Abstract
BACKGROUND
The nadir prostate-specific antigen (PSA) at 1 year (nPSA12) was investigated as an early estimate of biochemical and clinical outcome after radiotherapy (RT) alone for localized prostate cancer.
METHODS
From May 1989 to November 1999, 1000 men received 3D conformal RT alone (median, 76 Gy) with minimum and median follow-up periods of 26 and 58 months, respectively, from the end of treatment. The calculation of PSA doubling time (PSADT) was possible in 657 patients. Multivariate analyses (MVAs) via Cox proportional hazards regression were used to determine the association of nPSA12 to biochemical failure (BF; ASTRO definition), distant metastasis (DM), cause-specific mortality (CSM), and overall mortality (OM). Dichotomization of nPSA12 was optimized by evaluating the sequential model likelihood ratio and P-values.
RESULTS
In MVA, nPSA12 as a continuous variable was independent of RT dose, T-stage, Gleason score, pretreatment initial PSA, age, and PSADT in predicting for BF, DM, CSM, and OM. Dichotomized nPSA12 (≤2 versus >2 ng/mL) was independently related to DM and CSM. Kaplan-Meier 10-year DM rates for nPSA12 ≤2 versus >2 ng/mL were 4% versus 19% (P < .0001).
CONCLUSIONS
nPSA12 is a strong independent predictor of outcome after RTalone for prostate cancer and should be useful in identifying patients at high risk for progression to metastasis and death.
Keywords: prostate cancer, prostate-specific antigen nadir, 3D conformal radiotherapy, distant metastasis, cause-specific mortality
The nadir in prostate-specific antigen (nPSA) after radiotherapy (RT) has been shown to predict biochemical failure (BF),1-13 distant metastasis (DM),9,13-15 cause-specific mortality (CSM),14,15 and overall mortality (OM).15 However, the nPSA usually takes several years to occur, even as long as 8-10 years in some cases. As a consequence, the nPSA has little practical clinical value. A much more reasonable approach would be to use the lowest PSA achieved during a well-defined, relatively short (eg, ≤12 months) time interval after the completion of RT. There is little published that such early, time-limited measures of nPSA are significantly related to clinical failure, such as DM or mortality.16-19
The purpose of this study was to determine the relation of the nadir PSA within the first 12 months after RT (nPSA12) to overall nPSA, BF, DM, CSM, and OM. The 1-year time point was investigated because we have reported previously that the overwhelming majority of the drop in PSA after RT is during the first year.20,21
Our data here demonstrate that nPSA12 is a strong determinant of DM and death independently of RT dose, and has potential as an early surrogate endpoint in RT dose escalation trials.
MATERIALS AND METHODS
Between May 1989 and November 1999, 1000 men with T1-3 N0-X M0 (2002 AJCC) prostate cancer were treated with 3D conformal RT alone at the Department of Radiation Oncology at Fox Chase Cancer Center (Philadelphia, PA) and had a minimum follow-up of 24 months. The calculation of PSA doubling time (PSADT) was possible in 657 cases. All patients had biopsy-confirmed adenocarcinoma of the prostate and underwent pretreatment serum PSA testing. The T-stage was based on digital rectal examination. Median follow-up from the completion of RT was 58 months (range, 26-154).
The 3D-conformal RT technique used in this report has previously been reported.22-24 Briefly, patients were treated in the supine position in a custom-made cast for immobilization. In general, T1/T2a-b prostate cancer patients with Gleason score 2-6 received treatments to the prostate alone. Patients with more advanced prostate cancer, T2c/T3 or Gleason score 7-10, received 46-50 Gy to the prostate and surrounding periprostatic tissues (small pelvis field) followed by a boost to the prostate and seminal vesicles. Radiation dose is reported here as the International Commission on Radiation Units and Measurement (ICRU) reference dose.25 Dose was typically prescribed at the 95% isodose of the beam arrangements and normalized so that the planning treatment volume (PTV) was included within the 95% isodose line. All patients were treated with 10-18 MV photons. The median total radiation dose was 76 Gy (range, 67-84 Gy).
Follow-up history and physical examinations were performed at 6-month intervals initially (usually for 5 years) and then 6-12 months thereafter. If there was a rise in PSA seen at follow-up, PSA tests were performed typically at 3-month intervals until the question of biochemical failure was resolved. Clinical follow-up was defined as the interval from the completion of RT to the date of last known patient contact.
PSADT was based on PSA values obtained after post-RT PSA nadir and before any salvage treatment. Patients were required to have at least 2 posttreatment PSA values obtained at least 60 days apart. PSADT was calculated by modeling the linear relation between the log of PSA levels and time (ie, PSADT was calculated as the natural log of 2 divided by the slope of the relation between the log of PSA and the time of PSA measurement for each patient). There were 343 patients in whom PSADT could not be calculated; 6 of these had biochemical failure and the rest were disease-free. Of the 657 in whom PSADT was calculated, 254 had experienced biochemical failure.
nPSA12 was defined as the lowest PSA level achieved during the first year after completing RT. BF was defined according to the ASTRO consensus definition as 3 consecutive PSA rises backdated to the time midway between the nadir and first rise.26 DM was defined as patients with clinical, radiographic, or pathologic evidence of hematogenous spread. CSM was defined as death occurring in the setting of active disease (local progression, regional metastasis, or distant metastasis) with a rising PSA.15 Time to failure calculations were from the end of RT.
Pearson correlation coefficients were used to assess the relation between: 1) iPSA and nPSA12; 2) nPSA (the overall nPSA) and nPSA12; and 3) nPSA12 and PSADT.
Multivariate Cox proportional hazards models27 of outcome (BF, DM, CSM, OM) included the covariates iPSA (continuous), Gleason score (2-6 versus 7-10), RT dose (continuous), T-stage (T1-2 versus T3), age (continuous), PSADT (as continuous and as dichotomous >3 months), and nPSA12 (continuous). The final resulting full models were used to assess the optimal dichotomization of nPSA12 by evaluating the sequential model likelihood ratio chi-square values and P-values associated with each dichotomized nPSA12 variable. Univariate estimates of time-related outcome measures were based on Kaplan-Meier28 methodology and comparisons were accomplished using the log-rank test.
RESULTS
Table 1 summarizes the distribution of men by pretreatment age, Gleason score, T-stage, and iPSA. The population was relatively favorable, with 75% having a Gleason score of 2-6, 95% having T1-T2 disease, and 60% having a pretreatment initial PSA <10 ng/mL (overall median, 8.5 ng/mL). Table 2 shows various other patient characteristics. Median time to nPSA was 35 months and the median nPSA achieved was 0.4 ng/mL. The median nPSA12 achieved was 1.2 ng/mL. With a median follow-up of 58 months, 26% of patients had BF (n = 260), 5% had DM (n = 50), 2% died from prostate cancer (n = 17), and 13% died from any cause (n = 126).
TABLE 1.
Distribution of Patients by Age, Gleason Score, T Classification, and iPSA
| Subgroup | No. of patients (%) | |
|---|---|---|
| Age, y | <65 | 269 (27) |
| ≥65 | 731 (73) | |
| Gleason score | 2-6 | 749 (75) |
| 7 | 215 (21) | |
| 8-10 | 36 (4) | |
| T classification | T1-T2 | 947 (95) |
| T3 | 53 (5) | |
| iPSA, ng/mL | <10 | 598 (60) |
| 10-20 | 274 (27) | |
| >20 | 128 (13) | |
| Total | 1000 |
iPSA indicates initial pretreatment PSA.
TABLE 2.
Patient Characteristics (n = 1000)
| Group | Median (range) |
|---|---|
| Median RT dose, Gy | 76 (63-84) |
| Median follow-up, mo | 58 (26-154) |
| Median nPSA, ng/mL | 0.4 (<0.1-98.7) |
| Median time to nPSA, mo | 35.2 (2.53-126.9) |
| Median nPSA12, ng/mL | 1.2 (0.1-98.7) |
| Median PSADT, mo* | 21 (1-600) |
RT indicates radiation therapy; Gy, Gray; nPSA, nadir prostate-specific antigen; nPSA12, nPSA at 12 months after the completion of RT.
The PSADT was calculated in 657 patients.
Figure 1A is a scatterplot showing the correlation between nPSA and nPSA12 for the population (r = 0.975, P < .0001). There was also a significant correlation between nPSA12 and iPSA (Fig. 1B, r = 0.392; P < .0001), although the relation was much weaker. For those in whom PSADT was available (n = 657), there was no correlation between nPSA12 and PSADT (r = −0.03859; P = .32).
FIGURE 1.
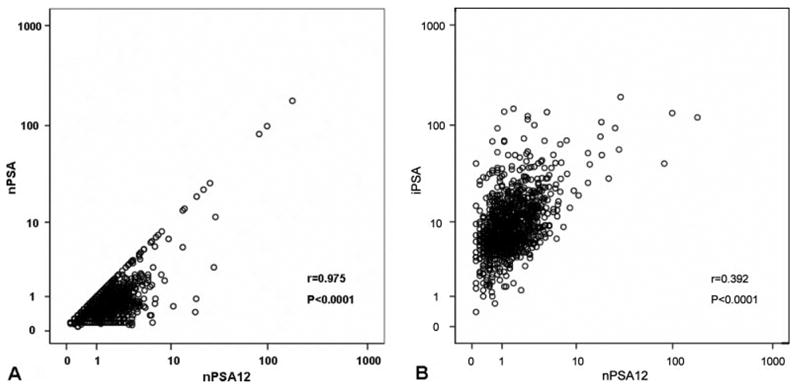
Scatterplot of (A) nadir prostate-specific antigen (nPSA) versus nadir PSA at 1 year (nPSA12) and (B) iPSA versus nPSA12 for all patients.
Multivariate Cox proportional hazard analyses were performed using the entire cohort (Table 3). The covariates included iPSA (continuous), Gleason score (2-6 versus 7-10), RT dose (continuous), T-stage (T1-2 versus T3), age (continuous), and nPSA12 (continuous). The nPSA12 was an independent predictor of BF (P = .0056), DM (P < .0001), CSM (P < .0001), and OM (P < .0001).
TABLE 3.
Multivariate Analyses for the Entire Cohort (n = 1000): nPSA12 as a Continuous Covariate
| RR (95% CI) | P | |
|---|---|---|
| Biochemical failure (n = 260)* | ||
| Increasing iPSA | 1.016 (1.011-1.020) | <.0001 |
| Gleason score ≥7 | 1.938 (1.475-2.546) | <.0001 |
| Increasing RT dose | 0.921 (0.887-0.957) | <.0001 |
| T3 | 2.122 (1.413-3.187) | .0003 |
| Increasing nPSA12 | 1.018 (1.005-1.031) | .0056 |
| Distant metastasis (n = 50)* | ||
| Gleason score ≥7 | 3.114 (1.718-5.646) | .0002 |
| Increasing RT dose | 0.894 (0.815-0.980) | .0169 |
| T3 | 6.027 (3.115-11.662) | <.0001 |
| Increasing nPSA12 | 1.037 (1.025-1.049) | <.0001 |
| Cause-specific mortality (n = 17)* | ||
| T3 | 4.532 (1.451-14.156) | .0093 |
| Increasing nPSA12 | 1.049 (1.033-1.065) | <.0001 |
| Overall mortality (n = 126)* | ||
| Gleason score ≥7 | 1.596 (1.069-2.383) | .0223 |
| Increasing RT dose | 0.912 (0.861-0.967) | .0018 |
| Increasing nPSA12 | 1.025 (1.014-1.037) | <.0001 |
| Age | 1.060 (1.031-1.091) | <.0001 |
RR indicates relative risk; iPSA, initial PSA; RT dose, radiotherapy dose in Gray; nPSA12, nadir PSA within 12 months of follow-up; Gleason Score.
Number of events for each endpoint in parentheses.
Table 4 shows the multivariate Cox proportional hazard model results in the population in which PSADT was available (n = 657). The covariates included iPSA (continuous), Gleason score (2-6 versus 7-10), RT dose (continuous), T-stage (T1-2 versus T3), age (continuous), PSADT (continuous), and nPSA12 (continuous). The nPSA12 was an independent predictor of BF (P = .0147), DM (P < .0001), CSM (P < .0001), and OM (P < .0001).
TABLE 4.
Multivariate Analyses for the PSADT Patient Subset (n = 657): nPSA12 as a Continuous Covariate
| Variable | HR (95% CI) | P |
|---|---|---|
| Biochemical failure (n = 254)* | ||
| Increasing PSADT | 0.971 (0.963-0.978) | <.0001 |
| Increasing iPSA | 1.012 (1.007-1.016) | <.0001 |
| Gleason score ≥7 | 2.026 (1.531-2.682) | <.0001 |
| Increasing RT dose | 0.912 (0.876-0.950) | <.0001 |
| Increasing nPSA12 | 1.015 (1.003-1.028) | .0147 |
| Distant metastasis (n = 49)* | ||
| T3 | 3.882 (2.028-7.429) | <.0001 |
| Increasing nPSA12 | 1.036 (1.022-1.051) | <.0001 |
| PSADT | 0.783 (0.730-0.839) | <.0001 |
| Cause-specific mortality (n = 17)* | ||
| Increasing nPSA12 | 1.046 (1.030-1.062) | <.0001 |
| T3 | 3.816 (1.223-11.903) | .0210 |
| Overall mortality (n = 89)* | ||
| Increasing nPSA12 | 1.026 (1.015-1.037) | <.0001 |
| Age | 1.072 (1.035-1.111) | .0001 |
| Increasing RT dose | 0.912 (0.848-0.980) | .0117 |
| Gleason score ≥7 | 1.663 (1.042-2.653) | .0329 |
HR indicates hazards ratio; 95% CI, 95% confidence interval; PSADT, PSA doubling time; iPSA, initial pretreatment PSA; RT dose, radiotherapy dose in Gy; nPSA12, nadir PSA within 12 months of follow-up; Gleason Score.
Number of events for each endpoint in parentheses.
To assess the optimal dichotomization of nPSA12, we evaluated the sequential model likelihood ratio chi-square values and P-values associated with each dichotomized nPSA12 variable (Table 5). The clinical utility of using a high nPSA12 cutpoint is questionable because the proportion of patients with unfavorable nPSA12s becomes vanishingly small (ie, high specificity and low sensitivity). As a compromise, we decided to use an nPSA12 cutpoint of 2 ng/mL; 27% of the patients had an nPSA12 >2.0 ng/mL. The 5- and 10-year BF rates for those with an nPSA12 >2 ng/mL were 36% and 46%, versus 26% and 30% for an nPSA12 ≤2 ng/mL (P = .0015). An nPSA12 >2 ng/mL was associated with an 8% risk of DM at 5 years, whereas for an nPSA12 of ≤2 ng/mL the risk was 2%. At 10 years the risk of DM for an nPSA12 >2 ng/mL was 19%, whereas for an nPSA12 of ≤2 ng/mL the risk was 4% (P ≤.0001; see Fig. 2). In terms of CSM, an nPSA12 of ≤2 ng/mL was associated with a <1% risk of CSM at 5 years, whereas for an nPSA12 >2 ng/mL of the risk was 3% (P = .0029; see Fig. 2). These numbers are about double at 10 years (2% and 7%). We did not find a statistically significant difference for OM (26% for an nPSA12 ≤2 ng/mL versus 35% for an nPSA12 >2 ng/mL at 10 years; P = .1781).
TABLE 5.
Full MVA Based on Covariates From Optimal Model in Table 3 With nPSA12 Cut at “X” ng/mL
| “X”ng/mL | N (%) > X | LRChi2 (P) for DM | LRChi2 (P) for CSM |
|---|---|---|---|
| 0.2 | 968 (97) | 43.5 (<.0001) | 6.2 (.0460) |
| 0.5 | 855 (86) | 42.9 (<.0001) | 5.0 (.0813) |
| 1.0 | 595 (60) | 47.1 (<.0001) | 6.4 (.0413) |
| 1.5 | 399 (40) | 54.8 (<.0001) | 9.6 (.0082) |
| 2.0 | 271 (27) | 60.9 (<.0001) | 12.5 (.0019) |
| 2.5 | 194 (19) | 63.6 (<.0001) | 10.7 (.0047) |
| 3.0 | 127 (13) | 63.9 (<.0001) | 12.2 (.0023) |
| 3.5 | 86 (9) | 60.1 (<.0001) | 9.6 (.0081) |
| 4.0 | 63 (6) | 60.4 (<.0001) | 11.4 (.0033) |
| 4.5 | 45 (5) | 58.8 (<.0001) | 13.3 (.0013) |
MVA indicates multivariate analyses; LRChi2, likelihood ratio chi-square value.
FIGURE 2.
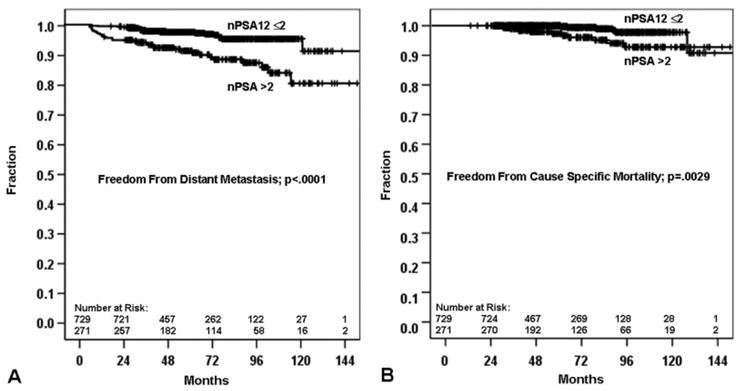
(A) Distant metastasis and (B) cause-specific mortality based on the 2.0-ng/mL nadir prostate-specific antigen at 1 year (nPSA12) cutpoint.
When tested as a dichotomous variable (≤2 versus > 2 ng/mL) in MVA using the entire patient cohort (n = 1000), nPSA12 was related to DM (P < .0001) and CSM (P = .0078) (Table 6). With the inclusion of PSADT as a continuous variable (n = 657), nPSA12 as a dichotomous variable remained significant for BF (P = .0392), DM (P < .0001), and CSM (P = .0094), but not OM. When PSADT was included as a dichotomous variable (≤3 versus >3 months), as described by D’Amico et al.29,30 nPSA12 >2 ng/mL remained independently predictive of DM (P < .0001).
TABLE 6.
Multivariate Analyses for nPSA12 as a Dichotomous Covariate (n = 1000; Cutpoint for nPSA12 = 2.0)
| HR (95% CI) | P | |
|---|---|---|
| Biochemical failure (n = 260)* | ||
| Increasing iPSA | 1.018 (1.015-1.022) | <.0001 |
| Gleason score ≥7 | 1.958 (1.491-2.570) | <.0001 |
| Increasing RT dose | 0.919 (0.885-0.955) | <.0001 |
| T3 | 2.130 (1.419-3.199) | .0003 |
| Distant metastasis (n = 50)* | ||
| Gleason score ≥7 | 3.205 (1.782-5.767) | .0001 |
| T3 | 5.657 (2.918-10.969) | <.0001 |
| nPSA12 >2 ng/mL | 3.400 (1.930-5.988) | <.0001 |
| Increasing RT dose | 0.904 (0.827-0.988) | .0262 |
| Cause-specific mortality (n = 17)* | ||
| nPSA12 >2 ng/mL | 3.881 (1.429-10.536) | .0078 |
| T3 | 4.037 (1.312-12.428) | .0150 |
| Overall mortality (n = 126)* | ||
| Gleason score ≥7 | 1.657 (1.113-2.466) | .0129 |
| Increasing RT dose | 0.913 (0.863-0.967) | .0019 |
| Age | 1.059 (1.030-1.089) | <.0001 |
HR indicates hazard ratio; CI, 95% confidence interval; iPSA, initial PSA; RT Dose, radiotherapy dose in Gy; nPSA12, nadir PSA within 12 months.
Number of events for each endpoint in parentheses.
DISCUSSION
The nadir PSA after radiation therapy has repeatedly been shown to be associated with prostate cancer outcome, including BF, DM, CSM, and OM.1-15 In general, the lower the nPSA, the more favorable the result. The problem with using nPSA clinically is that unless there is evidence of a rising PSA, it is difficult to know when the nPSA has occurred. The median time to an nPSA is 3 years, but new nadirs in men biochemically free of disease are many times seen 5 years or more after RT.
In terms of clinical applicability, with the goal of identifying men at high risk of failing later, a time-limited assessment of nadir/threshold PSA has promise. Cavanaugh et al.18 described strong associations of time-limited PSA thresholds to BF, CSM, and OM. When a time limit of 12 months was used, they found excellent correlations, using PSA thresholds of 2 or 3, to CSM and OM. Other significant relationships with outcome were seen when earlier evaluation points were used; however, the predicted and observed failure rates were less robust and would be suboptimal for early assessment of a clinical trial or in clinical practice. The same holds true for a recent report by Nickers et al.31 which described the predictive value of a PSA ≤4 ng/mL after 4 months of completing RT.
In our analysis we focused on the nPSA12 because at 1 year the mean nadir in PSA relative to the pretreatment value was 88%, whereas in patients with at least 4 years follow-up the mean nadir in PSA relative to the pretreatment value was 95%; thus, the nPAS12 represents 93% of the overall nadir in patients with long-term follow-up. The results are similar to those in our prior report from 1994,5 in an independent group of men treated definitively with RT. The relation of nPSA12 to nPSA is consistent and sound.
Like nPSA,14 the nPSA12 is a highly significant independent correlate of DM, CSM, and OM in multivariate analyses. As a continuous covariate, these associations with nPSA12 were striking. We then tested nPSA as a dichotomous covariate to identify a clinically meaningful cutpoint that could influence the decision to perform a workup (eg, consideration of prostate biopsy after RT, imaging for metastasis), initiate systemic therapy (androgen deprivation or chemotherapy), or have potential as a surrogate endpoint for clinical trials. A series of MVAs were performed with various nPSA12 cutpoints using DM and CSM as the endpoints. The most practical cutpoint was at 2 ng/mL; the risk of DM at 10 years was 4% versus 19% for an nPSA12 ≤2.0 versus >2.0 ng/mL (P < .0001). An nPSA12 >2.0 ng/mL was observed in over one-quarter (27%) of the patients in the full cohort (n = 1000).
Another posttreatment factor that has been associated with an increased risk of mortality after RT is a short PSADT.30 In a report by D’Amico et al.30 a PSADT of <3 months was seen in 20% of those with a rising PSA. Men with a posttreatment PSADT less than 3 months had an almost 20-fold increase in cancer-specific mortality compared with patients with a PSADT ≥3 months.
There are fundamental differences between the nPSA12 and PSADT for a rising PSA. The nPSA12 is available on all patients, whereas the post-RT PSADT many times cannot be determined unless there is a true rising PSA profile. We made an attempt to calculate PSADTon biochemical failures and nonfailures. The calculation of PSADTwas possible in 657 (65.7%) (Table 4), which consisted of 254 (39%) having had BF. Of these, 239 patients (36% of the PSADT group and 23.9% of the total population) had a PSADT >3 months. The nPSA12 continued to be significant in the MVAs, even though these analyses were weighted in favor of PSADT because of the greater degree of selection. The nPSA12 has much more general applicability and will be assessable earlier in the vast majority of patients.
In summary, the nPSA12 is an early predictor of BF, DM, and mortality that is independent of RT dose and other determinants of outcome after radiotherapy. The nPSA12 has potential as a surrogate early endpoint in clinical trials.
Footnotes
Presented at the 40th Annual Meeting of the American Society of Clinical Oncology, June 5-8, 2004, New Orleans, LA.
The authors thank Dr. Gerald E. Hanks for leadership in the establishment and maintenance of the Fox Chase Cancer Center database for the treatment of prostate cancer reported herein, and Ruth Peter, RN, for management of the prostate cancer database.
Supported in part by NIH grants CA101984 and CA006927 and by Varian Medical Systems, Palo Alto, CA.
References
- 1.Zietman AL, Tibbs MK, Dallow KC, et al. Use of PSA nadir to predict subsequent biochemical outcome after external beam radiation therapy for T1-2 adenocarcinoma of the prostate. Radiother Oncol. 1996;40:159–162. doi: 10.1016/0167-8140(96)01770-7. [DOI] [PubMed] [Google Scholar]
- 2.Lee WR, Hanlon AL, Hanks GE. Prostate specific antigen nadir following external beam radiation therapy for clinically localized prostate cancer: the relationship between nadir level and disease-free survival. J Urol. 1996;156:450–453. doi: 10.1097/00005392-199608000-00033. [DOI] [PubMed] [Google Scholar]
- 3.Critz FA, Levinson AK, Williams WH, Holladay DA, Holladay CT. The PSA nadir that indicates potential cure after radiotherapy for prostate cancer. Urology. 1997;49:322–326. doi: 10.1016/s0090-4295(96)00666-8. [DOI] [PubMed] [Google Scholar]
- 4.Critz FA, Williams WH, Holladay CT, et al. Post-treatment PSA < or = 0.2 ng/mL defines disease freedom after radiotherapy for prostate cancer using modern techniques. Urology. 1999;54:968–971. doi: 10.1016/s0090-4295(99)00346-5. [DOI] [PubMed] [Google Scholar]
- 5.Kavadi VS, Zagars GK, Pollack A. Serum prostate-specific antigen after radiation therapy for clinically localized prostate cancer: prognostic implications. Int J Radiat Oncol Biol Phys. 1994;30:279–287. doi: 10.1016/0360-3016(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 6.Kestin LL, Vicini FA, Ziaja EL, Stromberg JS, Frazier RC, Martinez AA. Defining biochemical cure for prostate carcinoma patients treated with external beam radiation therapy. Cancer. 1999;86:1557–1566. doi: 10.1002/(sici)1097-0142(19991015)86:8<1557::aid-cncr24>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Kestin LL, Vicini FA, Martinez AA. Practical application of biochemical failure definitions: what to do and when to do it. Int J Radiat Oncol Biol Phys. 2002;53:304–315. doi: 10.1016/s0360-3016(02)02707-4. [DOI] [PubMed] [Google Scholar]
- 8.Perez CA, Michalski JM, Lockett MA. Chemical disease-free survival in localized carcinoma of prostate treated with external beam irradiation: comparison of American Society of Therapeutic Radiology and Oncology Consensus or 1 ng/mL as endpoint. Int J Radiat Oncol Biol Phys. 2001;49:1287–1296. doi: 10.1016/s0360-3016(00)01492-9. [DOI] [PubMed] [Google Scholar]
- 9.Crook JM, Bahadur YA, Bociek RG, Perry GA, Robertson SJ, Esche BA. Radiotherapy for localized prostate carcinoma. The correlation of pretreatment prostate specific antigen and nadir prostate specific antigen with outcome as assessed by systematic biopsy and serum prostate specific antigen. Cancer. 1997;79:328–336. doi: 10.1002/(sici)1097-0142(19970115)79:2<328::aid-cncr16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Zelefsky M, Leibel S, Gaudin P, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41:491–500. doi: 10.1016/s0360-3016(98)00091-1. [DOI] [PubMed] [Google Scholar]
- 11.Crook J, Malone S, Perry G, Bahadur Y, Robertson S, Abdolell M. Postradiotherapy prostate biopsies: what do they really mean? Results for 498 patients. Int J Radiat Oncol Biol Phys. 2000;48:355–367. doi: 10.1016/s0360-3016(00)00637-4. [DOI] [PubMed] [Google Scholar]
- 12.Pollack A, Zagars GK, Antolak JA, Kuban DA, Rosen II. Prostate biopsy status and PSA nadir level as early surrogates for treatment failure: analysis of a prostate cancer randomized radiation dose escalation trial. Int J Radiat Oncol Biol Phys. 2002;54:677–685. doi: 10.1016/s0360-3016(02)02977-2. [DOI] [PubMed] [Google Scholar]
- 13.Ray ME, Thames HD, Levy LB, et al. PSA nadir predicts biochemical and distant failures after external beam radiotherapy for prostate cancer: a multi-institutional analysis. Int J Radiat Oncol Biol Phys. 2006;64:1140–1150. doi: 10.1016/j.ijrobp.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Hanlon AL, Diratzouian H, Hanks GE. Posttreatment prostate-specific antigen nadir highly predictive of distant failure and death from prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53:297–303. doi: 10.1016/s0360-3016(02)02717-7. [DOI] [PubMed] [Google Scholar]
- 15.Pollack A, Hanlon AL, Movsas B, Hanks GE, Uzzo R, Horwitz EM. Biochemical failure as a determinant of distant metastasis and death in prostate cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:19–23. doi: 10.1016/s0360-3016(03)00538-8. [DOI] [PubMed] [Google Scholar]
- 16.Cavanaugh SX, Kupelian PA, Fuller CD, et al. Early prostate-specific antigen (PSA) kinetics following prostate carcinoma radiotherapy: prognostic value of a time-and-PSA threshold model. Cancer. 2004;101:96–105. doi: 10.1002/cncr.20328. [DOI] [PubMed] [Google Scholar]
- 17.Johnstone PA, Williams SR, Riffenburgh RH. The 100-day PSA: usefulness as surrogate end point for biochemical disease-free survival after definitive radiotherapy of prostate cancer. Prostate Cancer Prostatic Dis. 2004;7:263–267. doi: 10.1038/sj.pcan.4500736. [DOI] [PubMed] [Google Scholar]
- 18.Cavanaugh SX, Fuller CD, Kupelian PA, et al. Time and PSA threshold model prognosticates long-term overall and disease-specific survival in prostate cancer patients as early as 3 months after external beam radiation therapy. Prostate Cancer Prostatic Dis. 2005;8:353–358. doi: 10.1038/sj.pcan.4500831. [DOI] [PubMed] [Google Scholar]
- 19.Feigenberg S, Horwitz E, Uzzo R, et al. Post-treatment PSA values within 6 months of 3D conformal radiotherapy for prostate cancer predict for distant metastases. Int J Radiat Oncol Biol Phys. 2004;60:S234–S235. [Google Scholar]
- 20.Zagars GK, Pollack A. The fall and rise of prostate-specific antigen. Kinetics of serum prostate-specific antigen levels after radiation therapy for prostate cancer. Cancer. 1993;72:832–842. doi: 10.1002/1097-0142(19930801)72:3<832::aid-cncr2820720332>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Zagars GK, Pollack A, Kavadi VS, von Eschenbach AC. Prostate-specific antigen and radiation therapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 1995;32:293–306. doi: 10.1016/0360-3016(95)00077-C. [DOI] [PubMed] [Google Scholar]
- 22.Horwitz EM, Hanlon AL, Pinover WH, Anderson PR, Hanks GE. Defining the optimal radiation dose with three-dimensional conformal radiation therapy for patients with nonmetastatic prostate carcinoma by using recursive partitioning techniques. Cancer. 2001;92:1281–1287. doi: 10.1002/1097-0142(20010901)92:5<1281::aid-cncr1449>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Jacob R, Hanlon AL, Horwitz EM, Movsas B, Uzzo RG, Pollack A. Role of prostate dose escalation in patients with greater than 15% risk of pelvic lymph node involvement. Int J Radiat Oncol Biol Phys. 2005;61:695–701. doi: 10.1016/j.ijrobp.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 24.Jacob R, Hanlon AL, Horwitz EM, Movsas B, Uzzo RG, Pollack A. The relationship of increasing radiotherapy dose to reduced distant metastases and mortality in men with prostate cancer. Cancer. 2004;100:538–543. doi: 10.1002/cncr.11927. [DOI] [PubMed] [Google Scholar]
- 25.Monti AF, Ostinelli A, Frigerio M, et al. An ICRU 50 radiotherapy treatment chart. Radiother Oncol. 1995;35:145–150. doi: 10.1016/0167-8140(95)01541-n. [DOI] [PubMed] [Google Scholar]
- 26.Cox J, Grignon D, Kaplan R, Parsons J, Schellhammer P. Consensus statement: guidelines for PSA following radiation therapy. Int J Radiat Oncol Biol Phys. 1997;37:1035–1041. [PubMed] [Google Scholar]
- 27.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:447–457. [Google Scholar]
- 29.D’Amico AV, Moul JW, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376–1383. doi: 10.1093/jnci/djg043. [DOI] [PubMed] [Google Scholar]
- 30.D’Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Prostate specific antigen doubling time as a surrogate end point for prostate cancer specific mortality following radical prostatectomy or radiation therapy. J Urol. 2004;172:S42–46. doi: 10.1097/01.ju.0000141845.99899.12. discussion S46-47. [DOI] [PubMed] [Google Scholar]
- 31.Nickers P, Albert A, Waltregny D, Deneufbourg JM. Prognostic value of PSA nadir ≤4 ng/mL within 4 months of high-dose radiotherapy for locally advanced prostate cancer. Int J Radiat Oncol Biol Phys. 2006;65:73–77. doi: 10.1016/j.ijrobp.2005.11.026. [DOI] [PubMed] [Google Scholar]


