Abstract
The primary reservoir for Streptococcus pneumoniae is the human nasopharynx, and colonization is often the initial step in pathogenesis. Recently we have demonstrated that pneumococcal colonization primes the immune response to subsequent vaccination with the pneumococcal conjugate vaccine (CPV). In this study we wished to determine if colonization stimulates the production of B cell memory that is activated following vaccination with CPV. To test this hypothesis, we colonized mice with S. pneumoniae serotype 14, adoptively transferred their B cells and CD4+ T cells into naïve recipients, and vaccinated the recipients with CPV. Our results indicate that pneumococcal colonization stimulates the production of memory B cells which are responsible for enhancing the immune response to CPV vaccination.
Keywords: Streptococcus pneumoniae, Pneumococcal conjugate vaccine, Adoptive transfer, Colonization
1. Introduction
Streptococcus pneumoniae is responsible for considerable morbidity and mortality worldwide and is a causative agent of otitis media, pneumonia, bacteremia, and meningitis. Pneumococcal vaccines have been developed that exploit the major virulence factor of the pneumococcus, the capsular polysaccharide (PPS). The 23-valent pneumococcal polysaccharide vaccine consists of purified PPSs and is recommended for use in immunocompromised individuals, the asplenic, and elderly adults [1]. Due to its poor immunogenicity in infants/children, the 7-valent pneumococcal conjugate vaccine (CPV) was developed containing PPSs covalently conjugated to CRM197, rendering it T cell dependent (TD) and highly efficacious in young children [2].
The primary ecological niche for S. pneumoniae is the human nasopharynx. Pneumococcal colonization is highly age dependent and over half of all children under the age of three have been colonized [3]. Colonization is self limiting, does not necessarily lead to disease, and can last from two to 18 weeks depending on the serotype. Recently, it has been demonstrated that CD4+ T cells are required for the clearance of colonization, while type specific antibodies and B cells are not essential [4, 5]. Moreover, studies have shown that colonization induces specific anti-PPS antibody and that most adults have significant levels of these antibodies prior to vaccination [6, 7].
Several studies have been performed to determine the influence of previous immunological exposure on subsequent polysaccharide-based vaccination. Work by Fernandez et al. has shown that, in mice, priming with the model T cell independent type 2 (TI-2) antigen dextran abolishes the immunoglobulin G (IgG) anti-dextran antibody response to subsequent vaccination with the conjugated (TD) form of dextran [8, 9]. Similarly, human studies have indicated that repeated vaccination with unconjugated meningococcal C polysaccharide (TI) leads to hyporesponsiveness [10, 11]. In addition, Southern et al. noted that vaccination with meningococcal C polysaccharide decreases the response to the conjugated form of the polysaccharide in adults [12]. These reports demonstrate the importance of the initial immunological exposure on subsequent vaccination with polysaccharide antigens.
Previously we demonstrated that colonization by S. pneumoniae, or cross-reactive organisms such as Group B Streptococcus type III, primes the immune response to subsequent CPV immunization [13]. Mice previously colonized with live S. pneumoniae serotype 14 (sp14) demonstrated significantly greater IgG responses to CPV vaccination compared to mice primed with phosphate-buffered saline (PBS). These results suggest that pneumococcal colonization stimulates the production of memory cells leading to increased vaccine responsiveness. In this study we sought to determine whether the immunologic memory observed following colonization was a memory B cell dependent phenomenon. To test this hypothesis we adoptively transferred B cells, CD4+ T cells or a combination of these cells from mice previously colonized by sp14 into naïve mice, and assessed the effect on vaccination. Our results suggest that the memory responsible for the increased response to CPV vaccination following colonization is a B cell dependent process.
2. Materials and Methods
2.1. Immunization Protocol
All protocols involving experimental animals were approved by the University of Toledo Institutional Animal Care and Use Committee. Six to 8 week old BALB/c mice (Taconic, Germantown, NY) were used throughout the study. For colonization studies, mice (n = 8) were inoculated with S. pneumoniae serotype 14 (sp14), S. pneumoniae serotype 19A (sp19A), S. pneumoniae serotype 19F (sp19F), or Group B Streptococcus (GBS) intranasally (i.n.). For priming and transfer studies, mice were primed with either live sp14 i.n. (n=36), phosphate-buffered saline (PBS) intra-peritoneally (i.p.) (n=36), or 50 μl CPV (containing 0.2 μg of each PPS) (Wyeth, Philadelphia, PA) i.p (n=36). Intranasal immunization was performed under anesthesia with Ketamine/Xylazine 80 mg/16 mg/kg. Colonization was achieved by administering 1 × 106 cfu live bacteria suspended in 10 μl of PBS i.n. To assess colonization, colonized mice (n=2) were sacrificed 4 weeks, 6 weeks, 8 weeks, and 10 weeks post-colonization. For cell transfer experiments, sp14-primed mice were sacrificed 9 weeks post-priming, and mice primed with either CPV or PBS were sacrificed 4 weeks post-priming based on previous studies [14]. All mice received 50 μl CPV i.p. two days and 4 weeks following cell transfer.
2.2. Nasal Washes
To determine the amount of bacterial nasopharyngeal colonization post-mortem nasal washes were performed by injecting 300 μl of a solution containing 5mM EDTA, 0.5% gelatin, and 0.05% Tween 20 in PBS retrograde via the trachea into the nasopharynx [15]. The resulting lavage fluid was collected at the nostrils. Bacterial loads were determined by serially diluting the lavage fluid and plating it on Todd-Hewitt Yeast Extract (THYE) agar plates.
2.3. B cell and CD4+ T cell isolation and Reconstitution
For cell isolation, mice were euthanized, their spleens were removed, perfused and a single cell suspension generated in 10% fetal bovine serum/RPMI. Spleens from mice primed with the same priming agent were combined for cell isolation. Cell isolation procedures were performed according to instructions from R&D Systems’ (Minneapolis, MN) MagCellect Mouse B cell/CD4+ T cell isolation kits. Briefly, the cells were suspended in M-Lyse buffer to eliminate red blood cells, and brought to a density of 2 × 108 cells per ml with cold MagCellect Buffer. Negative selection of murine B cells or CD4+ T cells was performed using MagCellect Mouse B Cell Biotinylated Antibody Cocktail or MagCellect Mouse CD4+ T Cell Biotinylated Antibody Cocktail respectively. Following incubation, MagCellect Streptavidin Ferrofluid was added to bind to the biotin-labeled unwanted cells. These cells were removed with the use of a magnet and the isolated cells were enumerated prior to injection into mice i.p. Mice (n=18) received either 1 × 106, 5 × 106, or 1 × 107 B cells, CD4+ T cells, or a combination of the two. Cell purity was verified using flow cytometry and was found to be between 90–93%, which was consistent with the manufacture’s specifications.
2.4. Pneumococcal Polysaccharide ELISA
A pneumococcal enzyme-linked immunosorbent assay (ELISA) was used to determine anti-PPS antibody levels as previously described [16]. Briefly, Nunc microtiter plates were coated with 20μg/ml PPS14 (ATCC, Rockville, MD). Murine sera were absorbed with 5μg/ml cell wall polysaccharide (CPS) and 10μg/ml PPS22F (ATCC) and incubated for 30 minutes [16]. Serial dilutions of absorbed sera were then added and incubated. Specific IgG antibody was detected using biotinylated goat anti-mouse IgG antisera (Southern Biotech, Birmingham, AL) and a streptavidin horse-radish peroxidase conjugate, which was developed with σ-phenylenediamine (Sigma, St. Louis, MO.) Standard serum specific for PPS14 were used for determination of specific antibody titers.
2.5. Statistical Analysis
Individual mouse titers were determined and geometric means and standard deviations were calculated for each group. Significant differences within and between groups were identified using the Mann-Whitney U-test with P values <0.05 considered significant.
3. Results
3.1 Duration of Streptococcal colonization
BALB/c mice were colonized with live streptococci and monitored for 10 weeks to assess the duration of colonization. Mice were inoculated with 1 × 106 cfu of S. pneumoniae serotype 14 (sp14), Group B Streptococcus type III (GBS), S. pneumoniae serotype 19F (sp19F), or S. pneumoniae serotype 19A (sp19A) intranasally, and were sacrificed 4, 6, 8, and 10 weeks post-colonization. By 4 weeks post-inoculation, mice colonized by GBS and sp19A did not have measurable levels of bacteria in their nasopharynx (figure 1). In contrast, mice colonized with sp14 had a geometric mean of 1400 cfu at 4 weeks post-inoculation while those colonized with sp19F had 5600 cfu. At 6 weeks post-administration the number of sp14 colonies recovered was reduced greatly, and the organism was cleared at 8 weeks post-inoculation. In contrast, sp19F was able to maintain colonization throughout the entire study (figure 1). Previous studies have shown that pneumococci maintain colonization at levels between 102 and 104 cfu per mouse [17]. Based on these results, we utilized sp14 for the duration of our studies as colonization and clearance were each confirmed.
Figure 1.

Extent of colonization for 3 different S. pneumoniae serotypes and Group B Streptococcus type III. Balb/c mice (n = 8) were colonized with 1 × 106 cfu, sacrificed at the indicated week, and bacterial loads were determined via nasal wash. Individual bacterial loads are in indicated for each mouse with open circles.
3.2 Adoptive transfer of primed cells into naïve mice
Prior to colonization, very low levels of anti-PPS14 specific antibody were present, and values approached the detection limit of our assay (data not shown). Intranasal colonization with sp14 produced a specific anti-PPS14 IgG antibody response that was significantly greater than priming with PBS two weeks post-priming (p < 0.001) (figure 2). Prior to the transfer of sp14-primed cells, the level of anti-PPS14 antibody increased to ~ 0.1 μg/ml and was significantly greater than the level at two weeks post-inoculation (p < 0.001). Priming with CPV resulted in significantly more antibody than priming with sp14 or PBS at both time points (all p < 0.001), however there was no difference between the levels of anti-PPS14 IgG antibody in CPV-primed mice at two weeks post-priming and immediately prior to transfer (figure 2).
Figure 2.

Anti-PPS14 IgG antibody response prior to priming, 2 weeks post-priming and prior to transfer. Geometric means with standard deviation are shown for each group at 2 weeks post-priming and immediately prior to transfer. Significant differences compared to PBS are indicated by (*) at week 2, and by (#) prior to transfer, and significant differences compared to sp14 week 2 are indicated by ($). P values < 0.05 were considered significant.
Following colonization splenic B cells, CD4+ T cells, or B cells with CD4+ T cells were isolated and transferred to naïve mice. The recipients were then vaccinated with CPV. Naïve mice received 1 × 106, 5 × 106, or 1 × 107 cells from primed mice. However, when these groups were compared no significant differences were observed (data not shown). Mice which received B, CD4+ T cells, or B cells and CD4+ T cells at a dose of 1 × 106 did not demonstrate significantly different effects compared to those mice which received the same cells at a higher dose. These results indicate that the transfer of 1 × 106 primed cells was sufficient to confer immunity to naïve mice. Therefore, we pooled the data from mice primed with the same antigen into one group, regardless of the dose of transferred cells given.
3.3 Adoptive transfer of primed B cells into naïve mice
Following vaccination, mice which received B cells from sp14-primed mice demonstrated a significantly greater anti-PPS14 IgG response than mice that received B cells from PBS-primed mice (p = 0.004) (figure 3a). Moreover, mice that received B cells from sp14-primed mice responded to vaccination more strongly than those mice which received B cells from CPV-primed mice, although this difference was not statistically significant. However, a second vaccination with CPV eliminated all of the differences between the groups (figure 3b). Nonetheless, these results suggest that pneumococcal colonization stimulates the production of memory B cells which are responsible for enhancing the immune response to CPV vaccination.
Figure 3.
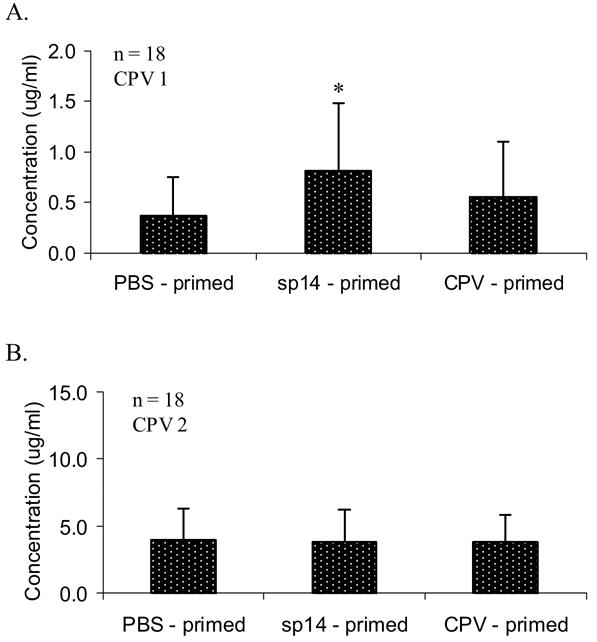
Anti-PPS14 IgG antibody titers in mice receiving B cells from PBS-primed, sp14-primed, or CPV-primed mice following initial CPV vaccination (A), and booster CPV vaccination (B). Geometric means with standard deviation are indicated for each group. Significant differences compared to PBS-primed mice are indicated by (*). P values < 0.05 were considered significant.
3.4 Adoptive transfer of primed CD4+ T cells into naïve mice
The role of CD4+ T cells in boosting the IgG response to CPV vaccination in mice that were previously colonized with sp14 was also examined. Adoptive transfer of CD4+ T cells from sp14-primed mice did not result in an increase of anti-PPS14 IgG in response to CPV vaccination compared to mice that received CD4+ T cells from PBS-primed mice (figure 4a). However, mice that received CD4+ T cells from CPV-primed mice demonstrated a significantly greater anti-PPS14 IgG response to CPV vaccination than mice receiving PBS-primed CD4+ T cells (p = 0.025) and mice receiving sp14-primed CD4+ T cells (p = 0.008). This trend continued to be observed 4 weeks following CPV vaccination (data not shown). However, a second vaccination with CPV eliminated the differences between mice receiving CD4+ T cells from PBS, CPV, and sp14-primed mice (figure 4b). These results demonstrate that CD4+ T cells from sp14-primed mice are not sufficient to confer the booster effect of sp14 colonization on CPV vaccination.
Figure 4.

Anti-PPS14 IgG antibody titers in mice receiving CD4+ T cells from PBS-primed, sp14-primed, or CPV-primed mice following initial CPV vaccination (A), and booster CPV vaccination (B). Geometric means with standard deviation are indicated for each group. Significant differences compared to PBS-primed mice are indicated by (*) and to sp14-primed mice by (#). P values < 0.05 were considered significant.
3.5 Adoptive transfer of primed B cells and CD4+ T cells into naïve mice
Primed B cells and CD4+ T cells were transferred together into naïve mice to study potential additive effects. Primed mice were sacrificed and both B cells and CD4+ T cells were isolated from the same splenic samples and transferred to naïve recipients. Surprisingly, following vaccination with CPV, no significant difference in anti-PPS14 IgG antibody titer was observed between mice primed with PBS and those primed with sp14 colonization (figure 5a). Recipients of sp14-primed cells had a lower anti-PPS14 IgG antibody titer than those that received cells primed with PBS and CPV. Following the second vaccination with CPV this trend continued and was now significant (figure 5b). Therefore, in our experimental system, a synergistic effect of transferring sp14-primed CD4+ T cells along with primed B cells was not observed, and the combination appeared to have a suppressive effect.
Figure 5.

Anti-PPS14 IgG antibody titers in mice receiving B cells and CD4+ T cells from PBS-primed, sp14-primed, or CPV-primed mice following initial CPV vaccination (A), and booster CPV vaccination (B). Geometric means with standard deviation are indicated for each group. Significant differences compared to PBS-primed mice are indicated by (*) and to CPV-primed mice by (#). P values < 0.05 were considered significant.
3.6 Comparison of transferring cells from mice primed with S. pneumoniae serotype 14 or the pneumococcal conjugate vaccine
Our findings indicate that transferring B cells from mice primed by sp14 colonization into naïve mice results in a significantly greater anti-PPS14 IgG antibody response compared to the transfer of B cells from PBS-primed mice following vaccination with CPV (figure 3a). In addition, the transfer of B cells from sp14-primed mice resulted in a greater post-vaccination anti-PPS14 IgG antibody titer compared to the transfer of CD4+ T cells (p = 0.034) and the combination of B cells with CD4+ T cells (p = 0.002) from sp14-primed mice (figure 6a). These results suggest that B cells produced following colonization with sp14 are sufficient to confer the increased ability to respond to vaccination with CPV seen in sp14-colonized mice. Moreover, we observed that the transfer of CPV-primed CD4+ T cells resulted in a significantly greater post-vaccination IgG antibody response than the transfer of PBS-primed CD4+ T cells (figure 4a). This increase in vaccine responsiveness following the transfer of CPV-primed CD4+ T cells was greater than the post-vaccination anti-PPS14 IgG response in mice which received CPV-primed B cells (p = 0.046) and the combination of CPV-primed B cells and CD4+ T cells (p = 0.043) (figure 6b). These results indicate that the resulting CD4+ T cell memory produced after vaccination with CPV is sufficient to boost the specific IgG response to subsequent CPV vaccination.
Figure 6.

Post-CPV vaccination anti-PPS IgG antibody titers in mice receiving sp14-primed (A) and CPV-primed (B) B cells, CD4+ T cells, and B cells with CD4+ T cells. Geometric means with standard deviation are indicated for each group. Significant differences compared to B cell recipients are indicated by (*), to CD4+ T cells recipients by (~), and to B cell with CD4+ T cell recipients by (#). P values < 0.05 were considered significant.
4. Discussion
The human nasopharynx is the major reservoir of S. pneumoniae, and colonization is often the initial step in pathogenesis [18]. A majority of individuals have been colonized by the pneumococcus, which correlates with the significant levels of anti-PPS specific antibody in adults [6, 3, 7]. Previously, we have demonstrated that streptococcal colonization can have a significant effect on subsequent pneumococcal vaccination [13]. Nasopharyngeal colonization with S. pneumoniae, in particular serotypes 14, 19A, and 19F, primes the immune response so that the magnitude of the antibody response is increased following vaccination with CPV. In this study, we tested the hypothesis that pneumococcal colonization stimulates the production of specific memory B cells which have the ability to respond rigorously to CPV vaccination.
Pneumococcal colonization in humans can be long-lasting and is highly variable depending on the individual and serotype. It has been shown in humans that the median duration of colonization with sp14 is 8 weeks [19]. In our model of nasopharyngeal carriage in BALB/c mice, we demonstrated that colonization persists through the sixth week post-inoculation. Previous work by Moreno et al. has shown that carriage of sp14 was consistent for 3–10 weeks in their model, and that colonized mice harbored 5 × 103 cfu of S. pneumoniae [20]. Our results are consistent with their findings, as well as with Wu et al. who demonstrated that sp14 carriage is stable for over 2 weeks and colonized mice contained 103–104 cfu of sp14 [17]. Based on these results, we colonized mice with sp14 and allowed them to clear colonization before they were sacrificed for adoptive transfer studies.
Pneumococcal colonization has been shown to stimulate the adaptive immune system in both humans and in mice. Previous studies in humans have shown that colonization elicits the production of serum IgG antibodies to both PPSs [6] and to pneumococcal surface-exposed proteins such as pneumococcal surface protein A (PspA) and choline binding protein A (CbpA) [21, 22]. These results indicate that colonization must stimulate the production of class-switched B cells and the activation of CD4+ helper T cells capable of producing specific IgG. The production of anti-PPS IgG antibody following pneumococcal colonization in mice has been demonstrated previously and confirmed in our model [13]. Moreover, work by Malley et al. and van Rossum et al. has shown that murine colonization stimulates the production of CD4+ T cells [23, 5]. Collectively they demonstrated that mice lacking these cells remained persistently colonized, similar to severe-combined immunodeficient mice. We therefore investigated which types of stimulated immune cells were responsible for priming the immune response to CPV in previously colonized mice.
To investigate the influence of sp14-primed B and CD4+ T cells on the immune response to CPV, we employed a murine adoptive transfer model. This model allowed us to assess the affect of primed immune cells on subsequent vaccination without host genetic variation or the need to irradiate the recipient mice. Previous studies by Roth and Mamula demonstrated that B cells transferred by intraperitoneal injection were equally distributed throughout the lymph nodes and spleen 48 hours after injection [24]. In addition, their work along with a study by Guttormsen et al. showed that up to 2 × 108 total cells could be transferred into naïve mice without complications [14]. In our study, no significant differences were observed between mice given 1 × 106, 5 × 106, or 1 × 107 primed cells. Consequently, we combined the group data as less than 1 × 106 cells were required to obtain a titration curve based on priming ability.
The cumulative results of our studies indicated that B cells present in the spleens of sp14-primed mice are sufficient to increase the magnitude of subsequent CPV vaccination. These B cells may be class-switched memory B cells that have migrated from the nasal mucosa to the spleen based on the time allotted to clear colonization and the isotype of the anti-PPS antibodies. Therefore, while B cells and anti-PPS antibodies are not required to clear colonization [4], they do play a role in priming the immune response to further vaccination. Furthermore, our results confirm the vital role of CD4+ T cells in the response to conjugated polysaccharide antigens [25]. The primed CD4+ T cells are able to respond to subsequent immunization with CPV more rapidly, resulting in an increase in anti-PPS14 IgG antibody production. Surprisingly however, the transfer of primed B cells and CD4+ T cells into the same mouse did not result in an increased antibody response, and actually lead to a decreased response in sp14-primed mice compared to PBS-primed mice. These studies suggest an antagonistic effect of co-transfer of these two subsets which could have led to the development of CD4+CD25+ regulatory T cells, which have been shown to arise in the periphery in certain situations and lead to decreased antibody production, however further studies are needed to elucidate this further [26].
In conclusion, our previous findings demonstrated the benefit of pneumococcal colonization prior to vaccination [13]. The results presented here support our previous work and indicate that this effect is due to the generation of PPS-specific memory B cells found in the spleen. These results demonstrate the effectiveness of stimulating the mucosal immune system prior to vaccination. Based on these findings, future pneumococcal vaccine regimens should be designed to stimulate the PPS-specific mucosal immune system prior to CPV vaccination to obtain an optimal antibody response.
Acknowledgments
We thank Sandra Romero-Steiner for providing S. pneumoniae strains and Hilde-Kari Guttormsen for providing the Group B Streptococcus strain. This study was supported by departmental funds provided by the Department of Medicine at the University of Toledo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Practices ACoI. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 1997;46:1–24. [PubMed] [Google Scholar]
- 2.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, Malinoski F, Madore D, Chang I, Kohberger R, Watson W, Austrian R, Edwards K. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 4.McCool TL, Weiser JN. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect Immun. 2004;72:5807–5813. doi: 10.1128/IAI.72.10.5807-5813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Rossum AM, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun. 2005;73:7718–7726. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musher DM, Groover JE, Reichler MR, Riedo FX, Schwartz B, Watson DA, Baughn RE, Breiman RF. Emergence of antibody to capsular polysaccharides of Streptococcus pneumoniae during outbreaks of pneumonia: association with nasopharyngeal colonization. Clin Infect Dis. 1997;24:441–446. doi: 10.1093/clinids/24.3.441. [DOI] [PubMed] [Google Scholar]
- 7.Kolibab K, Smithson SL, Shriner AK, Khuder S, Romero-Steiner S, Carlone GM, Westerink MA. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults. I. Antibody concentrations, avidity and functional activity. Immun Ageing. 2005;2:10. doi: 10.1186/1742-4933-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sverremark E, Fernandez C. Unresponsiveness following immunization with the T-cell-independent antigen dextran B512. Can it be abrogated? Immunology. 1998;95:402–408. doi: 10.1046/j.1365-2567.1998.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez M, Lindroth K, Sverremark E, Gonzalez Fernandez A, Fernandez C. The response in old mice: positive and negative immune memory after priming in early age. Int Immunol. 2001;13:1213–1221. doi: 10.1093/intimm/13.10.1213. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald NE, Halperin SA, Law BJ, Forrest B, Danzig LE, Granoff DM. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers: a randomized controlled trial. J Amer Med Assoc. 1998;280:1685–1689. doi: 10.1001/jama.280.19.1685. [DOI] [PubMed] [Google Scholar]
- 11.Jokhdar H, Borrow R, Sultan A, Adi M, Riley C, Fuller E, Baxter D. Immunologic hyporesponsiveness to serogroup C but not serogroup A following repeated meningococcal A/C polysaccharide vaccination in Saudi Arabia. Clin Diagn Lab Immunol. 2004;11:83–88. doi: 10.1128/CDLI.11.1.83-88.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Southern J, Deane S, Ashton L, Borrow R, Goldblatt D, Andrews N, Balmer P, Morris R, Kroll JS, Miller E. Effects of prior polysaccharide vaccination on magnitude, duration, and quality of immune responses to and safety profile of a meningococcal serogroup C tetanus toxoid conjugate vaccination in adults. Clin Diagn Lab Immunol. 2004;11:1100–1104. doi: 10.1128/CDLI.11.6.1100-1104.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabquer B, Smithson SL, Shriner A, Julie Westerink MA. The effect of T cell independent and cross-reactive antigen on the immune response to pneumococcal conjugate vaccination. Immunol Lett. 2006;106:187–190. doi: 10.1016/j.imlet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Guttormsen HK, Wetzler LM, Finberg RW, Kasper DL. Immunologic memory induced by a glycoconjugate vaccine in a murine adoptive lymphocyte transfer model. Infect Immun. 1998;66:2026–2032. doi: 10.1128/iai.66.5.2026-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westerink MAJ, Smithson SL, Srivastava N, Blonder J, Coeshott C, Rosenthal GJ. Projuvant™ (Pluronic F127®/chitosan) enhances the immune reponse to intranasally administered tetanus toxoid. Vaccine. 2002;20:711–723. doi: 10.1016/s0264-410x(01)00423-6. [DOI] [PubMed] [Google Scholar]
- 16.Wernette C, Frasch C, Madore D, Carlone G, Goldblatt D, Plikaytis B, Benjamin W, Quataert S, Hildreth S, Sikkema D, Kayhty H, Jonsdottir I, Nahm M. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol. 2003;10:514. doi: 10.1128/CDLI.10.4.514-519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu HY, Virolainen A, Mathews B, King J, Russell MW, Briles DE. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb Pathog. 1997;23:127–137. doi: 10.1006/mpat.1997.0142. [DOI] [PubMed] [Google Scholar]
- 18.Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. J Infect Dis. 1997;175:1440–1445. doi: 10.1086/516477. [DOI] [PubMed] [Google Scholar]
- 19.Crook DW, Brueggemann AB, Sleeman KL, Peto TEA. In: Pneumococcal Carriage. Tuomanen EI, editor. ASM Press; 2004. pp. 136–147. [Google Scholar]
- 20.Moreno RL, Sampson JS, Romero-Steiner S, Wong B, Johnson SE, Ades E, Carlone GM. A murine model for the study of immune memory in response to pneumococcal conjugate vaccination. Vaccine. 2004;22:3069–3079. doi: 10.1016/j.vaccine.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 21.McCool TL, Cate TR, Moy G, Weiser JN. The immune response to pneumococcal proteins during experimental human carriage. J Exp Med. 2002;195:359–365. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCool T, Cate T, Tuomanen E, Adrian P, Mitchell T, Weiser J. Serum immunoglobulin G response to candidate vaccine antigens during experimental human pneumococcal colonization. Infect Immun. 2003;71:5724. doi: 10.1128/IAI.71.10.5724-5732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A. 2005;102:4848–4853. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth R, Mamula MJ. Trafficking of adoptively transferred B lymphocytes in B-lymphocyte-deficient mice. J Exp Biol. 1997;200:2057–2062. doi: 10.1242/jeb.200.14.2057. [DOI] [PubMed] [Google Scholar]
- 25.Guttormsen HK, Sharpe AH, Chandraker AK, Brigtsen AK, Sayegh MH, Kasper DL. Cognate stimulatory B-cell-T-cell interactions are critical for T-cell help recruited by glycoconjugate vaccines. Infect Immun. 1999;67:6375–6384. doi: 10.1128/iai.67.12.6375-6384.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taams LS, Akbar AN. Peripheral generation and function of CD4+CD25+ regulatory T cells. Curr Top Microbiol Immunol. 2005;293:115–131. doi: 10.1007/3-540-27702-1_6. [DOI] [PubMed] [Google Scholar]


