections of polymixin B sulfate (a PKC and CaM kinase inhibitor) was shown to prevent avoidance learning (Walker and Gold, 1994). Genetically altered mice lacking the entire PKC β gene (PKC βII was the only isoform present in hippocampus and LA/BLA), exhibited robust impairments in both auditory and contextual fear conditioning (Weeber et al., 2000) (see also (Hosoda et al., 1989, Saito et al., 1989)).
Studies of patients with various lesions, with various functional imaging methodologies, and work with animals has led to a general consensus that the right amygdala is the predominant amygdala for fear learning (Gazzaniga et al., 1977, LeDoux et al., 1977a, LeDoux et al., 1977b, Baker and Kim, 2004). Hemispheric asymmetry has been described in a number of other contexts. For example, there are asymmetries in the distributions of serotonin in amygdala correlated with anxiety (Andersen and Teicher, 1999), dopamine in globus pallidus (Glick et al., 1982), and norepinephrine and dopamine in prefrontal cortex correlated with attention (Carlson et al., 1996). Increases or decreases in regional blood flow are often lateralized in medial temporal cortex and amygdala (Schneider et al., 1997, Schore, 2002, Baas et al., 2004). Even receptor molecules such as GABA and NMDA show asymmetric distributions (Guarneri et al., 1985, Guarneri et al., 1988, Ito et al., 2000, Kawakami et al., 2003). Interestingly, an initially asymmetric hemispheric distribution of GABA-gated chloride channels (based on convulsant drug binding) could be converted to a symmetric distribution in stressed animals (McIntyre et al., 1988). The issue of hemispheric asymmetry of specific proteins in the amygdala has not been addressed.
The role of PKC in the amygdala is just beginning to be defined. The studies with PKC inhibitors are specific for the amygdala (infusions are made directly into the amygdala), but the inhibitors are not specific for a particular PKC isoform, and some are not even specific for PKC (see above). Mutant mice are more specific for the isoform (e.g. PKC β, see above), but the mutations are not specific for a particular structure. We chose to focus on the βII isoform of protein kinase C because we felt the previous studies clearly pointed to this isoform as important in the amygdala (Hosoda et al., 1989, Saito et al., 1989, Weeber et al., 2000). Our own screen of PKC isoforms showed the βII isoform to be one of the most prominent (see Results). We chose to study this isoform by measuring the levels of PKC βII in the rat lateral and basolateral nuclei of the amygdala during classical fear conditioning using tones and footshocks. In an effort to detect protein level changes with the highest sensitivity, we chose to focus on an early time point after conditioning (1 hour). This time point limits our study to those proteins that undergo rapid changes in level as a function of conditioning paradigm, and reduces the likelihood of detecting changes that are secondary to less specific changes in amygdala neuron activity. We paid particular attention to differences in the hemispheric levels of this isoform, and show that: 1) the PKC βII isoform is asymmetrically distributed in unconditioned animals; 2) that the relative levels of the isoform change in response to classical conditioning; and 3) that context, not cue learning, is the best predictor of the changes in PKC βII levels.
MATERIALS AND METHODS
Animal handling before experiments
Male Sprague-Dawley albino rats (age: 1–2 months; weight: 200–300 g) were handled for up to one hour on each of three consecutive days. During each daily handling period, an animal was placed into the fear conditioning chamber for 15 minutes to habituate them to the conditioning context.
Fear conditioning and control paradigms
Our classical fear conditioning paradigm was a series of 20 second-duration, 5 kHz, 75 dB tones that each co-terminated with a 0.5 second, 0.5 mA foot shock applied through the floor of the conditioning apparatus (Coulbourn Instruments Habitest System, Allentown, PA). The interior dimensions of the conditioning chamber were 29.2 cm wide, 25.4 cm deep, and 29.2 cm high. The house lights of the isolation cubicle, tones, and shocks were all computer-controlled using electromechanical relays (Computer Boards, Inc., Middleboro, MA) and LabVIEW software. The background noise level was 60 dB inside the chamber. The full set of tones and shocks was given within a 14 to 22 minute period. Freezing behavior could be monitored visually through a 0.5 cm observation window in the front of the conditioning chamber, but also via the output of the video camera (Coulbourn Instruments H27-02 camera) on television monitor (Samsung CXJ 1364 13 in diagonal television with built-in video tape recorder). Behavior was videotaped for off-line review and measurements of freezing times.
Animals were randomly assigned to a fear conditioning group that received paired tones and shocks, or one of the control groups (see Table 1). The first control group (“box alone”) had only chamber exposure, consisting of a ≈20 minute session, during which no tones or shocks were presented. The second control group (“unpaired”) received a pseudo-conditioning paradigm consisting of a ≈20 minute session during which 5 tones and 5 shocks were presented, without overlap, where tones did not predict shocks, or vice versa. Analysis of the output of the program revealed that in each random sequence of tones and shocks, each session had one occurrence of tone following tone and shock following shock. A variant of the unpaired group was also used. In this group, called the “alternating” unpaired group, tones simply alternated with shocks with variable intervals between and tone – shock or shock – tone pair. Intervals between tones in the paired tone/shock paradigm were generated by setting the duration of each interval to a random fraction of the total time without tones (or shocks). In the unpaired tone/shock paradigm, intervals between tones and shocks were randomly generated separately and the two timing arrays were merged, thus permitting overlap of tones and shocks. In the alternating tone/shock paradigm, the total time without tones or shocks was divided into intervals, each of which was randomly split to define a delay for the tone, followed by a delay for the shock.
Table 1.
A summary of common fear conditioning training paradigms and the typically reported freezing behaviors that are observed in test sessions after training. The grayed training conditions are the three conditions used for this report. Habituation periods are useful for reducing the context conditioning in animals that receive paired tones and shocks. By habituating animals to the chamber before training, animals receiving paired tones and shocks show “purer” conditioning to tones, while animals receiving one of the unpaired tone/shock protocols show more pronounced context conditioning. Our two main control groups show either no freezing to context or cue, or freezing to both context and cue. Each resembles the paired tone/shock group in one aspect and differs in another. The alternating unpaired group can be used to show a different behavior entirely to the tone, i.e., animals can learn that the tone predicts safety.
| TRAINING CONDITIONS | Common Controls | ||||
|---|---|---|---|---|---|
| Paired tones & shocks | Box Alone | Unpaired(random tones & shocks, no overlap) | Unpaired(alternating tones & shocks, no overlap) | ||
| TESTING CONDITIONS | during tone or after tone | freezing | -- | freezing | (can learn tone is a safety signal) |
| to context | -- | -- | freezing | freezing | |
Behavioral monitoring and measurements
Behavior was monitored during all conditioning sessions. For a group of animals that was not used for biochemical studies, animals were brought back to the chamber at 1 hour to assess freezing behavior to the context and tones. A separate group of animals was used for two reasons: a) to eliminate delays caused by a testing session – we wanted to match our biochemical measures taken at 1 hour; and b) we did not want re-exposure to the chamber for testing to be a second learning experience. Our test session consisted of a period in the chamber during which no tones or shocks were delivered. This period ranged from 7 to 10 minutes and we measured the total times for freezing during the first 5 minutes by counting body freezing times and head + body freezing times from the video tapes of each session. The total time spent freezing was divided by the total observation time (5 minutes) to obtain a “fraction of time spent freezing.” After the initial period in the chamber (used to assess freezing to the context), two tones were delivered. The tones were separated by 3 to 5 minutes. We measured freezing during a 100 second sample period after each of the tones. In the Results, we report body + head freezing divided by total session time. Freezing times were tracked with custom software that used specific key presses to record start/stop times for various behaviors (freezing of body, freezing of body + head, rearing, walking, etc.) and computed times spent in each (courtesy of Dr. John Kubie).
Isolation of the amygdala
Animals used for biochemical analyses were removed from the conditioning chamber to a neutral holding facility (home cage in a different room) for 1 hour. After the specific post-condition time had elapsed, animals were sacrificed (halothane anesthesia and decapitation), their brains removed and immediately frozen whole with powdered dry ice. Isolation was accomplished by sectioning the intact brain coronally so as to preserve both right and left hemispheres in individual cuts. From 400 μm thick frozen sections that included the amygdala (Yamato Kohki microtome, Asaka, Japan), the lateral and basolateral regions were cut out with a #11 scalpel blade under a dissecting microscope. The lateral boundary of the dissected region was formed by the external capsule. The apex of a triangular sample containing lateral and basolateral nuclei of the amygdala was at the level of the rhinal fissure and corresponded with a split in the external capsule. The base of the triangular sample region was at the level of the ventralmost edge of the external capsule. The medial boundary was formed by the white matter tract that split from the external capsule at the triangle’s apex. Solid tissue samples were saved in centrifuge tubes at −80° C for further processing.
Tissue pieces were rapidly homogenized using Kontex conical glass tissue grinders in 50 μl of buffer (Tris HCl 50 mM pH 7.5, EDTA 1 mM, EGTA 1mM, 2-β-mercaptoethanol 5 mM) containing protease inhibitors (benzamidine 5 mM, leupeptin 0.1 mM, aprotinin 0.8 mM, and ABSF 0.5 mM). Homogenates were centrifuged twice: first, at 3000 g for 5 minutes to remove debris, and then at 100,000 g for 30 minutes (TLA-100.2 fixed angle rotor, Beckman Coulter, Fullerton, CA) to produce a cytosolic fraction (the supernatant) and a pellet containing surface and most organelle membranes and membrane fragments (membrane fraction) (Hrabetova and Sacktor, 1996, Libien et al., 2005). The protein concentration of each sample was determined by visible light spectrophotometry (Bradford assay, 585 nm). All samples were finally subjected to heating in SDS sample buffer at 70° C for 10 minutes.
Western blot analyses
For electrophoresis, equal aliquots of cytosolic or membrane fractions were loaded on 10% NuPage gels (Invitrogen, Carlsbad, CA). The volume of each aliquot loaded per lane was such that the aliquot had 4.5 micrograms of total protein. A point that is absolutely critical to stress is that the samples from the left and right hemispheres were always run on the same gels. This was done to minimize loading, staining and/or imaging differences that can occur between gels or membranes. After electrophoresis, proteins were transferred to polyvinylidene fluoride membranes (PVDF, Immobilon membranes, Pierce, Rockford, IL) and detected with primary antibodies. Specific antibodies for the conventional PKC’s (α, βI, βII, γ), the novel PKC’s (δ, ε, η, θ), and the atypical PKC’s (λ, ζ) were obtained from SantaCruz Biotechnology. A PKM ζ isoform-specific antibody was a gift from Dr. Ivan Hernandez, SUNY Downstate. Antibodies for β-actin were from Sigma (St. Louis, MO). Phosphorylated PKC α/βII antibodies (thr 638/641) were purchased from Cell Signaling (Beverly, MA).
Primary antibodies were monoclonal or polyclonal and diluted at 1:2000 or 1:1000, respectively in tris-buffered saline (TBS). Secondary antibodies were monoclonal or polyclonal and diluted at 1:10,000 or 1:7000, respectively. Secondary antibodies were obtained from Promega (Madison, WI).
A chemiluminescent reaction product was formed by reacting blots with peroxidase-conjugated secondary antibodies. The emitted light resulting from a peroxidase—peroxide—luminol—enhancer reaction (SuperSignal West Pico Chemiluminescence substrate kit, Pierce, Rockford, IL) was captured by a cooled 14-bit CCD camera (Hamamatsu C4880-24) controlled by computer (Kinetic Imaging Systems, UK). Image processing software (typically ImageJ, a public domain software package from NIH, http://rsb.info.nih.gov/ij/) was used to directly quantitate band densities from the acquired digital images. The software plots the average intensity of staining (across the width of the band, i.e., perpendicular the direction of protein movement in the electric field) as a function of distance along the lane. Each densitometric value, in arbitrary units, is the area beneath the intensity curve for a particular band. We selected the peak by defining a boundary at the start of the transition from background, thereby “subtracting” any background staining. It is important to note that exposures were always adjusted so that the intensity curve was never clipped (i.e., flat on top indicating saturation). In some portions of the Results, densitometric values are reported as ratios (e.g., difference measures) where values are unitless.
Statistics
One-way ANOVA’s were used to compare measurements as a function of conditioning protocol. The Scheffé test was applied post-hoc to all one-way ANOVAs. Animal freezing behaviors were also compared with non-parametric statistics (two sample Kolmogorov-Smirnov test). The freezing measures were correlated with biochemical data using Pearson correlations. All statistical tests were run in SPSS for Windows (versions 10 and 11). Graphs were made using SPSS, SigmaPlot for Windows (version 6), or Kaleidograph for Windows (version 3). In the Results, means are reported with standard errors (SEM). Additionally, for each ANOVA, the F-value, the total degrees of freedom and the significance level are reported.
RESULTS
The total number of male Sprague-Dawley rats (2 months old) used was 82. Our fear conditioning group (paired tone/shock) was compared with two other groups to form our principal data set: 1) animals who received no tones or shocks (box alone); and 2) animals that received randomly presented tones and shocks (unpaired tone/shock). An additional unpaired condition, where tones alternated with shocks (alternating tone/shock), was also tested, and formed a fourth training group. From each group of animals, some were taken for biochemical studies where, at 1 hour post-conditioning, animals were sacrificed by halothane anesthesia and decapitation, and the brain rapidly removed and processed for biochemistry (see Methods). A total of 60 rats were used for biochemical studies. Other animals were returned to the chamber at 1 hour and probed for context and cue learning. A total of 22 animals were used for behavioral studies.
Survey of PKC isoforms in the lateral and basolateral amygdala
We studied rats that were not assigned to any of the conditioning or control groups to define the isoforms of PKC present in the amygdala. As shown in Figure 1, immunoblots revealed detectable levels of nearly all isoforms in pooled samples from the left and right hemispheres. The bulk of each isoform was present in the cytosolic fraction.
Figure 1.
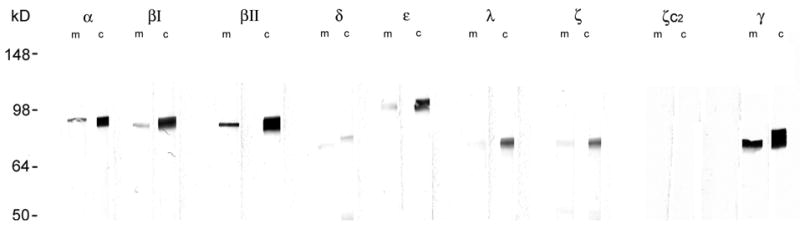
PKC isoforms of the lateral and basolateral nuclei of the rat amygdala. Western blot analyses of membrane (m) and cytosolic (c) fractions for 9 isoforms of Protein Kinase C. Pooled left and right hemisphere. Detectable levels of each isoform were present with cytosolic levels > membrane fraction levels. We were unable to detect any amount of PKM ζ with the PKM ζ-specific antibody (c2).
PKC βII levels in the LA/BLA of fear conditioned animals
After running cytosolic protein fractions on polyacrylamide gels, transfer to PVDF membranes and detection with specific antibodies, PKC βII was identified as a single nearly 80 kDa MW band (Fig. 1; Fig. 2, top left). Membrane fractions yielded a split band with a faint higher molecular weight component. Areas under densitometry curves (in arbitrary densitometric units) were measured for each band and normalized by total sample protein.
Figure 2.
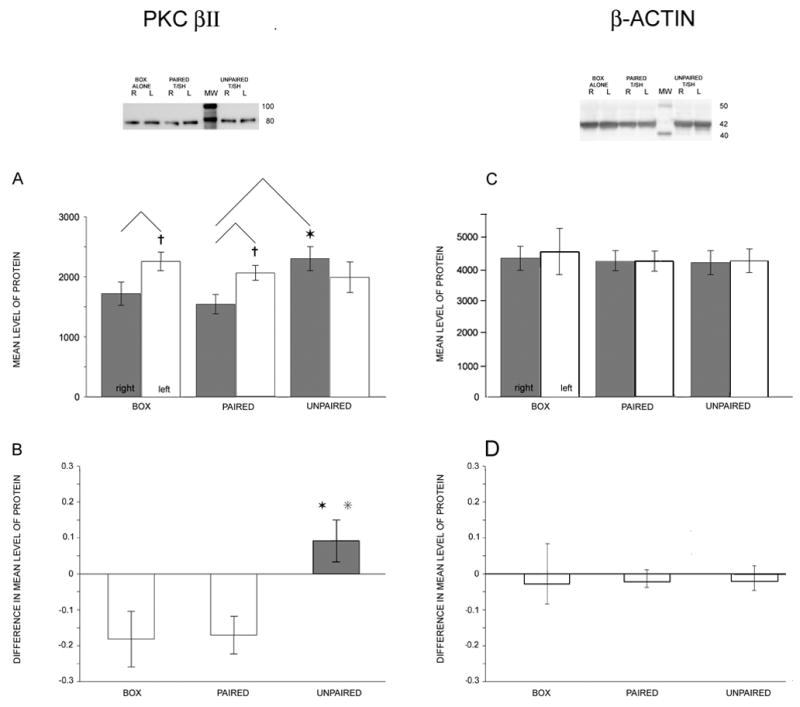
Hemispheric differences in LA/BLA PKC βII levels depend on conditioning protocol, but β-actin levels do not differ. Left, top: Western blot for PKC βII. From the left, matched (R, L) samples from a “box alone” animal (lanes 1, 2), a “paired tone/shock” animal (lanes 3, 4), the molecular weight marker (lane 5), and an “unpaired tone/shock” animal (lanes 6, 7). A: Plots of the mean densitometry readings taken from blots (normalized by total protein). Crosses above the left hemisphere (white) bars for the box alone (N=14) and paired tone/shock (N=19) groups indicate significantly higher PKC βII levels in left hemisphere (p=0.040, box; p=0.015, paired). Star above the right hemisphere (gray) bar in the unpaired tone/shock (N=10) group indicates statistically larger value compared with paired tone/shock from the same hemisphere (p=0.028, Scheffé post hoc). Mean ± SEM. B: Plots of difference measures ((R−L)/(R+L)). L>R (white bars) for the box alone and paired tone/shock groups. R>L (gray bar) for the unpaired group. Two stars over the unpaired bar indicate that this mean value was statistically larger than both the box alone and paired tone/shock values. Right, top: Western blots for β-actin from the same animals as shown in A (top). C: βactin levels did not differ between left and right hemispheres or between groups. D: Difference measures for β-actin.
Cytosolic PKC βII levels differed depending on the hemisphere and on the conditioning group. PKC βII levels in left and right hemisphere samples from box alone and paired tone/shock groups were different (paired t-tests, p=0.040, 0.015, respectively), but PKC βII levels in left and right hemisphere samples from the unpaired tone/shock group were similar (p= 0.170). The levels of PKC βII for each hemisphere and training condition are given in Table 2.
Table 2.
Data table for immunoblot analyses of PKC βII and β-actin levels and measures of freezing behavior to cue and context probe trials. Training conditions are shown as columns, measurements (mean ± SEM) are shown as rows. Significant differences between hemispheres or between training conditions are indicated in text and figures.
| TRAINING CONDITIONS | |||||
|---|---|---|---|---|---|
| BOX ALONE | PAIRED TONE/SHOCK | UNPAIRED TONE/SHOCK | ALTERNATING TONE/SHOCK | ||
| BIOCHEMISTRY | N | 14 | 19 | 10 | 17 |
| MEAN LEVEL OF PKC βII RIGHT | 1720 ± 194 | 1545 ± 161 | 2303 ± 200 | 2142 ± 117 | |
| MEAN LEVEL OF PKC βII LEFT | 2258 ± 154 | 2062 ± 126 | 1992 ± 256 | 2129 ± 100 | |
| DIFFERENCE IN MEAN LEVEL OF PKC βII | − 0.17 ± 0.07 | − 0.16 ± 0.05 | +0.09 ± 0.06 | − 0.01 ± 0.05 | |
| MEAN LEVEL OF β-ACTIN RIGHT | 4455 ± 398 | 4317 ± 335 | 4145 ± 491 | 4414 ± 428 | |
| MEAN LEVEL OF β-ACTIN LEFT | 4698 ± 613 | 4336 ± 272 | 4175 ± 491 | 4026 ± 418 | |
| DIFFERENCE IN MEAN LEVEL OF β-ACTIN | −0.052 ± 0.15 | −0.029 ± 0.06 | −0.029 ± 0.08 | 0.1931 ± 0.074 | |
| BEHAVIOR | N | 4 | 6 | 6 | 6 |
| FRACTIONAL FREEZING TIME TO CONTEXT | 0.084 ± 0.043 | 0.199 ± 0.043 | 0.713 ± 0.079 | 0.552 ± 0.081 | |
| FRACTIONAL FREEZING TIME TO CUE | 0.271 ± 0.095 | 0.822 ± 0.036 | 0.887 ± 0.025 | 0.534 ± 0.161 | |
We compared cytosolic PKC βII levels for each hemisphere across conditions. We obtained RIGHT hemisphere mean levels of PKC βII that were similar for the box alone and paired tone/shock groups. The unpaired tone/shock group had the highest mean level of PKC βII for the right hemisphere, a level that was significantly larger than the paired tone/shock group (ANOVA p=0.027, F=3.98, total df=42; unpaired larger than paired after post-hoc Scheffé correction, p=0.028; Fig. 2A, gray bars). LEFT hemisphere mean levels of PKC βII were similar across all groups (ANOVA not significant, F=0.629; Fig. 2A, white bars).
We also measured levels of cytosolic PKC α/βII in its phosphorylated state. There were no differences as a function of training condition in the levels of phosphorylated PKC α/βII in samples taken from either the right or the left hemisphere (ANOVA right hemisphere p=0.359, F=1.262; left hemisphere p=0.436, F=1.030).
Immunoblots of the membrane fractions of PKC βII showed a main band at a similar molecular weight to that for the cytosolic fraction and a faint, slightly higher molecular weight band. An analysis of differences in the PKC βII levels from the membrane fractions showed that neither the faint high MW nor the main low MW bands varied as a function of training conditions (ANOVA high MW p=0.794, F=0.234; low MW p=0.078, F=3.033; right hemisphere levels).
Even though the cytosolic PKC βII levels were sufficient to show differences as a function of training condition, we computed the relative hemispheric levels of cytosolic PKC βII as a way of reducing across-animal variability. These difference measures or “coefficients of asymmetry” ((R−L)/(R+L))(Bianki, 1985) had negative values when left hemisphere levels were larger than right hemisphere levels. Computed difference measures for the box alone and paired tone/shock groups had similar negative values, whereas the unpaired tone/shock group had a reversed R-L relation for PKC βII levels (Table 2; Fig. 2B). The difference measure for the unpaired condition was significantly more positive than both the box alone (p=0.035) and paired (p=0.026) conditions (one-way ANOVA, p=0.014, F=4.719; post-hoc Scheffé correction). The paired group did not differ from the box alone group (p=0.999).
Levels of β-actin were similar between left and right hemispheres within animals and between training conditions across animals. Mean levels for the right hemisphere did not differ (Fig. 1C; ANOVA p=0.959, F=0.10, total df=35; NS). Values for the left hemisphere samples were also similar across training conditions (ANOVA p=0.775, F=0.37; NS). The computed difference measures were nearly zero for all groups ANOVA p=0.155, F=1.87; NS; Fig. 2D).
Animals from the “alternating” unpaired group showed cytosolic PKC βII levels for amygdala samples from the right and left hemispheres that were symmetrical (Table 2; Fig. 3). If we added this group to an ANOVA with our 3 main groups, or if this group was substituted for our unpaired group, the resulting ANOVAs were significant for the right hemisphere values (p=0.020 with all 4 groups) and for the difference measures (p=0.012 with all 4 groups). As indicated by the difference measure, the alternating group was the closest to having equal levels of PKC βII in the right and left amygdala samples.
Figure 3.
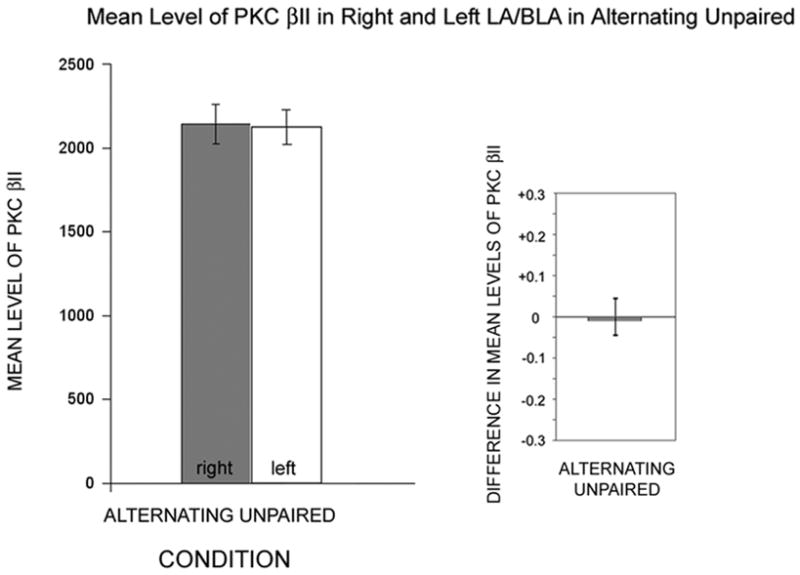
Hemispheric differences in LA/BLA PKC βII levels for animals in the alternating unpaired conditioning group. Left: Plots of the mean densitometry readings taken from blots (normalized by total protein), similar to that shown in Figure 1 for right hemisphere (gray) and left hemisphere (white). Right: Plots of difference measure ((R−L)/(R+L)) highlight the similarities in the levels between hemispheres.
Behavioral measures of learning in the fear conditioning paradigm
To correlate our biochemical findings with “learning,” without exposing animals to an additional learning session in the conditioning chamber, we used a separate subgroup of animals from each training condition to study the amount of time animals spent freezing at 1 hour post training in response to context or cue. A total of 22 animals were used (see Table 2). Of the group that received alternating tones and shocks for conditioning, all 6 animals were probed for context freezing, but 2 of the 6 were not tested with tones because of technical difficulties. The alternating tone/shock group size is therefore 4 for the tone probe trials.
Animals from the paired tone/shock and unpaired tone/shock groups had the highest fractional freezing times to the CUE when tested in probe trials at 1 hour after conditioning sessions ended (82%, 89%, respectively). Box alone animals showed the least time freezing to a test tone (<30%), and the alternating tone/shock group of animals showed an intermediate value (>50%; Table 2). The paired and unpaired tone/shock groups spent significantly more time freezing compared to the box alone group (ANOVA using all 4 groups; p=0.000, F=12.60, total df 19; p values after Scheffé post hoc corrections: box vs. paired 0.002, box vs. unpaired 0.001, paired vs. unpaired 0.935, box vs. alternating 0.244, paired vs. alternating 0.125, and unpaired vs. alternating 0.046). See Figure 4.
Figure 4.
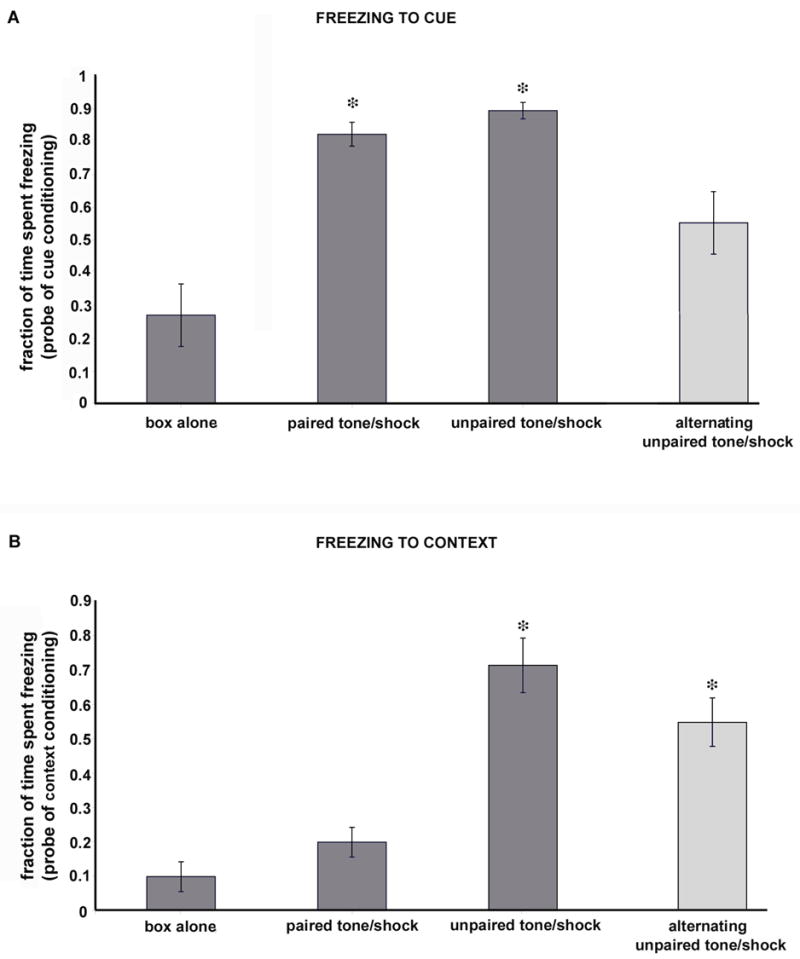
Fractions of time spent freezing to cue or context in probe trials to test learning in each conditioning group. A: Cue probe trials. Animals that received paired tones/shocks or randomly presented tones and shocks spent significantly more time freezing in response to a cue probe (single tone presentation) when compared with box alone animals (indicated by asterisks over bars). See also text for additional details. Note the intermediate time spent freezing for the alternating group. B: Context probe trials. Animals conditioned with unpaired tones and shocks or alternating tones and shocks spent significantly more time freezing during a context probe trial (placement in conditioning chamber for a period with no tones or shocks; asterisks). The low level of freezing to the context in animals that received paired tones and shocks is typical for animals that have been habituated to the chamber before conditioning trials begin.
Animals from the unpaired tone/shock group had the highest fractional freezing times to the CONTEXT (>70%) when tested in probe trials at 1 hour after conditioning sessions ended. Box alone animals spent the least time freezing (<10%), followed by the paired tone/shock group (<20%), and the alternating tone/shock group (<60%; see Table 2). Only the unpaired and the alternating tone/shock groups of animals showed significant freezing to the context (ANOVA p=0.000, F=17.54, df=21; p values after Scheffé post hoc corrections: box vs. paired 0.747, box vs. unpaired 0.000, paired vs. unpaired 0.000, box vs. alternating 0.003, paired vs. alternating 0.013, and unpaired vs. alternating 0.415).
The application of non-parametric analyses to cue and context freezing times yielded the same results. We compared groups with non-parametric tests (Kruskal Wallis repeated Wilcoxon signed rank test with Holms correction) as a precaution, given group sizes. The paired and unpaired groups had longer average freezing times to cue than the box group (two-tailed Kolmogorov-Smirnov: p=0.016 paired, 0.016 unpaired), whereas the alternating group of animals had an average freezing time that was no longer than box alone animals (p=0.211). In the comparison of freezing times to context, unpaired and alternating groups demonstrated longer average freezing times than box group animals (p=0.016, 0.016). The paired tone/shock group did not differ from the box group (p=0.236).
Correlation of freezing behaviors with PKC βII level changes
We compared freezing times to cue and context with difference measures for PKC βII (Fig. 5) and found that the difference measures paralleled freezing to context, not cue. A critical issue for our study is that we measured the freezing behaviors for each of our own training protocols. Correlating the freezing times from one group of animals with the biochemistry from a different, but identically trained, group of animals is reasonable because freezing times are very consistent for each condition. We plotted freezing time to cue or context for each training paradigm against the hemispheric differences in the levels of PKC βII. Freezing times to context were linearly related to the PKC βII levels (Pearson correlation = 0.990, two-tailed, p = 0.010). Freezing times to cue, by contrast, had no relation to the PKC βII levels (0.493, p=0.507).
Figure 5.
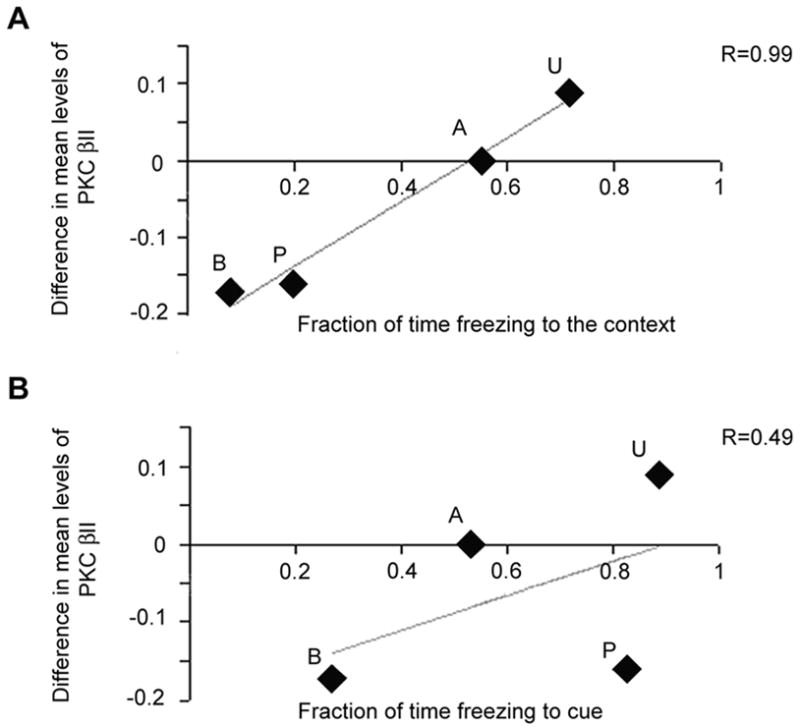
Correlation of freezing to cue and context with biochemical changes. Plots of the biochemical difference measures (ordinates) against the mean fraction of time freezing to either context or cue (abscissa in each plot). A: relation of biochemical changes to context freezing behavior. Simple linear regression line plotted and R value (0.99). Pearson correlation analyses (significant for biochemistry vs. context) in the text. B: relation of biochemical changes to cue freezing behavior. The R value from the linear regression analysis is much less (0.49) and the Pearson correlation analysis is not significant. Letters associated with each plotted point indicate the conditioning group: B=box alone, P=paired tone/shock, U=unpaired tones and shocks, and A=alternating tones and shocks.
DISCUSSION
The lateral and basolateral nuclei of the amygdala are critically important for fear conditioning, but the molecular events that accompany or support learning-related changes in these brain regions are not completely understood. We studied changes in the levels of PKC βII in the lateral and basolateral nuclei of amygdala in animals that were trained in a classical fear conditioning paradigm. We demonstrate that: a) the levels of PKC βII in the rat LA/BLA are different in the right and left hemispheres; and b) context learning, not cue learning, triggers changes in the hemispheric LA/BLA levels of PKC βII. Context, not cue, learning-related inputs to LA/BLA neurons caused cytosolic levels of PKC βII to increase in the right hemisphere, leading us to conclude that this enzyme is preferentially coupled to activation of hippocampal afferents (Wiltgen et al., 2006). This paper also provides the first evidence that a specific kinase system is activated by some inputs, but not others. However, the direct linkage mechanism between a synaptic input and increased levels of a specific kinase remains unknown.
PKC βII levels, cues and context
Several protein kinases have been identified as important for learning in studies of animals receiving paired tones and shocks or LTP in brain slices. Apart from the studies using PKC inhibitors mentioned earlier (see Introduction), PKA inhibition has been shown to block LTP in amygdala (Huang and Kandel, 1998). The phosphorylation/activation of MAP kinase, a convergence point for multiple intracellular signaling pathways, was shown to be necessary for fear conditioning (Schafe et al., 2000). Transiently increased cytosolic PKC activity was seen in CA1 in studies of LTP (Otani et al., 1993), and more sustained increases in PKC activity in both hippocampus and amygdala were seen after kindled seizures (Osonoe et al., 1994). Our work clearly identifies an association between cytosolic levels of PKC βII and context conditioning. The selective induction of PKC βII by a single input means either that different isoforms mediate memory at different synapses, or that the features of different synapses cause the cell to respond in various ways as these inputs are modified. We do not believe that the PKC βII level changes are the mechanism by which a memory is formed in amygdala neurons since both cue and context inputs show learning, but only context inputs are associated with changes in PKC βII levels.
How can there be an increase in cytosolic levels of PKC βII at 1 hour? PKC βII levels have been shown to increase in cell lysates from different muscle cell types within as little as 15 minutes following insulin exposure (Chalfant et al., 1995, Chalfant et al., 1998). Such rapid increases in cytosolic levels of PKC βII were argued to result from alternative splicing in the formation of mRNA for PKC βII, although activation of transcription is also a mechanism for controlling expression levels of the enzyme (Chalfant et al., 1995, Chalfant et al., 1998, Dempsey et al., 2000). By using a 1 hour time point for measuring PKC βII levels, we believe we have maximized the sensitivity of our measurements and, if anything, we would underestimate the final levels of PKC βII that might be generated.
The correlation between freezing to context and our difference measure for PKC βII levels is remarkably strong. Our behavioral findings of freezing to context and cue were the same as findings reported by others (Baker et al., 1981), in particular, strong context learning in the unpaired group. Our unpaired tone/shock animals were afraid of the box (context), and also the tone (cue). The paired tone/shock animals showed the highest levels of freezing to the tone and very low levels of freezing to the context. If one were to use the freezing times during cue and context probe trials to derive an estimate of learning for both cue and context, the largest combined freezing measure would occur in the animals of the unpaired tone/shock group (based on our results), followed by the paired tone/shock group, and finishing with the box alone group (even the box alone animals show learning because their amount of exploration goes down). Our PKC βII level changes did not correlate with such a measure, but instead clearly correlated specifically with context learning, suggesting that hippocampal formation inputs to the LA/BLA activate PKC βII whereas other cortical or thalamic inputs do not.
Paths clearly exist for the spread of activity, including synchronous activity, from hippocampal formation into the lateral nuclei of the amygdala (Brothers and Finch, 1985, McDonald and Mascagni, 1997, Ferry et al., 1999, Funahashi et al., 2000, Pitkanen et al., 2000). These same pathways can account for the increases in PKC βII activity between learning and seizures, but what distinguishes the hippocampal inputs from the thalamic and other cortical inputs that PKC βII levels change in response to hippocampal afferent inputs?
The mechanisms for LTP in the amygdala have been shown to include NMDA receptor dependent (Weisskopf and LeDoux, 1999, Lee et al., 2001, Bauer et al., 2002), voltage-gated calcium channel dependent (Weisskopf et al., 1999, Bauer et al., 2002) and even calcium permeable AMPA receptor dependent (Mahanty and Sah, 1998) forms. Data support the similarities of LTP with fear conditioning in the amygdala (Bauer et al., 2001, Tsvetkov et al., 2002). Every one of these mechanisms could provide a sufficient spike in the intracellular calcium to activate the conventional PKC’s such as the βII isoform. Immunohistochemical studies point to an overlap in the locations of cells expressing PKC βII and cells expressing NMDA receptors (Saito et al., 1989). PKC βII is located around Golgi apparatus and other perikaryal structures. An input that activates NMDA receptors would also activate PKC βII. Its localization near the Golgi apparatus suggests that it may be involved in the packaging or trafficking of receptors to the membrane as part of the learning. NMDA receptor blockade, however, impairs conditioning to both tone and context, and NMDA receptors have been argued to be important for basic synaptic transmission in the amygdala (Lee et al., 2001). These findings suggest that NMDA receptor activation by itself is not likely to be the underlying link between context-learning and PKC βII. Clearly, elucidation of the underlying link between particular afferent systems and particular enzyme isoforms will require additional work.
Why should PKC βII levels be different for different hemispheres?
Gazzaniga (Gazzaniga et al., 1977); see also (Hugdahl, 1996) viewed the right amygdala as the predominant one for emotional tasks. The right hemisphere also appears to be more important for contextual learning (Goosens and Maren, 2001, Scicli et al., 2004). Unilateral amygdala lesions made before training resulted in partial conditioning impairments with no left-right differences, but greater deficits in contextual fear occurred in animals with right-sided amygdala lesions made after training (Baker and Kim, 2004).
Others have described activation of the left amygdala in certain behaviors, such as viewing threatening situations (Phelps et al., 2001), when patients were sad (Schneider et al., 1997) or viewing sexually arousing stimuli (Hamann et al., 2004). There have even been suggestions that the hemispheres interact, tending to represent relatively emotionally positive or negative stimuli.
The basis for these differences could be anatomical in the sense that one amygdala receives denser or different inputs than the other, although quantitative data on the relative sizes of afferent systems to the left and right amygdala have not been reported. Lateralized differences could alternatively result from activity differences by amygdala neurons to similar inputs, or some combination of anatomy and activity. Principal cell differences could also reflect differences in inhibitory cells or inputs. To this point, left/right asymmetries in GABA-gated chloride channels have been described (Guarneri et al., 1985, Guarneri et al., 1988, McIntyre et al., 1988). Our own evidence from synaptic responses in brain slices suggests that the amounts of activity on the left and right LA/BLA are similar. Data on the firing properties of amygdala neurons in animals conditioned in different ways (e.g., paired vs. unpaired) show transient firing rate increases during acquisition of conditioned fear (Quirk et al., 1995, Quirk et al., 1997), in relation to the anticipation of noxious stimuli (Pare and Collins, 2000), and context dependent firing increases (Hobin et al., 2003). None of these studies discusses differences in firing rate changes across hemispheres. Clearly, studies of the differences between the hemispheres are critical for determining changes in a brain structure that relate to specific patterns or types of input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Serotonin laterality in amygdala predicts performance in the elevated plus maze in rats. Neuroreport. 1999;10:3497–3500. doi: 10.1097/00001756-199911260-00006. [DOI] [PubMed] [Google Scholar]
- Angenstein F, Riedel G, Reyman KG, Staak S. Transient translocation of protein kinase Cgamma in hippocampal long-term potentiation depends on activation of metabotropic glutamate receptors. Neuroscience. 1999;93:1289–1295. doi: 10.1016/s0306-4522(99)00315-2. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Brain Res Rev. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Baker AG, Mercier P, Gabel J, Baker PA. Contextual conditioning and the US preexposure effect in conditioned fear. J Exp Psychol Anim Behav Process. 1981;7:109–128. doi: 10.1037//0097-7403.7.2.109. [DOI] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Amygdalar lateralization in fear conditioning: evidence for greater involvement of the right amygdala. Behav Neurosci. 2004;118:15–23. doi: 10.1037/0735-7044.118.1.15. [DOI] [PubMed] [Google Scholar]
- Bauer EP, LeDoux JE, Nader K. Fear conditioning and LTP in the lateral amygdala are sensitive to the same stimulus contingencies. Nat Neurosci. 2001;4:687–688. doi: 10.1038/89465. [DOI] [PubMed] [Google Scholar]
- Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianki VL. Asimmetriya mozga zhivotnykh, [Asymmetry of the Brain of Animals, in Russian] Nauka, Leningrad [now Sankt-Peterburg] 1985 [Google Scholar]
- Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. I. Acoustic discharge patterns and frequency receptive fields. Exp Brain Res. 1994a;98:261–274. doi: 10.1007/BF00228414. [DOI] [PubMed] [Google Scholar]
- Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. II. Cells receiving convergent auditory and somatosensory inputs and cells antidromically activated by amygdala stimulation. Exp Brain Res. 1994b;98:275–286. doi: 10.1007/BF00228415. [DOI] [PubMed] [Google Scholar]
- Brothers LA, Finch DM. Physiological evidence for an excitatory pathway from entorhinal cortex to amygdala in the rat. Brain Res. 1985;359:10–20. doi: 10.1016/0006-8993(85)91407-6. [DOI] [PubMed] [Google Scholar]
- Carlson JN, Visker KE, Keller RW, Jr, Glick SD. Left and right 6-hydroxydopamine lesions of the medial prefrontal cortex differentially alter subcortical dopamine utilization and the behavioral response to stress. Brain Res. 1996;711:1–9. doi: 10.1016/0006-8993(95)01290-7. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Mischak H, Watson JE, Winkler BC, Goodnight J, Farese RV, Cooper DR. Regulation of alternative splicing of protein kinase C beta by insulin. J Biol Chem. 1995;270:13326–13332. doi: 10.1074/jbc.270.22.13326. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Watson JE, Bisnauth LD, Kang JB, Patel N, Obeid LM, Eichler DC, Cooper DR. Insulin regulates protein kinase CbetaII expression through enhanced exon inclusion in L6 skeletal muscle cells. A novel mechanism of insulin- and insulin-like growth factor-i-induced 5′ splice site selection. J Biol Chem. 1998;273:910–916. doi: 10.1074/jbc.273.2.910. [DOI] [PubMed] [Google Scholar]
- Dempsey EC, Newton AC, Mochly Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- Doron NN, LeDoux JE. Organization of projections to the lateral amygdala from auditory and visual areas of the thalamus in the rat. J Comp Neurol. 1999;412:383–409. [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ, Yipp J, De Oca B. Differential effects of the N-methyl-D-aspartate antagonist DL-2-amino-5-phosphonovalerate on acquisition of fear of auditory and contextual cues. Behav Neurosci. 1994;108:235–240. doi: 10.1037//0735-7044.108.2.235. [DOI] [PubMed] [Google Scholar]
- Ferry B, Wirth S, Di Scala G. Functional interaction between entorhinal cortex and basolateral amygdala during trace conditioning of odor aversion in the rat. Behav Neurosci. 1999;113:118–125. doi: 10.1037//0735-7044.113.1.118. [DOI] [PubMed] [Google Scholar]
- Funahashi M, Matsuo R, Stewart M. Propagation of synchronous burst discharges from entorhinal cortex to morphologically and electrophysiologically identified neurons of rat lateral amygdala. Brain Res. 2000;884:104–115. doi: 10.1016/s0006-8993(00)02854-7. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, LeDoux JE, Wilson DH. Language, praxis, and the right hemisphere: clues to some mechanisms of consciousness. Neurology. 1977;27:1144–1147. doi: 10.1212/wnl.27.12.1144. [DOI] [PubMed] [Google Scholar]
- Glick SD, Ross DA, Hough LB. Lateral asymmetry of neurotransmitters in human brain. Brain Res. 1982;234:53–63. doi: 10.1016/0006-8993(82)90472-3. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneri P, Guarneri R, La Bella V, Scondotto S, Scoppa F, Piccoli F. Lateral differences in GABA binding sites in rat brain. Neurochem Res. 1988;13:209–211. doi: 10.1007/BF00971534. [DOI] [PubMed] [Google Scholar]
- Guarneri P, Guarneri R, Zarcone D, Bettinazzi G, Amato L, Piccoli F. Lateral differences in the GABAergic system of the rat striatum. Ital J Neurol Sci. 1985;6:173–176. doi: 10.1007/BF02229188. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci. 2004;7:411–416. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Hirai T, Chida K. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem (Tokyo) 2003;133:1–7. doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda K, Saito N, Kose A, Ito A, Tsujino T, Ogita K, Kikkawa U, Ono Y, Igarashi K, Nishizuka Y, et al. Immunocytochemical localization of the beta I subspecies of protein kinase C in rat brain. Proc Natl Acad Sci U S A. 1989;86:1393–1397. doi: 10.1073/pnas.86.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabetova S, Sacktor TC. Bidirectional regulation of protein kinase M zeta in the maintenance of long-term potentiation and long-term depression. J Neurosci. 1996;16:5324–5333. doi: 10.1523/JNEUROSCI.16-17-05324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Hugdahl K. Classical conditioning and implicit learning: the right hemisphere hypothesis. In: Davidson RJ, Hugdahl K, editors. Brain asymmetry. MIT Press; Cambridge, MA: 1996. pp. 235–267. [Google Scholar]
- Ito I, Kawakami R, Sakimura K, Mishina M, Sugiyama H. Input-specific targeting of NMDA receptor subtypes at mouse hippocampal CA3 pyramidal neuron synapses. Neuropharmacology. 2000;39:943–951. doi: 10.1016/s0028-3908(99)00217-8. [DOI] [PubMed] [Google Scholar]
- Kawakami R, Shinohara Y, Kato Y, Sugiyama H, Shigemoto R, Ito I. Asymmetrical allocation of NMDA receptor epsilon2 subunits in hippocampal circuitry. Science. 2003;300:990–994. doi: 10.1126/science.1082609. [DOI] [PubMed] [Google Scholar]
- Kose A, Saito N, Ito H, Kikkawa U, Nishizuka Y, Tanaka C. Electron microscopic localization of type I protein kinase C in rat Purkinje cells. J Neurosci. 1988;8:4262–4268. doi: 10.1523/JNEUROSCI.08-11-04262.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Risse GL, Springer SP, Wilson DH, Gazzaniga MS. Cognition and commissurotomy. Brain. 1977a;100(Pt 1):87–104. doi: 10.1093/brain/100.1.87. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Wilson DH, Gazzaniga MS. A divided mind: observations on the conscious properties of the separated hemispheres. Ann Neurol. 1977b;2:417–421. doi: 10.1002/ana.410020513. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Choi JS, Brown TH, Kim JJ. Amygdalar NMDA receptors are critical for the expression of multiple conditioned fear responses. J Neurosci. 2001;21:4116–4124. doi: 10.1523/JNEUROSCI.21-11-04116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libien J, Sacktor TC, Kass IS. Magnesium blocks the loss of protein kinase C, leads to a transient translocation of PKC(alpha) and PKC(epsilon), and improves recovery after anoxia in rat hippocampal slices. Brain Res Mol Brain Res. 2005;136:104–111. doi: 10.1016/j.molbrainres.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Maren S, Ferrario CR, Corcoran KA, Desmond TJ, Frey KA. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur J Neurosci. 2003;18:3080–3088. doi: 10.1111/j.1460-9568.2003.03063.x. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Projections of the lateral entorhinal cortex to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1997;77:445–459. doi: 10.1016/s0306-4522(96)00478-2. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McIntyre TD, Trullas R, Skolnick P. Asymmetrical activation of GABA-gated chloride channels in cerebral cortex. Pharmacol Biochem Behav. 1988;30:911–916. doi: 10.1016/0091-3057(88)90119-0. [DOI] [PubMed] [Google Scholar]
- Murray AW, Fournier A, Hardy SJ. Proteolytic activation of protein kinase C: a physiological reaction? Trends in Biochemical Sciences. 1987;12:53–54. [Google Scholar]
- Newton AC. Analyzing protein kinase C activation. Methods Enzymol. 2002;345:499–506. doi: 10.1016/s0076-6879(02)45041-0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988;334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Ohno S, Nishizuka Y. Protein kinase C isotypes and their specific functions: prologue. J Biochem (Tokyo) 2002;132:509–511. doi: 10.1093/oxfordjournals.jbchem.a003249. [DOI] [PubMed] [Google Scholar]
- Osonoe K, Ogata S, Iwata Y, Mori N. Kindled amygdaloid seizures in rats cause immediate and transient increase in protein kinase C activity followed by transient suppression of the activity. Epilepsia. 1994;35:850–854. doi: 10.1111/j.1528-1157.1994.tb02522.x. [DOI] [PubMed] [Google Scholar]
- Otani S, Ben-Ari Y, Roisin-Lallemand MP. Metabotropic receptor stimulation coupled to weak tetanus leads to long-term potentiation and a rapid elevation of cytosolic protein kinase C activity. Brain Res. 1993;613:1–9. doi: 10.1016/0006-8993(93)90446-t. [DOI] [PubMed] [Google Scholar]
- Pare D, Collins DR. Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. J Neurosci. 2000;20:2701–2710. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Saito N, Kose A, Ito A, Hosoda K, Mori M, Hirata M, Ogita K, Kikkawa U, Ono Y, Igarashi K, et al. Immunocytochemical localization of beta II subspecies of protein kinase C in rat brain. Proc Natl Acad Sci U S A. 1989;86:3409–3413. doi: 10.1073/pnas.86.9.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Grodd W, Weiss U, Klose U, Mayer KR, Nagele T, Gur RC. Functional MRI reveals left amygdala activation during emotion. Psychiatry Res. 1997;76:75–82. doi: 10.1016/s0925-4927(97)00063-2. [DOI] [PubMed] [Google Scholar]
- Schonwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schore AN. Dysregulation of the right brain: a fundamental mechanism of traumatic attachment and the psychopathogenesis of posttraumatic stress disorder. Aust N Z J Psychiatry. 2002;36:9–30. doi: 10.1046/j.1440-1614.2002.00996.x. [DOI] [PubMed] [Google Scholar]
- Scicli AP, Petrovich GD, Swanson LW, Thompson RF. Contextual fear conditioning is associated with lateralized expression of the immediate early gene c-fos in the central and basolateral amygdalar nuclei. Behav Neurosci. 2004;118:5–14. doi: 10.1037/0735-7044.118.1.5. [DOI] [PubMed] [Google Scholar]
- Shi C, Davis M. Pain pathways involved in fear conditioning measured with fear-potentiated startle: lesion studies. J Neurosci. 1999;19:420–430. doi: 10.1523/JNEUROSCI.19-01-00420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- Walasek G, Wesierska M, Zielinski K. Conditioning of fear and conditioning of safety in rats. Acta Neurobiol Exp (Wars) 1995;55:121–132. doi: 10.55782/ane-1995-1067. [DOI] [PubMed] [Google Scholar]
- Walker DL, Gold PE. Intra-amygdala kinase inhibitors disrupt retention of a learned avoidance response in rats. Neurosci Lett. 1994;176:255–258. doi: 10.1016/0304-3940(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Atkins CM, Selcher JC, Varga AW, Mirnikjoo B, Paylor R, Leitges M, Sweatt JD. A role for the beta isoform of protein kinase C in fear conditioning. J Neurosci. 2000;20:5906–5914. doi: 10.1523/JNEUROSCI.20-16-05906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Bauer EP, LeDoux JE. L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci. 1999;19:10512–10519. doi: 10.1523/JNEUROSCI.19-23-10512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, LeDoux JE. Distinct populations of NMDA receptors at subcortical and cortical inputs to principal cells of the lateral amygdala. J Neurophysiol. 1999;81:930–934. doi: 10.1152/jn.1999.81.2.930. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Derr-Yellin EC, Kodavanti PR. Alterations in brain protein kinase C isoforms following developmental exposure to a polychlorinated biphenyl mixture. Brain Res Mol Brain Res. 2003;111:123–135. doi: 10.1016/s0169-328x(02)00697-6. [DOI] [PubMed] [Google Scholar]


