Abstract
This systematic review examined the evidence on the prevalence of the Hepatitis C virus (HCV) in non-injecting drug users (NIDUs) who sniff, smoke or snort drugs such as heroin, cocaine, crack or methamphetamine. The search included studies published from January 1989 to January 2006. Twenty-eight eligible studies were identified and the prevalence of HCV in these NIDU populations ranged from 2.3% to 35.3%. There was substantial variation in study focus and in the quality of the NIDU data presented in the studies. The results of our systematic review suggested that there are important gaps in the research of HCV in NIDUs. We identified a problem of study focus; much of the research did not aim to study HCV in users of non-injection drugs. Instead, NIDUs were typically included as a secondary research concern, with a principal focus on the problem of transmission of HCV in IDU populations. Despite methodological issues, HCV prevalence in this population is much higher than in a non-drug using population, even though some IDUs might have inadvertently been included in the NIDU samples. These studies point to a real problem of HCV in NIDU populations, but the causal pathway to infection remains unclear.
Keywords: HCV, non-injection drug use, crack cocaine, heroin, epidemiology, prevalence, drug use
1. Introduction
Hepatitis C virus (HCV) infection is a leading risk for liver disease, affecting more than 170 million people globally according to the World Health Organization (World Health Organization, 1998). Since the virus was first isolated in 1989, injection drug users (IDUs) have been found to be at high risk for contracting the virus (Hagan, 1998; Hocking et al., 2001; Roy et al., 2002).
However, the evidence regarding the relationship between HCV infection and non-injecting users of drugs such as crack, methamphetamines and powder cocaine or heroin is ambiguous and controversial. Some researchers have suggested that non-injecting drug users (NIDUs) are often injecting drug users (IDUs) who have failed to report their route of administration accurately (Judd et al., 2002), or that HCV transmission in NIDUs is associated with tattooing, (Howe et al., 2005) or unsafe sexual behavior (Gyarmathy et al., 2002). Hypothesized drug-related risk factors for acquisition of HCV in NIDUs include sharing of non-injection drug use equipment, such as straws or crack pipes (Gyarmathy et al., 2002; Howe et al., 2005; Tortu et al., 2004).
Quantifying the magnitude of the problem and understanding practices associated with HCV acquisition among NIDUs with more accuracy could lead to better informed and more effective prevention. Our goals were to perform a systematic review of the literature summarizing HCV prevalence in NIDUs and to critically evaluate evidence on risk of HCV in NIDUs by examining these data in relation to study methods and hypothesized risk factors.
2. Methods
2.1. Literature review
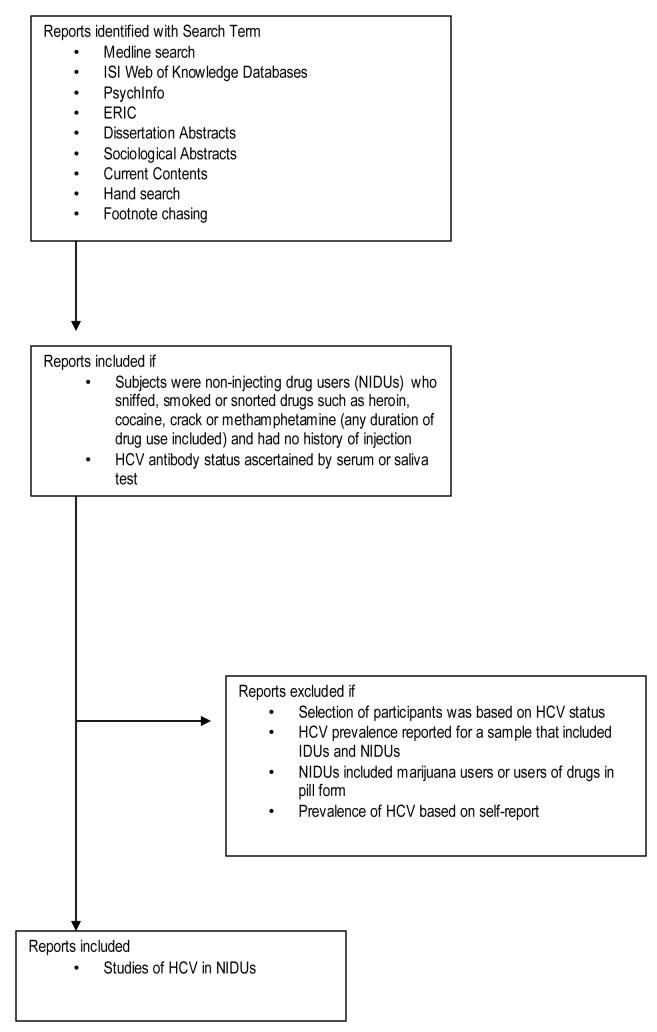
As part of the HCV Synthesis Project (a meta-analysis of HCV epidemiology and prevention in drug users), searches of published literature were carried out on MEDLINE, ISI Web of Knowledge databases (Thomson Scientific, 2006), PsychInfo, ERIC, Dissertation Abstracts, Sociological Abstracts, and Current Contents. We used the following search terms: (hepatitis C OR HCV) AND (intravenous drug abuse OR intravenous drug use OR drug misuse OR drug addict or injecting drug use OR drug abuse OR IDU) AND (prevention OR risk factor OR epidemiology OR prevalence OR incidence or seroprevalence OR seroincidence OR genotype OR co-infect*). Manual search methods included footnote chasing, conference abstract searches, and hand searching of journals on drug use, infectious disease and public health. The dates of publication included were January 1989 through January 2006. Figure 1 shows the process used to retrieve, screen and select reports for this analysis.
Figure 1.
Decision tree used to retrieve and select reports for synthesis of HCV infection in non-injection drug users.
Using our search methods, we identified two unpublished HCV prevalence estimates in NIDUs. However, no additional data regarding study methods and sample characteristics were available for these two studies, and the estimates were within the range of the published HCV prevalence estimates. Thus, these studies would not have contributed to our understanding of the variability of HCV in this population, and were excluded.
2.2. Inclusion criteria
For the present paper, our goal was to synthesize existing data on HCV infection in people who reported never injecting drugs but who did engage in non-injection drug use via sniffing, snorting or smoking heroin, cocaine, crack or methamphetamine--behaviors which could conceivably transmit HCV. Our initial intent was to include both prevalence and incidence studies, but our search identified only two small incidence studies. In the results section we cite the incidence data, but this synthesis focuses on studies of HCV prevalence in NIDUs.
The sample included studies of non-injectors for whom HCV transmission via NIDU behaviors was biologically plausible. For HCV infection, blood-to-blood exposure is considered to be a biologically plausible route of transmission (Sherlock, 1994). HCV transmission via smoking crack and methamphetamine is plausible because the pipe can get very hot and burn the lips, creating open sores. Thus, sharing these pipes could lead to blood to blood contact. Sniffing cocaine and other drugs could also lead to blood to blood contact. This behavior can cause bleeding in nasal passages as a result of erosion of the mucous membranes from exposure to cocaine or if the implement (a straw or tubing) is rigid or has sharp edges that cause small tears in the tissue. Sharing straws or other intranasal implements could also lead to blood to blood contact. Therefore, we believe the NIDUs in the studies we have included in this analysis could conceivably acquire HCV through such drug-related exposures. We did not set any minimum time threshold for exposure as indicated by duration of use of non-injection drugs; these studies include participants with duration of use ranging from 1 month to 30 years.
2.3. Exclusion criteria
Once NIDU studies had been identified, we applied a substantial number of exclusion criteria to increase precision in measurement of exposure and reduce bias in estimating HCV prevalence (Figure 1). We excluded studies in which selection of participants was based on HCV status, as this was our outcome of interest. Samples that included individuals who had ever injected drugs were excluded as previous injection drug use would tend to bias our results toward higher prevalence estimates, and obscure any association between HCV and non-injection risk factors. Samples that included users of drugs such as marijuana were excluded because smoking marijuana should not lead to blood to blood contact, as the pipe stem or “joint” does not typically get hot enough to burn lips. For the same reason, studies which included oral administration of drugs such as amphetamine, and club drugs such as ecstasy, were also excluded.
Our rationale for these criteria was to focus our review on samples where HCV acquisition via drug administration was biologically plausible. Applying these exclusion criteria eliminated some reports which are often cited as evidence of HCV prevalence in NIDUs (Conry-Cantilena et al., 1996; Fingerhood et al., 1993; Shirin et al., 2000; Wada et al., 1999). Additionally, we excluded reports that provided only aggregate estimates combining IDUs and never-injectors. Finally, to be certain that the outcome of HCV prevalence was adequately assessed in the studies under review, we included only studies that tested sera or saliva for antibodies to HCV; studies that used self-reporting to ascertain HCV antibody status of individual subjects were excluded.
2.4. Data coding
The content and structure of the coding form was developed by reviewing those used in other meta-analyses, for example, the CDC Prevention Research Synthesis Project (Sogolow et al, 2002). The coding form included items such as the type of study or studies included in the body of the report (prevalence, incidence or genotype), study methods, and demographics and other characteristics of the sample such as duration of drug use or type of drugs used. The coding form was piloted and the final version was complete within the first six months of the study. The coding was carried out by senior research assistants who had graduate training in research methodology, and received additional training in HCV epidemiology, drug use and meta-analytic methods by the investigators of the meta-analysis study.
A number of strategies were used in the course of this study to ensure reliable, valid and consistent coding of data. Each data report was coded by a research assistant and independently reviewed for accuracy and completeness by the Project Director and the Principal Investigator. After this review, the coders made any necessary changes to the coding before it was considered complete and ready for data entry. In all cases where there was lack of agreement in any coding decision, the study group members carried out discussions to clarify the interpretation until consensus was reached. A study manual provided instructions for coding each item, and noted special cases and their resolution, to provide guidelines for consistent coding.
2.5. Quality measurement
As suggested by the MOOSE (Meta-analysis of Observational Studies in Epidemiology) group (Stroup et al., 2000), we established ‘quality’ criteria to evaluate the evidence regarding the NIDUs in the studies in this review. We did not evaluate the studies' research designs with regard to their explicit aims, but rather examined the quality of evidence regarding the association of NIDU practices and HCV prevalence.
The quality criteria were developed by the research team and were comprised of two broadly defined sections. The “NIDU screening items” were intended to measure the efforts made by each study to classify and define non-injection drug users in their sample. These NIDU-specific items included whether one of the aims was to study NIDUs, whether there was a method for minimizing misclassification of IDUs as NIDUs and whether the definition of non-injection drug use the authors used was clearly stated. The other items in this quality checklist were intended to capture basic elements of observational research design. These measures of quality evaluated the studies' inclusion of demographics of the sample, treatment of confounders, inclusion/exclusion criteria, sample size, and consistency of data reporting.
The Project Director and a member of the research staff coded these items independently. Initially there was 89% agreement on the coding of these items. Where there was not agreement, the items were discussed by the group until consensus was reached.
2.6. Statistical methods
We report prevalence estimates from the studies of NIDUs in our sample along with 95 percent confidence intervals (CI); when confidence intervals did not appear in the reports they were calculated using the “epitools” (Aragon, 2005) package in the “R” statistical and graphical software (R Core development Team, 2005). We performed tests of heterogeneity in HCV prevalence on the data available in the articles under review. We used chi-square, which showed very strong evidence of heterogeneity: chi-square = 148.52, df = 24, p < 0.00001.
We plotted overall prevalence, prevalence in relation to sample size, and prevalence in relation to a “quality” score. For each of twelve quality criteria, coded as 0 or 1, we calculated mean and median score across all studies. Two summary scores were calculated (NIDU Screening Score, and Total Quality Score), to show both overall quality of the studies and which aspects of study quality were particularly problematic.
There was overlap between study samples in some cases, (Tortu et al., 2001; Tortu et al., 2004) and (Fuller et al., 2004 ; Howe et al., 2005; Koblin et al., 2003). For calculations of median HCV prevalence, we used the study with the largest NIDU sample (Howe et al., 2005; Tortu et al., 2001) and chose not to include the three others (Fuller et al., 2004; Koblin et al., 2003; Tortu et al., 2004) in order to avoid double counting overlapping samples. We have included all the published studies in Tables 1 and 3 and have included all studies with relevant risk factors in Table 2.
Table 1.
Characteristics of the NIDU studies in our systematic review (n=28)
| Citation | Location | Enrollment Dates | Recruitment Setting | NIDU definition/sample | HCV Test | Sample | % anti-HCV + | 95% C.I. | Chars of sample | % HIV+ | % HBV+ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Allwright et al., 2000 | Ireland | September - November 1998 | Prisons | Smoked heroin (in past 12 months), but did not inject | Saliva/ORTHO HCV 3.0, Saliva/RIBA HCV 3.0 | 119 NIDUs | 9.2 | 4.7 , 15.9% | 0 | 1.7* | |
| Baozhang et al., 1997 | Yunnan Province, China | unspecified | “community of drug addicts” | Oral heroin users | ELISA Shanghai… | 59 NIDUs | 20.3 | 11 , 32.8% | 45.8 | ||
| Broers et al., 1998 | Geneva, Switzerland | 1988 - 1995 | Drug treatment | Never injected drugs | Ortho ELISA 1.0 or Abbott HCV EIA 2.0 | 114 NIDUs | 7.0 | 3 , 13.4% | 78% Male, mean age 25.5 | 0 | 6.3 |
| Chang et al., 1999 | Kaohsiung, Taiwan | October 1994 - February 1996 | Drug treatment and Prison | Never injected drugs/in narcotics abstention program | Abbott HCV EIA 2.0 | 652 NIDUs | 14.7 | 12.1 , 17.7% | 100% male | ||
| Denis et al., 2000 | Belgium | 1995-1998 | Medical setting and Drug Treatment | Non-injection heroin users | MEIA or ELISA 2 or 3 and RIBA 2.0 | 85 NIDUs | 2.4 | 0.3 , 8.2% | apx. 75% male; mean age 24.8 | 0 | 8.3 |
| Feldman et al., 2000 | Brooklyn, NY | March 1990 - December 1991 | Medical Setting | 100% female; no history of IDU, used crack | Abbott HCV EIA unspec | 96 NIDUs | 5.2 | 1.7 , 11.7% | 100% female, at least one male sex partner in past year | 6.3 | 30.6* |
| Fuller et al., 2004 | Harlem and Bronx, NY | August 2000 - August 2003 | Storefront/Street Outreach | 15-40 years old; used heroin, crack, or cocaine 10 years or less; no history of IDU and used drugs at least once/week in the past two months | Ortho 2.0 ELISA & RIBA 3.0 | 683 NIDUs | 4.4 | 3.0 , 6.2% | |||
| Galperim et al., 2004 | Porto Alegre, Southern Brazil | February 1998 - March 1999 | Drug Treatment | Intranasal cocaine use | COBAS CORE EIA II | 44 NIDUs | 2.3 | .001, 12.0% | |||
| Garten et al., 2004 | Guangxi Province, China | 1999 and 2000 | Storefront/Street Outreach | Non-injection heroin users | ELISA 3.0 | 106 NIDUs | 28.3 | 20 , 37.9% | |||
| Gyarmathy et al., 2002 | New York, NY | March 1996 - March 2001 | Storefront/street outreach | Used non-injected heroin in past 30 days & never injected drugs | Abbott HCV EIA 2.0 | 337 NIDUs | 12.5 | 9.1 , 16.5% | 69% male, 37% Hispanic | 8.6 | 23.4* |
| Harsch et al., 2000 | Milwaukee, WI | Unspecified | Research Clinic | Used non-injection cocaine and HCV negative or untested | Abbott HCV EIA 2.0 | 80 NIDUs | 13.8 | 7.1 , 23.3% | [see sample] | ||
| Hershow et al., 1998 | Chicago, IL | October 1994 - November 1995 | Medical Settings | HIV positive or at risk of HIV and used crack, cocaine or heroin | Abbott HCV EIA 2.0 & RIBA 2.0 | 89 NIDUs | 18.0 | 10.6 , 27.5% | 100% female | ||
| Howe et al., 2005 | New York, NY | August 2000- ?? | Storefront/Street Outreach | Noninjection use of heroin or cocaine [2-3× in past month but for no more than 10 years]; Ages 15-40 | Ortho 2.0 ELISA and Chiron RIBA 3.0 | 722 NIDUs | 3.9 | 2.6 , 5.6% | Median Age=30, IQR=24-35; 70% Male; 90% Black, Hisp; annual income < $5000 (83%); 80% ever homeless | 9.8 | |
| Koblin et al., 2003 | Manhattan and Bronx, NY | August 2000 - April 2002 | Storefront/Street Outreach | Noninjection use of heroin or cocaine 2-3 times in past month but for no more than 10 years | Ortho 2.0 ELISA & RIBA 3.0 | 276 NIDUs | 4.7 | 2.5 , 7.9% | 62% male, 37% under 30, 56% Black and mean age 30.8 | ||
| Lai et al., 2001 | Guangxi Province, China | February 1998 - May 1998 | Public health clinic | Noninjection use of heroin | Ortho 3.0 ELISA | 40 NIDUs | 32.5 | 18.6 , 49.1% | |||
| Maayan et al, 1994 | Jerusalem, Israel | December 1988- March 1989 | Drug Treatment | Never used injection drugs and in treatment | Abbott HCV EIA 2.0 | 131 NIDUs | 4.5 | 1.7, 9.7% | >90% heroin smokers | 0 | 32.1* (3% coinfected |
| Mathei et al., 2005 | Antwerp and Limburg Belgium | 1999 - 2000 | Drug Treatment/MMT sample | Never injected and not previously tested for HCV | Abbott HCV EIA 3.0 and RIBA 3.0 | 85 NIDUs | 18.8 | 11.2 , 28.8% | 18.8* | ||
| Njoh et al., 1997 | Jeddah, Saudi Arabia | February 1995 - August 1996 | Drug Treatment | Never used injection drugs and in treatment | Abbott HCV EIA 3.0 | 494 NIDUs | 10.5 | 8.0 , 13.6% | 62% of sample used drugs 5 years or more/100% male | ||
| Nyamathi, et al., 2002 | Los Angeles, CA | 1995 - 1999 | Homeless shelters and street outreach | Never used injection drugs, homeless and used crack | Modified ORTHO HCV ELISA 3.0 & RIBA SIA | 459 NIDUs | 14.6 | 11.5, 18.2% | Homeless adults | ||
| Quaglio et al., 2003a and 2003b | Veneto Region, Italy | February 2001 - August 2001 | Drug Treatment | Never injected drugs and receiving treatment for opiate addiction | Organon EIA | 130 NIDUs | 20.0 | 13.5 , 27.9% | 82% male, mean age 30, mean yrs NIDU=9 | 1.5 | 13.1 |
| Santana Rodriguez et al., 1998 | Gran Canaria, Spain | June 1993 - June 1994 | Drug Treatment Center | Never injected drugs and used heroin and/or cocaine | Abbott HCV EIA 2.0 & Immunoblot | 260 NIDUs | 35.3 | 29.6 , 41.5% | mean age=27.3; 78% Male | 2.7 | 20.7* |
| Shrestha et al., 1998 | Nepal | March 1997 - September 1997 | Community Organization | Oral drug abusers | SMITEST HCV ELISA & Ortho HCV ELISA 2 | 20 NIDUs | 30.0 | 11.9 , 54.3% | 100% 20-29 year old males | 5.0 | 15.0 |
| Strasfeld et al., 2003 | Bronx, NY | November 1999 - December 2000 | Drug Treatment/mmt | 100% HIV +, current or former opiate addicts who never injected | ORTHO HCV ELISA 3.0 | 157 NIDUs | 31.8 | 24.6 , 39.7% | |||
| **Tortu et al., 2001 | Lower East Side, New York, NY USA | March 1996 - July 1998 | Street Outreach | NIDU heroin in past 30 days | Abbott HCV EIA 2.0 | 358 NIDUs | 16.8 | 13.0 , 21.0% | 70% male | ||
| **Tortu et al., 2001 | East Harlem, NY, NY USA | October 1997 - June 1999 | Street Outreach | NIDU heroin, cocaine or crack in the past 30 days; Heterosexually active at least once/past six months | Abbott HCV EIA 2.0 | 171 NIDUs | 17.0 | 11.7 , 23.4% | 100% female sample | ||
| Tortu et al., 2004 | East Harlem, NY, NY USA | October 1997 - June 1999 | Street Outreach | NIDU heroin, cocaine or crack in the past 30 days: Heterosexually active at least once/past six months | Abbott HCV EIA 2.0 | 123 NIDUs | 19.5 | 12.9 , 27.6% | 54% Afr-Am, 100% female | 14.6 | |
| Van Ameijden et al., 1993 | Amsterdam, Netherlands | Dec. 1985 - Sept. 1989 | STD Clinic and Methadone program | Never injected drugs; No homosexual contact | Ortho HCV ELISA 1.0 | 36 NIDUs | 11.0 | 3.1 , 26.1% | 0 | 19.4* | |
| Ward et al., 2000 | London, UK | 1995-1996 | “Clinic Sample” | Sex worker crack users/never injected drugs | unspecified | 40 NIDUs | 5-6% (approx) | 0.6 , 16.9% | 100% female sample |
Tested for HBc--all others tested for a combination of markers
The Tortu et al. 2001 article is one report which contains 2 separate studies
Table 3.
Mean and median scores of quality items across NIDU studies in our sample
| Mean | Median | |
|---|---|---|
| NIDU screening items* | ||
| 1. Was one of the stated aims to study the disease in NIDU's | 0.57 | 1 |
| 2. Is there a method for minimizing misclassification bias | 0.32 | 0 |
| 3. Was NIDU definition and method of drug use clear? | 0.75 | 1 |
| Subtotal: NIDU Screening Score** | 1.64 | 1.5 |
| Other items* | ||
| 4. Aside from any inclusion criteria, were there any demographics/characteristics of the sample for the NIDU sample? | 0.39 | 0 |
| 5. Did the authors control/stratify for any relevant (drug-user related) confounders/co-variates for the NIDU sample? | 0.32 | 0 |
| 6. Did the authors assess any possible biologic mechanisms for disease transmission attributable to non-injection use? | 0.29 | 0 |
| 7. Were inclusion criteria well-described? | 0.93 | 1 |
| 8. Were exclusion criteria well-described? | 0.61 | 1 |
| 9. Were data collected in a similar manner for all participants? | 1.00 | 1 |
| 10. Was outcome (HCV) assessed using both antibody test and confirmatory test? | 0.32 | 0 |
| 11. Was sample size sufficient (>=100 NIDU participants) | 0.61 | 1 |
| 12. Were the number of subjects and percentages consistent? | 1.00 | 1 |
| Total quality scores*** | 7.11 | 7 |
Each item was scored 0 or 1 (absent, present) for each report
Items 1-3. Total summed scores ranged from 0 to 3.
Items 1-12. Total summed scores ranged from 3 to 12.
Table 2.
HCV prevalence by drug-related risk factors in the NIDU studies in our sample
| Duration of use | Sharing of pipes or other oral implements |
Sharing of straws or other intranasal implements |
Sharing of both pipes and straws |
|---|---|---|---|
|
*Heroin use:† <=5 years: 7/118 (5.9%) >5 years: 35/218 (16.1%) (Gyarmathy) |
*Shared crack pipe: No: 2/46 (4.3%) Yes: 5/19 (26.3%) (Gyarmathy) |
*Shared rolled bank note for heroin use: No: 8/89 (9.0%) Yes: 4/12 (33.3%) (Gyarmathy) |
*Ever shared both intranasal and oral implements: No: 7/65 (10.8%) Yes: 17/58 (29.3%) (Tortu, 2004) |
| Crack use: <=5 years: 26/209 (12.4%) >5 years: 16/128 (12.5%) (Gyarmathy) |
*Shared crack pipe when blood present, past 6 months: No: 27/717 (3.8%) Yes: 1/ 3 (33.3%) (Howe) |
Shared straws or dollar bill to sniff/snort cocaine, past 6 months: No: 17/468 (3.6%) Yes: 11/254 (4.3%) (Howe) |
Ever shared both intranasal and oral implements w/IDU: No: 22/118 (18.6%) Yes: 2/5 (40.0%) (Tortu, 2004) |
|
*Duration NIDU: <10 years: 10/80 (12.5%) >10 years: 16/50 (32.0%) (Quaglio, study b) |
Ever shared crack implements No: 6/47 (12.8%) Yes: 18/76 (23.7%) (Tortu, 2004) |
Sharing straws or dollar bill to sniff/snort heroin, past 6 months: No: 24/592 (4.1%) Yes: 4/130 (3.1%) (Howe) |
|
| Ever shared crack implements w/IDU No: 20/107 (18.7%) Yes: 4/16 (25%) (Tortu, 2004) |
Shared straw/dollar bill to sniff/snort heroin w/cocaine, past 6 months: No: 25/672 (3.7%) Yes: 3/50 (6.0%) (Howe) |
||
| Ever shared oral implements No: 6/47 (12.8%) Yes: 18/76 (23.7%) (Tortu, 2004) |
Ever shared intranasal implements: No: 5/39 (12.8%) Yes: 19/84 (22.6%) (Tortu 2004) |
||
| Ever shared oral implements w/IDU No: 21/108 (19.4%) Yes: 3/15 (20%) (Tortu, 2004) |
Ever shared intranasal implements w/IDU: No: 19/108 (17.6%) Yes: 5/15 (33.3%) (Tortu 2004) |
||
| Ever shared heroin implements: No: 13/72 (18.1%) Yes: 11/40 (27.5%) (Tortu 2004) |
|||
| Ever shared heroin implements w/IDU: No: 18/110 (16.4%) Yes: 6/13 (46.2%) (Tortu 2004) |
|||
| Ever shared cocaine implements: No: 6/48 (12.5%) Yes: 18/75 (24.0%) (Tortu 2004) |
|||
| Ever shared cocaine implements w/IDU: No: 23/115 (20.0%) Yes: 1/8 (12.5%) (Tortu 2004) |
Among those NIDUs with 5 years or less duration of use, 5.9% were HCV positive
Among those NIDUs with over 5 year duration of use, 16.1% were HCV positive
p<.05
3. Results
3.1 Study characteristics
We found 28 published reports that met our criteria (Allwright et al., 2000; Baozhang et al., 1997; Broers et al., 1998; Chang et al., 1999; Denis et al., 2000; Feldman et al., 2000; Fuller et al., 2004 ; Galperim et al., 2004; Garten et al., 2004; Gyarmathy et al., 2002; Harsch et al., 2000; Hershow et al., 1998; Howe et al., 2005; Koblin et al., 2003; Lai et al., 2001; Maayan et al., 1994; Mathei et al., 2005; Njoh et al., 1997; Nyamathi et al., 2002; Quaglio et al 2003a; Quaglio et al 2003b; Santana Rodriguez et al., 1998; Shrestha et al., 1998; Strasfeld et al., 2003; Tortu et al, 2001; Tortu et al., 2004; Van Ameijden et al., 1993; Ward et al., 2000). One report included two separate studies, both of which were included (Tortu et al., 2001) and which appear in Table 1 in two separate rows; two reports were by the same author and indicated the same overall seroprevalence and sample size, therefore we counted this as one study (Quaglio et al. 2003a & 2003b). Thus our sample included 28 reports and 28 studies. The 28 studies in this review are listed in table 1 with data on geographic location, enrollment dates, recruitment setting, sample size, definition of NIDU subjects, study design, HCV test method, anti-HCV seroprevalence information, prevalence of HIV and/or HBV in the sample, and characteristics of the NIDU sample. These studies represent several different regions including the USA (n=12), Western Europe (n=8), Asia (n=5), two in the Middle East (Israel & Saudi Arabia), and one in Brazil.
Study methodology varied substantially, particularly with respect to recruitment setting. Investigators of eleven studies did at least some of their recruitment in substance abuse treatment settings, while two of the studies took place in prisons. Other recruitment locations included storefront/street outreach (n=9), community organizations (n=1), the “community of addicts” (n=1) and medical/clinic settings (n=6). Note that some studies recruited from more than one location, so that the sum of the number of recruitment locations exceeds the total number of studies. When we examined variation in HCV prevalence in relation to recruitment settings we did not observe any association.
Drugs used by the NIDUs in the samples also varied greatly, with seven studies including only heroin users, three studies comprised of crack or cocaine users, and the remainder of the studies recruiting a combination of heroin, crack, methamphetamine and cocaine users. When we examined the relationship between drug type and HCV prevalence, there was no association, perhaps because many samples included multiple drug types. The sample sizes ranged from 20 - 722 NIDUs. The median number of NIDU participants in these studies was 119.
3.2. Sample characteristics
Fewer than half the studies reported descriptive characteristics for NIDUs. Those that did give characteristics reported that their samples were predominantly male (62 percent-100 percent male), although there were five studies that either recruited only females or restricted their analysis to female subjects (Feldman et al., 2000; Hershow et al., 1998; Tortu et al., 2004; Tortu et al., 2001; Ward et al., 2000). The NIDUs in the majority of the reports we located were relatively young; among those studies that reported age information, the mean age of the subjects varied between 25 and 30 years old.
3.3. HCV prevalence and prevalence of other infectious diseases
Prevalence of HCV in these studies ranged from 2.3 to 35.3 percent, with a median of approximately 14 percent. Studies reporting high prevalence (over 20 percent) tended to have small to modest sample sizes (n=20 to 260; median=83). This correlation between sample size and prevalence may be due to sampling error or publication bias, but may be related to other factors such as geography—four of six high prevalence studies took place in East Asia. Approximately half of the NIDU studies reported HIV and/or HBV infection. Four studies reported 0 percent HIV infection. Overall HIV prevalence in the studies ranged from 0-14.6 percent. Three studies reported on the relationship between HIV and HCV. There was no linear relationship between HIV and HCV when we examined all studies with HIV prevalence data.
Prevalence of seromarkers for HBV prevalence ranged from 1.7 -45.8 percent (Table 1). One study reported that subjects with HBV were significantly more likely (Odds Ratio (OR) =10.35, 95 percent CI=2.75-38.99) than HBV negatives to be infected with HCV (Quaglio et al., 2003a).
3.4. Risk factors
Table 2 presents HCV prevalence estimates from the studies of NIDUs in relation to categories of drug-related risk factors which were hypothesized to be most informative in terms of exposure risk. Three studies examined duration of use in NIDUs (Chang et al., 1999; Gyarmathy et al., 2002; Quaglio et al., 2003). One study (data not shown) reported high prevalence (22 percent) for NIDUs using for one month or less (Chang et al., 1999), though prevalence decreased as duration of use increased. Another study found that the risk of HCV increased threefold after 5 years of use (16.1 vs. 5.9 percent; Adjusted Odds Ratio (AOR) =3.80, 95 percent CI =1.4, 10.6 (data not shown)) (Gyarmathy et al., 2002). The third study also found a relationship between duration of use and HCV infection; those non-injectors using more than 10 years were significantly more likely than those using less than 10 years to have HCV (32 vs. 12.5 percent; p<.05) (Quaglio et al., 2003b).
Two studies (Tortu et al., 2004; Howe et al., 2005) differed in their findings with respect to sharing of non-injection drug use equipment; these data were difficult to compare given that one examined recent sharing behavior and one analyzed ever-sharing behavior. Specifically, one study found that any lifetime sharing of implements was associated with HCV--ever shared both oral and intranasal drug-use implements (HCV prevalence 29.3% vs. 10.8%; AOR=2.83 (AOR not shown); 95 percent CI=1.04, 7.72) and ever shared noninjected heroin implements with injector (HCV prevalence 27.5% vs. 18.1%; AOR=3.06 (AOR not shown); 95 percent CI=0.87, 10.70) (Tortu et al., 2004). In contrast, the study that examined more recent behavior found no association between HCV prevalence and sharing straws: shared straw or dollar bill to sniff/snort heroin, past 6 months (3.1 vs. 4.1 percent) and shared straw or dollar bill to sniff/snort heroin with cocaine, past six months (6.0 vs. 3.7 percent) (Howe et al., 2005).
Two studies (Gyarmathy et al., 2002; Howe et al., 2005) examined sexual risk behaviors in NIDUs and had different findings (data not shown). One found no association between HCV infection and “high risk” partners: unprotected sex with someone with HIV; with men who had sex with men; and with other NIDUs (Gyarmathy et al., 2002). The other found that having a sex partner with self-reported HCV was associated with HCV infection (12.5 vs. 3.5 percent) (Howe et al., 2005).
These same two studies also examined tattooing, household or personal exposures, and transfusion exposure (Howe et al., 2005; Gyarmathy et al., 2002) (data not shown). One study found that having tattoos was associated with HCV infection (18.2% with tattoos vs. 9.2% of other NIDUs; AOR=2.2, 95 percent CI=1.0, 4.7) (Gyarmathy et al., 2002), while the other found that having a tattoo done by a friend/relative/acquaintance compared with no tattoo (7.3% vs. 3.4%; AOR=3.61; 95 percent CI=1.15, 11.26) was associated with HCV infection (Howe et al., 2005). The Howe study also reported an inverse association between HCV and the sharing of personal hygiene items. These studies adjusted for drug-related risk behaviors in the calculation of these odds.
3.5. Cohort Studies
We were able to identify only 2 HCV incidence studies in this population. One U.S. study found an incidence of 0.4 per 100 Person Years of Observation (PY) (Fuller et al., 2004), while a study based in China calculated 8 HCV seroconversions per 100 PY (Garten et al., 2004). The authors of the China study reported problems assessing route of administration and they hypothesized that this high incidence of HCV may have been related to a misclassification of participants with respect to route of drug administration.
3.6. Quality
We utilized 12 items related to study quality to assess the NIDU studies in our sample. Each item was scored for its presence (score=1) or absence (score=0) in a given study. When we summed the 12 items for each study, the overall scores of each individual study ranged from 3 to 12, with a median score of 7.
As a whole, the studies had some areas of strength. As shown in table 3, all or almost all of the studies described their inclusion criteria in a detailed manner, collected their data in a similar manner for all participants and showed consistency in their numbers and percentages.
Mean quality scores for the NIDU studies are shown in Table 3. The five indicators of quality that appeared least frequently in the NIDU studies were: minimizing misclassification bias; including demographics/characteristics of the sample for the NIDU sample; controlling for any drug-related confounders; assessing any biologic mechanisms for transmission; and use of both an antibody and a confirmatory test.
Because many of these studies had an IDU focus, often there were relatively few noninjectors in the sample. Perhaps because of these small numbers, many studies did not indicate demographics or other characteristics of the NIDU sample outside the general inclusion criteria. Only eight studies indicated the use of specific criteria for ascertaining NIDU status and minimizing misclassification of IDUs as NIDUs (e.g. checking for track marks or re-confirming route of administration several times within a study instrument). Of the eight studies that examined risk factors for HCV exposure, only four of them utilized multivariate techniques to examine possible risk factors for HCV in NIDU populations.
Confirmatory testing of HCV ELISA testing is recommended in populations with a low prevalence of HCV (Alter et al., 2003). Since NIDUs may meet the definition of a low-prevalence population (<10 percent), we examined whether HCV prevalence varied in relation to the use of a confirmatory RIBA test to verify HCV antibody status. Of those studies that used a RIBA test, prevalence ranged from 2.4 percent to 35.3 percent with a median of approximately 14 percent. Of those studies that used only antibody testing to assess positivity, prevalence ranged from 4.5 - 32.5 percent with a median prevalence of approximately 17 percent.
We were interested in evaluating the evidence around appropriate identification of NIDUs, which constitutes a potential source of bias in measuring prevalence accurately. We identified three items which pertained to the identification of NIDUs--these are items 1 through 3 in Table 3: 1-Was one of the stated aims to study HCV in NIDU's; 2-Was there a method given for minimizing misclassification bias and 3-Was NIDU definition/inclusion criteria and method of drug use clearly stated in the report?
For each study, we gave a score of “1” to the studies that adhered to these criteria and “0” to those that did not. We then summed these three scores. Using this scheme, we were able to attribute a numeric value to each report—from“0” for studies that possessed none of these qualities (n=5) to “3” for those that contained all of these elements (n=6). Figure 2 shows the range and median of the prevalence of HCV in each group. The range is indicated by the horizontal bottom line or dot and the horizontal top line or dot. The dot indicates that this upper or lower range value is an outlier, while the horizontal line shows that the top or bottom value is within the expected range. The median is indicated by the thick black line in the rectangle. As shown in figure 2, the studies with the highest NIDU screening score (value = 3) had the narrowest range of prevalence measures (2.3 -17 percent) and a median HCV prevalence of 9 percent. The other three groups had substantially broader ranges (studies scoring “0”: 5 to 30 percent; studies scoring “1”: 4.5 to 32.5 percent; and studies scoring “2”: 2.4 to 35.3 percent). Medians ranged from 10.5 - 20 percent.
Figure 2.
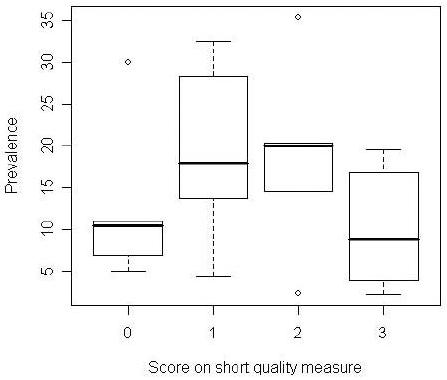
Plots of prevalence by quality scores; medians and ranges by quality. The range is indicated by the horizontal bottom line or dot and the horizontal line or dot. The dot indicates that this lower or upper value is an outlier, while the line indicates that this lower or upper value is within the expected range. The median value for each quality score is indicated by the thick black line in the rectangle. Each rectangle encompasses the 25th - 75th percentile for the corresponding quality score.
4. Discussion
4.1. Summary of findings
Our systematic review identified 28 published studies of HCV in self-reported NIDUs. Prevalence of HCV in never-injecting drug users ranged from 2.3 - 35.3 percent in studies from several regions with samples that included between 20 and 722 NIDU participants. When we restricted the sample to reports that were least likely to misclassify NIDUs, prevalence estimates ranged between 2.3 and 17 percent. Whether the higher observed prevalence rates (up to 35 percent) in studies that were less focused on NIDUs is attributable to misclassification of IDUs as NIDUs is uncertain. This finding suggests that future NIDU studies should apply rigorous and standard screening methods including inspection for evidence of track marks, and describe specific protocols used to screen subjects for route of drug administration data. Otherwise, evaluating the evidence regarding risk of HCV infection in never-injector populations will remain speculative.
4.2. Gaps and methodological issues
The results of our systematic review suggest that there are substantial gaps in the research of HCV in NIDUs. There was little consistency in methods used to determine NIDU status, and it was often challenging to glean this and other methodological information from the reports. Additionally, because many of the studies did not provide prevalence estimates by drug type, we do not know if there are different degrees of risk for specific drug types. For this review, there was a problem of study focus; much of the research we identified did not aim to study HCV in users of non-injection drugs. Instead, NIDUs were included as a secondary research concern, with a principal focus on the problem of transmission of HCV in IDU populations. Misclassification of route of administration was also a concern—it was conceivable that in some studies individuals who had identified themselves as non-injectors may have actually been IDUs. One of the NIDU studies in our review included in their baseline prevalence estimates subjects who revealed themselves to be IDUs in later waves of a cohort study (Garten et al., 2004). The rationale for this decision may be related to the article's focus on injectors, and may have contributed to an inflation of prevalence and incidence estimates among NIDUs in that study. Clearly, reporting of more detailed descriptions of screening and recruitment methods used in these studies would permit more precise classification, and might aid in the detection of variation in HCV prevalence in relation to study methods.
4.3. Risk factors
Few of the studies included in our review systematically examined or reported on HCV prevalence in relation to drug-related risk behaviors. Most of the studies identified in our review were descriptive studies of HCV prevalence in NIDUs and other drug users. Therefore, we were constrained in our ability to examine and statistically synthesize covariates which may be associated with HCV in these users. We were able to identify a limited number of studies that attempted to assess duration of drug use, sexual, or tattooing behaviors related to HCV. The findings of these studies regarding equipment-sharing, sexual behavior and tattooing may or may not be replicable in other NIDU populations.
To our knowledge, the association between HCV and risk factors related to non-injection drug use has not been examined in large studies outside the United States. Studies based in New York City have analyzed a variety of risks for HCV. Some of these studies have found evidence for sharing of non-injection equipment as a possible route of HCV transmission, while others have found that tattooing, older age, and having friends/associates infected with HCV are risk factors of interest. Tattooing, age and knowing people with HCV may represent duration of time spent in the drug use scene. Based on our review, it remains unclear whether HCV in NIDUs can be attributed to drug rather than sex-related or other risk behaviors.
4.4. Confirmatory testing
One potential complication related to the measure of HCV prevalence in NIDU populations in these studies is the lack of confirmatory HCV testing in most of these studies. Only eight studies in our sample reported using RIBA confirmatory tests. In their screening guidelines, the CDC notes that in populations with low prevalence of infection (<10 percent), the proportion of false-positives with antibody tests averages 35 percent (Alter et al., 2003). It is unclear what proportion of false positives would apply to a true NIDU population, but based on the CDC estimates, sole reliance on an EIA test to assess HCV antibody status may inflate prevalence estimates.
4.5. Limitations
In most studies, limitations included potential misclassification of the exposure and the lack of adjustment for confounding to assess association between risk behaviors and HCV prevalence. Thus we cannot draw firm conclusions about the role of non-injecting drug use in HCV. However, there is evidence to support the plausibility of HCV transmission via NIDU routes, including the isolation of HCV in the nasal secretions of a non-injection drug user (McMahon and Tortu, 2003).
The rate and circumstances of seroconversion in NIDU populations remain unclear as well. Only two of the studies we located (Fuller et al., 2004; Garten et al 2004) measured seroconversion, and the incidence rate in the study in China (8 per 100PY) was undermined by the likely misclassification of some IDUs as NIDUs. Further, the small number of seroconversions limited the examination of HCV incidence in relation to risk factors.
While incidence studies in NIDUs may require large samples, following baseline HCV negative NIDUs over time could guide researchers in understanding (1) NIDU risk behaviors that are most likely associated with seroconversion to HCV, and (2) circumstances leading to NIDU transition to IDU, which will increase the risk for contracting blood-borne viruses (Fuller et al., 2004; Neaigus et al., 2001). Neaigus et al.'s study suggests that certain NIDU characteristics or behaviors may merit further investigation in an effort to prevent or delay transitioning to injection.
5. Conclusions
Despite this relative scarcity of information, those who work with drug user populations are creating interventions to protect non-injection users of illicit drugs. For example, a study from Canada (Shannon et al., 2006) describes efforts in Vancouver to encourage safe crack cocaine smoking. Those who work with NIDUs would benefit from stronger evidence to help inform their efforts to reduce drug-related harm.
We conclude that current studies have not clearly demonstrated whether or not NIDU-specific behaviors are associated with HCV infection. We suggest that future research be targeted to examine NIDU-specific risks and exposures. Despite quality problems, HCV prevalence among this population is higher than in a non-drug using population, even assuming some IDUs were inadvertently included in the samples. These studies point to a real problem of HCV in NIDU populations. With better classification and risk factor data we might be able to better grasp the mechanisms of transmission in this population and design appropriate interventions for this at-risk population.
Acknowledgments
This research was funded by NIDA Grant Number R01 DA018609. Sommer Rentmeesters provided research assistance on the initial construction of the tables. The authors would like to thank colleagues at the Center for Drug Use and HIV Research for their support and assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allwright S, Bradley F, Long J, Barry J, Thornton L, Parry JV. Prevalence of antibodies to hepatitis B, hepatitis C, and HIV and risk factors in Irish prisoners: results of a national cross sectional survey. BMJ. 2000;321:78–82. doi: 10.1136/bmj.321.7253.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter MJ, Kuhnert WL, Finelli L. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2003;52:1–13. 15; quiz CE1-4. [PubMed] [Google Scholar]
- Aragon T. Epitools: Epidemiology Tools. R package version 0.4-7. 2005 http://www.epitools.net. Accessed September 28, 2006.
- Baozhang T, Kaining Z, Jinxing K, Ruchang X, Ming L, Caixia Z, Li T. Infection with human immunodeficiency virus and hepatitis viruses in Chinese drug addicts. Epidemiol Infect. 1997;119:343–347. doi: 10.1017/s0950268897007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broers B, Junet C, Bourquin M, Deglon JJ, Perrin L, Hirschel B. Prevalence and incidence rate of HIV, hepatitis B and C among drug users on methadone maintenance treatment in Geneva between 1988 and 1995. AIDS. 1998;12:2059–66. doi: 10.1097/00002030-199815000-00018. [DOI] [PubMed] [Google Scholar]
- Chang CJ, Lin CH, Lee CT, Chang SJ, Ko YC, Liu HW. Hepatitis C virus infection among short-term intravenous drug users in southern Taiwan. Eur J Epidemiol. 1999;15:597–601. doi: 10.1023/a:1007662315835. [DOI] [PubMed] [Google Scholar]
- Conry-Cantilena C, VanRaden M, Gibble J, Melpolder J, Shakil AO, Viladomiu L, Cheung L, DiBisceglie A, Hoofnagle J, Shih JW. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med. 1996;334:1691–6. doi: 10.1056/NEJM199606273342602. [DOI] [PubMed] [Google Scholar]
- Denis B, Dedobbeleer M, Collet T, Petit J, Jamoulle M, Hayani A, Brenard R. High prevalence of hepatitis C virus infection in Belgian intravenous drug users and potential role of the “cotton-filter” in transmission: the GEMT Study. Acta Gastroenterol Belg. 2000;63:147–53. [PubMed] [Google Scholar]
- Feldman JG, Minkoff H, Landesman S, Dehovitz J. Heterosexual transmission of hepatitis C, hepatitis B, and HIV-1 in a sample of inner city women. Sex Transm Dis. 2000;27:338–42. doi: 10.1097/00007435-200007000-00007. [DOI] [PubMed] [Google Scholar]
- Fingerhood MI, Jasinski DR, Sullivan JT. Prevalence of hepatitis C in a chemically dependent population. Arch Intern Med. 1993;153:2025–2030. [PubMed] [Google Scholar]
- Fuller CM, Ompad DC, Galea S, Wu Y, Koblin B, Vlahov D. Hepatitis C incidence--a comparison between injection and noninjection drug users in New York City. J Urban Health. 2004;81:20–4. doi: 10.1093/jurban/jth084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperim B, Cheinquer H, Stein A, Fonseca A, Lunge V, Ikuta N. Intranasal cocaine use does not appear to be an independent risk factor for HCV infection. Addiction. 2004;99:973–7. doi: 10.1111/j.1360-0443.2004.00761.x. [DOI] [PubMed] [Google Scholar]
- Garten RJ, Lai S, Zhang J, Liu W, Chen J, Vlahov D, Yu XF. Rapid transmission of hepatitis C virus among young injecting heroin users in Southern China. Int J Epidemiol. 2004;33:182–8. doi: 10.1093/ije/dyh019. [DOI] [PubMed] [Google Scholar]
- Gyarmathy VA, Neaigus A, Miller M, Friedman SR, Des Jarlais DC. Risk correlates of prevalent HIV, hepatitis B virus, and hepatitis C virus infections among noninjecting heroin users. J Acquir Immune Defic Syndr. 2002;30:448–456. doi: 10.1097/00042560-200208010-00011. [DOI] [PubMed] [Google Scholar]
- Hagan H. Hepatitis C virus transmission dynamics in injection drug users. Subst Use Misuse. 1998:1197–1212. doi: 10.3109/10826089809062214. [DOI] [PubMed] [Google Scholar]
- Harsch HH, Pankiewicz J, Bloom AS, Rainey C, Cho JK, Sperry L, Stein EA. Hepatitis C virus infection in cocaine users--a silent epidemic. Community Ment Health J. 2000;36:225–33. doi: 10.1023/a:1001988613235. [DOI] [PubMed] [Google Scholar]
- Hershow RC, Kalish LA, Sha B, Till M, Cohen M. Hepatitis C virus infection in Chicago women with or at risk for HIV infection: Evidence for sexual transmission. Sex Transm Dis. 1998;25:527–532. doi: 10.1097/00007435-199811000-00006. [DOI] [PubMed] [Google Scholar]
- Hocking J, Crofts N, Aitken C, MacDonald M. Epidemiology of the hepatitis C virus among injecting drug users. In: Crofts N, Dore G, Locarnini S, editors. Hepatitis C: An Australian perspective. IP Communications; Victoria, Australia: 2001. pp. 260–295. [Google Scholar]
- Howe CJ, Fuller CM, Ompad DC, Galea S, Koblin B, Thomas D, Vlahov D. Association of sex, hygiene and drug equipment sharing with hepatitis C virus infection among non-injecting drug users in New York City. Drug Alcohol Depend. 2005;79:389–95. doi: 10.1016/j.drugalcdep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Judd A, Hickman M, Rhodes T. Transmission of hepatitis C--are noninjecting cocaine users at risk? Subst Use Misuse. 2002;37:573–5. [PubMed] [Google Scholar]
- Koblin BA, Factor SH, Wu Y, Vlahov D. Hepatitis C virus infection among noninjecting drug users in New York City. J Med Virol. 2003;70:387–390. doi: 10.1002/jmv.10407. [DOI] [PubMed] [Google Scholar]
- Lai S, Liu W, Chen J, Yang J, Li ZJ, Li RJ, Liang FX, Liang SL, Zhu QY, Yu XF. Changes in HIV-1 incidence in heroin users in Guangxi Province, China. J Acquir Immune Defic Syndr. 2001;26:365–70. doi: 10.1097/00126334-200104010-00014. [DOI] [PubMed] [Google Scholar]
- Maayan S, Shufman EN, Engelhard D, Shouval D. Exposure to hepatitis B and C and to HTLV-1 and 2 among Israeli drug abusers in Jerusalem. Addiction. 1994;89:869–74. doi: 10.1111/j.1360-0443.1994.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Mathei C, Robaeys G, van Damme P, Buntinx F, Verrando R. Prevalence of hepatitis C in drug users in Flanders: determinants and geographic differences. Epidemiol Infect. 2005;133:127–36. doi: 10.1017/s0950268804002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon JM, Tortu S. A potential hidden source of hepatitis C infection among noninjecting drug users. J Psychoactive Drugs. 2003;35:455–60. doi: 10.1080/02791072.2003.10400492. [DOI] [PubMed] [Google Scholar]
- Neaigus A, Miller M, Friedman SR, Hagen DL, Sifaneck SJ, Ildefonso G, des Jarlais DC. Potential risk factors for the transition to injecting among non-injecting heroin users: a comparison of former injectors and never injectors. Addiction. 2001;96:847–60. doi: 10.1046/j.1360-0443.2001.9668476.x. [DOI] [PubMed] [Google Scholar]
- Njoh J, Zimmo S. Prevalence of antibodies to hepatitis C virus in drug-dependent patients in Jeddah, Saudi Arabia. East Afr Med J. 1997;74:89–91. [PubMed] [Google Scholar]
- Nyamathi AM, Dixon EL, Robbins W, Smith C, Wiley D, Leake B, Longshore D. Risk factors for hepatitis C virus infection among homeless adults. J Gen Intern Med. 2002;17:134–143. doi: 10.1046/j.1525-1497.2002.10415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglio G, Lugoboni F, Pajusco B, Sarti M, Talamini G, Lechi A, Mezzelani P, Des Jarlais DC. Factors associated with hepatitis C virus infection in injection and noninjection drug users in Italy. Clin Infect Dis. 2003;37:33–41. doi: 10.1086/375566. [DOI] [PubMed] [Google Scholar]
- Quaglio GL, Lugoboni F, Pajusco B, Sarti M, Talamini G, Mezzelani P, Des Jarlais DC. Hepatitis C virus infection: prevalence, predictor variables and prevention opportunities among drug users in Italy. J Viral Hepat. 2003;10:394–400. doi: 10.1046/j.1365-2893.2003.00448.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2005. ISBN 3-900051-07-0, URL: http://www.R-project.org. Accessed September 28, 2006. [Google Scholar]
- Roy K, Hay G, Andragetti R, Taylor A, Goldberg D, Wiessing L. Monitoring hepatitis C virus infection among injecting drug users in the European Union: A review of the literature. Epidemiol Infect. 2002;129(3):577–585. doi: 10.1017/s0950268802007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana Rodriguez OE, Male Gil ML, Hernandez Santana JF, Liminana Canal JM, Martin Sanchez AM. Prevalence of serologic markers of HBV, HDV, HCV and HIV in non-injection drug users compared to injection drug users in Gran Canaria, Spain. Eur J Epidemiol. 1998;14:555–561. doi: 10.1023/a:1007410707801. [DOI] [PubMed] [Google Scholar]
- Shannon K, Ishida T, Morgan R, Bear A, Oleson M, Kerr T, Tyndall MW. Potential community and public health impacts of medically supervised safer smoking facilities for crack cocaine users. Harm Reduct J. 2006;3:1. doi: 10.1186/1477-7517-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock DS. Chronic hepatitis C. Dis Mon. 1994 Mar;40(3):117–96. [PubMed] [Google Scholar]
- Shirin T, Ahmed T, Iqbal A, Islam M, Islam MN. Prevalence and risk factors of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus infections among drug addicts in Bangladesh. J Health Popul Nutr. 2000;18:145–150. [PubMed] [Google Scholar]
- Shrestha SM, Subedi NB, Shrestha S, Maharjan KG, Tsuda F, Okamoto H. Epidemiology of hepatitis C virus infection in Nepal. Trop Gastroenterol. 1998;19:102–4. [PubMed] [Google Scholar]
- Sogolow E, Peersman G, Semaan S, Strouse D, Lyles CM. The HIV/AIDS Prevention Research Synthesis Project: scope, methods, and study classification results. J Acquir Immune Defic Syndr. 2002;30(Suppl 1):S15–29. [PubMed] [Google Scholar]
- Strasfeld L, Lo Y, Netski D, Thomas DL, Klein RS. The association of hepatitis C prevalence, activity, and genotype with HIV infection in a cohort of New York City drug users. J Acquir Immune Defic Syndr. 2003;33:356–64. doi: 10.1097/00126334-200307010-00010. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Tortu S, Neaigus A, McMahon J, Hagen D. Hepatitis C among noninjecting drug users: A report. Subst Use Misuse. 2001;36:523–534. doi: 10.1081/ja-100102640. [DOI] [PubMed] [Google Scholar]
- Tortu S, McMahon JM, Pouget ER, Hamid R. Sharing of noninjection drug-use implements as a risk factor for hepatitis C. Subst Use Misuse. 2004;39:211–24. doi: 10.1081/ja-120028488. [DOI] [PubMed] [Google Scholar]
- Thomson Scientific. Science Citation Index Accessed April 16, 2006. URL: http://isiknowledge.com.
- van Ameijden EJ, van den Hoek JA, Mientjes GH, Coutinho RA. A longitudinal study on the incidence and transmission patterns of HIV, HBV and HCV infection among drug users in Amsterdam. Eur J Epidemiol. 1993;9:255–262. doi: 10.1007/BF00146260. [DOI] [PubMed] [Google Scholar]
- Wada K, Greberman SB, Konuma K, Hirai S. HIV and HCV infection among drug users in Japan. Addiction. 1999;94:1063–1070. doi: 10.1046/j.1360-0443.1999.947106311.x. [DOI] [PubMed] [Google Scholar]
- Ward H, Pallecaros A, Green A, Day S. Health issues associated with increasing use of “crack” cocaine among female sex workers in London. Sex Transm Infect. 2000;76:292–3. doi: 10.1136/sti.76.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Weekly Epidemiologic Record. 1998;73(20):145–153. [Google Scholar]