Short Summary
A method for selective detection of apoptotic cells by ligation of DNA probes to the ends of specific double-stranded DNA breaks in tissue sections was recently reported (1). Unfortunately, this method produces high levels of background staining, due to nonspecific attachment of the probes to the tissue sections, and thereby requires extensive and repeated washing. In the current study we redesigned the oligoprobes in a manner that virtually eliminated background staining. This new design substantially reduces the cost of the probe preparation, making it a convenient and robust methodology to detect apoptosis.
Keywords: apoptosis, DNA fragmentation, hairpin oligonucleotides, T4 DNA ligase, background staining, DNase I
A ligase-based assay for selective detection of apoptosis in tissue sections utilizing oligonucleotide probes was recently reported (1). This assay detects apoptosis when it is accompanied by internucleosomal DNA cleavage with the production of multiple double-strand breaks and electrophoretic ladder-type DNA fragmentation. Detection of double-strand breaks in apoptotic DNA in this assay is via ligation of labeled double-stranded DNA fragments to the ends of DNase I type breaks (1). Unlike conventional terminal transferase-based labeling (TUNEL), the assay stains apoptotic but not necrotic or transiently damaged cells (1,2). The major drawback of the assay, limiting its usefulness, is high background staining caused by nonspecific binding of the probe to the cells in the tissue sections.
Several modifications were previously introduced to reduce background labeling: 1) synthetic hairpin shaped oligonucleotides were substituted for PCR derived probes to obtain uniform, well defined, and highly purified probes (2); 2) long, stringent, high temperature washes were used after ligation (1), or prior to ligation (2). However, these approaches increased the time required to perform the assay, and did not sufficiently decrease the background staining. Modifications described in the present article, however, have virtually eliminated background staining.
Previously used oligonucleotide probes were synthesized with 20 base-long loops that contained five nucleotides modified by the attachment of biotin. The probes also had a 10 base pair-long stem, with a short 3’ overhang which could be ligated to double-strand breaks in DNA (Fig. 1, panel A) (1, 2). The placement of a nonradioactive label in the loop away from the stem of the hairpin was intended to prevent potential interference of the large biotin groups with the enzymatic linkage of the probe to the section. However, the single-stranded loop consisted of unpaired nucleotides, and was charged and therefore highly reactive. Nonspecific binding could be caused by interactions between a negatively charged single-stranded loop of the probe and positively charged protein molecules, or by nonspecific hybridization to cellular RNA or single-stranded regions in cellular DNA, present in damaged or necrotic cells. In addition, the flexible loop structure could easily twist, and thus prevent all of the tagged nucleotides from being detected. The presence of several closely positioned biotinylated nucleotides was also redundant, and likely created steric hindrance problems. With these issues in mind we designed a new oligonucleotide probe (Fig. 1, panel B).
Figure 1. Configuration and sequence of biotin-labeled oligonucleotide probes for detection of double-strand breaks: looped hairpin (A) and loopless hairpin (B).

Both probes are designed so that the terminus of the stem has a characteristic structure, in this case a single 3’ A overhang. The loop (A) contains five deoxythymidine derivatives (t) labeled with biotin. The “loopless” probe contains a single biotin positioned at the very end of the hairpin opposite the ligatable end (B). X in the stem corresponds to biotinylated deoxythymidine derivative or to biotin TEG derivative.
In the new probe design, the highly reactive single-stranded loop was eliminated. This modification virtually eliminated nonspecific binding of the probe to tissue sections. In order to avoid steric hindrance problems and to create better conditions for the reaction between biotin and streptavidin in probe detection, the number of biotins was reduced from five to one. The single biotin was positioned at the opposite end of the hairpin stem, at a maximal distance from the ligatable end. The biotin was incorporated into the probe either by using amino modifier C6 deoxythymidine with subsequent attachment of biotin bis-bis-aminohexanoyl N-hydroxysuccinimide ester (Synthetic Genetics, San Diego, CA), or by chemical insertion of biotinTEG phosphoramidite (Glen Research, Sterling, VA) directly into the oligonucleotide backbone (performed by Oncor, Inc., Gaithersburg, MD). Both types of labeling gave similar results.
In situ ligation using oligo probes of the previous (“looped”) and the new (“loopless”) designs was performed on 6μm thick tissue sections of dexamethasone-treated rat thymus, a classical model of apoptosis (3). Sections were deparaffinized with xylene, rehydrated in graded alcohol concentrations, briefly washed in water and then treated with proteinase K (50 μg/mL ) in 0.1M PBS for 15 minutes. In preliminary experiments, we found that ligation still proceeds without proteinase K treatment, but with somewhat lower efficiency. Sections were rinsed with water and then 80μl of the ligation buffer without the probe and ligase was applied to each section for 15 min. Pre-incubation with ligation buffer ensured even saturation of the section prior to addition of the enzyme and the probe, and was shown, in preliminary experiments, to increase the ligation efficiency. The ligation buffer contained 66 mM-Tris HCl, pH 7.5, 5mM MgCl2, 0.1mM dithioerythritol, 1 mM ATP, and 15% polyethylene glycol (8000 MW; Sigma, St. Louis, MO). The buffer was aspirated and the full ligation mix containing the ligation buffer with the hairpin probe (35 μg/mL ) and T4 DNA ligase (250 U/mL) (Boehringer-Mannheim, Indianapolis, IN) was applied onto the sections. In a mock control reaction solution, an equal volume of 50% glycerol in water was substituted for T4 DNA ligase. Sections were covered with glass coverslips and placed in a humidified box for 16 hours, at room temperature (23º C). In preliminary experiments this time and temperature were shown to be optimal for in situ ligation. Sections were then washed in water (3 x 10min).
4 μg/mL avidin-fluorescein conjugate (Vector Laboratories, Burlingame, CA) was added to the sections in 50 mmol/L sodium bicarbonate, 15 mmol/L sodium chloride, pH 8.2 for 45 minutes. The sections were washed in the same buffer three times for 10 min, mounted in Vectashield (Vector Laboratories) and observed by fluorescent microscopy.
For the detection of free DNA 3’hydroxyls with terminal transferase, we used the procedure previously published (4) but used Texas Red as the label rather than biotin. After completion of the ligation reaction and 3x10 min washes in water, a mixture comprised of 30 mmol/L Tris/HCl, pH 7.2, 140 mmol/L sodium cacodylate, 1 mmol/L cobalt chloride, 0.1 mmol/L dithiothreitol, 8 μmol/L Texas Red dUTP (Molecular probes, Eugene, OR), and 800 U/mL terminal transferase (Boehringer-Mannheim, Indianapolis, IN) (20 μl per section) was added to the sections. The sections were incubated for 1 hour at 37º C. After washing in water (3 x 10 min), the sections were mounted in Vectashield (Vector Laboratories) and observed by fluorescent microscopy.
Figure 2 is a composite micrograph showing background staining in apoptotic cells at high magnification using fluorescent detection. It demonstrates that the looped probe has higher background, caused by ligase-independent attachment to both cytoplasmic and nuclear compartments. Staining with the loopless probe shows localization of the signal in the areas of apoptotic chromatin condensation on the nuclear membrane. The double staining procedure with visualization of both 3’ hydroxyls and full double-strand breaks demonstrates colocalization of both ligase and terminal transferase based signals in the same nuclei with more intense staining of chromatin precipitated on the nuclear membrane by in situ ligation. This data is consistent with our previous results (1,2).
Figure 2. Comparison of the looped and loopless hairpin-shaped probes in dexamethasone treated thymus using fluorescent detection.
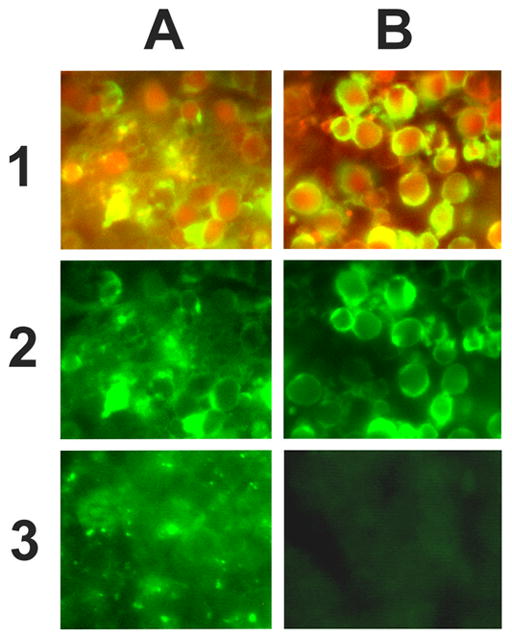
6 μm thick rat thymus sections, 24h after dexamethasone injection, were stained using looped or loopless probes. Control sections had T4 ligase omitted from the procedure. Images were acquired using an Olympus IX 70 fluorescent microscope with Texas Red and fluorescein bandpass filters. For double staining red (TUNEL) and green (in situ ligation) images were superimposed in Adobe Photoshop 4.0. High background staining was observed when the looped probe was used with or without ligase (1A, 2A, 3A). No background staining was present when the loopless probe was used (1B, 2B, 3B). Since terminal transferase labeling was shown to be specific for apoptotic cells in dexamethasone treated thymus (4) it was used in a double staining procedure to colocalize the terminal transferase based signal, and in situ ligation using looped and loopless probes.
1A - Double staining using terminal transferase based detection of free 3’hydroxyls (red fluorescence) and in situ ligation with looped probe (green fluorescence). Superimposition of both signals creates a range of colors from bright green to yellow. Hazy nonspecific background staining can be clearly seen.
2A - Apoptotic cells in dexamethasone-treated thymus labeled with the looped probe (green fluorescence).
3A - Background staining (mock ligation reaction, no T4 ligase added) with the looped probe.
1B – Double staining using terminal transferase based detection of free 3’hydroxyls (red fluorescence) and in situ ligation with loopless probe (green fluorescence). Superimposition of both signals creates a range of colors from bright green to yellow. Apoptotic morphology of stained cells can be clearly seen.
2B - Apoptotic cells in dexamethasone-treated thymus labeled with the loopless probe (green fluorescence).
3B - Background staining (mock ligation reaction, no T4 ligase added) with the loopless probe.
The new probe design, analyzed by fluorescent microscopy, has resulted in substantial reduction of nonspecific background, without the need for long, stringent washes. It has also made the assay more cost–effective, by using a much shorter probe, and reducing the number of biotins in the probe without loss of sensitivity.
In conclusion, our findings indicate that ligase mediated apoptosis detection in tissue sections can be enhanced and simplified by using a newly designed loopless hairpin oligonucleotide probe. The reproducibility of the probe preparation, the economy of the reagents, possibility of double-staining with terminal transferase and the simplicity of the assay are factors that make the method attractive for use by other laboratories.
Acknowledgments
We thank Dr. Bill James of Intergen, Gaithersburg, MD for supplying biotin TEG labeled probes, and Hop Ngo for technical help. This research was supported by grant R01 CA78912-01 from the National Cancer Institute, National Institutes of Health, by grant 004949-054 from the Texas Higher Education Coordinating Board, and by grants from The Taub Foundation, The Henry J.N. Taub Fund for Neurosurgical Research, The George A. Robinson, IV Foundation, The Blanche Greene Estate Fund of The Pauline Sterne Wolff Memorial Foundation, and The Koppelman Fund of The Neurological Research Foundation.
References
- 1.Didenko VV, Hornsby PJ. Presence of double-strand breaks with single-base 3’overhangs in cells undergoing apoptosis but not necrosis. J Cell Biol. 1996;135:1369–1376. doi: 10.1083/jcb.135.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Didenko VV, Tunstead JR, Hornsby PJ. Biotin-labeled hairpin oligonucleotides: probes to detect double-strand breaks in DNA in apoptotic cells. Am J Pathol. 1998;152:897–902. [PMC free article] [PubMed] [Google Scholar]
- 3.Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 4.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]


