Abstract
During the past several decades, there has been a significant increase in the understanding of the biology, clinical behavior, and prognostic factors of renal cell carcinoma (RCC). Such progress has led to greater sophistication in the diagnosis and classification of RCC. Here, we review recent advances in our knowledge of the biologic characteristics of RCC that have resulted in notable achievements in staging, prognosis, patient selection, and treatment.
Key words: Angiogenesis, Genetics, Molecular markers, Renal cell carcinoma, Targeted treatment, Tumor-node-metastasis classification
The incidence of renal cell carcinoma (RCC) has been increasing steadily over the past decades. It is estimated that more than 38,000 Americans developed RCC in 2006, and more than 12,000 are expected to die from the disease.1 The diagnostic trend is mainly due to the widespread use of noninvasive abdominal imaging procedures, which detect incidental renal lesions.2 The majority of these incidentally detected tumors are at low stages and low grades and are amenable to curative surgical treatments; therefore, they carry a good prognosis.3,4 However, a stable proportion of 20% to 30% of patients still present with metastatic disease, and 20% to 30% of the patients who undergo curative surgery will develop metastatic disease during follow-up.5 Despite the recent arrival of targeted drugs, metastatic RCC remains an incurable condition for the majority of these patients.
RCC is a unique solid tumor that continues to intrigue basic and clinical scientists alike. In recent years, new discoveries and developments in genetics and molecular markers have led to a better understanding of the underlying pathways driving RCC biology and have subsequently led to new classifications and drugs that have had an impact on diagnosis, classification, patient selection, and therapy.6 The discovery of the von Hippel-Lindau (VHL) tumor suppressor gene and the hypoxia-induced pathway in clear cell RCC has provided a valuable substrate for the application of new strategies to diagnosis, patient selection, and targeted therapy.7 Herein, we review the strides in classification and underlying pathways in RCC.
Histology and Staging: From Tumor-Node-Metastasis to Integrated Staging
Categorizing tumors into subgroups with similar pathologic features may be helpful in understanding the disease, selecting therapy, and predicting prognosis. RCC is composed of various cell types and growth patterns. Historically, RCC was regarded as a single entity. Today, RCC is more accurately recognized as a family of cancers in which each results from a distinct genetic abnormality with unique morphologic features, but all are derived from renal tubular epithelium.
In 1986, Thoenes and colleagues8 established what became known as the Mainz classification of renal tumors. Based on their studies, the authors proposed a classification based on cell type and growth pattern that recognized clear cell, chromophilic, chromophobe, spindle-shaped, oncocytic, and unclassified tumor cell types, and solid, tubulopapillary, and mixed growth patterns. Throughout the 1990s, advances in the understanding of the genetic alterations underlying the pathogenesis of RCC reinforced the concept that the distinct subtypes of RCC each have their own associated genetic lesions. Under consideration of cytologic, growth pattern, and genetic features, the Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) introduced a new classification in 1997 that distinguishes clear cell, papillary, chromophobe, collecting duct, and unclassified RCC.9 These differences reflect a greater sophistication in tumor analysis based on cytology, histology, genetic aberrations, glycogen content, electron microscopy of cytoplasmic microvesicles, and immunohistochemistry of intermediate filament proteins, which strongly suggest that these subtypes in fact arose in distinct regions of the renal tubule despite their basic cytoplasmic staining properties. Their proposed classification is summarized in Table 1.
Table 1.
Current Classification of Renal Cell Tumors and Frequency in a Surgical Series of 1600 Consecutive Patients With Renal Cell Tumor at UCLA
| Tumor | Percentage |
|---|---|
| Benign neoplasms | 7 |
| Papillary and | 3 |
| metanephric adenoma | |
| Oncocytoma | 61 |
| Angiomyolipoma | 29 |
| Other | 7 |
| Malignant neoplasms | 93 |
| Clear cell renal | 82 |
| carcinoma | |
| Papillary renal | 11 |
| carcinoma | |
| Chromophobe renal | 5 |
| carcinoma | |
| Collecting duct | <1 |
| carcinoma | |
| Renal cell carcinoma, | 2 |
| unclassified |
These histologic subtypes are associated with distinct genetic alterations. Principally, changes in the genes can be “loss-of-function” mutations in tumor suppressor genes and “gain-of-function” mutations in proto-oncogenes, which are then called oncogenes. Research regarding heredity of clear cell RCC has led to the identification of the relevant gene locus on the short arm of chromosome 3.10–12 Additionally, these aberrations have been found in nonhereditary, sporadic clear cell RCC. This loss-of-function mutation led to the assumption of the existence of a tumor suppressor gene, and subsequent research led to the identification of the VHL gene.13 In papillary RCC, trisomies of chromosomes 7 and 17 and a loss of chromosome Y are typical findings.14–17 Occurrence of a relevant trisomy is usually associated with activation of a proto-oncogene, which is then called a gain-of-function mutation. Finally, the MET proto-oncogene on chromosome 7q was identified in hereditary papillary RCC.18,19 However, mutations in the MET proto-oncogene are found in only a small proportion of patients with sporadic papillary RCC. Launonen and associates20 reported on a novel familial renal cancer syndrome called “hereditary leiomyomatosis and renal cell cancer,” which involves the FH gene encoding fumarate hydratase, an enzyme responsible for catalysis in the conversion of fumarate to malate. This syndrome is characterized by cutaneous and uterine leiomyomas and aggressive type 2 papillary RCC.20,21 Another familial RCC syndrome called Birt-Hogg-Dube syndrome has been described.22 This syndrome is a genodermatosis also characterized by an increased risk for multiple or bilateral RCC, particularly chromophobe RCC and renal oncocytomas.23 Table 2 summarizes the most relevant chromosomal aberrations among clear cell, papillary, and chromophobe RCC.
Table 2.
Typical Genetic Aberrations of Clear Cell, Papillary, and Chromophobe Renal Cell Carcinoma
| Typical | |
|---|---|
| Subtype | Aberration |
| Clear cell | 3p– |
| Papillary* | Trisomies (3q, 7, 12, |
| 16, 17, 20) | |
| Chromophobe | Monosomies (1, 2, 6, |
| 10, 13, 17, 21) |
Staging of RCC is performed worldwide according to the tumor-node-metastasis (TNM) classification, which was most recently modified in 2002 (Table 3).24 The main change was the subdivision of tumor stage T1 into T1a and T1b based on a tumor size cutoff of 4 cm. For tumor grading, several different staging systems exist.25 The most frequently used system is the Fuhrman grading scheme, which distinguishes 4 grades.26 Grade 1 carcinomas have round, uniform nuclei approximately 10 µm in diameter with minute or absent nuclei. In grade 2, the nuclei are slightly irregular, with diameters of approximately 15 µm and visible nucleoli on 400-fold magnification. The nuclei in grade 3 are moderately irregular, with diameters of at least 20 µm and large nucleoli visible on 100-fold magnification. Grade 4 nuclei are markedly irregular and pleomorphic, with clumped chromatin. TNM stage and grade are considered to be the most important prognostic factors in RCC.27,28
Table 3.
2002 TNM Classification of Renal Cell Carcinoma
| Primary Tumor (T) | |
|---|---|
| T1a | Tumor 4 cm or less in greatest dimension, limited to the kidney |
| T1b | Tumor more than 4 cm but not more than 7 cm in greatest dimension, |
| limited to the kidney | |
| T2 | Tumor more than 7 cm in greatest dimension, limited to the kidney |
| T3a | Tumor directly invades adrenal gland or perirenal and/or renal sinus fat but |
| not beyond Gerota’s fascia | |
| T3b | Tumor grossly extends into the renal vein or its segmental (muscle-containing) |
| branches, or the vena cava below the diaphragm | |
| T3c | Tumor grossly extends into the vena cava above the diaphragm or invades the |
| wall of the vena cava | |
| T4 | Tumor invades beyond Gerota’s fascia |
| Regional Lymph Nodes (N) | |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single regional lymph node |
| N2 | Metastasis in more than 1 regional lymph node |
| Distant Metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
From American Joint Committee on Cancer.24
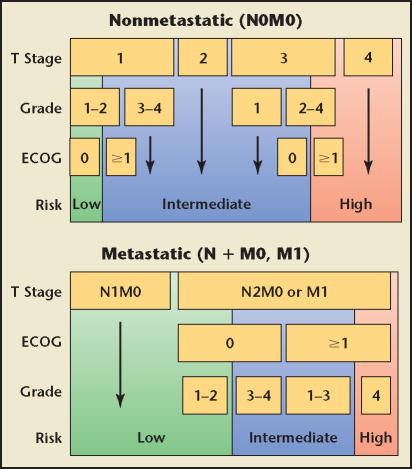
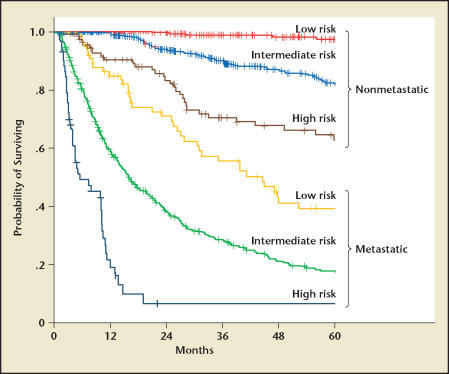
The clinical behavior of RCC, however, results from complex interactions among multiple factors. This realization has led to an increasing interest in using integrated staging systems to predict outcome by combining several prognostic variables. TNM stage, Fuhrman grade, and the patient’s performance status according to the Eastern Cooperative Oncology Group (ECOG) criteria29 comprise the University of California Integrated Staging System (UISS), which originally stratified patients into 5 different categories.30 The UISS was later modified into a simplified system containing 6 categories, 3 for nonmetastatic and 3 for metastatic disease (Figure 1).5 The UISS classifies patients into 3 different risk categories: low, intermediate, and high. An updated analysis of the UISS shows that the 2- and 5-year survival rates for these categories were 99% and 97% (nonmetastatic [NM] low risk), 93% and 81% (NM intermediate risk), 82% and 63% (NM high risk), 69% and 39% (metastatic [M] low risk), 37% and 17% (M intermediate risk), and 7% (M high risk) (Figure 2). This system permits the selection of high-risk patients most suitable for adjuvant treatment trials and the assignment of patients with metastatic disease to different therapeutic strategies. For example, patients in the metastatic high-risk group had a median survival of 6 months, which leads to the conclusion that the current treatment strategy consisting of nephrectomy and immunotherapy/angiogenesis-targeted therapy is ineffective. Hence, these patients might be better candidates for agents targeting the mammalian target of rapamycin (mTOR) pathway31 or experimental therapies. The SSIGN (stage, size, grade, and necrosis) score is based on the Mayo Clinic experience that included 1801 patients with surgically treated clear cell RCC.32 The score consists of TNM stage, tumor size, Fuhrman grade, and presence/absence of necrosis. Kattan and coworkers33 introduced an instrument for patients with surgically resected nonmetastatic RCC that incorporates patients’ symptoms, histologic subtype, tumor size, and T stage into a nomogram that predicts the risk of disease recurrence after nephrectomy. The UISS and the SSIGN score have been validated by external institutions.34–36
Figure 1.
The University of California Integrated Staging System. ECOG, Eastern Cooperative Oncology Group. Data from Zisman A et al.5
Figure 2.
Kaplan-Meier survival estimates (disease-specific survival) according to the University of California Integrated Staging System risk group.
Molecular Markers: From Bench to Bedside
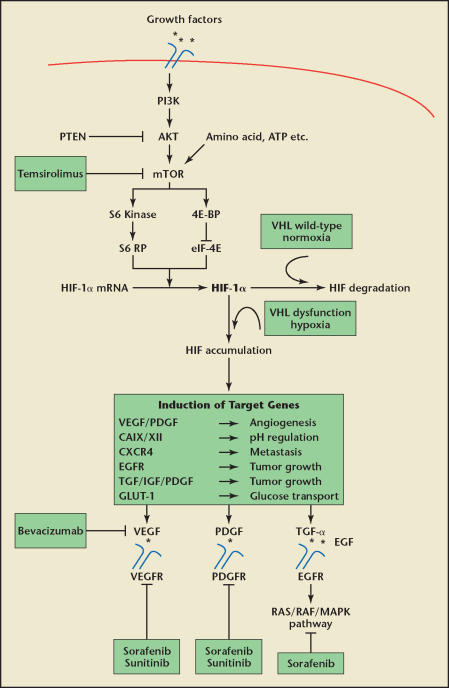
The following paragraphs discuss the most relevant pathways in RCC. A summary of these pathways and their inhibitors currently used in RCC is depicted in Figure 3.
Figure 3.
Regulation of hypoxia-inducible factor-1α (HIF-1α) and drug interventions currently in use. Activation of receptor tyrosine kinase (RTK), such as Rous Sarcoma oncogene (SRC) and the human epidermal growth factor receptor 2 (HER2), insulinlike growth factor (IGF), and epidermal growth factor (EGF) receptors, stimulate the PI3K-AKT-mTOR pathway. These pathways lead to the phosphorylation of S6 kinase and 4E-BP1. S6 kinase and 4E-BP1 lead to the translation of HIF-1α messenger RNA (mRNA). The hypoxia-induced pathway is linked to the VHL dysfunction in renal cell carcinoma (RCC). HIF regulates the expression of an array of genes that encode proteins regulating angiogenesis, pH, metastatic spread, tumor growth, and glucose transport. The drugs currently preferred for the targeted treatment of metastatic RCC are depicted here. ATP, adenosine triphosphate; EGFR, EGF receptor; mTOR, mammalian target of rapamycin; PDGF, platelet-derived growth factor; PDGFR, PDGF receptor; PI3K, phosphoinositide 3-kinase, TGF-α, transforming growth factor-α; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Carbonic Anhydrase IX and the Hypoxia-Induced Pathway
Carbonic anhydrase IX (CAIX) is the most significant molecular marker in RCC to date.37 CAIX is a transmembrane enzyme that plays a role in the adaptation of the tumor to hypoxic conditions by catalyzing the reversible reaction of carbonic acid to carbon dioxide and water, thereby regulating the temporal pH value. CAIX expression in normal tissue is limited to the gastrointestinal tract, gallbladder, and pancreatic ducts,38 whereas overexpression of CAIX has been seen in many tumors, including RCC. In addition to hypoxia, CAIX expression is regulated through VHL.39 Both hypoxia and loss of the function of the VHL protein lead to increased cellular levels of hypoxia-inducible factor-1α (HIF-1α) and subsequently to an up-regulation of the CAIX expression.39,40 Because clear cell RCC is genetically linked to loss of the VHL function and is commonly associated with a hypoxic tumor microenvironment, CAIX serves as a strong biomarker for clear cell RCC. In addition to up-regulation of CAIX, HIF-1α also increases the expression of other proteins, such as the proangiogenic vascular endothelial growth factor (VEGF-A), platelet-derived growth factor (PDGF), transforming growth factor-α (TGF-α), epidermal growth factor receptor (EGFR), insulin-like growth factor (IGF), and glucose transporters.
CAIX serves as an important predictor for survival and response to immunotherapy among patients with metastatic RCC. Bui and colleagues41 were able to show that CAIX expression is an independent prognostic factor for patients with metastatic clear cell RCC. In their study, CAIX was expressed in 94% of the clear cell RCCs. Univariate recursive partitioning analysis defined a threshold of 85% cells expressing CAIX as the optimum to stratify patients according to disease-specific survival. Using this cutoff, patients with an expression of 85% or less had a 3.1-fold increased risk (95% CI, 2.0–4.8) of death from RCC than patients with an expression greater than 85%. Expanding on an observation noted in this study, Atkins and associates42 showed that CAIX expression is also associated with response to interleukin-2 (IL-2)-based immunotherapy. Seventy-eight percent of the responders had high CAIX expression compared with 51% of the nonresponders. Furthermore, long-term survival of more than 5 years was seen only in patients with high CAIX expression. These findings have important implications for patient selection regarding therapeutic approaches for metastatic RCC. Patients with high CAIX expression (> 85%) might be optimal candidates for IL-2-based immunotherapy as a first-line therapy, which remains the only therapeutic option with a chance for a durable, complete remission.
Besides its use as a biomarker, CAIX is used as a therapeutic target in 2 different strategies. One strategy is to target CAIX using an anti-CAIX antibody. Bleumer and coworkers43 recently reported on 35 patients with metastatic RCC who were treated with the CAIX antibody WX-G250 combined with low-dose IL-2. They showed a clinical benefit in 8 patients (23%), including 3 with partial response and 5 with disease stabilization. An international multicenter phase III trial testing the effect of WX-G250 in an adjuvant setting is currently enrolling patients. The second strategy is to use a vaccination stimulating the host immune system to generate CAIX targeting cytotoxic T lymphocytes (CTLs). Uemura and colleagues44 showed safety and efficacy of a CAIX vaccine in HLA-A24-positive patients with cytokine-refractory metastatic RCC. Of 23 enrolled patients, 3 had partial response and 6 had stable disease. The median survival time was 21 months in this setting. In the Kidney Cancer Program laboratory at UCLA, a granulocyte-macrophage colony-stimulating factor (GM-CSF)-CAIX fusion gene has been created.45–47 Transduced in peripheral blood monocytes, it has been successful in generating CTLs capable of lysing CAIX-expressing cancer cells.45 Currently, a clinical grade GM-CSF-CAIX vaccine is being manufactured with assistance from the National Cancer Institute’s Rapid Access to Intervention Development Program. In conclusion, CAIX is a strong molecular marker and holds much promise as a therapeutic target for the future.
Angiogenesis
Clear cell RCC is genetically linked to factors regulating angiogenesis, such as VEGF, PDGF-B, and TGF-α, of which VEGF is the strongest proangiogenic protein.6,48,49 The importance of angiogenesis in the biology and therapy of RCC is now well established, based on several decades of worldwide research. RCC, like all cancers, must induce angiogenesis to supply nutrients and oxygen required for progressive tumor growth. In-growth of new blood and lymphatic vessels also provides access to the vasculature and thereby promotes metastasis.50–52
VEGF has several isoforms. VEGFA is involved in angiogenesis, whereas VEGF-C and VEGF-D are more prominent in regulating lymphangiogenesis. VEGF receptor 1 (VEGFR-1) and VEGFR-2 are the primary VEGF receptors, whereas VEGFR-3 is more involved in lymphangiogenesis.53–55 The role of these proteins has been evaluated in recent studies. Leppert and associates56 reported a tissue microarray-based study on 382 patients with RCC. Immunohistochemistry was performed with antibodies directed against VEGF-A, VEGF-C, VEGF-D, VEGFR-1, VEGFR-2, and VEGFR-3; the mean expression percentage within the tumor epithelium in clear cell and papillary RCC was 37 and 57, 71 and 75, 51 and 41, 54 and 58, 49 and 37, and 13 and 3, respectively. An additional analysis of these patients was reported by Lam and coworkers,57 who clearly showed the predictive ability for both presence of metastasis and disease-specific survival of these proteins of the VEGF family. In their analysis, low endothelial expression of VEGFR-3 was independently associated with both lymph node metastasis and poor prognosis. The observed high expression of VEGF in clear cell RCC has been shown by other groups.58,59
Hence, inhibition of angiogenesis is a promising approach for targeting metastatic RCC. This has led to the development of inhibitors of angiogenesis such as bevacizumab, sorafenib (BAY 43-9006), and sunitinib (SU11248). Antiangiogenic agents act at various steps of the angiogenesis pathway, inhibiting tumor growth and new vessel growth. Bevacizumab is a monoclonal antibody against VEGF-A. In a randomized phase II trial, patients who had received high-dose bevacizumab had a significant prolongation of progression-free survival (hazard ratio, 2.55; P < .001) compared with placebo. However, the drug did not improve overall survival.60 Sorafenib is an orally bioavailable multikinase inhibitor that targets the VEGF receptor (VEGFR), PDGFR-β, and Raf kinase. Sorafenib has been shown to be effective and tolerable in recent phase II and phase III trials.61,62 In a phase III trial, sorafenib produced responses in only 2% but stable disease in another 78% of the patients, which led to a significant improvement in progression-free survival compared with placebo.62 Sunitinib inhibits VEGFR and PDGFR-β. Sunitinib also showed significant activity, with a response rate of approximately 25% and prolonged survival when compared with interferon-α.63–65 In December 2005 and January 2006, the Food and Drug Administration approved both agents for the treatment of advanced RCC. These new potent agents are replacing IL-2-based immunotherapy as first-line treatment for many patients following nephrectomy.
mTOR Pathway
The mammalian target of rapamycin (mTOR) pathway has a central role in the regulation of cell growth, and increasing evidence suggests its dysregulation in cancer.66 Receiving input from multiple signals, including growth factors, hormones, nutrients, and other stimulants or mitogens, the pathway stimulates protein synthesis by phosphorylating key translation regulators, such as ribosomal S6 kinase. The mTOR pathway also contributes to many other critical cellular functions, including protein degradation and angiogenesis. A UCLA tissue microarray-based study showed that the mTOR pathway is most affected in patients with clear cell RCC and poor prognostic factors, such as high nuclear grades.67
Hence, use of inhibitors of this pathway may represent a new strategy for the targeted treatment of RCC. At present, there are at least 3 mTOR inhibitors that have been widely characterized in preclinical models and that are in clinical development as anticancer agents: temsirolimus (CCI-779), AP23573, and RAD001, which are esters of rapamycin that improve bioavailability and formulation. Rapamycin and esters first bind to FK506-binding protein 12 (FKBP12). The FKBP12/rapamycin complex then binds mTOR, inducing a G1 growth arrest rather than apoptosis. Completed clinical trials show safety and efficacy of mTOR-targeting therapy in patients with RCC. In a randomized phase II trial of the mTOR inhibitor temsirolimus, Atkins and colleagues68 observed objective responses in 7% and a clinical benefit rate (complete and partial response, minor responses, stable disease) in about 50% of individuals with metastatic RCC. As noted above, mTOR inhibitors induce G1 arrest rather than apoptosis. Therefore, disease stabilization might be higher and objective response rates might be lower. Data presented at the 2006 meeting of the American Society of Clinical Oncology from a study using temsirolimus in a high-risk patient population demonstrated an impressive 50% improvement in median survival from 7.3 to 10.9 months,31 indicating that this new drug might be the firstline treatment of choice in patients with high-risk metastatic RCC.
Raf Kinase Pathway
Another important pathway in RCC is the Ras/Raf/MAPK pathway. Signaling starts by binding of a ligand to 1 of the 4 erbB proteins; the most prominent is erbB1, which is also called the EGFR. The EGFR consists of 3 domains: 1 ligand-binding domain, 1 transmembrane domain, and 1 cytoplasmic domain, which has tyrosine kinase activity. After binding of the ligands, EGF, or TGF-α, the EGFR becomes phosphorylated on the tyrosine residues. The EGFR then interacts with docking proteins, which subsequently allow the Ras protein to bind guanosine triphosphate and become active. The Ras protein then binds to Raf kinase and activates its kinase function. Subsequently, multiple kinases (MEK, MAPK, MNK) are involved in this pathway, which finally leads to regulation of translation and transcription of important proteins, such as S6 kinase. Targeting this pathway might also be an attractive intervention.69–71
MET Proto-Oncogene, Nuclear Factor-κB
The majority of these new drugs are reserved for patients with clear cell RCC because studies have indicated that patients with non-clear cell RCC are less amenable to the new drugs. VEGF is expressed in lesser amounts in non-clear cell RCC,58 and the mTOR pathway seems to be less affected in non-clear cell tumors,67 providing strong evidence that antiangiogenic and mTOR-targeting therapy might be less effective. As explained in a previous paragraph, however, the results of Leppert and colleagues56 suggest that patients with papillary RCC might also be candidates for treatment with angiogenesis inhibitors.
Significant achievements in basic research regarding the MET proto-oncogene have led to a new treatment strategy for patients with papillary RCC. The MET proto-oncogene encodes a transmembrane receptor with tyrosine kinase activity (c-Met), which interacts with hepatocyte growth factor (HGF). Mutation of the MET proto-oncogene was frequently observed in hereditary papillary RCC but also in a subset of the sporadic tumors.72–74 Additionally, trisomy of chromosome 7, the chromosome that contains the MET and HGF genes, is the most frequently found aberration in sporadic papillary RCC. In addition to papillary RCC, HGF and the MET protein expression are frequently observed in clear cell RCC75,76 and were noted as strong indicators for survival among these patients.77 Further, it has been shown that VHL inactivation induces phosphorylation of the MET protein.78 Taken together, studies on targeting HGF, the receptor, and the activity of tyrosine kinase offer promise for both clear cell and papillary RCC.79 Another strategy for non-clear cell RCC might be to target nuclear factor-κB (NF-κB). NF-κB interacts as a transcription factor, turning on genes that cause production of proteins that are essential for cell growth and survival. The efficient VHL protein, present in non-clear cell RCC, down-regulates NF-κB,80 and maximum NF-κB down-regulation is required for maximum efficacy of the drug bortezomib.81 Following in vitro studies showing that tumors with efficient VHL, such as papillary RCC, have a higher response to bortezomib,82 this new treatment strategy is currently being explored in clinical trials.
Combining Clinical Factors and Molecular Markers
The potential of tissue and gene microarray technology to affect the predictive accuracy of RCC prognosis and treatment is enormous. These arrays allow the simultaneous analysis of hundreds of tumor specimens for their expression of thousands of markers and genes. It is worth noting that, even at the present time, the results of these techniques can augment traditional clinical and pathologic factors.
At UCLA, a tissue microarray on 150 patients with metastatic clear cell RCC was constructed to develop a prognostic model combining clinical and molecular markers.83 For this analysis, the tumor specimens were stained for Ki67, p53, gelsolin, CAIX, CAXII, phosphatase and tensin homologue deleted on chromosome 10 (PTEN), epithelial cell adhesion molecule, and vimentin. Clinical and marker models were carried out using Cox regression analysis. The models were corrected by bootstrapping and the corresponding c-indices were compared using Harrell’s U-test statistics. In multivariate Cox regression analysis, T category, ECOG performance status, CAIX, PTEN, p53, and vimentin were independent prognostic factors of disease-specific survival. Moreover, the results showed that the marker model (c-index 0.64) was significantly better than the clinical model (c-index 0.62). Further, an additional increase in the prognostic accuracy was determined when combining both models to a clinical/marker model (c-index 0.68). The markers were than integrated in a nomogram that was calibrated to provide 2-year and 4-year survival rates and median survival. Based on these criteria, the clinician may identify the suitability of patients for varying treatments, such as patients with high CAIX expression for IL-2-based immunotherapy.
Conclusions
Better understanding of RCC biology is revolutionizing the approach to its surgical and medical management. The ability to individualize patient treatment is starting to have a significant impact on clinical strategies. In the future, gene expression technology will greatly expedite the process of making such strategies a reality.
Main Points.
Staging of renal cell carcinoma (RCC) is performed worldwide according to the TNM (tumor, nodes, metastasis) classification. RCC’s clinical behavior, however, results from complex interactions among many factors. This realization has led to more integrated staging systems, such as the University of California Integrated Staging System and the SSIGN (stage, size, grade, and necrosis) score.
Carbonic anhydrase IX (CAIX) is the most significant molecular marker in RCC to date. Besides serving as an important predictor for survival and response to immunotherapy among patients with metastatic RCC, CAIX holds much promise as a therapeutic target.
Inhibition of angiogenesis is a promising approach for targeting metastatic RCC that has led to the development of agents such as bevacizumab, sorafenib, and sunitinib.
The mTOR (mammalian target of rapamycin) pathway has a central role in the regulation of cell growth, and increasing evidence suggests its dysregulation in cancer. Hence, the use of inhibitors of this pathway may represent a new strategy for the targeted treatment of RCC.
Another important pathway in RCC is the Ras/Raf/MAPK pathway, which also represents an attractive target for intervention.
Significant achievements in basic research on the MET proto-oncogene have led to a new treatment strategy for patients with papillary and clear cell RCC.
Tissue and gene microarray technology may someday have an enormous impact on the predictive accuracy of RCC prognosis and treatment. These arrays allow the simultaneous analysis of hundreds of tumor specimens for their expression of thousands of markers and genes. Even now, the results of these techniques can augment traditional clinical and pathologic findings.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166:1611–1623. [PubMed] [Google Scholar]
- 3.Patard JJ, Rodriguez A, Rioux-Leclercq N, et al. Prognostic significance of the mode of detection in renal tumours. BJU Int. 2002;90:358–363. doi: 10.1046/j.1464-410x.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- 4.Tsui KH, Shvarts O, Smith RB, et al. Renal cell carcinoma: prognostic significance of incidentally detected tumors. J Urol. 2000;163:426–430. doi: 10.1016/s0022-5347(05)67892-5. [DOI] [PubMed] [Google Scholar]
- 5.Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–4566. doi: 10.1200/JCO.2002.05.111. [DOI] [PubMed] [Google Scholar]
- 6.Pantuck AJ, Zeng G, Belldegrun AS, Figlin RA. Pathobiology, prognosis, and targeted therapy for renal cell carcinoma: exploiting the hypoxia-induced pathway. Clin Cancer Res. 2003;9:4641–4652. [PubMed] [Google Scholar]
- 7.Lam JS, Leppert JT, Figlin RA, Belldegrun AS. Role of molecular markers in the diagnosis and therapy of renal cell carcinoma. Urology. 2005;66(5 suppl):1–5. doi: 10.1016/j.urology.2005.06.112. [DOI] [PubMed] [Google Scholar]
- 8.Thoenes W, Störkel S, Rumpelt HJ. Histopathology and classification of renal cell tumors (adenomas, oncocytomas and carcinomas). The basic cytological and histopathological elements and their use for diagnostics. Pathol Res Pract. 1986;181:125–143. doi: 10.1016/S0344-0338(86)80001-2. [DOI] [PubMed] [Google Scholar]
- 9.Störkel S, Elbe JN, Adlakha K, et al. Classification of renal cell carcinoma Workgroup No. 1. Cancer. 1997;80:987–989. doi: 10.1002/(sici)1097-0142(19970901)80:5<987::aid-cncr24>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Cohen AJ, Li FP, Berg S, et al. Hereditary renal-cell carcinoma associated with a chromosomal translocation. N Engl J Med. 1979;301:592–595. doi: 10.1056/NEJM197909133011107. [DOI] [PubMed] [Google Scholar]
- 11.Pathak S, Strong LC, Ferrell RE, Trindade A. Familial renal cell carcinoma with a 3;11 chromosome translocation limited to tumor cells. Science. 1982;217:939–941. doi: 10.1126/science.7112106. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs G, Brusa P, De Riese W. Tissue-specific expression of a constitutional 3;6 translocation: development of multiple bilateral renal-cell carcinomas. Int J Cancer. 1989;43:422–427. doi: 10.1002/ijc.2910430313. [DOI] [PubMed] [Google Scholar]
- 13.Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs G. Molecular cytogenetics of renal cell tumors. Adv Cancer Res. 1993;62:89–124. doi: 10.1016/s0065-230x(08)60316-4. [DOI] [PubMed] [Google Scholar]
- 15.Kovacs G. Papillary renal cell carcinoma. A morphologic and cytogenetic study of 11 cases. Am J Pathol. 1989;134:27–34. [PMC free article] [PubMed] [Google Scholar]
- 16.van den Berg E, Dijkhuizen T, Oosterhuis JW, et al. Cytogenetic classification of renal cell cancer. Cancer Genet Cytogenet. 1997;95:103–107. doi: 10.1016/s0165-4608(96)00289-0. [DOI] [PubMed] [Google Scholar]
- 17.van den Berg E, van der Hout AH, Oosterhuis JW, et al. Cytogenetic analysis of epithelial renal-cell tumors: relationship with a new histopathological classification. Int J Cancer. 1993;55:223–227. doi: 10.1002/ijc.2910550210. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang Z, Park WS, Pack S, et al. Trisomy 7-harbouring non-random duplication of the mutant MET allele in hereditary papillary renal carcinomas. Nat Genet. 1998;20:66–69. doi: 10.1038/1727. [DOI] [PubMed] [Google Scholar]
- 19.Fischer J, Palmedo G, von Knobloch R, et al. Duplication and overexpression of the mutant allele of the MET proto-oncogene in multiple hereditary papillary renal cell tumours. Oncogene. 1998;17:733–739. doi: 10.1038/sj.onc.1201983. [DOI] [PubMed] [Google Scholar]
- 20.Launonen V, Vierimaa O, Kiuru M, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98:3387–3392. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlinson IP, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 22.Birt AR, Hogg GR, Dube WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol. 1977;113:1674–1677. [PubMed] [Google Scholar]
- 23.Pavlovich CP, Grubb RL, 3rd, Hurley K, et al. Evaluation and management of renal tumors in the Birt-Hogg-Dube syndrome. J Urol. 2005;173:1482–1486. doi: 10.1097/01.ju.0000154629.45832.30. [DOI] [PubMed] [Google Scholar]
- 24.American Joint Committee on Cancer, authors. AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002. Kidney; pp. 323–325. [Google Scholar]
- 25.Goldstein NS. The current state of renal cell carcinoma grading. Cancer. 1997;80:977–980. [PubMed] [Google Scholar]
- 26.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Gelb AB. Renal cell carcinoma—current prognostic factors. Cancer. 1997;80:981–986. [PubMed] [Google Scholar]
- 28.Lam JS, Shvarts O, Leppert JT, et al. Renal cell carcinoma 2005: new frontiers in staging, prognostication and targeted molecular therapy. J Urol. 2005;173:1853–1862. doi: 10.1097/01.ju.0000165693.68449.c3. [DOI] [PubMed] [Google Scholar]
- 29.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 30.Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19:1649–1657. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 31.Hudes G, Carducci M, Tomczak P, et al. A phase 3, randomized, 3-arm study of temsirolimus (TEMSR) or interferon-alpha (IFN) or the combination of TEMSR + IFN in the treatment of first-line, poor-risk patients with advanced renal cell carcinoma (adv RCC) J Clin Oncol. 2006;24:LBA4. [Google Scholar]
- 32.Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 33.Kattan MW, Reuter V, Motzer RJ, et al. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166:63–67. [PubMed] [Google Scholar]
- 34.Patard JJ, Kim HL, Lam JS, et al. Use of the University of California Los Angeles integrated staging system to predict survival in renal cell carcinoma: an international multicenter study. J Clin Oncol. 2004;22:3316–3322. doi: 10.1200/JCO.2004.09.104. [DOI] [PubMed] [Google Scholar]
- 35.Ficarra V, Martignoni G, Lohse C, et al. External validation of the Mayo Clinic Stage, Size, Grade and Necrosis (SSIGN) score to predict cancer specific survival using a European series of conventional renal cell carcinoma. J Urol. 2006;175:1235–1239. doi: 10.1016/S0022-5347(05)00684-1. [DOI] [PubMed] [Google Scholar]
- 36.Han KR, Bleumer I, Pantuck AJ, et al. Validation of an integrated staging system toward improved prognostication of patients with localized renal cell carcinoma in an international population. J Urol. 2003;170:2221–2224. doi: 10.1097/01.ju.0000096049.64863.a1. [DOI] [PubMed] [Google Scholar]
- 37.Leppert JT, Lam JS, Pantuck AJ, et al. Carbonic anhydrase IX and the future of molecular markers in renal cell carcinoma. BJU Int. 2005;96:281–285. doi: 10.1111/j.1464-410X.2005.05615.x. [DOI] [PubMed] [Google Scholar]
- 38.Ivanov S, Liao SY, Ivanova A, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivanov SV, Kuzmin I, Wei MH, et al. Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc Natl Acad Sci U S A. 1998;95:12596–12601. doi: 10.1073/pnas.95.21.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grabmaier K, De Weijert MCA, Verhaegh GW, et al. Strict regulation of CAIX(G250/MN) by HIF-1alpha in clear cell renal cell carcinoma. Oncogene. 2004;23:5624. doi: 10.1038/sj.onc.1207764. [DOI] [PubMed] [Google Scholar]
- 41.Bui MH, Seligson D, Han KR, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802–811. [PubMed] [Google Scholar]
- 42.Atkins M, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714–3721. doi: 10.1158/1078-0432.CCR-04-2019. [DOI] [PubMed] [Google Scholar]
- 43.Bleumer I, Oosterwijk E, Oosterwijk-Wakka JC, et al. A clinical trial with chimeric monoclonal antibody WX-G250 and low dose interleukin-2 pulsing scheme for advanced renal cell carcinoma. J Urol. 2006;175:57–62. doi: 10.1016/S0022-5347(05)00040-6. [DOI] [PubMed] [Google Scholar]
- 44.Uemura H, Fujimoto K, Tanaka M, et al. A phase I trial of vaccination of CA9-derived peptides for HLA-A24-positive patients with cytokine-refractory metastatic renal cell carcinoma. Clin Cancer Res. 2006;12:1768–1775. doi: 10.1158/1078-0432.CCR-05-2253. [DOI] [PubMed] [Google Scholar]
- 45.Mukouyama H, Janzen NK, Hernandez JM, et al. Generation of kidney cancer-specific antitumor immune responses using peripheral blood monocytes transduced with a recombinant adenovirus encoding carbonic anhydrase 9. Clin Cancer Res. 2004;10:1421–1429. doi: 10.1158/1078-0432.ccr-03-0067. [DOI] [PubMed] [Google Scholar]
- 46.Hernandez JM, Bui MH, Han KR, et al. Novel kidney cancer immunotherapy based on the granulocyte-macrophage colony-stimulating factor and carbonic anhydrase IX fusion gene. Clin Cancer Res. 2003;9:1906–1916. [PubMed] [Google Scholar]
- 47.Tso CL, Zisman A, Pantuck A, et al. Induction of G250-targeted and T-cell-mediated antitumor activity against renal cell carcinoma using a chimeric fusion protein consisting of G250 and granulocyte/monocyte-colony stimulating factor. Cancer Res. 2001;61:7925–7933. [PubMed] [Google Scholar]
- 48.Igarashi H, Esumi M, Ishida H, Okada K. Vascular endothelial growth factor overexpression is correlated with von Hippel-Lindau tumor suppressor gene inactivation in patients with sporadic renal cell carcinoma. Cancer. 2002;95:47–53. doi: 10.1002/cncr.10635. [DOI] [PubMed] [Google Scholar]
- 49.Gunningham SP, Currie MJ, Han C, et al. Vascular endothelial growth factor-B and vascular endothelial growth factor-C expression in renal cell carcinomas: regulation by the von Hippel-Lindau gene and hypoxia. Cancer Res. 2001;61:3206–3211. [PubMed] [Google Scholar]
- 50.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 51.Bouck N, Stellmach V, Hsu SC. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 52.Rini BI, Small EJ. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J Clin Oncol. 2005;23:1028–1043. doi: 10.1200/JCO.2005.01.186. [DOI] [PubMed] [Google Scholar]
- 53.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 55.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777–794. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leppert JT, Lam JS, Yu H, et al. Targeting the vascular endothelial growth factor pathway in renal cell carcinoma: A tissue array based analysis. J Clin Oncol. 2005;23:4536. [Google Scholar]
- 57.Lam JS, Leppert JT, Yu H, et al. Expression of the vascular endothelial growth factor family in tumor dissemination and disease free survival in clear cell renal cell carcinoma. J Clin Oncol. 2005;23:4538. [Google Scholar]
- 58.Ljungberg BJ, Jacobsen J, Rudolfsson SH, et al. Different vascular endothelial growth factor (VEGF), VEGF-receptor 1 and -2 mRNA expression profiles between clear cell and papillary renal cell carcinoma. BJU Int. 2006;98:661–667. doi: 10.1111/j.1464-410X.2006.06387.x. [DOI] [PubMed] [Google Scholar]
- 59.Jacobsen J, Grankvist K, Rasmuson T, et al. Expression of vascular endothelial growth factor protein in human renal cell carcinoma. BJU Int. 2004;93:297–302. doi: 10.1111/j.1464-410x.2004.04605.x. [DOI] [PubMed] [Google Scholar]
- 60.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 62.Escudier B, Szczylik C, Eisen T, et al. Randomized phase III trial of the Raf kinase and VEGFR inhibitor sorafenib (BAY 43-9006) in patients with advanced renal cell carcinoma (RCC) J Clin Oncol. 2005;23:4510. [Google Scholar]
- 63.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 64.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 65.Motzer RJ, Hutson TE, Tomczak P, et al. Phase III randomized trial of sunitinib malate (SU11248) versus interferon-alpha (IFN-α) as first-line systemic therapy for patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2006;24:LBA3. [Google Scholar]
- 66.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Figlin RA, Seligson DB, Wu H, et al. Characterization of the mTOR pathway in renal cell carcinoma and its use in predicting patient selection for agents targeting this pathway. J Clin Oncol. 2005;23:4539. [Google Scholar]
- 68.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 69.Stadler WM. Targeted agents for the treatment of advanced renal cell carcinoma. 2005;104:2323–2333. doi: 10.1002/cncr.21453. [DOI] [PubMed] [Google Scholar]
- 70.van Spronsen DJ, Mulders PF, De Mulder PH. Novel treatments for metastatic renal cell carcinoma. Crit Rev Oncol Hematol. 2005;55:177–191. doi: 10.1016/j.critrevonc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med. 2006;38:200–211. doi: 10.1080/07853890600551037. [DOI] [PubMed] [Google Scholar]
- 72.Schmidt L, Junker K, Nakaigawa N, et al. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene. 1999;18:2343–2350. doi: 10.1038/sj.onc.1202547. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt L, Junker K, Weirich G, et al. Two North American families with hereditary papillary renal carcinoma and identical novel mutations in the MET proto-oncogene. Cancer Res. 1998;58:1719–1722. [PubMed] [Google Scholar]
- 74.Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 75.Pisters LL, el-Naggar AK, Luo W, et al. C-met proto-oncogene expression in benign and malignant human renal tissues. J Urol. 1997;158:724–728. doi: 10.1097/00005392-199709000-00009. [DOI] [PubMed] [Google Scholar]
- 76.Natali PG, Prat M, Nicotra MR, et al. Overexpression of the met/HGF receptor in renal cell carcinomas. Int J Cancer. 1996;69:212–217. doi: 10.1002/(SICI)1097-0215(19960621)69:3<212::AID-IJC11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 77.Miyata Y, Kanetake H, Kanda S. Presence of phosphorylated hepatocyte growth factor receptor/c-Met is associated with tumor progression and survival in patients with conventional renal cell carcinoma. Clin Cancer Res. 2006;12:4876–4881. doi: 10.1158/1078-0432.CCR-06-0362. [DOI] [PubMed] [Google Scholar]
- 78.Nakaigawa N, Yao M, Baba M, et al. Inactivation of von Hippel-Lindau gene induces constitutive phosphorylation of MET protein in clear cell renal carcinoma. Cancer Res. 2006;66:3699–3705. doi: 10.1158/0008-5472.CAN-05-0617. [DOI] [PubMed] [Google Scholar]
- 79.Peruzzi B, Bottaro DP. Targeting the c-Met signaling pathway in cancer. Clin Cancer Res. 2006;12:3657–3660. doi: 10.1158/1078-0432.CCR-06-0818. [DOI] [PubMed] [Google Scholar]
- 80.Qi H, Ohh M. The von Hippel-Lindau tumor suppressor protein sensitizes renal cell carcinoma cells to tumor necrosis factor-induced cytotoxicity by suppressing the nuclear factor-kappaB-dependent antiapoptotic pathway. Cancer Res. 2003;63:7076–7080. [PubMed] [Google Scholar]
- 81.An J, Sun Y, Fisher M, Rettig MB. Maximal apoptosis of renal cell carcinoma by the proteasome inhibitor bortezomib is nuclear factor-kappaB dependent. Mol Cancer Ther. 2004;3:727–736. [PubMed] [Google Scholar]
- 82.An J, Fisher M, Rettig MB. VHL expression in renal cell carcinoma sensitizes to bortezomib (PS-341) through an NF-kappaB-dependent mechanism. Oncogene. 2005;24:1563–1570. doi: 10.1038/sj.onc.1208348. [DOI] [PubMed] [Google Scholar]
- 83.Kim HL, Seligson D, Liu X, et al. Using tumor markers to predict the survival of patients with metastatic renal cell carcinoma. J Urol. 2005;173:1496–1501. doi: 10.1097/01.ju.0000154351.37249.f0. [DOI] [PubMed] [Google Scholar]
- 84.Gunawan B, von Heydebreck A, Fritsch T, et al. Cytogenetic and morphologic typing of 58 papillary renal cell carcinomas: evidence for a cytogenetic evolution of type 2 from type 1 tumors. Cancer Res. 2003;63:6200–6205. [PubMed] [Google Scholar]