Abstract
Purpose
The α/β ratio for prostate cancer is postulated to be between 1 and 3, giving rise to the hypothesis that there may be a therapeutic advantage to hypofractionation. The dosimetry and acute toxicity are described in the first 100 men enrolled in a randomized trial.
Patients and Methods
The trial compares 76 Gy in 38 fractions (Arm I) to 70.2 Gy in 26 fractions (Arm II) using intensity modulated radiotherapy. The planning target volume (PTV) margins in Arms I and II were 5 mm and 3 mm posteriorly and 8 mm and 7 mm in all other dimensions. The PTV D95% was at least the prescription dose.
Results
The mean PTV doses for Arms I and II were 81.1 and 73.8 Gy. There were no differences in overall maximum acute gastrointestinal (GI) or genitourinary (GU) toxicity acutely. However, there was a slight but significant increase in Arm II GI toxicity during Weeks 2, 3, and 4. In multivariate analyses, only the combined rectal DVH parameter of V65 Gy/V50 Gy was significant for GI toxicity and the bladder volume for GU toxicity.
Conclusion
Hypofractionation at 2.7 Gy per fraction to 70.2 Gy was well tolerated acutely using the planning conditions described.
Keywords: IMRT, Dosimetry, Hypofractionation, Toxicity
INTRODUCTION
Brenner and Hall (1) calculated the α/β ratio for prostate cancer to be about 1.5 using freedom from biochemical failure (FFBF) data in men treated by external beam radiotherapy (1.8–2.0 Gy) and permanent 125-I seed implant. They and others have confirmed that the α/β ratio is in the 1.5 range in other patient datasets; however, there is a wide range of reported α/β ratios and estimates of the associated error in the assumptions made (2–11). The results of a randomized trial would provide more tangible evidence for the value of the α/β ratio in prostate cancer.
Radiotherapy dose has consistently been observed to be a determinant of FFBF in retrospective, sequential prospective, and randomized series. A premise for this trial was to build on the prior randomized dose escalation trial of 70 vs. 78 Gy (12), administered in 2.0 Gy fractions and prescribed to the isocenter (i.e., International Commission on Radiation Units and Measurements point doses). The 8-Gy difference in dose between the arms resulted in a significant improvement in FFBF that was most pronounced for men with a pretreatment prostate-specific antigen (PSA) >10 ng/mL. The rationale for the hypofractionation scheme described herein was that there would be a therapeutic gain if the α/β ratio for prostate cancer was 1.5 and for the rectum ≥4.0 for late effects (13–17). The preliminary results from the Cleveland Clinic suggest that this may be true (18). Our study compares 76 Gy in 2.0 Gy fractions with 70.2 Gy in 2.7 Gy fractions, prescribed to the planning target volume (PTV). Assuming a prostate cancer α/β ratio of 1.5, the delivery of 70.2 Gy in 26 fractions would be biologically equivalent to 84.4 Gy in 2.0 Gy fractions. Using the same 26 fraction regimen and assuming an α/β ratio for late effects of 4.0 for the rectum, the biologically equivalent dose in 2-Gy fractions would be ≤78 Gy. The hypothesis was that FFBF would be increased without increasing late toxicity. In this report, the dosimetry and acute side effects for the first 100 men randomized are described.
PATIENTS AND METHODS
Protocol design
The protocol was designed to randomize men with intermediate-to high-risk prostate cancer to 76 Gy in 38 fractions at 2.0 Gy per fraction (Arm I, conventional fractionation intensity-modulated radiation therapy [CIMRT]) vs. 70.2 Gy in 26 fractions at 2.7 Gy per fraction (Arm II, hypofractionation intensity-modulated radiation therapy [HIMRT]). The hypofractionation arm was hypothesized to be equivalent to 84.4 Gy in 2 Gy fractions assuming an α/β ratio of 1.5. This design was formulated to test whether dose escalation via hypofractionation results in a significant improvement in FFBF, without increasing late complications. The principal hypothesis was that the 8-Gy escalation in biologic dose between Arms I and II would result in a 15% gain in FFBF from 70% to 85%.
The estimated 5-year FFBF rate for intermediate-risk patients treated with radiotherapy alone to 76 Gy at 2 Gy per fraction is 70% (12, 19). The 5-year FFBF rate for high-risk patients treated with 76 Gy plus 2 years of androgen deprivation is also estimated to be about 70% (20).
Classification of risk, eligibility, and stratification
Men with Stage T1-3 adenocarcinoma of the prostate and Gleason score ≥5 were eligible if they had intermediate to high-risk features. Intermediate risk was defined as Gleason score 7, pre-treatment initial PSA (iPSA) >10–20 ng/mL, or ≥3 biopsy cores of Gleason score ≥5, as long as no high-risk features were present. High risk was defined as Gleason score 8–10, Gleason score 7 in ≥4 cores, cT3 disease, or an iPSA >20 ng/mL. Up to 4 months of androgen deprivation with a luteinizing hormone releasing hormone (LHRH) agonist or antiandrogen before randomization was permitted. For those with intermediate risk who were receiving androgen deprivation when enrolled, androgen deprivation was discontinued, whereas for those with high risk (also referred to as unfavorable) androgen deprivation was to be continued for 2 years.
Patients were ineligible if they had a prior history of androgen deprivation for >4 months before randomization, an iPSA of >80 ng/mL, prior pelvic radiotherapy, prior radical prostatectomy, or prior malignancy other than nonmetastatic skin cancers or early stage small lymphocytic lymphoma within the last 5 years. Patients were stratified by iPSA (≤10, >10, ≤20, or >20 ng/mL), Gleason score (5–7 or 8–10), and whether or not long-course androgen deprivation was planned (high or intermediate risk).
Simulation
Patients were simulated in the supine position in an α-cradle (Smithers Medical Products, Inc., North Canton, OH) with a Plexiglas holder to immobilize the feet. An enema per rectum was given before simulation to empty the rectum as much as possible. The patients were asked to have a moderately full bladder.
Target and normal structure definition and margins
Computed tomography and magnetic resonance imaging (MRI)-based simulations were routinely performed unless there was a medical contraindication to performing an MRI (e.g., pacemaker). Images were acquired from the top of the ilium to below the ischial tuberosities. A slice thickness of 3 mm was used from the bottom of the sacroiliac joints to the ischial tuberosities and 1-cm cuts above and below these levels. The scans were loaded into a planning computer and fused based on bony anatomy using either chamfer matching or maximization of mutual information methods. The computed tomography images were used to outline the external contour and femoral heads. All other contours, including the bladder, rectum, prostate, seminal vesicles, and lymph nodes were outlined using the MRI images.
The structures outlined included the prostate, proximal seminal vesicles (at least the first approximately 9 mm for intermediate-risk patients and >9 mm for high-risk patients), distal seminal vesicles, rectum (entire contents) from the ischial tuberosities to the sigmoid flexure, bladder (entire contents), femurs down to the superior aspect of the lesser trochanter, and the external contour. The penile bulb and corporal bodies were outlined for reference; no dose constraints were placed on these structures. The pelvic lymph nodes were added as a target structure in men with high-risk features after the results from Radiation Therapy Oncology Group (RTOG) protocol 94-13 became available (21). Our policy now is to outline and treat the external iliac, obturator, and proximal internal iliac lymph nodes (Fig. 1) using the vessels as a guide. The lymph nodes are outlined up to the bifurcation of the common iliac vessels. As described by Price et al. (22), six artificial ring-shaped structures were also defined to aid in reducing dose to the normal tissues.
Fig. 1.
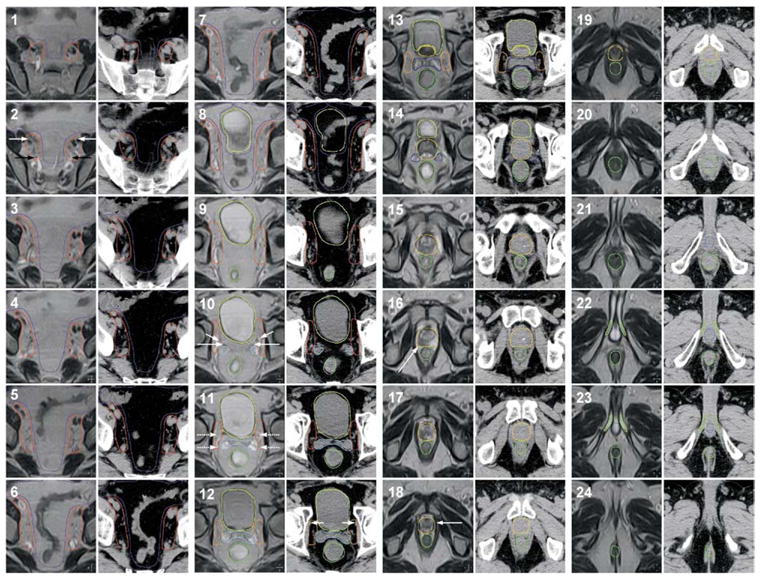
Illustration of the target and normal tissue volumes. Magnetic resonance and computed tomography images were obtained at 3-mm intervals and fused. Every other image slice (every 6 mm) is displayed. The structures outlined are displayed as follows: urinary bladder, yellow; rectum, dark green; prostate, orange; proximal seminal vesicles, dark blue; distal seminal vesicles, light blue; periprostate lymph nodes, mustard; pelvic lymph nodes, red; bowel (area of potential small bowel and distal colon/sigmoid), purple; penile bulb, royal blue; corporal bodies, light green. The following structures are labeled: external iliac vessels (panel 2, white arrow); internal iliac vessels (panel 2, black arrow); ureter (panel 10, short white arrow); vas deferens (panel 10, long white arrow); vesicoprostatic venous plexus (panel 11, dashed white arrow); obturator vessel (panel 12, short white arrow); intraprostatic mass (panel 16, long white arrow); prostatic apex (panel 18, arrow).
The gross tumor volume (GTV) was the prostate, any extraprostatic spread identified on imaging, and the proximal seminal vesicles. The primary clinical target volume (CTV1) encompassed the GTV, although an additional approximately 6–9 mm was added inferiorly below the last slice on which the prostate apex was seen on MRI because of lack of capsule in this region. The contour was generous (an extra 1–2 mm) around obvious areas of bulky/extraprostatic tumor seen on MRI. The CTV1 for intermediate-risk patients included the prostate and proximal seminal vesicles (approximately 9 mm); these structures were usually outlined separately to facilitate daily B-mode acquisition and targeting ultrasound alignment (NOMOS, Cranberry Township, PA) and then grouped as the CTV1 for planning. In high-risk patients, the CTV1 included at least 50% of the seminal vesicles (all gross disease extending to the seminal vesicles received the full dose), in addition to the prostate and any extraprostatic extension. In the high-risk patients, the CTV2 comprised the distal portions of the seminal vesicles and the CTV3 comprised the periprostatic, periseminal vesicle, external iliac, obturator, and internal iliac lymph nodes (Fig. 1). The PTV1s were planned to receive a D95% of the prescription dose or higher. The PTV2s and PTV3s were planned to receive a D95% of ≥56 Gy in the CIMRT arm and ≥52–50 Gy in the HIMRT arm (initially it was 52 Gy, but was changed to 50 Gy after the protocol opened to better equate the subclinical dose for the two treatment arms biologically). The PTV2 and PTV3 doses were applied over the full number of fractions (38 for Arm I and 26 for Arm II).
The PTV1, PTV2, and PTV3 margins were consistent within each arm, but were different for the two treatment groups. For CIMRT, the desired PTVs were 8 mm in all dimensions except posteriorly (the prostate-rectal interface for PTV1), in which the margin was 5 mm. For HIMRT, the desired PTVs were 7 mm in all dimensions except posteriorly, in which the margin was 3 mm. The PTV margins were smaller for the HIMRT arm to reduce the potential increased complication risk from hypofractionation. This strategy was based in part on the Cleveland Clinic experience using a similar technique (18) and the rationale that the 90% line in the HIMRT plans would fall in about the same position as the 100% line in the CIMRT arms. The effective PTV in the CIMRT plans (i.e., where the prescription line was located relative to the CTVs as monitored on a transverse slice by slice basis) was 8–13 mm in all dimensions, except posteriorly, in which the effective PTV was 3–8 mm. The effective PTV in the HIMRT plans was 5–10 mm around the CTVs in all dimensions, except posteriorly, in which the PTV was 2–6 mm.
IMRT plan evaluation and acceptance
Step-and-shoot IMRT was planned using the Corvus (NOMOS) treatment planning system. A series of dose–volume histograms were generated and analyzed to determine the adequacy of the plan. At least 95% of the PTV (D95%) was to receive the prescribed dose; a variation was noted if <95% to 90% of the PTV received the prescribed dose and a protocol violation was noted if <90% of the PTV received the prescribed dose. There were no variations for the PTV D95% (median, 100%). The maximum dose heterogeneity allowable in the PTV was 20%. There were 8 patients in the HIMRT (overall median heterogeneity = 17.2%) and 5 patients in the CIMRT (median overall = 15.9%) groups that had dose gradients above 20%. These were considered variations (dose gradient > 20–25%); no violations (dose gradient > 25%) were observed. Because the dose is prescribed to the minimum isodose line encompassing the PTV, the dose variability was seen in portions of the target volume receiving higher than the specified dose.
The normal tissue planning limits for the bladder and rectum were set based on prior studies (23, 24). The plan was deemed acceptable under the following conditions. Less than or equal to 17% and 35% of the rectum should receive ≥65 Gy (V65 Gy) and ≥40 Gy (V40 Gy), respectively, for the conventionally fractionated patients (Arm I, 76 Gy total dose). The bladder V65 Gy and V40 Gy was ≤25% and ≤50% in Arm I patients. The rationale for these cutpoints has been described previously (25–27). The criteria for the bladder were relaxed because a meaningful dosimetric cutpoint has not been defined.
For Arm II, the rectal V50 Gy and V31 Gy were ≤17% and ≤35%. The bladder V50 Gy and V31 Gy were ≤25% and ≤50%. The derivation of the V50 Gy and V31 Gy criteria for the Arm II patients was based on very conservative extrapolations from the V65 Gy and V40 Gy parameters used for Arm I patients. The α/β ratio for late effects was assumed to be the same as that for prostate cancer tumor control (α/β ratio 1.5) and was probably too conservative, as described in the Discussion section.
If the volume of the rectum or bladder exceeded the dose limits described by <7.5%, this was classified as a variation. The inclusion of rectal volumes beyond these constraints was considered a protocol violation. The inclusion of bladder volumes beyond these constraints was considered a secondary protocol variation; it was not considered a protocol violation because a distinct bladder dose–volume histogram relationship has not been defined previously. The variations and violations in Arm I (standard fractionation) were as follows: rectal V65 Gy, one variation (0.3% above constraint) and no violations; rectal V40 Gy, two variations (1.4% and 2.5% above constraint); bladder V65 Gy, 5 variations and two secondary variations (median, 4.8% above constraint; range, 0.2–18.6%); bladder V40 Gy, four variations and three secondary variations (median, 7.2% above constraint; range, 2.9–26.3%). The variations and violations in Arm II (hypofractionation) were as follows: rectal V50 Gy, 17 variations and no violations (median, 2.3% above constraint; range, 0.1–5.4%); rectal V31 Gy 10 variations and no violations (median, 4.0% above constraint; range, 0.1–6.5%); bladder V50 Gy, 12 variations and 12 secondary variations (median, 7.6% above constraint; range, 0.2–35.3%); and bladder V31 Gy, 3 variations and 11 secondary variations (median, 20.9% range, 2.0–41.9% above constraint).
Endpoint and statistics
The primary endpoint of the study is FFBF using the American Society for Therapeutic Radiology and Oncology consensus guidelines (28). In this communication, we describe the acute side effects of radiotherapy using modified RTOG and Late Effects Normal Tissue Task Force (LENT) criteria, modeled after that described by Hanlon et al. (29) and later Storey et al. (23).
The acute gastrointestinal (GI) side effect grading scale is as follows. Grade I: Increased frequency or change in quality of bowel habits needing ≤2 antidiarrheals per week; Rectal discomfort not requiring analgesics; Mild rectal bleeding not needing medication: Grade II; Diarrhea needing more than two antidiarrheals per week; Mucous discharge requiring one sanitary pad per day; Rectal pain needing analgesics or occasional narcotics; Rectal bleeding needing Anusol HC or other medication; Rectal bleeding or other GI symptoms requiring a treatment break of ≤1 week: Grade III: Diarrhea needing more than two antidiarrheals per day or parenteral support; Severe mucous discharge requiring more than one sanitary pad per day; Rectal pain requiring frequent narcotics (≥1/day) for more than a week; GI bleeding requiring one transfusion; Rectal bleeding or GI symptoms requiring a treatment break of >1 week: Grade IV; Acute or subacute obstruction; GI bleeding requiring more than one transfusion; Fistula or perforation; Abdominal pain or tenesmus requiring bowel diversion.
The acute genitourinary (GU) grading scale is: Grade I: Frequency or nocturia twice pretreatment habit or non-narcotic medication (e.g., alpha blocker) once per day over baseline; Dysuria not needing medication; Microscopic or infrequent gross hematuria not needing medication: Grade II: Frequency or nocturia less frequent than hourly; Dysuria or bladder spasm needing an anesthetic (Pyridium or occasional narcotics); Hematuria or GU symptoms requiring medication or a treatment break of ≤1 week. Infrequent gross hematuria needing medical intervention. Urinary obstruction requiring temporary catheterization (including Foley or self-catheterization) for ≤1 week: Grade III: Frequency or nocturia hourly or more; Dysuria, pain, or spasm needing narcotics >1 dose/day for >1 week; Hematuria or GU symptoms requiring a treatment break >1 week; Gross hematuria requiring one transfusion; Urinary obstruction from prostate inflammation or clots requiring catheterization (including Foley or self-catheterization or suprapubic) for >1 week: Grade IV: Hematuria needing more than one transfusion; Hospitalization for sepsis from obstruction, ulceration, or necrosis of the bladder.
Two-sample t tests were used to assess differences between dosimetric parameters according to treatment arms. Confirmatory analyses were performed using nonparametric Wilcoxon tests. Similar methodology was used to evaluate differences in International Prostate Symptom Scores according to treatment groups. Stepwise ordinal logistic regression modeling was used to determine independent predictors of changes in GU and GI toxicity, relative to pretreatment function assessed using the same grading scale. The variable for the change in acute toxicity was coded as follows: no change in toxicity acutely was coded as a 0; an increase in toxicity from Grade 0 to Grade 1 was coded as a 1; from Grade 1 to Grade 2 as a 2; from Grade 2 to Grade 3 as a 3; from Grade 0 to Grade 2 as a 3; from Grade 1 to Grade 3 as a 3; and Grade 0 to Grade 3 as a 4. Covariates included: maximum dose received by the bladder (continuous), maximum dose received by the rectum (continuous), rectal volume (continuous), bladder volume (continuous), rectal V65 Gy/V50 Gy (continuous), rectal V40 Gy/V31 Gy (continuous), bladder V65 Gy/V50 Gy (continuous), bladder V40 Gy/V31 Gy (continuous), PTV1 volume (continuous), PTV1 mean dose (continuous), PTV1 maximum dose (continuous), androgen deprivation therapy (no vs. yes), iPSA (continuous), T-stage (T1–T2 vs. T3), risk group (intermediate vs. high), and treatment group (Arm I vs. Arm II).
RESULTS
Table 1 shows the distribution of patients in the study cohort subdivided by treatment arm and Gleason score, T-stage, iPSA, whether or not neoadjuvant androgen deprivation was given before protocol enrollment and risk group assignment. There were no significant differences in the makeup of the patients in the treatment arms.
Table 1. Patient characteristics.
| Type | Subgroup | Arm I* | Arm II* |
|---|---|---|---|
| Gleason score | 5–6 | 15 (30%) | 24 (48%) |
| 7 | 24 (48%) | 18 (36%) | |
| 8–10 | 11 (22%) | 8 (16%) | |
| T stage | T1–T2 | 43 (86%) | 43 (86%) |
| T3 | 7 (14%) | 7 (14%) | |
| iPSA | <10 ng/mL | 30 (60%) | 26 (52%) |
| 10–20 ng/mL | 15 (30%) | 16 (32%) | |
| >20 ng/mL | 5 (10%) | 8 (16%) | |
| AD | No | 28 (56%) | 28 (56%) |
| Yes | 22 (44%) | 22 (44%) | |
| Risk group | Intermediate | 32 (64%) | 33 (66%) |
| High | 18 (36%) | 17 (34%) |
Abbreviations: iPSA = initial pretreatment PSA; AD = androgen deprivation (intermediate-risk patients were allowed to have up to 4 months of neoadjuvant AD prior randomization and high risk patients were planned to receive 2 years of AD).
Number (percent) shown. There were no significant differences between the arms.
Table 2 displays a summary of the CTV1, PTV1, and rectal, bladder, and femoral head dosimetric parameters. The prescribed PTV1 dose in Arm I was 76 Gy and the mean dose was 81.1 Gy. The prescribed PTV1 dose in Arm II was 70.2 Gy and the mean was 73.8 Gy. As would be expected, there were significant differences in the PTV D95, mean, maximum, and minimum doses, with Arm I patients receiving higher RT doses. There were no statistical differences between the arms in the CTV1 volumes, PTV1 volumes, rectal volumes, and bladder volumes. There were statistically higher volume percentages of the rectum treated to more than both the high (V50 Gy) and low (V31 Gy) dose cutpoints in Arm II, as compared with Arm I (V65 Gy and V40 Gy). A similar pattern was observed for the bladder. Likewise, for the femoral heads, the percent volumes treated above the constraints (V50 Gy for Arm I and V40 Gy for Arm II) were higher in Arm II.
Table 2. Dosimetric parameters for the PTV1, rectum, bladder, and femoral heads.
| Mean ± SEM
|
||
|---|---|---|
| Parameter | Arm I | Arm II |
| CTV1 volume (cm3) | 54.2 ± 3.1 | 65.0 ± 4.1 |
| PTV1 volume (cm3) | 145.1 ± 5.8 | 158.4 ± 7.5 |
| Rectal volume (cm3) | 58.8 ± 2.4 | 57.4 ± 2.7 |
| Bladder volume (cm3) | 261.0 ± 18.8 | 254.9 ± 18.7 |
| PTV1 D95% (Gy)* | 76.0 ± 0.2 | 70.4 ± 0.1 |
| PTV1 mean dose (Gy)* | 81.1 ± 0.3 | 73.8 ± 1.0 |
| PTV1 max dose (Gy)* | 88.4 ± 0.3 | 82.4 ± 0.3 |
| PTV1 min dose (Gy)* | 62.8 ± 1.2 | 55.7 ± 1.2 |
| Rectal V65/50 Gy (%)* | 11.7 ± 0.4 | 15.9 ± 0.5 |
| Rectal V40/31 Gy (%)* | 28.1 ± 1.2 | 29.8 ± 0.9 |
| Bladder V65/50 Gy (%)* | 16.1 ± 1.2 | 27.1 ± 1.8 |
| Bladder V40/31 Gy (%)* | 34.8 ± 2.2 | 45.6 ± 2.7 |
| Bladder max dose (Gy)* | 86.8 ± 0.3 | 80.6 ± 0.3 |
| Rectal max dose (Gy)* | 86.4 ± 0.3 | 79.4 ± 0.3 |
| Right femoral head V50/40 Gy (%)* | 1.0 ± 0.3 | 3.1 ± 0.5 |
| Left femoral head V50/40 Gy (%)* | 1.4 ± 0.4 | 3.4 ± 0.5 |
Abbreviations: Arm I = conventional fraction intensity-modulated radiation therapy; Arm II = hypofractionation intensity-modulated radiation therapy; CTV = clinical tumor volume; PTV = planning target volume; D95% = dose to 95% of the volume.
p < 0.05 between groups, two-sided Wilcoxon test.
The dosimetric parameters related to the distal seminal vesicles and pelvic lymph nodes for the unfavorable patients are shown in Table 3. The lymph nodes were not included in the intermediate-risk patients and in a few of the high-risk patients initially treated on the protocol. As would be expected, the seminal vesicle and lymph node PTV D95% and mean doses were significantly higher in the Arm I patients.
Table 3. Dosimetric parameters for the distal seminal vesicles and lymph nodes in unfavorable patients.
| Mean ± SEM
|
||
|---|---|---|
| Parameter | Arm I | Arm II |
| SV-CTV2 volume (cm3) | 10.7 ± 1.6 | 11.9 ± 1.9 |
| SV-PTV2 volume (cm3) | 51.8 ± 4.8 | 55.1 ± 4.5 |
| SV-PTV2 D95% (Gy)* | 62.3 ± 0.6 | 56.1 ± 0.5 |
| SV-PTV2 mean dose (Gy)* | 74.6 ± 0.8 | 67.9 ± 0.5 |
| LN-CTV3 volume (cm3) | 36.4 ± 4.8 | 36.1 ± 5.5 |
| LN-PTV3 volume (cm3) | 135.9 ± 15.1 | 142.7 ± 15.3 |
| LN-PTV3 D95% (Gy)* | 58.3 ± 0.4 | 54.5 ± 0.5 |
| LN-PTV3 mean dose (Gy)* | 68.3 ± 0.6 | 64.3 ± 0.3 |
Abbreviations: SV-PTV2 = seminal vesicle planning target volume; D95% = dose to 95% of the volume; LN-PTV3 = lymph node planning target volume; SV = seminal vesicles; LN = lymph nodes; PTV = planning target volume; CTV = clinical target volume.
p < 0.05 between groups, nonparametric Wilcoxon test.
The maximum acute GU toxicity that was observed during treatment and at the first 3-month follow-up visit is displayed in Table 4. The majority in both Arms I and II experienced Grade 1–2 GU side effects during radiotherapy. There were slightly more Arm II patients with Grade 3 reactions (one in Arm I and four in Arm II); this difference was not statistically significant. At the first 3-month follow-up visit, the majority experienced no GU side effects with <10% experiencing Grade 2 toxicity. There was no statistical difference in acute toxicity at the 3 month follow-up visit. As another assessment of urinary function, the International Prostate Symptom Score (30) was determined before treatment and at the 3-month follow-up visit (Table 5). There was no statistically significant difference between Arms I and II. Figure 2 shows that there were no significant differences between the CIMRT and HIMRT treatment groups in the average weekly on-treatment changes in acute GU toxicity. There were 8% of those in Arm I and 16% in Arm II taking medication to improve urinary function (e.g., alpha blocker, antispasmodic, analgesic such as Pyridium) before treatment. At the 3-month follow-up, there were 14% and 16% in Arms I and II taking medication to improve urinary function; the difference in change was not significant.
Table 4. Maximum acute genitourinary toxicity.
| Grade
|
|||||
|---|---|---|---|---|---|
| Group | Timing | 0 | I | II | III |
| Arm I | During radiation therapy | 8 (16%) | 14 (28%) | 27 (54%) | 1 (2%) |
| Arm II | During radiation therapy | 4 (8%) | 22 (44%) | 20 (40%) | 4 (8%) |
| Arm I | At 3-month follow-up | 30 (61%) | 15 (31%) | 4 (8%) | 0 |
| Arm II | At 3-month follow-up | 32 (64%) | 15 (30%) | 3 (6%) | 0 |
There were no significant differences between the arms.
Table 5. International prostate symptom score evaluation.
| Mean ± SEM
|
||
|---|---|---|
| Group | Before radiation therapy | After radiation therapy* |
| Arm I | 7.3 ± 0.8 | 6.8 ± 0.7 |
| Arm II | 8.4 ± 1.0 | 6.9 ± 0.7 |
There were no significant differences between the arms.
At first 3-month follow-up.
Fig. 2.
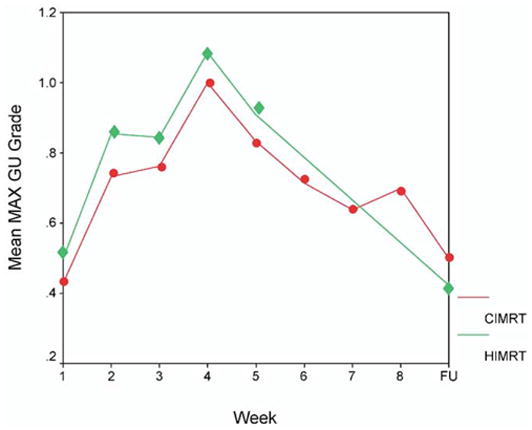
Mean maximum genitourinary (GU) toxicity is plotted by week of treatment. The y axis is scaled from 0 to 1.2. The toxicity at the 3 month follow-up is displayed at the far right on the x-axis (labeled FU). The conventional fractionation intensity-modulated radiation therapy (76 Gy in 38 fractions; solid circles) patients are compared with the hypofractionation intensity-modulated radiation therapy (70.2 Gy in 26 fractions; solid diamonds) patients. CIMRT = conventional fractionation intensity-modulated radiation therapy; HIMRT = hypofractionated intensity-modulated radiation therapy.
The maximum GI toxicities observed during treatment and at the first 3-month follow-up visit are displayed in Table 6. There were no statistically significant differences in acute GI toxicity during treatment; however, there were slightly more men with Grade 2 reactions in Arm II (9 vs. 4 in Arm I). At the first 3-month follow-up visit, about 85% experienced no GI side effects with <5% experiencing Grade 2 side effects. Figure 3 displays the average weekly on-treatment changes in GI morbidity, demonstrating that at Weeks 2, 3, and 4 of treatment, there was a slight, but significant increase in mean maximum toxicity seen in the HIMRT-treated men.
Table 6. Maximum acute gastrointestinal toxicity.
| Grade
|
|||||
|---|---|---|---|---|---|
| Group | Timing | 0 | I | II | III |
| Arm I | During radiation therapy | 26 (52%) | 20 (40%) | 4 (8%) | 0 |
| Arm II | During radiation therapy | 21 (42%) | 20 (40%) | 9 (18%) | 0 |
| Arm I | At 3-month follow-up* | 42 (86%) | 6 (12%) | 1 (2%) | 0 |
| Arm II | At 3-month follow-up | 42 (84%) | 8 (16%) | 0 | 0 |
There were no significant differences between the arms.
One patient had an unrelated abdominoperineal resection and could not be assessed.
Fig. 3.
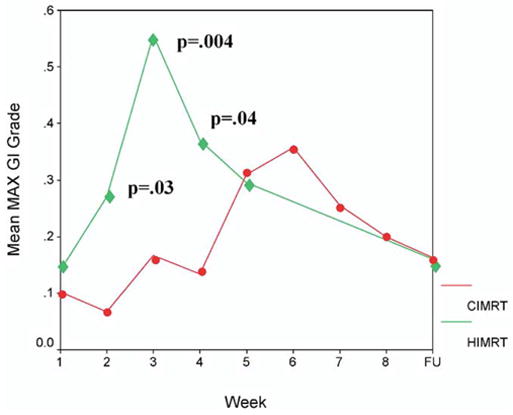
Mean maximum gastrointestinal (GI) toxicity is plotted by week of treatment. The y axis is scaled from 0 to 0.6. The toxicity at the 3-month follow-up is displayed at the far right (labeled FU). The conventional fractionation intensity-modulated radiation therapy (76 Gy in 38 fractions; solid circles) patients are compared to the hypofractionation intensity-modulated radiation therapy (70.2 Gy in 26 fractions; solid diamonds) patients. Statistically different points are labeled with the respective p value. CIMRT = conventional fractionated intensity-modulated radiation therapy; HIMRT = hypofractionated intensity-modulated radiation therapy.
Multivariate analysis via logistic regression was used to determine covariates associated with an increase in acute GI and GU morbidity over pretreatment symptoms. When possible, the covariates were included as continuous variables. The only covariate shown to be related to increased acute rectal reactions was the composite DVH V65 Gy/V50 Gy parameter (Table 7). The rectal high dose percent volume constraints, the V65 Gy and V50 Gy for Arms I and II, were put together as a single continuous variable. The higher the percentages of rectum exposed to these threshold doses, the greater the risk of a Grade 2 or higher acute rectal reaction. Neither treatment arm, nor any of several other dosimetric or volume parameters were significant. Risk group designation (intermediate vs. high risk), and the administration of androgen deprivation were not significant.
Table 7. Multivariate analysis of maximum acute gastrointestinal toxicity.
| Variable | Chi-square | RR (95% CI) | p value |
|---|---|---|---|
| Rectal V65/50 | 3.9 | 1.109 (1.002–1.228) | 0.046 |
Covariates not significant: bladder maximum dose; rectal maximum dose; rectal volume; rectal V40/31; PTV1 volume; PTV1 mean dose; PTV1 maximum dose; iPSA; AD (no vs. yes); T-stage (T1–T2 vs. T3); risk group (intermediate vs. high); treatment group (Arm I vs. Arm II). Covariates were continuous unless indicated otherwise.
The increase in acute toxicity over pretreatment status was used (see Patients and Methods) in stepwise ordinal logistic regression.
Table 8 displays the logistic regression results for covariates related to increased acute GU morbidity. A smaller bladder volume at planning was independently associated with an increase in acute effects.
Table 8. Multivariate analysis of maximum acute genitourinary toxicity.
| Variable | Chi-square | RR (95% CI) | p value |
|---|---|---|---|
| Bladder volume | 6.0 | 0.996 (0.994–0.999) | 0.010 |
Covariates not significant: bladder maximum dose; rectum maximum dose; rectal volume; bladder V65/V50; bladder V40/31; PTV1 mean dose; PTV1 maximum dose; iPSA; AD (no vs. yes); T-stage (T1–T2 vs. T3); risk group (intermediate vs. high); treatment group (Arm I vs. Arm II). Covariates were continuous unless indicated otherwise.
The increase in acute toxicity over pretreatment status was used (see Patients and Methods) in stepwise ordinal logistic regression.
DISCUSSION
Dose escalation and hypofractionation
The biochemical response of prostate cancer to RT dose escalation is pronounced. The randomized trials from the M.D. Anderson Cancer Center (12) and Proton Radiation Oncology Group (31), and the supportive prospective sequential and retrospective series provide rather convincing evidence that RT doses above 75.6 Gy to the PTV are essential in men at intermediate-to-high risk. Given the prohibitive cost of extending IMRT treatments to 9 weeks or more and the different radiobiologic properties of prostate cancer and the surrounding normal tissues, hypofractionation is an attractive strategy that should be investigated in randomized trials.
Hypofractionation for prostate cancer has been used for many years without substantial toxicity (32–35, 18, 36). An extreme example is an older regimen of 36 Gy given in 6 Gy fractions administered twice weekly (33). More commonly, 3–3.5 Gy fractions have been used. A similar hypofractionation strategy was recently tested in a randomized trial of 66 Gy in 33 fractions vs. 52.5 Gy in 20 fractions (37). In the preliminary report of this trial, there was no statistically significant difference in biochemical failure. The Christie, Royal Marsden, and Princess Margaret Hospitals are all looking at ≥3 Gy fractions to 57–66 Gy (35, 36, 38).
In the trial described here, the PTV1 dose of 70.2 Gy in 26 fractions is equivalent to 84.4 Gy in 2 Gy fractions using an α/β ratio of 1.5 (1, 6). Recently, Brenner (17) summarized the evidence indicating that the α/β ratio for late rectal effects is 5.4. Thus the equivalent dose in 2 Gy fractions for rectal late effects, at least to the anterior rectal wall, would be 76.9 Gy. The approach used in developing the dosimetric constraints applied in this study was that hypofractionation was no safer than standard fractionation. Considering that the rectal dosimetric constraints were calculated using an α/β ratio of 1.5 for the rectum, and that the biologically equivalent dose for late effects to the rectum is probably similar for both protocol arms, adherence to the protocol constraints for the rectum, and possibly the bladder as well, could result in fewer late rectal reactions.
The α/β ratio for late genitourinary side effects is not known and may be different. However, Table 2 shows that the proportions of the rectum and bladder treated to the ≥50 Gy (V50 Gy) and ≥31 Gy (V31 Gy) in Arm II were higher than to the V65 Gy and V40 Gy in Arm I. In addition, there were significantly more protocol variations for the rectal and bladder V50 and V31 Gy in Arm II than in Arm I, indicating that the constraints used were more difficult to adhere to and possibly too strict. These factors and the use of smaller PTV margins in the HIMRT arm should be considered in interpreting the results that overall acute effects were not significantly different between the treatment arms.
Incidence of acute toxicity
The acute effects observed for the patients treated herein were comparable for the most part to those reported by others (23, 39–49), although there were some differences. We describe about a 48% rate of Grade 2 or higher maximum genitourinary reactions (Table 4), whereas the average in the other reports is 35% (range, 28–56%). The slightly higher than average incidence of genitourinary reactions may be related to our use of a modified RTOG scale, the inclusion of lymph nodes in the high-risk patients and that mean biologic doses to the prostate, and hence urethra, were in excess of 80 Gy. The drop in Grade 2 or higher acute genitourinary toxicity to <10% by 3 months after the completion of radiotherapy is noteworthy. In terms of Grade 2 or higher maximum gastrointestinal reactions, the average reported by others is about 30% (range, 14–52%) (23, 39–48). Our finding of about 13% (Table 6) is at the low end. By 3 months after completion of radiotherapy, only 1 patient still had Grade 2 gastrointestinal toxicity.
We observed a slightly higher frequency of gastrointestinal acute toxicity in the hypofractionation arm during Weeks 2–4 of radiotherapy, although the maximum mean grade of reactions was less than 0.6. Yang et al. (50) described weekly GI toxicity in men treated with three-dimensional conformal radiotherapy to 65–70 Gy for prostate cancer and found slightly higher mean reactions that peaked between Weeks 4–6 of treatment. Peeters et al. (49) also found that peak GI toxicity was seen in the latter weeks of treatment with standard fractionation to 68–78 Gy. In our study, the peak mean GI reactions were at 3 weeks for Arm II and 5–6 weeks for Arm I.
Lukka et al. (37) compared 66 Gy in 33 fractions to 52.2 Gy in 20 fractions and found increases in both acute urinary (5.1 to 9.2%) and rectal (2.8 to 4.3%) toxicity in the men randomized to the hypofractionation arm. Kupelian et al. (51) contrasted the acute toxicity in men with prostate cancer treated sequentially using three-dimensional conformal radiotherapy to 78 Gy in 39 fractions and later with IMRT to 70 Gy in 28 fractions. They found comparable rates of Grade 2 or higher acute urinary (20% conformal vs. 21% IMRT) and rectal (19% conformal vs. 14% IMRT) toxicity. Kitamura et al. (52) used the same hypofractionation scheme of 70 Gy in 28 fractions combined with real-time tumor tracking and reported even lower acute reactions. With the limited data available, there is no consistent pattern of the degree of acute reactions from the hypofractionated treatment of prostate cancer, probably because of differences in treatment methods, target and normal tissue definitions, and normal tissue constraints used.
Dose–volume histogram associations
Dose–volume histogram parameters were included in logistic regression analyses to identify correlates of acute normal tissue toxicity. The only significant determinant of increased gastrointestinal reactions was the high-dose rectal constraint (V65 Gy for Arm I and V50 Gy for Arm II; see Table 7); the complication risk was greater when the volume of rectum receiving over these doses was higher. A consideration in interpreting these data are that for the HIMRT patients the V50 cutpoint was stricter because the extrapolation from the V65 cutpoint for the CIMRT patients was based on an α/β ratio of 1.5 for late rectal toxicity. Nuyttens et al. (41), Karlsdottir et al. (47), and Peeters et al. (49) found that patients in the higher dose–volume groups had more acute GI toxicity. Others have not found any relationships between acute GI toxicity and dose–volume parameters (23, 40, 46, 53, 54).
Acute genitourinary toxicity was found to be most dependent on the bladder volume at planning (Table 8); the complication risk was greater when the bladder volume was smaller. Karlsdottir et al. (47) and Michalski et al. (54) observed that the proportion of the bladder receiving over a reference dose was an independent correlate of acute GU reactions. Beckendorf et al. (45) reported that acute GU reactions were dependent on the volume of the CTV1 and PTV1. Others have not observed any target volume or normal tissue dose–volume dosimetric relationships with acute GU toxicity (23, 41, 46, 49, 53).
In conclusion, there was a small, but significant increase in acute GI reactions at Weeks 2–4 of treatment in the HIMRT arm. Overall, there was little difference in acute morbidity between the standard and hypofractionation randomization arms of the IMRT-based treatments used here, although PTV margins were slightly smaller in the hypofractionation arm. Dose–volume criteria were related to treatment-related increases in acute GI and GU reactions. Longer follow-up is needed to determine the significance of these associations with late toxicity.
Acknowledgments
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. The authors thank Teri White, R.N., and Elaine Callahan, R.N., for their help with the implementation of the protocol and Ruth Peter, R.N., Th.M., for database management.
Footnotes
Supported in part by National Cancer Institute Grants CA101984-01 and CA-00692.
References
- 1.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095–1101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 2.King CR, Mayo CS. Is the prostrate alpha/beta ratio of 1.5 from Brenner & Hall a modeling artifact. Int J Radiat Oncol Biol Phys. 2000;47:536–539. doi: 10.1016/s0360-3016(00)00442-9. [DOI] [PubMed] [Google Scholar]
- 3.D’Souza WD, Thames HD. Is the alpha/beta ratio for prostate cancer low? Int J Radiat Oncol Biol Phys. 2001;51:1–3. doi: 10.1016/s0360-3016(01)01650-9. [DOI] [PubMed] [Google Scholar]
- 4.Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys. 2001;50:1021–1031. doi: 10.1016/s0360-3016(01)01607-8. [DOI] [PubMed] [Google Scholar]
- 5.King CR, Fowler JF. A simple analytic derivation suggests that prostate cancer alpha/beta ratio is low. Int J Radiat Oncol Biol Phys. 2001;51:213–214. doi: 10.1016/s0360-3016(01)01651-0. [DOI] [PubMed] [Google Scholar]
- 6.Brenner DJ, Martinez AA, Edmundson GK, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6–13. doi: 10.1016/s0360-3016(01)02664-5. [DOI] [PubMed] [Google Scholar]
- 7.King CR, Fowler JF. Yes, the alpha/beta ratio for prostate cancer is low or “methinks the lady doth protest too much. about a low alpha/beta that is”. Int J Radiat Oncol Biol Phys. 2002;54:626–627. doi: 10.1016/s0360-3016(02)02922-x. author reply 627–628. [DOI] [PubMed] [Google Scholar]
- 8.Carlone M, Wilkins D, Nyiri B, et al. Comparison of alpha/beta estimates from homogeneous (individual) and heterogeneous (population) tumor control models for early stage prostate cancer. Med Phys. 2003;30:2832–2848. doi: 10.1118/1.1612946. [DOI] [PubMed] [Google Scholar]
- 9.Fowler JF, Ritter MA, Fenwick JD, et al. How low is the alpha/beta ratio for prostate cancer? In regard to Wang, et al. IJROBP. 2003;55:194–203. doi: 10.1016/s0360-3016(03)00364-x. [DOI] [PubMed] [Google Scholar]; Int J Radiat Oncol Biol Phys. 2003;57:593–595. author reply 595–596. [Google Scholar]
- 10.Nahum AE, Movsas B, Horwitz EM, et al. Incorporating clinical measurements of hypoxia into tumor local control modeling of prostate cancer: Implications for the alpha/beta ratio. Int J Radiat Oncol Biol Phys. 2003;57:391–401. doi: 10.1016/s0360-3016(03)00534-0. [DOI] [PubMed] [Google Scholar]
- 11.Wang JZ, Guerrero M, Li XA. How low is the alpha/beta ratio for prostate cancer? Int J Radiat Oncol Biol Phys. 2003;55:194–203. doi: 10.1016/s0360-3016(02)03828-2. [DOI] [PubMed] [Google Scholar]
- 12.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: Results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 13.van der Kogel AJ, Jarrett KA, Paciotti MA, et al. Radiation tolerance of the rat rectum to fractionated X-rays and pimesons. Radiother Oncol. 1988;12:225–232. doi: 10.1016/0167-8140(88)90265-4. [DOI] [PubMed] [Google Scholar]
- 14.Deore SM, Shrivastava SK, Supe SJ, et al. Alpha/beta value and importance of dose per fraction for the late rectal and recto-sigmoid complications. Strahlenther Onkol. 1993;169:521–526. [PubMed] [Google Scholar]
- 15.Gasinska A, Dubray B, Hill SA, et al. Early and late injuries in mouse rectum after fractionated X-ray and neutron irradiation. Radiother Oncol. 1993;26:244–253. doi: 10.1016/0167-8140(93)90266-b. [DOI] [PubMed] [Google Scholar]
- 16.Dubray BM, Thames HD. Chronic radiation damage in the rat rectum: An analysis of the influences of fractionation, time and volume. Radiother Oncol. 1994;33:41–47. doi: 10.1016/0167-8140(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 17.Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60:1013–1015. doi: 10.1016/j.ijrobp.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Kupelian PA, Reddy CA, Carlson TP, et al. Preliminary observations on biochemical relapse-free survival rates after short-course intensity-modulated radiotherapy (70 Gy at 2.5 Gy/fraction) for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53:904–912. doi: 10.1016/s0360-3016(02)02836-5. [DOI] [PubMed] [Google Scholar]
- 19.Pollack A, Hanlon AL, Horwitz EM, et al. Prostate cancer radiotherapy dose response: An update of the Fox Chase experience. J Urol. 2004;171:1132–1136. doi: 10.1097/01.ju.0000111844.95024.74. [DOI] [PubMed] [Google Scholar]
- 20.Pollack A, Kuban DA, Zagars GK. Impact of androgen deprivation therapy on survival in men treated with radiation for prostate cancer. Urology. 2002;60:22–30. doi: 10.1016/s0090-4295(02)01564-9. [DOI] [PubMed] [Google Scholar]
- 21.Roach M, 3rd, DeSilvio M, Lawton C, et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003;21:1904–1911. doi: 10.1200/JCO.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Price RA, Murphy S, McNeeley SW, et al. A method for increased dose conformity and segment reduction for SMLC delivered IMRT treatment of the prostate. Int J Radiat Oncol Biol Phys. 2003;57:843–852. doi: 10.1016/s0360-3016(03)00711-9. [DOI] [PubMed] [Google Scholar]
- 23.Storey MR, Pollack A, Zagars G, et al. Complications from radiotherapy dose escalation in prostate cancer: Preliminary results of a randomized trial. Int J Radiat Oncol Biol Phys. 2000;48:635–642. doi: 10.1016/s0360-3016(00)00700-8. [DOI] [PubMed] [Google Scholar]
- 24.Huang EH, Pollack A, Levy L, et al. Late rectal toxicity: Dose-volume effects of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002;54:1314–1321. doi: 10.1016/s0360-3016(02)03742-2. [DOI] [PubMed] [Google Scholar]
- 25.Pollack A, Hanlon A, Horwitz EM, et al. Radiation therapy dose escalation for prostate cancer: A rationale for IMRT. World J Urol. 2003;21:200–208. doi: 10.1007/s00345-003-0356-x. [DOI] [PubMed] [Google Scholar]
- 26.Buyyounouski MK, Horwitz EM, Price RA, et al. IMRT for prostate cancer. In: Bortfeld T, Schmidt-Ullrich R, de Neve W, editors. IMRT handbook: Concepts & clinical applications. Heidelberg, Germany: Springer-Verlag; 2005. in press. [Google Scholar]
- 27.Pollack A, Price R, Dong L, et al. Intact prostate cancer: Overview. In: Mundt AJ, Roeske JC, editors. Intensity modulated radiation therapy: A clinical perspective. Ontario, Canada: BC Decker; 2005. pp. 459–463. [Google Scholar]
- 28.Cox J, Grignon D, Kaplan R, et al. Consensus statement: Guidelines for PSA following radiation therapy. Int J Radiat Oncol Biol Phys. 1997;37:1035–1041. [PubMed] [Google Scholar]
- 29.Hanlon AL, Schultheiss TE, Hunt MA, et al. Chronic rectal bleeding after high dose conformal treatment of prostate cancer warrants modification of existing morbidity scales. Int J Radiat Oncol Biol Phys. 1997;38:59–63. doi: 10.1016/s0360-3016(97)00234-4. [DOI] [PubMed] [Google Scholar]
- 30.Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 31.Zietman A, DeSilvio M, Slater JD, et al. A randomized trial comparing conventional dose (70.2 GyE) and high dose (79.2 GYE) conformal radiation in early stage adenocarcinoma of the prostate: Results of an interim analysis of PROG 95-09. Int J Radiat Oncol Biol Phys. 2004;60(Suppl):S131–132. [Google Scholar]
- 32.Read G, Pointon RC. Retrospective study of radiotherapy in early carcinoma of the prostate. Br J Urol. 1989;63:191–195. doi: 10.1111/j.1464-410x.1989.tb05163.x. [DOI] [PubMed] [Google Scholar]
- 33.Collins CD, Lloyd-Davies RW, Swan AV. Radical external beam radiotherapy for localised carcinoma of the prostate using a hypofractionation technique. Clin Oncol (R Coll Radiol) 1991;3:127–132. doi: 10.1016/s0936-6555(05)80831-3. [DOI] [PubMed] [Google Scholar]
- 34.Duncan W, Warde P, Catton CN, et al. Carcinoma of the prostate: Results of radical radiotherapy (1970–1985) Int J Radiat Oncol Biol Phys. 1993;26:203–210. doi: 10.1016/0360-3016(93)90198-5. [DOI] [PubMed] [Google Scholar]
- 35.Catton CN, Chung P, Haycocks T, et al. Hypofractionated intensity modulated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002;54(Suppl):188. [Google Scholar]
- 36.Livsey JE, Cowan RA, Wylie JP, et al. Hypofractionated conformal radiotherapy in carcinoma of the prostate: Five-year outcome analysis. Int J Radiat Oncol Biol Phys. 2003;57:1254–1259. doi: 10.1016/s0360-3016(03)00752-1. [DOI] [PubMed] [Google Scholar]
- 37.Lukka H, Hayter C, Warde P, et al. A randomized trial comparing two fractionation schedules for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;57(Suppl):S126. doi: 10.1200/JCO.2005.06.153. [DOI] [PubMed] [Google Scholar]
- 38.Mott JH, Livsey JE, Logue JP. Development of a simultaneous boost IMRT class solution for a hypofractionated prostate cancer protocol. Br J Radiol. 2004;77:377–386. doi: 10.1259/bjr/66104316. [DOI] [PubMed] [Google Scholar]
- 39.Chou RH, Wilder RB, Ji M, et al. Acute toxicity of three-dimensional conformal radiotherapy in prostate cancer patients eligible for implant monotherapy. Int J Radiat Oncol Biol Phys. 2000;47:115–119. doi: 10.1016/s0360-3016(00)00422-3. [DOI] [PubMed] [Google Scholar]
- 40.Teh BS, Mai WY, Uhl BM, et al. Intensity-modulated radiation therapy (IMRT) for prostate cancer with the use of a rectal balloon for prostate immobilization: Acute toxicity and dose-volume analysis. Int J Radiat Oncol Biol Phys. 2001;49:705–712. doi: 10.1016/s0360-3016(00)01428-0. [DOI] [PubMed] [Google Scholar]
- 41.Nuyttens JJ, Milito S, Rust PF, et al. Dose-volume relationship for acute side effects during high dose conformal radiotherapy for prostate cancer. Radiother Oncol. 2002;64:209–214. doi: 10.1016/s0167-8140(02)00185-8. [DOI] [PubMed] [Google Scholar]
- 42.Ryu JK, Winter K, Michalski JM, et al. Interim report of toxicity from 3D conformal radiation therapy (3D-CRT) for prostate cancer on 3DOG/RTOG 9406, level III (79.2 Gy) Int J Radiat Oncol Biol Phys. 2002;54:1036–1046. doi: 10.1016/s0360-3016(02)03006-7. [DOI] [PubMed] [Google Scholar]
- 43.Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: Early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111–1116. doi: 10.1016/s0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- 44.Lebesque J, Koper P, Slot A, et al. Acute and late GI and GU toxicity after prostate irradiation to doses of 68 Gy and 78 Gy: Results of a randomized trial. Int J Radiat Oncol Biol Phys. 2003;57:S152. [Google Scholar]
- 45.Beckendorf V, Guerif S, Le Prise E, et al. The GETUG 70 Gy vs. 80 Gy randomized trial for localized prostate cancer: Feasibility and acute toxicity. Int J Radiat Oncol Biol Phys. 2004;60:1056–1065. doi: 10.1016/j.ijrobp.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 46.De Meerleer G, Vakaet L, Meersschout S, et al. Intensity-modulated radiotherapy as primary treatment for prostate cancer: Acute toxicity in 114 patients. Int J Radiat Oncol Biol Phys. 2004;60:777–787. doi: 10.1016/j.ijrobp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 47.Karlsdottir A, Johannessen DC, Muren LP, et al. Acute morbidity related to treatment volume during 3D-conformal radiation therapy for prostate cancer. Radiother Oncol. 2004;71:43–53. doi: 10.1016/j.radonc.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Michalski JM, Winter K, Purdy JA, et al. Toxicity after three-dimensional radiotherapy for prostate cancer with RTOG 9406 dose level IV. Int J Radiat Oncol Biol Phys. 2004;58:735–742. doi: 10.1016/S0360-3016(03)01578-5. [DOI] [PubMed] [Google Scholar]
- 49.Peeters ST, Heemsbergen WD, van Putten WL, et al. Acute and late complications after radiotherapy for prostate cancer: Results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys. 2005;61:1019–1034. doi: 10.1016/j.ijrobp.2004.07.715. [DOI] [PubMed] [Google Scholar]
- 50.Yang FE, Vaida F, Ignacio L, et al. Acute toxicity in radiotherapy of prostate cancer: Results of a randomized study with and without beam’s-eye view three-dimensional conformal therapy. Radiat Oncol Investig. 1996;4:231–238. [Google Scholar]
- 51.Kupelian PA, Willoughby TR. Short-course, intensity-modulated radiotherapy for localized prostate cancer. Cancer J. 2001;7:421–426. [PubMed] [Google Scholar]
- 52.Kitamura K, Shirato H, Shinohara N, et al. Reduction in acute morbidity using hypofractionated intensity-modulated radiation therapy assisted with a fluoroscopic real-time tumor-tracking system for prostate cancer: Preliminary results of a phase I/II study. Cancer J. 2003;9:268–276. doi: 10.1097/00130404-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Pollack A, Zagars GK, Starkschall G, et al. Conventional vs. conformal radiotherapy for prostate cancer: Preliminary results of dosimetry and acute toxicity. Int J Radiat Oncol Biol Phys. 1996;34:555–564. doi: 10.1016/0360-3016(95)02103-5. [DOI] [PubMed] [Google Scholar]
- 54.Michalski JM, Purdy JA, Winter K, et al. Preliminary report of toxicity following 3D radiation therapy for prostate cancer on 3DOG/RTOG 9406. Int J Radiat Oncol Biol Phys. 2000;46:391–402. doi: 10.1016/s0360-3016(99)00443-5. [DOI] [PubMed] [Google Scholar]


