Abstract
The ability to select an appropriate response among competing alternatives is a fundamental requirement for successful performance of a huge variety of everyday tasks. Recent research suggests that a frontal-parietal network of brain regions (including dorsal prefrontal, dorsal premotor and superior parietal cortices) mediate response selection for spatial material. Most of this research has used blocked experimental designs. Thus, the frontal-parietal activity reported may be due either to tonic activity across a block or to processing occurring at the trial level. Our current event-related fMRI study investigated response selection at the level of the trial in order to identify possible response selection sub-processes. In the study, participants responded to a visually presented stimulus with either a spatially compatible or incompatible manual response. On some trials, several seconds prior to stimulus onset, a cue indicated which task was to be performed. In this way we could identify separate brain regions for task preparation and task performance, if they exist. Our results showed that the frontal-parietal network for spatial response selection activated both during task preparation as well as during task performance. We found no evidence for preparation specific brain mechanisms in this task. These data suggest that spatial response selection and response preparation processes rely on the same neurocognitive mechanisms.
Keywords: attention, cognitive control, neuroimaging, response selection, working memory
1. Introduction
Selecting appropriate responses to environmental stimuli is critical for human goal-directed behavior. Research suggests that a network of posterior and prefrontal brain regions mediate these response selection processes. The particular regions mediating task performance within this network may vary depending on the tasks and task modalities used (Hazeltine, Bunge, Scanlon, & Gabrieli, 2003; Pardo, Pardo, Janer, & Raichle, 1990; Schumacher, Elston, & D’Esposito, 2003; but see Jiang & Kanwisher, 2003). Nevertheless, for spatial response selection there is broad agreement that dorsal prefrontal cortex (dPFC), dorsal premotor cortex (dPMC), and superior parietal cortex (SPC) are involved (Dassonville et al., 2001; Iacoboni, Woods, & Mazziotta, 1996; Jiang & Kanwisher, 2003; Merriam et al., 2001; Schumacher & D’Esposito, 2002; Schumacher et al., 2003; Schumacher, Hendricks, & D’Esposito, 2005).
For example, in Schumacher et al. (2003) we showed that right dPFC, bilateral dPMC, and medial and left SPC were sensitive to manipulations of spatial response selection difficulty. In that study we manipulated difficulty in two ways: we varied stimulus-response (SR) numerosity and compatibility. For SR numerosity, participants made compatible key-presses to the horizontal position of a stimulus cue. That is, the correct response spatially corresponded, left to right, to the stimulus location. Across blocks, two, four, or six possible SR rules were used. For SR compatibility, participants made incompatible key-presses to the spatial position of a cue. The correct response was based on an arbitrary mapping from stimulus to response that the participant learned prior to scanning. Consistent with increases in reaction time (RT), brain activation increased in right dPFC, bilateral dPMC, and medial and left SPC with spatial response-selection difficulty (both for numerosity and compatibility). Because these regions showed increased activity across different manipulations of spatial response selection difficulty (i.e., one increasing the number of SR rules and the other increasing the interference between them), they likely mediate spatial response selection specifically, rather than other ancillary differences between the task conditions (e.g., differences in stimulus encoding or response programming).
Many other studies of spatial response selection have activated some or all of this frontal-parietal brain network (Dassonville et al., 2001; Iacoboni et al., 1996; Jiang & Kanwisher, 2003; Merriam et al., 2001; Schumacher & D’Esposito, 2002; Schumacher et al., 2003; Schumacher et al., 2005). One limitation of these studies is their use of blocked experimental designs. In experiments like these, blocked designs do not allow one to dissociate distinct response selection sub-processes that may be mediated by the different brain regions.
A study of verbal/non-spatial response selection using the Stroop task avoided this limitation with an event-related design and identified neurocognitively distinct response selection processes (MacDonald, Cohen, Stenger, & Carter, 2000). In that study, at the beginning of each trial, participants were cued either with the word “word” or “color” indicating whether they were to read the presented word or identify the color of the ink in which it appeared. MacDonald et al. found that left dPFC was more active to the color than the word cue, and that anterior cingulate cortex (ACC) was more active to the stimulus when participants responded to its color than when they read the word. These results may suggest that ACC mediates the selection of the appropriate response among competing alternatives (i.e., correct color instead of word) and that left dPFC is involved in preparing the information processing system to respond to the more difficult of the two task conditions (i.e., color naming).
Consistent with this finding, left inferior frontal junction (IFJ), the region at the junction between the inferior frontal sulcus and the inferior precentral sulcus, has been reported to be sensitive to task cuing. In two studies, activity in this region (as well as right inferior frontal gyrus, IFG; and right intra-parietal sulcus, IPS) was shown to be sensitive to a stimulus cue for the subsequent performance of a non-spatial task (Brass & von Cramon, 2002, 2004). These results suggest that these brain regions may mediate the activation of task appropriate SR rules or other task preparation processes. A similar result has been reported for right anterior PFC, which has been shown to activate to a cue signaling an upcoming spatial or non-spatial working memory task (Sakai & Passingham, 2003).
Thus, there is evidence for neurocognitively distinct task preparatory and task performance processes from studies using spatial and non-spatial tasks. Additionally, there is evidence that the regions involved in the frontal-parietal network for spatial response selection (i.e., dPFC, dPMC, and SPC) may mediate distinct neurocognitive processes as well. Firstly, these regions have distinct patterns of connectivity. In non-human primates, there are dense reciprocal connections between these three regions. Dorsal PFC, however, is also connected to temporal, limbic, and subcortical brain regions (Cavada & Goldman-Rakic, 1989; Goldman-Rakic, Selemon, & Schwartz, 1984; Petrides, 2005; Petrides & Pandya, 1984; Wise, Boussaoud, Johnson, & Caminiti, 1997). These anatomical differences may lead to functional differences in the cognitive processing mediated by these regions. Secondly, research suggests that dPMC and SPC neurons likely mediate visuospatial-motoric integration (Wise et al., 1997). Dorsal PMC neurons, for example, are more active when monkeys perform visually guided motor tasks than when they perform tasks with no visual cues (Mushiake, Inase, & Tanji, 1991). Similarly, neurons in the lateral intra-parietal area are sensitive to spatially relevant movements (Bracewell, Mazzoni, Barash, & Andersen, 1996; Mazzoni, Bracewell, Barash, & Andersen, 1996). Finally, dPFC has been hypothesized to be involved in higher-order cognitive control and working memory processes, which modulate processing in premotor, parietal, and other posterior brain regions (Fuster, 2001; Miller & Cohen, 2001). Thus, neuroanatomical and neurophysiological data suggest distinct processing in dPFC, dPMC, and SPC.
Cognitive theories also postulate separate response selection sub-processes. Kornblum, Hasbroucq, and Osman (1990) proposed two routes for response selection for tasks with incompatible SR mappings. These routes are: a) an automatic response activation route that activates the response corresponding to the cue location (compatible response) and b) a controlled selection route that identifies the correct response based on the SR mappings stored in memory.
More recently, Eimer, Hommel, and Prinz proposed a similar two-process response selection model (Eimer, 1995; Eimer, Hommel, & Prinz, 1995). The first process, automatic response activation, is a direct route from stimulus location to response preparation that circumvents working memory. The second process, controlled selection, is an indirect route from stimulus to response mediated by working memory. The direct route is sufficient to make compatible responses but the indirect route is required for incompatible SR mappings. A precuing procedure measuring event-related potentials showed that activation of the automatic response preceded stimulus presentation and was subsequently replaced by the correct response in a spatially incompatible task (Eimer, 1995).
To test neurocognitive distinctions within the brain network for spatial response selection, the current experiment modified the blocked SR compatibility manipulation we have used previously (Schumacher et al., 2003; Schumacher et al., 2005). Instead of manipulating task difficulty across separate blocks of trials, here participants performed compatible and incompatible trials in an event-related design. The task procedure used is shown in Figure 1. On some trials, participants were cued at the beginning of each trial which task (i.e., compatible or incompatible) they would perform on that trial; other trials were uncued. After a delay, the task stimulus appeared and participants responded accordingly.
Figure 1.
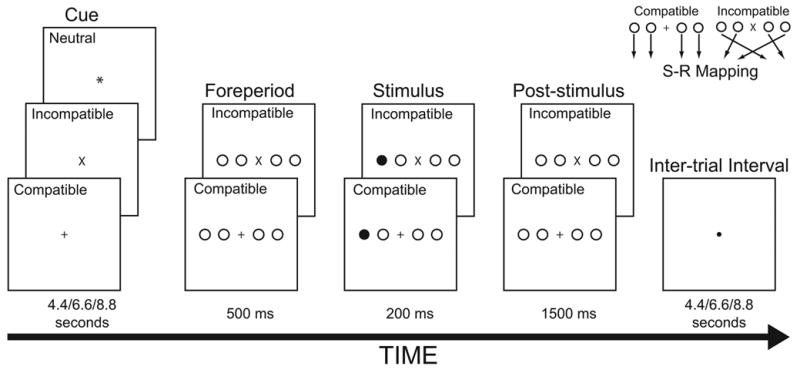
Schematic of experimental tasks. Participants performed an incompatible or compatible stimulus-response mapping task on each trial. The mappings are shown in the upper right of the figure. A centrally located task cue (neutral, incompatible, or compatible) appeared between 4.4 and 8.8 sec prior to stimulus onset. These trial-types are identified in the figure. The words, neutral, compatible, and incompatible did not appear onscreen during the experiment.
In this way, we can separate brain activity to the task cue during the cue period, which may reflect automatic SR activation, from the activity to the task stimulus during the stimulus period, which may reflect the selection of the appropriate response based on a particular stimulus.
Because the current study is similar to one by MacDonald et al. (2000), we may predict similar results. This prediction is additionally warranted because regions active in their study (ACC, left PMC and left dPFC) have also been implicated in spatial response selection (Iacoboni et al., 1996; Jiang & Kanwisher, 2003; Schumacher & D’Esposito, 2002). However, there are several contrary findings that lead us to question this prediction. Firstly, as stated previously, several studies report distinct brain networks for response selection depending on stimulus modality (Hazeltine et al., 2003; Schumacher et al., 2003). For example, in Schumacher et al., we reported an additional experiment in which we manipulated response selection difficulty for a non-spatial task. In that study left dPFC, left temporal, and left inferior parietal cortices were sensitive to non-spatial response selection difficulty. Additionally, ACC is inconsistently activated by manipulations of spatial response selection. Some studies report increased activity in ACC for harder spatial tasks (Schumacher & D’Esposito, 2002); but others report no evidence for its involvement, even when using a relatively sensitive region-of-interest analysis (Jiang & Kanwisher, 2003). Thus, the activity reported by MacDonald et al. may be specific to selection for verbal material, or specific to the Stroop task.
Therefore, the current event-related study manipulating spatial response selection may produce a different pattern of cue and stimulus related activity than previously reported for other task domains. In any case, this experiment should identify neurocognitive differences between spatial perceptual-motor task preparation and spatial perceptual-motor task performance, if differences exist.
2. Results
2.1 Behavioral Results
A within-subjects analysis of variance with Task Difficulty (compatible and incompatible) and Cue Condition (cued and uncued) as variables was performed on the mean RTs from correct trials. There were reliable main effects of Task Difficulty, F(1,10) = 74.76, p < .001 and Cue Condition, F(1,10) = 8.51, p<.05, and a reliable interaction, F(1,10) = 18.07, p < .01. As shown in Figure 2, mean RTs were longer for incompatible than compatible trials. Mean RTs were also longer for uncued than cued trials, but most of this effect was due to the RT difference between the cued and uncued conditions on incompatible trials.
Figure 2.
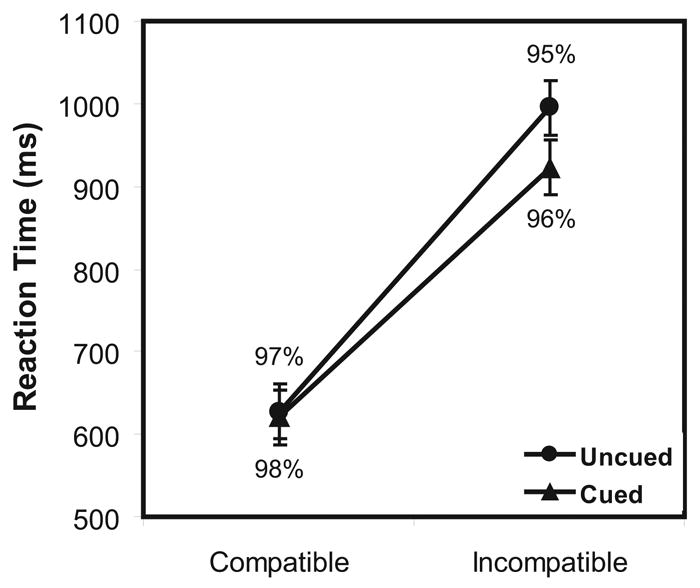
Mean reaction times with standard errors and mean accuracy rates for the Incompatible and Compatible stimulus-response mapping tasks when the task was cued and uncued.
A similar analysis of variance was performed on an arcsine transformation of the proportion correct (Howell, 1987). There were no reliable effects of Task Difficulty, F(2,10) = 2.59, p > .10, Cue Condition, F(2,10) = 1.39, p > .25, and no interaction between them, F(2,10) = 0.56, p > .70. The mean percent correct for each condition are also shown in Figure 2.
2.2 Whole-Brain Activation Results
The Table and Figure 3 show regions with reliably more activity in the incompatible than compatible trials separately for the cue and stimulus trial periods when corrected for multiple comparisons across the entire brain (Genovese, Lazar, & Nichols, 2002). No other comparisons produced reliable whole-brain activity. Further analyses were conducted on the subset of these regions thought to be theoretically interesting.
Table.
Sites of Peak Activation when Cued* from Cue and Stimulus Trial Periods for the Incompatible versus the Compatible Tasks.
| Incompatible vs. Compatible Activity | MNI Coordinates | ||||
|---|---|---|---|---|---|
| Brain Region | Brodmann’s Area | T-value | x | y | z |
| Cue Trial Period | |||||
| Left Dorsal Prefrontal | 45/46 | 7.72** | −40 | 34 | 26 |
| Cuneus/Precuneus | 7/19 | 10.76 | −10 | −80 | 42 |
| Stimulus Trial Period | |||||
| Left Dorsal Premotor | 6 | 6.37 | −22 | −6 | 54 |
| Right Dorsal Premotor+ | 6 | 5.45 | 24 | 2 | 64 |
| Right Dorsal Premotor | 6 | 5.99 | 18 | −20 | 64 |
| Left Dorsal Prefrontal | 46 | 5.68 | −24 | 38 | 24 |
| Right Dorsal Prefrontal | 45/46 | 4.53** | 42 | 32 | 28 |
| Anterior Cingulate | 32 | 4.92 | 14 | 24 | 22 |
| Anterior Cingulate | 24 | 5.18 | 16 | 16 | 26 |
| Anterior Cingulate | 24 | 6.44 | 2 | 16 | 28 |
| Left Superior Parietal | 7 | 12.91 | −12 | −66 | 50 |
| Left Superior Parietal | 7/40 | 4.65 | −36 | −56 | 38 |
| Left Inferior Parietal | 40 | 5.04 | −58 | −28 | 26 |
| Precuneus | 7 | 11.07 | −2 | −72 | 44 |
| Cuneus | 7/19 | 9.99 | 14 | −76 | 38 |
| Right Inferior Temporal+ | 20 | 10.21 | 26 | −18 | −4 |
| Right Lateral Occipital | 18 | 5.51 | 26 | −90 | 10 |
| Right Caudate Nucleus | 5.15 | 8 | 18 | −4 | |
| Right Insula+ | 5.08 | 38 | 4 | −4 | |
| Left Thalamus | 5.20 | −12 | −18 | 6 | |
| Right Thalamus | 10.17 | 10 | −12 | 0 | |
| Right Thalamus | 5.97 | 10 | −2 | 4 | |
No reliable activity for the uncued condition for either trial period.
Not shown in Figure 3
Reliable at .05<pFDR<.06.
Figure 3.
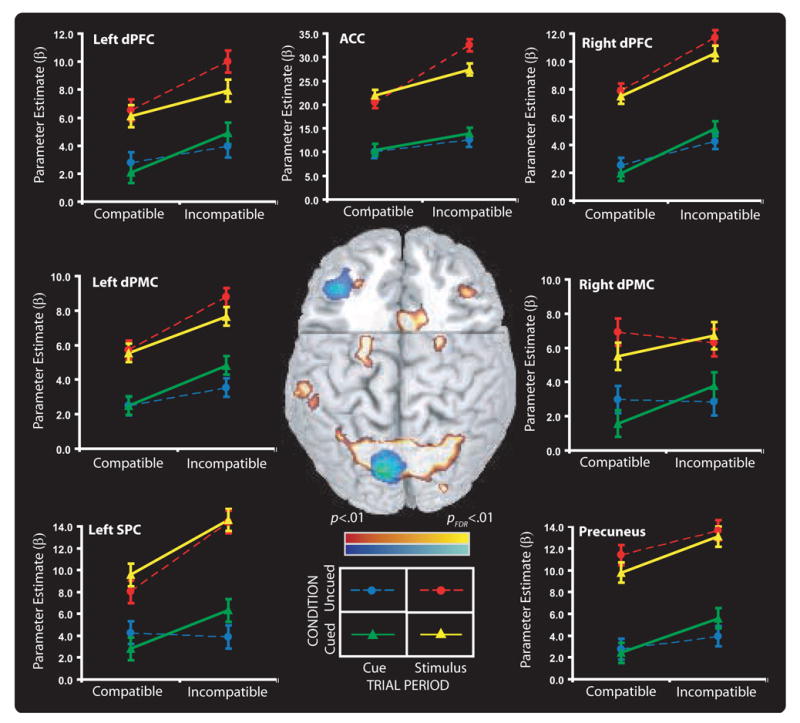
Extent of activity for cortical regions from the Table superimposed on a spatial normalized brain, with frontal section removed at (y = 10, z = 26). Voxels with incompatible vs. compatible task related activity greater than p<.01 (uncorrected) contiguous to peak activity are shown. Regions showing a compatibility effect during the cue period are shown in cool colors and regions showing a compatibility effect active during the stimulus period are shown in warm colors. The line graphs plot mean activity and standard errors relative to baseline for each task during both trial periods when cued or uncued for the regions-of-interest from left dPFC and from Schumacher et al. (2003) and MacDonald et al. (2000).
2.3 Region-of-Interest (ROI) Activation Results
For each participant and ROI, mean β-values were extracted separately for each experimental condition relative to the baseline: Trial Period (cue and stimulus), Cue Condition (cued and uncued), and Task Difficulty (compatible and incompatible). This led to eight activation values for each participant for each ROI. These data are also shown in Figure 3. Separate within-subjects analyses of variance with Trial Period, Cue Condition, and Task Difficulty as variables were performed with these β-values as data. The data from the stimulus period only was also analyzed separately to identify Cue Condition effects during this trial period. Finally, Task Difficulty was investigated during the Cue Period when participants were cued for the task to determine if task cueing affected activity during this trial period.
Although there were some differences across ROIs, two results were largely consistent across the response selection ROIs. First, there was more activity during the stimulus than the cue period. This is not surprising given that participants performed the task during the stimulus period and only prepared for it during the cue. The more interesting result is that the incompatible task produced more activity than the compatible one during both the stimulus and cue periods. None of the other effects or interactions was consistently reliable across ROIs. The next section provides an exhaustive listing of the effects of the experimental factors on brain activity across the ROIs.
2.3.1. Region-of-Interest in Left dPFC
As shown in the Table, two regions in left dPFC showed reliable effects of task difficulty in the cue and stimulus periods. The activation peak in the left dPFC region during the cue period is 1.6 cm more lateral to the activation peak during the stimulus period. This difference may reflect neurally distinct preparation and performance processes in left dPFC. Alternatively, this may reflect variability in peak activation produced by a unitary neural process. To test this, the activity in these regions was compared with a within-subjects analysis of variance including Region as a variable as well as the experimental variables: Trial Period, Cue Condition, and Task Difficulty. If these regions reflect distinct underlying processes, then there should be reliable interactions between the regions and the effects of the experimental variables. Contrary to this prediction, region did not interact with any experimental variable. The F-value was greater than 1.0 only for the interaction between Region and Trial Period, F(1,10)=1.98, p=.19. Thus, there was little evidence that these regions reflect distinct neural processors. These ROIs were therefore combined for further analyses.
There was a reliable main effect of Trial Period on left dPFC activity, F(1,10)=5.91, p<.05. Stimulus Period activity was greater than activity in the Cue Period. There was no reliable effect of Cue Condition, F(1,10)=1.94, p=.20. As expected, because this region was selected on the basis of the task difficulty effect, the effect of Task Difficulty was reliable, F(1,10)=12.21, p=.01. There were no reliable interactions.
When the stimulus period was analyzed separately, Task Difficulty approached reliability, F(1,10)=4.55, p<.06. There was no reliable effect of Cue Condition and no interaction, F(1,10)=0.37, p=.56, and no reliable interaction, F(1,10)=0.17, p=.69. For the cue period, again there was a reliable effect of Task Difficulty (incompatible > compatible task) when the task was cued, t(10)=4.30, p<.01.
2.3.2 Regions-of-Interest from Schumacher et al. (2003)
To characterize the effects of our conditions on regions specifically related to spatial response selection, we investigated brain activity in ROIs from a previous study with the same and other similar spatial choice-reaction tasks (Schumacher et al., 2003).
Right dPFC (x=42; y=32; z=24)
There were reliable main effects of Trial Period and Task Difficulty on right dPFC activity, F(1,10)=13.72, p<.005, and F(1,10)=10.62, p<.01, respectively. Stimulus period activity was greater than activity in the cue period; and the incompatible task produced more activity than the compatible one. The effect of Cue Condition was not reliable, F(1,10)=.42, p=.53 and there were no reliable interactions.
When the stimulus period was analyzed separately, only Task Difficulty produced a reliable effect, F(1,10)=8.25, p<.05; with incompatible trials producing more activity than compatible ones. Neither the effect of Cue Condition nor its interaction with Task Difficulty was reliable: F(1,10)=1.99, p=.19, F(1,10)=.60, p=.46, respectively. For the cue period, there was a reliable effect of Task Difficulty (incompatible > compatible task) when the task was cued, t(10)=3.09, p<.05.
Left dPMC (x=−30; y=−8; z=58)
There was a reliable main effect of Trial Period on left dPMC activity, F(1,10)=5.41, p<.05. Stimulus period activity was greater than activity in the cue period. There was a reliable effect of Task Difficulty, F(1,10)=7.50, p<.05. The incompatible task produced more activity than the compatible one. There was no reliable effect of Cue Condition F<1 and there were no reliable interactions.
When the stimulus period was analyzed separately, only Task Difficulty (incompatible > compatible task) produced a reliable effect, F(1,10)=5.08, p<.05. Neither the effect of Cue Condition nor its interaction with Task Difficulty was reliable: F(1,10)=1.01, p=.34, F(1,10)=.90, p=.37, respectively. For the cue period, the effect of Task Difficulty (incompatible > compatible task) when the task was cued approached reliability, t(10)=2.16, p<.06.
Right dPMC (x=18; y=4; z=58)
The effect of Trial Period (stimulus > cue period) on right dPMC activity approached reliability, F(1,10)= 3.67, p<.09. Only the interaction between Cue Condition and Task Difficulty also approached reliability, F(1,10)=4.84, p<.06. Incompatible trials were more active than compatible ones when cued, but not when uncued. Neither the effect of Cue Condition nor Task Difficulty was reliable: F(1,10)=.56, p=.47, F(1,10)=.73, p=.41, respectively.
There were no reliable effects when the stimulus period was analyzed separately: Cue Condition, F(1,10)=1.00, p=.34; Task Difficulty, F(1,10)=.07, p=.80; interaction, F(1,10)=1.98, p=.19. For the cue period, there was a reliable effect of Task Difficulty (incompatible > compatible task) when the task was cued, t(10)=5.77, p<.05.
Left SPL (x=−16; y=−70; z=44)
There were reliable main effects of Trial Period (stimulus > cue period) and Task Difficulty (incompatible > compatible task) on left SPL activity, F(1,10)=14.99, p<.005, and F(1,10)=9.65, p<.05, respectively. The effect of Cue Condition was not reliable, F(1,10)=1.02, p=.34. The only reliable interaction was between Trial Period and Task Difficulty, F(1,10)=5.48, p<.05. Incompatible trials produced more activity than compatible ones during the stimulus but not the cue period.
When the stimulus period was analyzed separately, only Task Difficulty (incompatible > compatible) produced a reliable effect, F(1,10)=9.14, p<.05. Neither the effect of Cue Condition nor its interaction with Task Difficulty was reliable: F(1,10)=.85, p=.38, F(1,10)=.68, p=.43, respectively. For the cue period, there was a reliable effect of Task Difficulty (incompatible > compatible task) when the task was cued, t(10)=3.50, p<.01.
Precuneus (x=−8; y=−54; z=48)
There were reliable main effects of Trial Period (stimulus > cue period) and Task Difficulty (incompatible > compatible task) on precuneus activity, F(1,10)=16.23, p<.005, and F(1,10)=6.51, p<.05, respectively. The effect of Cue Condition was not reliable, F(1,10)=.09, p=.77. The only reliable interaction was between Cue Condition and Task Difficulty, F(1,10)=5.48, p<.05. Incompatible trials produced more activity than compatible ones when cued, but not when uncued.
When the stimulus period was analyzed separately, the effect of Cue Condition (uncued > cued condition) approached reliability, F(1,10)=4.34, p<.07. The effect Task Difficulty (incompatible > compatible task) was reliable, F(1,10)=6.21, p<.05. The interaction between these effects was not reliable, F(1,10)=.35, p=.57. For the cue period, there was a reliable effect of Task Difficulty (incompatible > compatible task) when the task was cued, t(10)=2.68, p<.05.
2.3.3 Region-of-Interest from MacDonald et al. (2000)
To investigate the effect of our experiment on ACC activity, a 5-mm spherical ROI was created centered on activation peak from MacDonald et al. (x=4; y=−1; z=47).1 There were reliable main effects of Trial Period (stimulus > cue period) and Task Difficulty (incompatible > compatible task) on ACC activity, F(1,10)=13.18, p<.01, and F(1,10)=10.92, p<.01, respectively. The effect of Cue Condition was not reliable, F(1,10)=.36, p=.56. There was a reliable interaction between Trial Period and Task Difficulty, F(1,10)=8.17, p<.05. Incompatible trials produced more activity than compatible ones during the stimulus but not the cue period. The three-way interaction approached reliability F(1,10)=4.25, p<.07.
When the stimulus period was analyzed separately, Task Difficulty (incompatible > compatible task) produced a reliable effect, F(1,10)=14.48, p<.01. The interaction between Cued Condition and Task Difficulty was also reliable, F(1,10)=8.56, p<.05. Uncued incompatible trials produced more activity than cued incompatible trials, but there was no effect of Cue condition on compatible trials. The effect of Cue Condition was not reliable, F(1,10)=2.00, p=.19. For the cue period, there was no reliable effect of Task Difficulty when the task was cued, t(10)=1.56, p=.15.
2.3.4 Summary of Activation Results in Response Selection Regions
The stimulus period produced more activity than the cue period in all ROIs. The incompatible task produced more activity than the compatible one in all ROIs except right dPMC. The incompatible task produced more activity than the compatible one in nearly every analysis: a) when both trial periods were analyzed together; b) when the stimulus period was analyzed alone; and c) during the cue period when the cued trials were analyzed separately (except for ACC). When the stimulus period was analyzed separately, Cue Condition produced marginally reliable effects in Precuneus, with uncued trials producing more activity than cued ones; and a reliable interaction with Task Difficulty in ACC. As can be seen in Figure 3, the trend for more activity on uncued than cued trials is apparent in other ROIs as well.
2.3.5 Regions-of-Interest for Task Preparation
Brass and von Cramon (2002; 2004) suggest that left IFJ (x=−37; y=4; z=35); right IFG (x=56; y=22; z=36); and right IPS (x=41; y=−48; z=51)1 and Sakai and Passingham (Sakai & Passingham, 2003) suggest that right anterior PFC (x=36; y=44; z=6) mediate task preparatory processes. If these regions mediate task preparation in our task, then Cue Condition should affect activity during the cue period. To investigate this, 5-mm spherical ROIs were created centered on activation peaks from these studies. As shown in Figure 4, there was no reliable effect of Cue Condition on activity in any of these regions during the cue period: IFJ, F(1,10)=1.35, p=.27; IFG, F(1,10)=.22, p=.65; IPS, F(1,10)=2.79, p=.13; anterior PFC, F(1,10)=3.32, p=.10.
Figure 4.
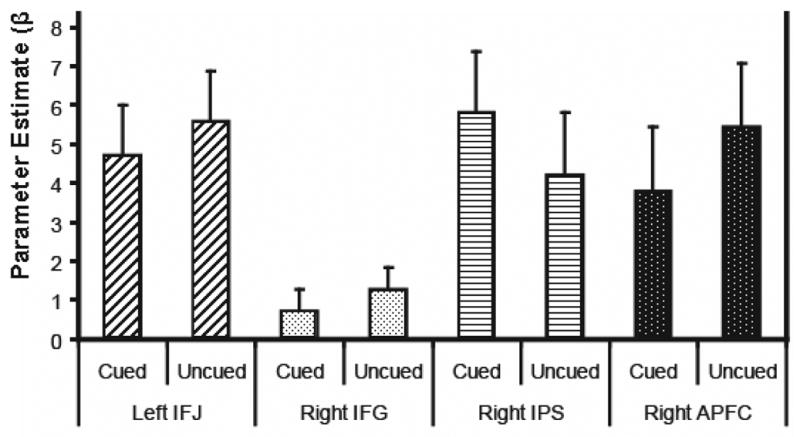
Mean activity and standard errors relative to baseline for cued and uncued trials during the cue period for brain regions associated with task preparation (Brass & von Cramon, 2004; Sakai & Passingham, 2003): IFJ = inferior frontal junction; IFG = inferior frontal gyrus; IPS = intra-parietal sulcus; APFC = anterior prefrontal cortex. No reliable effect of the task cue was found in these regions during the cue period.
3. Discussion
This study used an event-related experimental design to investigate neurocognitive dissociations in the frontal-parietal network for spatial response selection. The use of an event-related design allowed us to investigate the response in these regions during task preparation as well as task performance. Based on previous neuroimaging and neurophysiological research, we predicted that regions in this network may respond differently during the cue and stimulus trial periods. Contrary to this prediction, during both trial periods, brain regions previously implicated in spatial response selection (ROIs from right dPFC, dPMC, and SPC from Schumacher et al., 2003) activated more to the incompatible than compatible cue. For the stimulus period, this result replicates previous studies. The result during the cue period is novel. The only difference between the task conditions was that a centrally presented x or a + appeared on the screen to indicate either the incompatible or compatible task. Thus, the increased activity to the incompatible cue (i.e., the x) likely reflects task preparation processing. Furthermore, none of these regions showed a reliable interaction between Trial Period and Task Difficulty. The frontal-parietal network of brain regions for spatial response selection activates both during task performance and preparation. Thus, the same brain regions that mediate spatial response selection also mediate spatial response preparation. We found no evidence for separate neurocognitive processors for task performance and preparation among these regions
Our study is a conceptual replication of one using the Stroop task that found specific regions of activity for the cue period (MacDonald et al., 2000). That study reported evidence for specific regions mediating task preparation (left dPFC) and task performance (ACC). To the authors, these results suggested that left dPFC mediates the maintenance and representation of task context and attentional task demands and ACC mediates an evaluative process that monitors for response conflict or competition on each trial.
We were unable to replicate the trial period dissociation reported by MacDonald et al. (2000). We found task difficulty effects in left dPFC during both cue and stimulus trial periods. These regions were very close to the left dPFC region found by MacDonald et al. to be affected by task difficulty (x=−41; y=17; z=31).1 However, the ACC activity in the current study is broadly consistent with the MacDonald et al. result. Anterior cingulate cortex was the only region to show a reliable interaction between trial period and task difficulty. There was a compatibility effect during the stimulus period only. Additionally, the three-way interaction between trial period, cue condition, and task difficulty approached reliability in ACC as well. Activity was less during the stimulus period when the incompatible task was cued than when it was uncued. During the stimulus period, activity increased more for incompatible than compatible trials when the tasks were not cued than when they were. This is what one would expect if this region monitored for response conflict and the cue served to increase the activity of one rule set over the other, and thus decrease conflict when the stimulus was presented.
We found that brain regions previously implicated in spatial response selection also mediate task preparation. To more closely examine neural processing related to task preparation in brain areas outside the frontal-parietal network for spatial response selection, we extracted the task-related activity from four brain regions previously implicated in task preparation (Brass & von Cramon, 2002, 2004; Sakai & Passingham, 2003). These frontal and parietal brain regions were found to activate to task cues and the authors proposed that this activity reflected control processing specific to task preparation. If this is the case, then these regions should have shown an effect of Cue Condition in our experiment. That is, there should be more activity during the cue period when the task is cued than when uncued. Contrary to that prediction, none of these regions showed this pattern (see Figure 4). Even in brain regions previously implicated in task preparation, there is no evidence for a selective effect during the cue period for any of the frontal or parietal regions investigated. Nor did the whole-brain analysis reveal any regions with cue specific activity. Thus, we found no evidence for neurocognitively distinct task preparation processing.
3.1. Implications for Cognitive Processing: Relationship between Response Selection and Working Memory
Given the behavioral evidence for cognitively distinct SR rule activation and response selection processes (Eimer, 1995; Eimer et al., 1995; Kornblum et al., 1990), our current data suggest that these cognitive processes are mediated by the same neural processors. Consistent with this interpretation, as shown in Figure 3, for the incompatible task there was more activity during the stimulus period on uncued than cued trials. This suggests that some cognitive processing may precede the stimulus on cued trials, but not on uncued ones. One interpretation is that the SR rules are activated when the task was cued, but that activation process necessarily occurs during the stimulus period on uncued trials.
The frontal-parietal network activated here for spatial response preparation and response selection is very similar to the network activated in an experiment investigating spatial working memory (Rowe, Toni, Josephs, Frackowiak, & Passingham, 2000). In that experiment, dPMC and SPC regions activated when participants held three spatial locations in working memory (WM) over a delay. Right dPFC was active when participants selected one of the locations from memory.
We propose that the overlap in activity in these regions during task preparation and performance in our current study reflects the underlying role played by spatial WM in spatial response selection. Specifically, during task preparation; participants activate and maintain the SR rules for the cued task in spatial WM. This involves maintaining a representation of the potential stimulus locations as well as the appropriate responses to them. This results in more cue-related activity for the incompatible task than the compatible one. We propose that it is the SR rules that are maintained in WM, and not just the potential stimulus and response locations. This is because although both the incompatible and compatible tasks involve the same number of potential stimulus and response locations, they differ in the number of SR rules. There are up to four SR rules for the incompatible task: one for each stimulus location and response pairing; but only one SR rule for the compatible task (viz., press the button corresponding to the stimulus location). Once the task stimulus appears, the appropriate SR rule is then selected and executed.
Rowe et al. (2000) found that right dPFC was active only during the selection of an item in spatial WM. We found both cue-related and stimulus-related activity in right dPFC. It is not clear why we failed to replicate this dissociation. Our stimuli and responses were more consistent across trials (only four potential SR rules) than were theirs. Perhaps this consistency allowed controlled response-selection processes to be activated and partially selected during task preparation in our study.
Despite this inconsistency, we found substantial overlap between activity in a task that required maintenance and selection from spatial WM (Rowe et al., 2000) and in a choice-reaction task that required maintenance and selection of SR rules stored in long-term memory. This overlap is consistent with our proposal that spatial rule activation and spatial response selection are mediated through spatial WM processes.
3.2. Implications for Cognitive Processing: Relationship between Current Study and Task Switching
We found no neural evidence for preparation-specific processing, despite the behavioral evidence for such processes (Meiran, 1996; Rubinstein, Meyer, & Evans, 2001). For example, using Sternberg’s (1969) additive factor logic, Rubinstein et al. reported evidence for distinct executive control processes for goal shifting and rule-activation. We found no evidence for a brain region mediating goal-shifting. However, one important difference between studies implicating goal-shifting and other task preparation control processes and the current one is that most of the previous studies used experimental procedures designed to investigate the effect of task switching. That is, comparing trials where participants either repeated the same task from the previous trial or switched to a different one (Meiran, 1996; Rogers & Monsell, 1995; Rubinstein et al., 2001). Here we compared uncued and cued incompatible and compatible trials regardless of the previous trial condition. Additionally, half the trials involved long (4.4 to 8.8 sec) intervals between both the task cue and the task stimulus and between adjacent trials. Even with these long intervals, there was a small (18 ms) unreliable effect of switching on mean RT (p > .10), which did not interact reliably with any other variables. Thus, this procedure is likely to have been insensitive to additional control processes required to reconfigure processing for a new task situation. Yet, other studies using procedures sensitive to task switching have also reported that regions active during task preparation are also active during task performance (Ruge et al., 2005; Wylie, Javitt, & Foxe, 2006). Thus, it may be that preparation-specific processes (e.g., goal shifting) are mediated by task performance regions in these procedures as well.
3.3. Conclusions
One might expect the task preparation control processes proposed by Brass and von Cramon (2002; 2004) and Sakai and Passingham(2003) to mediate task preparation in this procedure. There was nearly a 75 ms effect of cuing for the incompatible condition, which suggests cognitive processing proceeded during the cue period. Additionally, MacDonald et al. (2000) report task preparation specific activity in a procedure very similar to ours. Thus, the lack of evidence for brain regions selectively activated during the cue period, coupled with the presence of cue-related activity in brain regions known to mediate task performance, suggests that that, at least for these tasks, preparation control processes are also mediated through the existing spatial WM system (Schneider & Logan, 2005).
Procedural differences between the experiments may be an important factor for why Brass and Von Cramon (2002; 2004) and Sakai and Passingham (2003) found neurally distinct brain regions for task preparation. The tasks in the current study each involved a relatively small set of SR rules. The experiments by Brass and von Cramon and Sakai and Passingham involved tasks with more SR rules or where the SR rules were known at the time of the cue (e.g., determine if an upcoming digit was odd or even or memorize a set of upcoming spatial positions). Perhaps additional task preparation control processes only activate in these situations. When the known number of potential SR rules is small, they are activated in WM in preparation for task performance and no additional task preparation is required. These tasks differences, however, cannot explain the lack of overlap in task preparation activity between the Brass and Von Cramon and Sakai and Passingham experiments.
The current study helps to unify disparate cognitive processes (e.g., SR rule activation and response selection) within the spatial WM system. These results suggest that different aspects of cognitive control may be mediated the same neurocognitive mechanisms.
4. Experimental Procedure
4.1. Participants
Eleven healthy right-handed volunteers (5 females; ages 19–27 years) participated in this experiment. All participants were recruited from the University of California community and gave their informed consent.
4.2. Behavioral Procedure
Stimuli were projected onto a screen in white on a black background. Participants viewed the screen through a mirror mounted on the head radiofrequency (RF) coil while lying on their back in a magnetic resonance scanner. Participants made their responses with the index and middle fingers of their left and right hands using an in-line 4-button response pad.
A schematic of the trial conditions is shown in Figure 1. Participants performed two choice-reaction tasks in the experiment. At the beginning of each trial, a cue appeared in the center of the fixation display. On one-third of the trials the cue was an x, which indicated an incompatible trial, on one-third of the trials the cue was a +, which indicated a compatible trial, and on one-third of the trials the cue was an *, which did not cue the task to be performed. Every cue remained on screen for 4.4 seconds on half the trials, and 6.6 or 8.8 seconds for a quarter of the trials each.2 After the cue period, the foreperiod display appeared onscreen for 500 ms. For trials in which the task was cued, the foreperiod display consisted of a horizontal array of four circles, two on either side of the cue stimulus. For trials with a neutral cue, the foreperiod display for trials of the incompatible condition appeared for half the trials and the foreperiod display for the compatible condition appeared for the other half. All stimuli (i.e., circles and fixation stimulus) were equidistant from each other and the entire display subtended roughly 3° horizontal visual angle. Following the foreperiod display, a filled white disk (i.e., the task stimulus) replaced one of the display circles for 200 ms after which a post-stimulus display appeared, which was identical to the foreperiod display, and remained onscreen for an additional 1500 ms.
The disk appeared equally often in each of the four possible locations. For the compatible task, participants pressed a button with their left middle, left index, right index, or right middle finger if the task stimulus appeared on the far left, middle left, middle right or far right position, respectively. For the incompatible task, participants pressed a button with their left middle, left index, right index, or right middle finger if the task stimulus appeared on the middle left, far right, far left, or middle right position respectively. Following the post-stimulus fixation display, a ● appeared indicating the inter-trial interval. The inter-trial interval lasted for 4.4 seconds on half the trials, and 6.6 and 8.8 seconds for a quarter of the trials each.2 Each experimental run included 48 trials. The cue duration, inter-trial interval duration, and task stimulus location were randomized. Each participant performed six experimental runs.
To encourage optimal performance, participants were paid $10 an hour plus a monetary bonus based on points earned for their performance. Three hundred points were awarded for each correct response and 1 point was deducted for every 10 ms taken to respond correctly; 300 points were deducted per incorrect response. Participants earned $1 for every 10,000 points they scored. They were fully informed about the reward system before the experiment began. At the end of each experimental run, participants’ average accuracy and RT for each task was presented onscreen as were the total points earned for the experiment. Participants practiced the tasks for two experimental runs prior to functional scanning.
4.3. Functional MRI Procedure
Imaging was performed using a 4.0 Tesla Varian Inova scanner equipped with a fast gradient system for echoplanar imaging. A standard RF head coil was used with foam padding to restrict head motion comfortably. A 2-shot gradient echo, echoplanar sequence (TR = 2200 ms, TE = 28 ms, matrix size = 64 × 64, FOV = 22.4 cm) was used to acquire data sensitive to the blood oxygen level dependent signal. Each functional volume contained 20-3.5 mm axial slices with a 0.5 mm gap between slices. Each experimental run was preceded by 5 sec of dummy gradient RF pulses to achieve a steady state of tissue magnetization. Each run lasted about 11 min 29 sec (313 volumes/run). Two high-resolution structural T1-weighted scans were also acquired. The first collected 20 axial slices in the same plane as the echoplanar images (TR = 200 ms, TE = 5 ms, matrix size = 256 × 256, FOV = 22.4 cm). The second was a 3D MPFLASH scan (TR = 9 ms, TE = 4.8 ms, TI = 300 ms).
4.4. Functional MRI Data Processing and Analyses
During reconstruction, two images were created for each scan by linearly interpolating each adjacent scan. All additional data pre-processing and analysis was conducted using SPM2 (http://www.fil.ion.ucl.ac.uk/spm/). First, data were corrected for head-motion artifacts with a least squares approach using a six-parameter, rigid-body transformation algorithm (Friston et al., 1995; Friston, Williams, Howard, Frackowiak, & Turner, 1996); then they were smoothed with a 6 mm full-width half-maximum Gaussian kernel.
Data were analyzed with a modified general linear model (Worsley & Friston, 1995). For each participant, we created a design matrix with eight discrete covariates. We modeled the task (incompatible and compatible) and the cue (cued or uncued) separately for the cue and stimulus trial periods. These covariates were convolved with an idealized hemodynamic response function. A high-pass filter removed frequencies below .0078 Hz.
The cue period covariates were centered on the first scan of the cue period. Because half the trials included only a 4.4 sec cue, any task preparation processing will likely occur during this scan. For longer cue periods, preparation may have degenerated, but that will not affect activity to our cue-period covariates.
For each participant, contrast images were computed for the incompatible vs. compatible conditions at both the cue and stimulus trial periods both when the task was cued and when it was uncued. Additional contrast images for each of the eight covariates vs. baseline (i.e., the inter-trial interval) were also computed.
Each participant’s brain and contrast images were normalized to the Montreal Neurological Institute reference brain. Group level random effect analyses then were conducted on the following contrasts: a) incompatible vs. compatible when cued for the cue period, b) incompatible vs. compatible when cued for the stimulus period, and c) incompatible vs. compatible when uncued for the stimulus period.
4.5. Region-of-Interest Analyses
Task difficulty effects on brain activity in left dPFC have been inconsistently reported in previous studies of spatial response selection (Dassonville et al., 2001; Iacoboni et al., 1996; Jiang & Kanwisher, 2003; Merriam et al., 2001; Schumacher & D’Esposito, 2002; Schumacher et al., 2003; Schumacher et al., 2005). Two sites in left dPFC produced a task difficulty effect in this experiment (see Table). Regions-of-interest were created from the reliable clusters of activity surrounding each of these activation peaks.
To characterize the effects of our conditions on regions specifically related to spatial response selection, we investigated brain activity in ROIs from a previous study with the same and other similar spatial choice-reaction tasks (Schumacher et al., 2003).
As described previously, in Schumacher et al. (2003), participants performed spatial choice-reaction tasks that differed across four levels of response-selection difficulty. That study conducted whole-brain statistical analyses and found five brain regions (i.e., right dPFC, bilateral dorsal dPMC, and two regions in SPC: left superior parietal lobule and precuneus) to be monotonically affected by our parametric manipulation of spatial response-selection difficulty.
For the current analyses, ROIs for each of the areas identified from Schumacher et al. (2003) included the sites of peak activity and contiguous voxels with a t-value corresponding to p < .01.
Additional ROIs were also included based on other findings reported in the literature. We created four 5-mm spherical ROIs centered on activation peaks from two studies of theoretical interest. One in ACC, found to be active in the similar study by MacDonald et al. (2000); and four ROIs shown two be active during task preparation: three from Brass and von Cramon (2004), in left IFJ, right IFG, and right IPS and one from Sakai and Passingham (2003) in right anterior PFC.
Acknowledgments
The authors thank the members of CoNTRoL and the D’Esposito Laboratory for their helpful comments and technical assistance. This work was supported by grants from the National Institute of Health and the Veterans Administration (MH63901 and NS40813).
Footnotes
Voxel coordinates converted from Talairach to MNI coordinates with MRIcro (www.mricro.com) prior to analysis.
The jittering of stimuli by varying the cue-stimulus and inter-trial interval in this way has been shown to be effective in isolating brain activity for separate trials and trial periods (Ollinger, Corbetta, & Shulman, 2001; Ollinger, Shulman, & Corbetta, 2001).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bracewell RM, Mazzoni P, Barash S, Andersen RA. Motor intention activity in the macaque’s lateral intraparietal area 2. Changes of motor plan. Journal of Neurophysiology. 1996;76(3):1457–1464. doi: 10.1152/jn.1996.76.3.1457. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. The role of the frontal cortex in task preparation. Cerebral Cortex. 2002;12(9):908–914. doi: 10.1093/cercor/12.9.908. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Decomposing components of task preparation with functional magnetic resonance imaging. Journal of Cognitive Neuroscience. 2004;16(4):609–620. doi: 10.1162/089892904323057335. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with frontal lobe. The Journal of Comparative Neurology. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Lewis SM, Zhu XH, Ugurbil K, Kim SG, Ashe J. The effect of stimulus-response compatibility on cortical motor activation. Neuroimage. 2001;13:1–14. doi: 10.1006/nimg.2000.0671. [DOI] [PubMed] [Google Scholar]
- Eimer M. Stimulus-response compatibility and automatic response activation: Evidence from psychophysiological studies. Journal of Experimental Psychology: Human Perception and Performance. 1995;21(4):837–854. doi: 10.1037//0096-1523.21.4.837. [DOI] [PubMed] [Google Scholar]
- Eimer M, Hommel B, Prinz W. S-R compatibility and response selection. Acta Psychologica. 1995;90:301–313. [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;2:165–189. [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex - An update: time is of the essence. Neuron. 2001;30(2):319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual Pathways Connecting the Dorsolateral Prefrontal Cortex with the Hippocampal-Formation and Parahippocampal Cortex in the Rhesus-Monkey. Neuroscience. 1984;12(3):719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Bunge SA, Scanlon MD, Gabrieli JDE. Material-dependent and material-independent selection processes in the frontal and parietal lobes: an event-related fMRI investigation of response competition. Neuropsychologia. 2003;41(9):1208–1217. doi: 10.1016/s0028-3932(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Howell DC. Statistical Methods for Psychology. 2. Boston: Duxbury Press; 1987. [Google Scholar]
- Iacoboni M, Woods RP, Mazziotta JC. Brain-behavior relationships: Evidence from practice effects in spatial stimulus-response compatibility. Journal of Neurophysiology. 1996;76(1):321–331. doi: 10.1152/jn.1996.76.1.321. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Kanwisher N. Common neural substrates for response selection across modalities and mapping paradigms. Journal of Cognitive Neuroscience. 2003;15(8):1080–1094. doi: 10.1162/089892903322598067. [DOI] [PubMed] [Google Scholar]
- Kornblum S, Hasbroucq T, Osman A. Dimensional overlap: Cognitive basis for stimulus-response compatibility -- A model and taxonomy. Psychological Review. 1990;97:253–270. doi: 10.1037/0033-295x.97.2.253. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Bracewell RM, Barash S, Andersen RA. Motor intention activity in the macaque’s lateral intraparietal area I. dissociation of motor plan from sensory area. Journal of Neurophysiology. 1996;76:1439–1456. doi: 10.1152/jn.1996.76.3.1439. [DOI] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of processing mode prior to task performance. Journal of Experimental Psychology-Learning Memory and Cognition. 1996;22(6):1423–1442. [Google Scholar]
- Merriam EP, Colby CL, Thulborn KR, Luna B, Olson CR, Sweeney JA. Stimulus-response incompatibility activates cortex proximate to three eye fields. Neuroimage. 2001;13(5):794–800. doi: 10.1006/nimg.2000.0742. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. Journal of Neurophysiology. 1991;66:705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI - II. Analysis. Neuroimage. 2001;13(1):218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI - I. The method. Neuroimage. 2001;13(1):210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proceedings of the National Academy of Sciences. 1990;87(1):256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philosophical Transactions of the Royal Society B-Biological Sciences. 2005;360(1456):781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the Frontal-Cortex from the Posterior Parietal Region in the Rhesus-Monkey. Journal of Comparative Neurology. 1984;228(1):105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General. 1995;124:207–231. [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RSJ, Passingham RE. The prefrontal cortex: Response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Rubinstein JS, Meyer DE, Evans JE. Executive control of cognitive processes in task switching. Journal of Experimental Psychology: Human Perception and Performance. 2001;27(4):763–797. doi: 10.1037//0096-1523.27.4.763. [DOI] [PubMed] [Google Scholar]
- Ruge H, Brass M, Koch I, Rubin O, Meiran N, von Cramon DY. Advance preparation and stimulus-induced interference in cued task switching: further insights from BOLD fMRI. Neuropsychologia. 2005;43(3):340–355. doi: 10.1016/j.neuropsychologia.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal interactions reflect future task operations. Nature Neuroscience. 2003;6(1):75–81. doi: 10.1038/nn987. [DOI] [PubMed] [Google Scholar]
- Schneider DW, Logan GD. Modeling task switching without switching tasks: A short-term priming account of explicitly cued performance. Journal of Experimental Psychology: General. 2005;134(3):343–367. doi: 10.1037/0096-3445.134.3.343. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, D’Esposito M. Neural implementation of response selection in humans as revealed by localized effects of stimulus-response compatibility on brain activation. Human Brain Mapping. 2002;17(3):193–201. doi: 10.1002/hbm.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Elston PA, D’Esposito M. Neural evidence for representation-specific response selection. J Cogn Neurosci. 2003;15(8):1111–1121. doi: 10.1162/089892903322598085. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Hendricks MJ, D’Esposito MD. Sustained involvement of a frontal-parietal network for spatial response selection with practice of a spatial choice-reaction task. Neuropsychologia. 2005;43(10):1444–1455. doi: 10.1016/j.neuropsychologia.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Sternberg S. The discovery of processing stages: extensions of Donders’ method. Acta Psychologica. 1969;30:276–315. [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annual Review of Neuroscience. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited - again. Neuroimage. 1995;2:173–182. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Javitt DC, Foxe JJ. Jumping the gun: Is effective preparation contingent upon anticipatory activation in task-relevant neural circuitry? Cerebral Cortex. 2006;16(3):394–404. doi: 10.1093/cercor/bhi118. [DOI] [PubMed] [Google Scholar]


