Abstract
Lymph nodes function as filters of tissues and tissue fluids and are sites of origin and production of lymphocytes for normal physiological functions. As part of this normal function, they react to both endogenous and exogenous substances with a variety of specific morphological and functional responses. Lesions can be both proliferative and nonproliferative, and can be treatment-related or not. The histological evaluation of lymph nodes is necessary in order to understand the immunotoxic effects of chemicals with the resulting data providing an important component of human risk assessment. It is the challenge of the toxicologic pathologist to interpret the pathology data within the complete clinical evaluation of the entire animal. Daily insults, ageing and toxins can alter the normal histology and primary function of lymph nodes. Therefore it is important to distinguish and differentiate lesions that occur naturally during normal development and ageing from those that are induced by xenobiotics. To achieve this goal, comparison with strain- age- and sex-matched controls is crucial.
Keywords: Lymph node, immunotoxic, xenobiotic, hyperplasia, angiectasis, lymphoma
Introduction
The lymph nodes are organized lymphoid organs that contain lymphocytes within a fine reticular stroma. The structures within a lymph node include the capsule, subcapsular sinus, cortex (B cell zone with follicles and germinal centers), paracortex (T cell zone), medullary sinuses, medullary cords and hilus. In order to ensure that all of these components are evaluated, particular attention should be paid to tissue collection and orientation. Lymph nodes should routinely be examined grossly and microscopically since they may reflect lesions in organs and tissues they drain. Moreover, special attention should be given to lymph nodes that are more likely to be exposed to a test compound (Haley et al., 2005; Ruehl-Fehlert et al., 2005). The bronchial and mediastinal lymph nodes should be examined for compounds administered via inhalation whereas orally administered compounds would most likely affect the mandibular (superficial cervical) and mesenteric lymph nodes. For dermal or subcutaneous exposures, the most proximal draining peripheral node or nodes (auricular, axillary, inguinal and popliteal) should be examined.
The range of normal histological findings within groups of lymph nodes due to normal immune functions should also be considered. For example, sinus histiocytosis is a normal finding in mesenteric lymph nodes and the macrophages may contain endogenous pigment (hemosiderin, lipofuscin) or exogenous pigments reflecting antigen uptake from the digestive tract. The mandibular lymph nodes are exposed to antigens from the oropharyngeal region and will typically have well-developed secondary follicles and considerable numbers of plasma cells within the medullary cords. For systemic effects of compounds, examination of lymph nodes distal to the site of application may provide additional information. Unless draining a site of application, the popliteal and axillary lymph nodes are often not stimulated and can give information on the resting state of the lymph node. However these lymph nodes can be highly variable, small and difficult to adequately sample. It is also useful to have knowledge of the regions drained by specific nodes for determining the origin of metastatic neoplasms. Detailed information on the location of specific lymph nodes and patterns of lymphatic drainage in the rat are described by Tilney (1971) and Sainte-Marie et al. (1982). Information on anatomy and nomenclature of murine lymph nodes is provided by Van den Broeck et al. (2006). Finally, a careful and detailed examination of the lymph nodes may give valuable clues to the possible mechanism of action of the test material. For a thorough description of the normal structure, function and histology of the lymph node, refer to the article by Willard-Mack (2006). For detailed information on the enhanced histopathological evaluation of the lymph node, refer to the article by Elmore (2006). The following figures and descriptions illustrate and discuss some of the typical lesions observed in lymph nodes.
Lymphoid Necrosis
Lymphoid necrosis may either be focal, multifocal or diffuse within a lymph node and there can be differences in the presence and severity of lymphoid necrosis between lymph nodes in the same animal, depending on the inciting factor and the effectiveness of the immune response. Lymphocyte necrosis is characterized by cell swelling with chromatin clumping, karyorrhexis or karyolysis and in more severe lesions, abundant eosinophilic cellular debris (Figures 1A–C). Necrosis is frequently accompanied by inflammatory cells including neutrophils and phagocytic macrophages with intracytoplasmic cellular debris (Figures 1D–E). This form of necrosis should be distinguished from apoptosis in which there is individual cell death characterized by cell shrinkage, nuclear pyknosis and fragmentation with apoptotic bodies and tingible body macrophages. This type of cell death normally occurs within the germinal centers of secondary follicles where it is an important homeostatic mechanism. Apoptosis can also be produced by a variety of injurious stimuli when given at low doses, but these same stimuli may be capable of producing necrosis at higher doses. Examples are heat, irradiation, hypoxia and cytotoxic cancer drugs (i.e., cyclophosphamide). Dexamethasone, a glucocorticoid, can also cause lymphocyte apoptosis in the lymph node, but the thymus is the more sensitive organ (Elmore, 2006).
Figure 1.


Figures 1A–E are from the mandibular lymph node of a male cynomolgus monkey that received a test article in a four week oral gavage study with a 4-week recovery period. Figures 1A–C illustrate lymphoid necrosis within the cortex and regions of the paracortex and medulla. The areas of necrosis are eosinophilic at low magnifications (Figures 1A–B). At higher magnification (Figure 1C) the loss of lymphocytes can be seen in conjunction with eosinophilic cellular debris and dark pyknotic nuclear debris. Macrophages are the predominant inflammatory cells in this region. Figure 1D and the higher magnification in Figure 1E are from a different region of the same lymph node with a more robust inflammatory response, predominately neutrophils. Figures 1F and 1G are low and high magnifications of the mediastinal lymph node from a 90-day-old female F344 rat that was treated with a high dose of urethane. There is moderate lymphoid depletion, characterized by a decrease in the number and size of follicles with few to no germinal centers as well as depletion of paracortical lymphocytes. With a depletion of paracortical lymphocytes, the stromal cells may become more prominent, as illustrated by the arrow in Figure 1G. Photomicrographs 1A–E are courtesy of Drs. Hans Harleman and Kathryn Bowenkamp.
Lymphoid depletion or atrophy is the sequela to chronic necrosis or apoptosis. It can occur in any lymph node and there can be differences in the presence and severity of lymphoid depletion between lymph nodes in the same animal. Lymphoid depletion is typically characterized by a decrease in the number and size of follicles with few to no germinal centers and/or depletion of paracortical lymphocytes (Figure 1F). With a depletion of paracortical lymphocytes, the stromal cells may become more prominent (Figure 1G).
Massive necrosis of the lymph nodes is uncommon and may be induced by obstruction of the blood flow (infarction). This lesion is characterized by diffuse coagulation necrosis with loss of cell nuclei but, early on, with well preserved cell outlines. Caseous necrosis is also an uncommon lesion but can be produced by infection with tuberculosis organisms and fungi in nonhuman primates. It can also be seen in the centers of rapidly growing neoplasms. Grossly, there is semisolid, gray to pale yellow tissue. Microscopically, there is an amorphous mass of granular eosinophilic material with no cell outlines as well as no identifiable nuclei.
Lymphatic Sinus Ectasia
Lymphatic sinus ectasia can involve both the medullary and subcapsular sinuses. Diffuse sinus ectasia is typically associated with lymphoid atrophy. This lesion can be found in control animals, especially in the mesenteric and mediastinal lymph nodes of ageing mice. Figure 2 is an example of medullary sinus ectasia. This lesion is characterized by the presence of dilated or cystic sinuses lined by lymphendothelium and filled with pale eosinophilic/amphophilic material (presumably lymph) that has a delicate lacy appearance (Figure 2B). A few lymphocytes, plasma cells and macrophages can be found admixed with the lymph.
Figure 2.

This is an example of medullary sinus ectasia from a control 2-year-old female F344 rat. The low magnification image shows a large ectatic medullary sinus (Figure 2A). At higher magnification the lymphendothelium lining the sinus can be visualized (arrow, Figure 2B). The pale eosinophilic lymph that fills the dilated sinus has a delicate lacy appearance (Figure 2B). A few lymphocytes, plasma cells and macrophages can sometimes be found admixed with the lymph.
Synonymous names for this lesion are lymphangiectasia, lymphatic cysts, cystic lymphatic ectasia and sinus dilatation. A group of pathologists that are associated with the National Toxicology Program were surveyed for the preferred terminology for this lesion. The 2 favored terms were “lymphatic sinus ectasia” and “lymphangiectasia.” Lymphatic sinus ectasia more clearly defines the lesion location.
Vascular Lesions
When defining vascular lesions in the lymph node, various terminologies can be used, each with a very specific definition. Angiectasis is defined in Dorland’s Medical Dictionary as “abnormal, usually gross dilatation and often lengthening of a blood vessel or lymphatic” and its synonyms are hemangiectasis, hemangiectasia, vasodilation and vasodilatation. In the lymph node, blood vessel angiectasis is characterized by the dilatation and congestion of thin veins within the cortex, medulla, capsule, hilus or surrounding connective tissue. Angiectasis is most often seen in the mesenteric lymph nodes of rats and mice, including the B6C3F1 strain and may or may not be accompanied by hemorrhage (Ward et al., 1999). This lesion can be distinguished from a hematoma by the presence of endothelial lining cells and from early hemangioma by the absence of large neoplastic endothelial cells.
Intrasinusoidal erythrocytes (sinus erythrocytosis) can result from a lymph node draining a region of hemorrhage. This can also be an artifact that results from euthanasia or tissue dissection during necropsy, especially in the bronchial or mediastinal lymph nodes. The trimming and sectioning of lymph node tissue can also dislodge erythrocytes from congested blood vessels, which can then appear in the sinusoids or in the perinodal region (Figures 3A–B). Lymph nodes draining sites of hemorrhage can also have intrasinusoidal erythrocytes but, depending on chronicity, they can be accompanied by variable numbers of hemosiderin-laden macrophages, erythrophagocytosis and inflammatory cells (Figures 3C–D).
Figure 3.



Figures 3A and 3B are images of the bronchial lymph node of a control 3-month-old Sprague–Dawley rat. The trimming and sectioning of this tissue dislodged erythrocytes from congested blood vessels which then appeared in the sinusoids (Figure 3A, short arrow and Figure 3B) and in the perinodal region (Figure 3A, long arrow). Figures 3C and 3D are images of the mediastinal lymph node from a 3-month-old Wistar rat with a lesion of pulmonary hemorrhage. In this case the chronic nature of the lesion is indicated by the presence of erythrophagocytosis and hemosiderin-laden macrophages (Figure 3D). Figures 3E–H are images of lymph nodes from mice with dilated vascular spaces filled with red blood cells. In this case it is difficult to differentiate lymph node angiectasis from moderate to marked sinus erythrocytosis because dilated and blood filled lymphatic vessels can resemble dilated blood vessels. They are both lined by flattened cells and both occur throughout the lymph node. Evaluation of the other organ systems for congestion and a survey for regions of hemorrhage in the drainage field could help to more clearly define this lesion. Figures 3I–L are images of the mesenteric lymph node from a 24-month-old male B6C3F1 mouse treated with a high dose of N-methylolacrylamide. This lymph node has a range of lesions that includes nodal and perinodal angiectasia with congestion (Figures 3I–K), sinus erythrocytosis (Figures 3I–J), perinodal vascular proliferation with angiectasis and congestion (Figure 3K) and hemorrhage within the nodal parenchyma (Figure 3L). Figures 3M–P are images of the mesenteric lymph nodes from two 24-month-old male B6C3F1 mice that were treated with a high dose of methylimidazole. There are blood-filled vascular spaces (blood vessels and/or lymphatics) throughout both of the lymph nodes. There are also congested arterioles in the surrounding tissue of one lymph node (Figure 3M) and perivascular hemorrhage associated with an arteriole in the perinodal adipose tissue of the other lymph node (Figures 3O–P). Figures 3E and 3F photomicrographs are courtesy of Drs. C. Frith and J. Ward. Figures 3G and 3H photomicrographs are courtesy of Dr. Michael Leach.
Lesions of lymph node vascular angiectasis can be difficult to differentiate from moderate to marked sinus erythrocytosis because dilated blood vessels can resemble dilated and blood-filled lymphatic vessels. Both are lined by flattened cells and both occur throughout the lymph node. In trying to resolve this issue, a more holistic diagnostic approach could be taken. For example, evaluation of the other organ systems for congestion could rule in/out blood vessel congestion as a possible diagnosis. A survey for regions of hemorrhage in the drainage field for the node could help to rule in/out sinus erythrocytosis. Figures 3E–H are examples of blood-filled vascular spaces that would require this type of holistic approach to more clearly define the lesion.
A wide range of vascular lesions in treated animals can include sinus congestion, sinus erythrocytosis, nodal and perinodal angiectasia with congestion, hemorrhage within the nodal parenchyma, perinodal vascular proliferation, and perivascular hemorrhage (Figures 3I–P). The diagnosis of treatment-related vascular lesions in the lymph node should be made with consideration of the animal’s overall health status and the presence or absence of vascular lesions in other organs and tissues. A survey of lesion terminology for the two lymph nodes in Figures 3M–P was obtained from a group of pathologists. Without any prior knowledge of treatment-related lesions and pathogenesis of the lymph node lesions in these mice, there was disagreement as to the most appropriate terminology. Sinus erythrocytosis and congestion emerged as the two favored terms. However, most agreed that information on other organs, potential areas of hemorrhage in the drainage field and time from death to necropsy were needed in order to make an accurate diagnosis.
Telangiectasia and angiomatosis are two terms that would not typically be used to define the lesions of vascular ectasia in the rodent lymph node because in human medicine they are terms that define neoplastic lesions. Telangiectasia is defined as “an abnormal dilatation of capillary vessels and arterioles that often forms an angioma” and angiomatosis is defined as “a diseased state of the vessels with the formation of multiple angiomas.” An angioma is considered a tumor that is made up of chiefly blood or lymph vessels. However, when blood-filled vessels are present in the lymph node, it is important to distinguish them from vascular tumors such as hemangiomas and hemangiosarcomas.
Pigment
Pigment is a common finding within the cytoplasm of sinusoid macrophages in both control and treated animals. The most common pigments are hemosiderin and ceroid/lipofusin. Hemosiderin is an iron-containing golden brown granular material and macrophages containing this pigment are most likely found within the medullary cords and lymphatic sinuses of nodes with sinus erythrocytosis. Lipofuscin is also a golden brown, finely granular pigment but it is derived chiefly from the breakdown products of lipids, usually those derived from cell membranes. Ceroid is a variant of lipofuscin that is acid-fast and autofluorescent. The numbers of hemosiderin- or ceroid/lipofuscin-laden macrophages can be increased with associated macrophage hyperplasia in nodes draining various lesions (inflammatory, necrotic, neoplastic, etc.). These pigments are difficult to distinguish from each other with a conventional hematoxylin and eosin stain. In order to differentiate the two, an iron stain (Perl’s iron stain, Prussian blue reaction) can be used to stain hemosiderin blue. A variety of stains and methods can be used to identify ceroid/lipofuscin including Sudan Black B, Schmorl’s reaction, Oil red O, carbol lipofuscin stain, Periodic acid-Schiff, Ziehl-Neelsen acid fast stain, autofluorescence or the lysosomal acid phosphatase and esterase stains. Melanin is another endogenous pigment that can be found within lymph nodes. This is a normal finding in black-skinned mice and is not considered a lesion. For positive identification of melanin, Schmorl’s method can be used which stains the melanin granules blue-green. DOPA-oxidase is an enzyme histochemical method that can also be used and is very specific for melanin.
A variety of inhaled, ingested, injected, and topically applied chemicals can induce sinus histiocytosis in associated lymph nodes with macrophages that contain inert or insoluble pigmented test substance (Goginpath et al., 1987). Tail tattoo pigment, which is inert and non-polarizable, can sometimes be found as aggregates of scattered brown/black material in the lymph nodes adjacent to the tattoo. Regional lymph nodes draining test article application sites should always be inspected for the presence of exogenous pigments (Figures 4A–B) but the parent compound, or its metabolite, can sometimes localize in specific tissues in the body (Figures 4C–F).
Figure 4.


Figures 4A and 4B are low and high images of a mediastinal lymph node from a male F344 rat treated with 2.5 mg of nickel oxide. Note the minimal scattered aggregates of brown/black pigment and associated lymphoid hyperplasia. Figures 4C–F are from a 2-year-old female F344 rat that received a high dose of 2,4 diaminophenol 2 HCL. The pancreatic lymph node contains a finely granular brown pigment (Figures 4C–D) that was negative for iron. This material was presumed to be the parent compound or its metabolite. The same pigment was present in the lamina propria of the duodenum (Figures 4E–F). Figures 4G–I are images of the bronchial lymph node from a 2-year-old female F344 rat that received a low dose of molybdenum trioxide. Although the red blood cells are difficult to see in these images, there is sinus erythrocytosis, erythrophagocytosis and intra-histiocytic accumulations of a golden brown globular pigment consistent with hemosiderin.
Amyloidosis
With the light microscope and hematoxylin and eosin stain, amyloid appears as amorphous, eosinophilic and hyalinized extracellular material. With large accumulations, amyloid will encroach on adjacent tissue causing pressure atrophy. Congo red is the most common stain used to evaluate amyloid, imparting an apple-green birefringence when polarized. Amyloidosis occurs in a number of strains of mice and other rodents, but is not seen in the lymph nodes of rats. Amyloidosis occurs in a low incidence in most mouse strains but in CD1 mice, there appears to be a genetic predisposition (Frith and Chandra, 1991). Lymph node amyloidosis is more prevalent in female CD1 mice and the degree of amyloidosis increases with age with 20–30% of the lymph nodes affected by 24 months of age. In these mice, amyloid deposition occurs in a variety of tissues, including the lymph node. The mesenteric lymph node is most commonly affected and amyloid predominately accumulates within the subcapsular sinuses with progressive extension into the paracortical areas of the lymph node (Figures 5A–D). Early lesions typically occur in the periphery of the node.
Figure 5.

These are images of lymph node amyloidosis in CD1 mice. The mesenteric lymph node is most commonly affected in this strain of mouse and amyloid predominately accumulates within the subcapsular sinuses (Figure 5A, arrows). Figures 5B and 5C illustrate the characteristic homogenous, amorphous, eosinophilic and extracellular nature of the amyloid with progressive extension into the paracortical areas of the lymph node. Figure 5D illustrates the pale eosinophilic nature of the amyloid when stained with Congo red. Photomicrographs 5A–C are courtesy of Dr. Michael Leach. Photomicrograph 5D is courtesy of Drs. C. Frith and J. Ward.
Lymphadenitis
Inflammatory cells can be found in lymph nodes draining sites of inflammation, necrosis, neoplasia, etc. or they can be the result of the administration of an irritating test compound. Inflammatory cells can also be present within a lymph node in response to primary lymphocyte necrosis. The type of lymphadenitis can vary depending on the inciting factor (foreign body, bacteria, etc.) and the response can vary from acute to granulomatous (Figure 6). In acute lymphadenitis, neutrophils and immature myeloid cells can be found within the sinuses and medullary cords. Lymphoid hyperplasia or atrophy may also occur in association with nodal inflammation. Inflammatory infiltrates should be distinguished from conditions of extramedullary hematopoiesis (EMH) (Figures 6F–J) and granulocytic leukemia.
Figure 6.





The images in Figures 6A–E illustrate the histological changes in a lymph node from a 1-month-old male FVB/n mouse that is draining on overlying skin lesion from the ventral neck region caused by a bite from a cage mate. The arrow in Figure 6A and higher magnifications in Figures 6B and 6C, show a focal full thickness epidermal lesion with necrosis, neutrophils and crusting. Figure 6D shows transmigration of neutrophils and macrophages (arrows) down through the collagenous layers of the underlying dermis, destined for the subjacent lymphatics (arrowhead). Figure 6E is a high magnification of the subcapsular region of the underlying draining lymph node with macrophages and neutrophils (arrows). Figures 6F–J are images of a mandibular lymph node from an approximately 3–6-year-old male cynomolgus monkey that received twice daily oral doses of a test article for 39 weeks. There is extramedullary hematopoiesis (EMH) within the medullary region. Although there are a few erythroid cells (arrow), the predominant cell type is the neutrophil with a mixture of both immature band forms and mature segmented neutrophils (Figure 6J). This example underscores the difficulty in differentiating EMH from lymphadenitis. Figure 6K illustrates a large focal organized granulomatous lesion (chronic abscess) within a mouse lymph node. Figures 6L and 6M are higher magnifications of the region indicated by the arrow in Figure 6K. At the higher magnifications, the necrosis, mixture of inflammatory cells and fibrosis can be appreciated. Figures 6N–R are images of a mandibular lymph node from a 9–10 month old male Beagle dog that received a once daily oral dose of test article for two weeks. This lesion was diagnosed as pyogranulomatous lymphadenitis due to the abundant neutrophils and macrophages. Figures 6F–J and 6N–R photomicrographs are courtesy of Drs. Hans Harleman and Kathryn Bowenkamp. Figures 6K–M photomicrographs are courtesy of Drs. C. Frith and J. Ward.
With EMH, there is typically a mixture of megakaryocytes and other hematopoietic elements whereas granulocytic leukemia will have high numbers of immature myeloid cells with multiple organ involvement. The term “chronic lymphadenitis” and “granulomatous lymphadenitis” should be reserved for lymph nodes with chronic abscesses or granulomatous lesions that partially or completely efface the normal nodal architecture as opposed to increased numbers of histiocytes within the subcapsular and medullary sinuses (sinus histiocytosis) (Figures 6K–R). Abscesses may be acute or chronic and are characterized by a central region of necrosis associated with predominately neutrophils. In the later stages, they are surrounded by variable amounts of granulation tissue which can progress to fibrous connective tissue (Figures 6K–M). Pyogranulomatous lymphadenitis is characterized by an abundance of neutrophils and macrophages partially or completely effacing the normal nodal architecture.
Lymphocyte Hyperplasia
The description of reactive hyperplastic lesions, including lymphocyte hyperplasia, in rodent lymph nodes has been described (Ward, 1990). In normal rodents, lymphocyte hyperplasia may be evident to varying degrees depending on the location of the lymph node, health status of the animal, age of the animal, and plane of section of the node. Mesenteric lymph nodes, in particular, may show a wide variation in degree of reactive lymphocyte hyperplasia between animals due to stimulation by antigens in the intestinal tract. If an increase in lymphocytes is suspected to be treatment-related, then this potential for variability underscores the need to compare with control tissues. If a treatment-related effect is suspected, then enhanced histopathology may be performed to more clearly define the nature and degree of this lesion (Elmore, 2006).
Lymphocyte hyperplasia can involve both the B-cell-rich follicles and the T-cell-rich paracortex and can be indicative of a humeral or cell-mediated response, respectively (Figure 7). Lymphoid hyperplasia is generally a reactive or immune response and is not considered to be a preneoplastic lesion in the lymph node. Stimulated (reactive) follicles, also called secondary follicles, are usually larger than the unstimulated primary follicles and will have a paler staining germinal center with large lymphoblasts and increased numbers of apoptotic lymphocytes and tingible body macrophages. The mantle zone surrounding the germinal center is composed of small to medium-sized darker staining B lymphocytes. Hyperplastic follicles are identified by an increase in number and size of follicles and conversion to secondary follicles. Hyperplasia of the paracortex is characterized by an increase in the cell density and, depending on the degree of hyperplasia, an increase in the paracortical area.
Figure 7.

Figures 7A and 7B illustrate lymphoid hyperplasia with a follicular pattern in a mandibular lymph node from a 22-month-old C57bl/6 mouse. At low magnification (Figure 7A) it is difficult to determine if this is a case of lymphocyte proliferation or follicular lymphoma. However, at higher magnification (Figure 7B), the heterogeneous nature of the lymphocyte proliferation indicates hyperplasia rather than neoplasia. There are paler staining B lymphoblasts (Figure 7B, long arrow) within the follicular germinal centers interspersed with and surrounded by the smaller, more mature lymphocytes (Figure 7B, short arrow). For comparison, the mandibular lymph node from a female B6C3F1 mouse with lymphoma is depicted in Figure 7C. At higher magnification (Figure 7D) the homogeneous nature of the neoplastic lymphocytes is illustrated.
Plasma Cell Hyperplasia
Plasma cells are usually increased in number in response to antigenic stimulation that requires antibody production. Therefore B cell hyperplasia can occur simultaneously with plasma cell hyperplasia. Marked plasma cell hyperplasia, or plasmacytosis, is a common finding in rodents, particularly in the submandibular lymph nodes. The medullary cords normally contain plasma cells and their precursors as the dominant cell types and these cords are the primary sites of plasma cell hyperplasia. In cases of marked plasma cell hyperplasia the node can be greatly enlarged, composed almost entirely of plasma cells, exhibit partial effacement of normal nodal architecture, and can be difficult to differentiate from neoplasia. Findings that support hyperplasia are a lack of cortical and capsular infiltration, atypical plasma cells and metastases (Figure 8). Depending on the degree and chronicity of antigenic stimulation, some plasma cells may contain Russell’s bodies. Plasma cell precursors (immunoblasts or plasmablasts) may also be present among the more mature plasma cells.
Figure 8.

This is a peripancreatic lymph node from a 15-month-old C57bl/6 mouse with marked plasma cell hyperplasia involving the medullary and paracortical regions. Although there is marked infiltration of the medullary and paracortical regions by this population of plasma cells, the intact nature of the follicles (Figure 8B, arrows) and the uniform and differentiated cytomorphology of the plasma cells (Figure 8C) indicate that this is plasma cell hyperplasia rather than neoplasia.
Macrophage Hyperplasia
Macrophage hyperplasia usually results from proliferation of resident sinusoidal macrophages (Figure 9A) but can also be seen as aggregates of macrophages within any region of the lymph node. Macrophage aggregates can be peripherally located around the paracortex (Figures 9B–D) or within the cortical, paracortical and medullary regions (Figures 9E–G). Macrophage hyperplasia can also be a feature of lymph nodes that drain a site of test article application (Figures 9H–I). Specific patterns (intrasinusoidal, cortical, paracortical, medullary) of macrophage hyperplasia in the same node within a dose group would be consistent with a treatment-related effect.
Figure 9.



Figure 9A is an example of a mesenteric lymph node from a female B6C3F1 mouse that was treated with a high dose of 4,4′-thiobis-(6-T-butyl-M-cresol). Note the proliferation of sinusoidal macrophages (sinus histiocytosis). However, aggregates of macrophages can also be seen within any region of the lymph node. Figures 9B–D are images of a lymph node from of a male B6C3F1 mouse treated with sodium dichromate dihydrate that illustrate macrophage aggregates peripherally located around the paracortex (arrows). Figures 9E–G are images of a lymph node from a 2-year-old F344 rat with macrophage aggregates within the cortical, paracortical and medullary regions. Figures 9H–I are images of the mesenteric lymph node from a 90-day-old male F344 rat in a subchronic elmiron study. These images illustrate a proliferation of macrophages with vacuolated cytoplasm within the medullary region. These types of hyperplasia can be a feature of lymph nodes that drain a site of test article application. Specific patterns of macrophage hyperplasia in the same node within a dose group would be consistent with a treatment related effect. Inhalation studies with particulates may result in increased accumulations of macrophages within regional draining lymph nodes. An inhalation study of talc resulted in accumulations of macrophages, most containing talc particles, in the peribronchial lymphoid tissue of the lung and in the bronchial (Figures 9J and 9K) and mediastinal lymph nodes of a 2-year-old male F344 rat. Polarization of the bronchial lymph node revealed short linear fragments of talc within the macrophage cytoplasm (Figures 9L and 9M).
Proliferation (hyperplasia) of resident macrophages can easily be confused with increased numbers of intrasinusoidal macrophages that enter through the efferent lymphatics draining an area with high numbers of macrophages. Comparison of the lesions within the lymph node with the organs and tissues that that particular node drains helps to differentiate the two. Mesenteric lymph nodes are constantly stimulated by intra-intestinal antigens and can therefore have large numbers of intrasinusoidal macrophages as well as multifocal aggregates of macrophages within the cortex and paracortex. Also, there can be considerable individual variation among animals. Comparison of a group of treated animals with control animals would help to determine if this is a treatment-related finding. Special attention should be given to lymph nodes associated with the route of administration of a test compound. For inhalation studies, bronchial and mediastinal lymph nodes should be examined and orally administered compounds may result in lesions in the submandibular and mesenteric lymph nodes. Prior knowledge of the physical and chemical properties of the test compound may help to identify phagocytized test article material. For example, insoluble particulate matter may be seen as intracytoplasmic refractile material when polarized (Figures 9J–M).
When histiocytes occur as aggregates within the sinusoids, the common term for this finding is “sinus histiocytosis.” When aggregates of histiocytes occur within the lymph node parenchyma, the terms “granulomatous inflammation,” “granulomatous lymphadenitis,” “histiocyte aggregates/infiltrates,” and “macrophage aggregates/infiltrates” have been used interchangeably. However, the degree of macrophage accumulation should help to determine if the term “granulomatous” is used. If there are histiocyte aggregates with a minimal to mild severity grade, then histiocyte or macrophage aggregates/infiltrates would be appropriate. If the severity is moderate or marked with partial or complete effacement of nodal architecture, then the term “granulomatous” would be more appropriate.
Extramedullary Hematopoiesis
Although the spleen is the principle site of extramedullary hematopoiesis (EMH) in the rodent, it can sometimes be present in the lymph node. EMH is typically a physiological response to a dramatic loss or increased need for additional blood cells from conditions such as hemorrhage or severe inflammation. It is characterized by a mixture of myelocytic, erythrocytic and megakaryocytic cells and is primarily present in the medullary cords (Figure 10).
Figure 10.

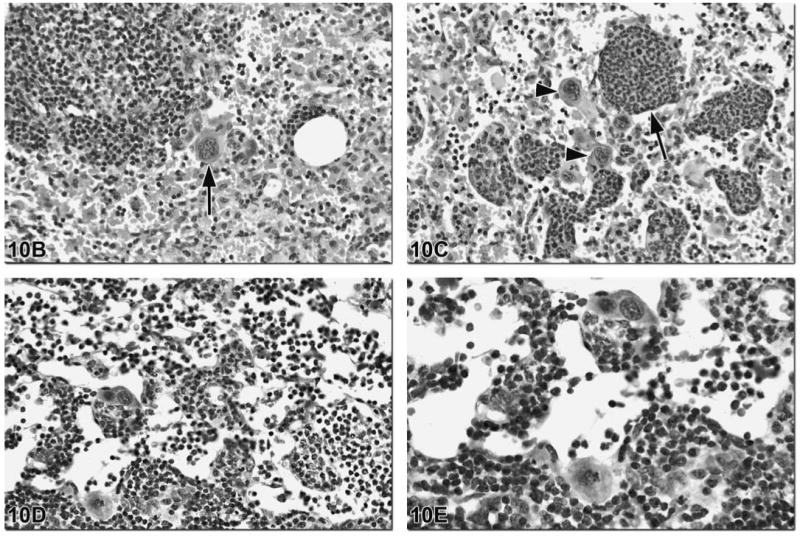
These images illustrate various types of EMH present in rodent lymph nodes. Figure 10A is an image of the mesenteric lymph node of a control male B6C3F1 mouse. Sinus erythocytosis can be seen in this low magnification image. The presence of megakaryocytes (arrows) indicates that EMH is also present. This may be in response to chronic blood loss in the body and the need for additional platelets. Figures 10B and 10C are images of the mesenteric lymph node from a 24-month-old male B6C3F1 mouse that was treated with a high dose of N-methylolacrylamide. In addition to sinus erythrocytosis, the arrow in Figure 10B and the arrowheads in Figure 10C indicate megakaryocytes within the sinuses. The medullary cords are filled with myeloid precursors, indicative of granulopoiesis (Figure 10C, arrow). Figures 10D and 10E are images of the mesenteric lymph node of a 24-month-old male B6C3F1 mouse from a control group. In addition to megakaryocytes, there are myeloid and erythroid precursors within the medullary cords and sinuses.
Lymphoma and MCL
There are a variety of subclassifications of lymphoma including small lymphocyte, lymphoblastic, plasma cell, immunoblastic, follicular center and marginal zone lymphomas. A consensus system for classification of mouse lymphoid neoplasms according to their histopathologic and genetic features has been proposed as a way to model human hematopoietic diseases in mice (Morse et al., 2002). However, discussion and description of each type is beyond the scope of this paper.
Lymphoma is the most common primary neoplasm arising in lymph nodes and, in the F344 rat, must be distinguished from mononuclear cell leukemia (MCL). In the B6C3F1 mouse, lymphomas often arise in mesenteric lymph nodes, spleen and Peyer’s patches (Ward et al., 1999). Lymphoma typically consists of monomorphic sheets of neoplastic lymphocytes (Figure 11A). Lymphocyte apoptosis is a common feature of lymphoma giving the lymph node a “starry sky” appearance at low magnification (Figure 11B). At higher magnification tingible body macrophages are visualized with intracytoplasmic apoptotic bodies, which represent nuclear debris (Figure 11C). General diagnostic features of lymphoid neoplasia include the size of the lymph node, the loss of normal architecture, the presence of a monomorphic population of lymphocytes, capsular invasion and perinodal fat invasion (Figures 11D–G). It should be noted that lymphoid tissue is a common finding within the perinodal fat and should not be considered neoplastic invasion without other features of lymphoma present in the node.
Figure 11.
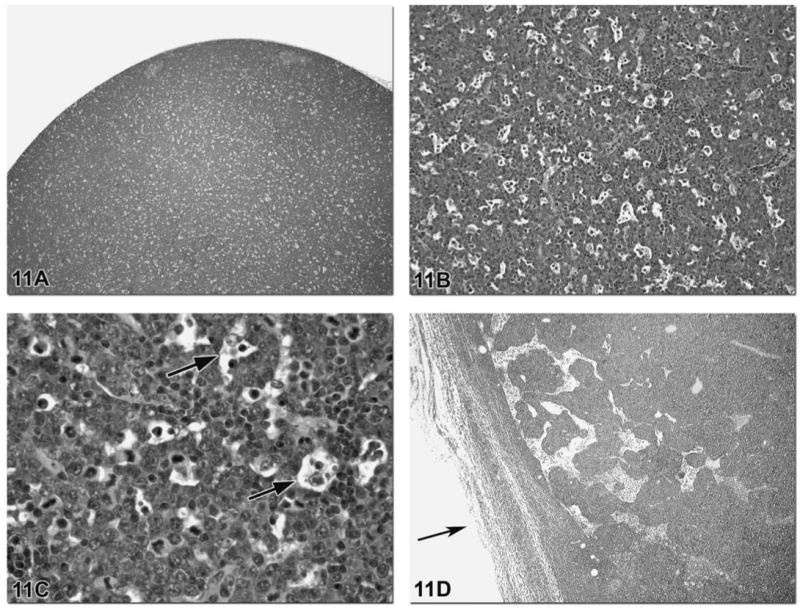

Lymphosarcoma typically consists of monomorphic sheets of neoplastic lymphocytes as depicted in this lymph node from a TGAC (FVB/N) hemizygous mouse (Figure 11A). Lymphocyte apoptosis is a common feature of lymphosarcoma giving the lymph node a “starry sky” appearance at low magnification (Figure 11B). At higher magnification tingible body macrophages are visualized with intracytoplasmic apoptotic bodies (nuclear debris) (Figure 11C, arrows). General diagnostic features of lymphoid neoplasia include the size of the lymph node, the loss of normal architecture, the presence of a monomorphic population of lymphocytes, capsular invasion and perinodal fat invasion. However, these can also be features of MCL. Figures 11D–H are images of a lymph node from a F344 rat and illustrate a case of MCL with capsular invasion (Figure 11D and 11E), perinodal fat invasion (Figure 11F) and a monomorphic population of neoplastic lymphocytes (Figure 11G). Diffuse splenic red pulp involvement is the primary feature of MCL and will help to differentiate MCL from lymphosarcoma. The presence of leukemia in other tissues such as lung (Figure 11H), liver and kidney is a common feature of advanced MCL.
The diagnostic features of lymphoma can also be features of MCL. However, the cytoplasm of MCL cells may have a characteristic eosinophilic granular appearance with a hematoxylin and eosin stain or show almost no staining and the nuclear staining can range from pale to densely basophilic. Diffuse splenic red pulp involvement is the primary feature of MCL and will help to differentiate MCL from lymphoma. The presence of leukemia in other tissues such as lung (Figure 11H), liver and kidney is a common feature of advanced MCL. For more detailed information on MCL, refer to the paper by Suttie (2006).
Metastatic Lesions
Metastatic lesions in lymph nodes can arise from neoplastic blood-born emboli from tumors that are not in close proximity to the node. Metastatic neoplasias can also be found in the lymph nodes draining the region affected by the tumor. Therefore, if metastatic neoplasia is found within a lymph node and the primary tumor has not yet been identified, the location of the primary tumor may be found by evaluating tissues within the lymphatic draining field of that particular node. Although not an exhaustive list, Table 1 lists several lymph nodes and corresponding metastases in the F344 rat based on anatomic location and draining field (Stefanski et al., 1990). Detailed information on the location of specific lymph nodes and patterns of lymphatic drainage in the rat and mouse have been described (Tilney, 1971; Sainte-Marie et al., 1982; Van den Broeck et al., 2006). The photomicrographs and descriptions for Figures 12–17 illustrate and discuss various metastatic lesions in rodent lymph nodes.
Table 1.
Lymph nodes and corresponding metastases.
|
Figure 12.
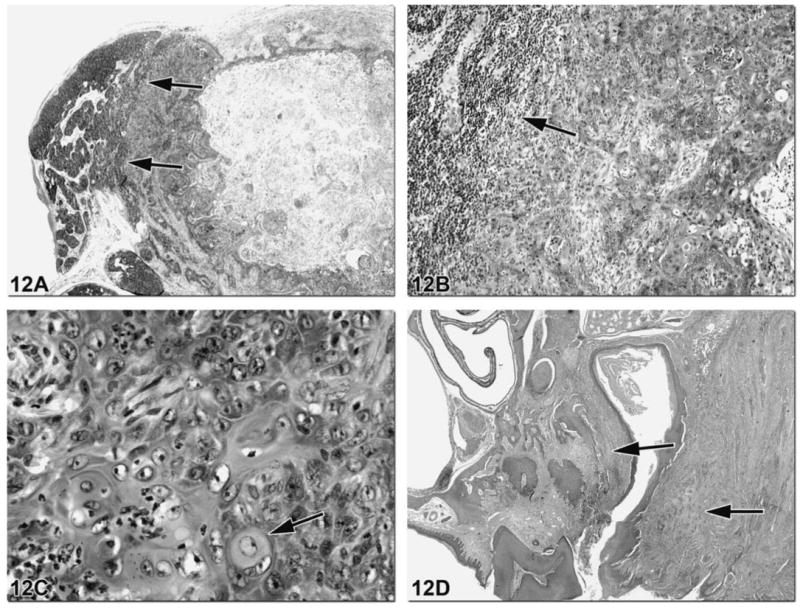
Metastatic lesions can sometimes be found in the lymph node that drains a region affected by neoplasia. Figures 12A and 12B illustrate a mandibular lymph node from a F344 rat with effacement of approximately 80% of the normal node architecture by a large metastatic lesion. There is compression of the remaining normal lymphoid tissue to the left of the mass (arrows). At higher magnification, islands and trabeculae of neoplastic squamous epithelial cells are evident with eosinophilic keratin production (Figure 12C, arrow). Figure 12D shows the primary site of this neoplasm, the maxillary gingiva, which is a region drained by the mandibular lymph node.
Figure 17.

Figures 17A and 17B show a lymph node from a female B6C3F1 mouse with sheets of round cells effacing the normal node architecture. Evaluation of the cells at the periphery of the lesion reveal neoplastic cells with classic features of plasma cells (eccentric nucleus and prominent golgi apparatus) (Figure 17C). Plasma cell tumors can be primary neoplasms of the lymph nodes or can be metastatic lesions. In the mouse, prolonged adjuvant stimulation can result in the development of plasma cell tumors within the peritoneum (Potter and Robertson, 1960; McIntire and Princler, 1969). Tumors of plasma cells result from the monoclonal proliferation of B cells.
Figure 13.


Figure 13A illustrates a mesenteric lymph node from a B6C3F1 mouse with effacement of the cortex and paracortex by neoplastic cells. Higher magnification reveals sheets of neoplastic cells with abundant foamy eosinophilic cytoplasm, multinucleated giant cells (Figure 13B) and a high mitotic index (Figure 13C) indicative of histiocytic sarcoma. The primary site of this tumor was the skin (Figure 13D). Hyaline droplets within the renal tubular epithelial cells are a common sequela of histiocytic sarcoma. Figure 13E depicts these bright eosinophilic intra-epithelial hyaline droplets (arrow) as well as an interstitial accumulation of neoplastic cells admixed with inflammatory cells. These droplets are predominately located in the renal proximal tubules in the P2 segment and contain lysozyme, a secretory product of monocytes and macrophages (Hard and Snowden, 1991). Strain differences in the incidence of histiocytic sarcoma-associated hyaline droplets have been reported and the B6C3F1 mouse may be a higher incidence strain (Ward and Sheldon, 1993). In Wistar rats, histiocytic sarcoma will typically arise in the subcutis and metastasize to distant sites whereas in the Sprague Dawley rat the liver and lung are the common sites of primary tumor. In the F344 rat, this tumor is usually found in multiple tissues with the liver, uterus or other abdominal organs the most common sites (Elwell et al., 1990).
Figure 14.

Figure 14A depicts a mediastinal lymph node from a female B6C3F1 mouse with effacement of most of the node architecture by a neoplastic mass. The arrow indicates a small amount of resident lymphocytes within this field of view. There are focal areas with arborizing papillary growths of thin connective tissue lined by cuboidal epithelium seen at higher magnification (Figure 14B). Figure 14C shows the primary tumor is a bronchoalveolar carcinoma with normal pulmonary tissue on the lower right, indicated by the arrow.
Figure 15.

Figures 15A and 15B are images from the popliteal lymph node of a female B6C3F1 that drained a hind leg with a neoplastic mass. There is effacement of the cortical, paracortical and medullary regions by a neoplastic mass as well as invasion of the capsule and perinodal adipose tissue (Figure 15A). Higher magnification shows an eosinophilic “strap cell” indicative of rhabdomyosarcoma (Figure 15B, arrow), a tumor of striated muscle origin. Microscopic focusing of the tissue can allow striations to be seen within the strap cell cytoplasm. Figures 15C and 15D are images from a male F344 rat lymph node, also with a metastatic rhabdomyosarcoma. In addition to strap cells, immunohistochemical and ultrastructural features can also aid in the diagnosis of rhabdomyoscaromas. The most useful ultrastructural feature is the presence of myofilaments (actin and myosin filaments). They are typically arranged in parallel bundles, or myofibrils, with recognizable electron dense Z bands. These are the equivalent of the cross striations seen with light microscopy. Immunohistochemistry is the preferred diagnostic method to confirm the diagnosis of rhabdomyosarcoma. The most useful antibodies are those against vimentin, desmin, actin, myoglobin, myosin and titin. Since various antigens are expressed differently, it is important to use a panel of antibodies for immunohistochemical characterization of these tumors.
Figure 16.

Figures 16A–C are from a female F344 rat lymph node. There is total effacement of nodal architecture by a metastatic neoplastic mass (Figure 16A). There is capsular invasion and the arrows in Figures 16A and 16B show the only remaining lymphocytes within the lymph node. The neoplastic cells contain abundant eosinophilic cytoplasm with indistinct cell borders (Figure 16C). The site of the primary tumor was the ovary (Figure 16D). The cytomorphological features of the neoplastic cells in Figures 16E (arrow) and 16F are similar to the metastatic neoplastic cells within the lymph node (Figure 16C). The primary tumor location and the cellular features of the tumor are indicative of a yolk sac carcinoma. This is a malignant tumor of germ cell origin that occurs in the ovaries and testes of humans and rodents. The tumor arises from primitive germ cells and develops into extra-embryonic tissue resembling the yolk sac.
Footnotes
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
References
- Elmore SA. Enhanced histopathology of the lymph nodes. Toxicol Pathol. 2006;34:634–47. doi: 10.1080/01926230600939997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell MR, Stedman MA, Kovatch RM. Skin and subcutis. In: Boorman GA, Eustis SL, Elwell MR, Montgomery CA Jr, MacKenzie WF, editors. Pathology of the Fisher Rat: Reference and Atlas. Vol. 272. Academic Press, Inc; San Diego, CA: 1990. [Google Scholar]
- Frith CH, Chandra M. Incidence, distribution, and morphology of amyloidosis in Charles River CD-1 mice. Toxicol Pathol. 1991;19:123–27. doi: 10.1177/019262339101900206. [DOI] [PubMed] [Google Scholar]
- Goginpath C, Prentice DE, Lewis DJ. Atlas of Experimental Toxicological Pathology. MTP Press Limited; Springer, Boston: 1987. pp. 122–36. [Google Scholar]
- Haley P, Perry R, Ennulat D, Frame S, Johnson C, Lapointe JM, Nyska A, Snyder P, Walker D, Walter G. STP position paper: best practice guideline for the routine pathology evaluation of the immune system. Toxicol Pathol. 2005;33:404–7. doi: 10.1080/01926230590934304. [DOI] [PubMed] [Google Scholar]
- Hard GC, Snowden RT. Hyaline droplet accumulation in rodent kidney proximal tubules: An association with histiocytic sarcoma. Toxicol Pathol. 1991;19:88–97. doi: 10.1177/019262339101900202. [DOI] [PubMed] [Google Scholar]
- McIntire KR, Princler GL. Prolonged adjuvant stimulation in germ-free BALB-c mice: development of plasma cell neoplasia. Immunology. 1969;17:481–7. [PMC free article] [PubMed] [Google Scholar]
- Morse HC, Anver MR, Fredrickson TN, Haines DC, Harris AW, Harris NL, Jaffe ES, Kogan SC, MacLennan ICM, Pattengale PK, Ward JM. Bethesda proposals for classification of lymphoid neoplasms in mice. Blood. 2002;100:246–58. doi: 10.1182/blood.v100.1.246. [DOI] [PubMed] [Google Scholar]
- Potter M, Robertson CL. Development of plasma-cell neoplasms in BALB/c mice after intraperitoneal injection of paraffin-oil adjuvant, heart-killed Staphylococcus mixtures. J Natl Cancer Inst. 1960;25:847–61. doi: 10.1093/jnci/25.4.847. [DOI] [PubMed] [Google Scholar]
- Ruehl-Fehlert C, Bradley A, George C, Germann PG, Bolliger AP, Schultee A. Harmonization of immunotoxicity guidelines in the ICH process–pathology considerations from the guideline Committee of the European Society of Toxicological Pathology (ESTP) Exp Toxicol Pathol. 2005;57:1–5. doi: 10.1016/j.etp.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Sainte-Marie G, Peng FS, Belisle C. Overall architecture and pattern of lymph flow in the rat lymph node. Am J Anat. 1982;164:275–309. doi: 10.1002/aja.1001640402. [DOI] [PubMed] [Google Scholar]
- Stefanski SA, Elwell PC, Stromberg PC. Spleen, lymph nodes and thymus. In: Boorman GA, Eustis SL, Elwell MR, Montgomery CA Jr, MacKenzies WF, editors. Pathology of the Fisher Rat: Reference and Atlas. Academic Press, Inc; San Diego, CA: 1990. pp. 383–8. [Google Scholar]
- Suttie AW. Histopathology of the spleen. Toxicol Pathol. 2006;34:466–503. doi: 10.1080/01926230600867750. [DOI] [PubMed] [Google Scholar]
- Tilney NL. Patterns of lymphatic drainage in the adult laboratory rat. J Anat. 1971;109:369–83. [PMC free article] [PubMed] [Google Scholar]
- Van den Broeck W, Derore A, Simoens P. Anatomy and nomenclature of murine lymph nodes: Descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Meth. 2006;312(1)(2):12–9. doi: 10.1016/j.jim.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Ward JM. Classification of reactive lesions in lymph nodes. In: Jones TC, Ward JM, Mohr U, Hunt RD, editors. Monographs on Pathology of Laboratory Animals. Springer-Verlag, Berlin; Heidelberg and New York: 1990. pp. 155–161. Hematopoietic System. [Google Scholar]
- Ward JM, Sheldon W. Expression of mononuclear phagocyte antigens in histiocytic sarcoma of mice. Vet Pathol. 1993;30:560–565. doi: 10.1177/030098589303000610. [DOI] [PubMed] [Google Scholar]
- Ward JM, Mann PC, Morishima H, Frith CH. Thymus, spleen and lymph nodes. In: Maronpot RR, editor. Pathology of the Mouse. Cache River Press; Vienna, IL: 1999. [Google Scholar]
- Willard-Mack CL. Normal structure, function, and histology of the lymph nodes. Toxicol Pathol. 2006;34:409–24. doi: 10.1080/01926230600867727. [DOI] [PubMed] [Google Scholar]


