Abstract
Recent evidence reveals that the immune system is under the direct control of the vagus nerve via the “cholinergic anti-inflammatory pathway.” Stimulation of vagus nerve activity significantly inhibits cytokine levels in animal models, and cholinergic agents inhibit cytokine release by human macrophages. Moreover, when vagus nerve activity is decreased or absent, cytokines are overproduced. Atherosclerosis is an inflammatory disease characterized by elevated levels of CRP and IL-6, but the relationship between cardiac vagal activity and cytokine levels in healthy humans is not well understood. Here we measured RR interval variability, an index of cardiac vagal modulation, and CRP and IL-6 in 757 subjects participating in a subset of the year 15 data collection in the CARDIA study of the evolution of risk factors in young adults. Univariate analysis revealed that all indices of RRV were strongly and inversely related to IL-6 (log pg/mL b = −0.08 and −0.17 for HF and LF power, P < 0.001 respectively) and CRP (log mg/L b = −0.14 and −0.26 for HF and LF power, P < 0.001 respectively) levels. In the multivariate model including gender, race, age, smoking, physical activity, SBP, BMI, and disease, the inverse relationship between RRV and inflammatory markers, although slightly attenuated, remained significant. These findings are consistent with the hypothesis that diminished descending vagal anti-inflammatory signals can allow cytokine overproduction in humans.
INTRODUCTION
Advances in understanding the biology of inflammation indicate that an over-expression of cytokines contributes to the development of tissue injury and damage. Excessive production of proin-flammatory cytokines mediates the development of autoimmune diseases in humans, including rheumatoid arthritis and inflammatory bowel disease. Therapies that directly target cytokines, for example, TNF and IL-1, can ameliorate the signs and symptoms of disease and have enjoyed widespread clinical use (1–3). Redundant counter-regulatory mechanisms normally control the magnitude of the cytokine response and function to prevent the development of tissue damage. These anti-inflammatory mechanisms include the pituitary adrenocortical axis, the release of soluble cytokine receptors, and the production of anti-inflammatory mediators that inhibit inflammation.
Recent work has implicated the central nervous system in the direct counter-regulation of cytokine release. The cholinergic anti-inflammatory pathway has been defined on the basis that signals transmitted along the vagus nerve into the organs of the reticuloendothelial system inhibit cytokine release and prevent disease. A large body of evidence in animal studies indicates that acetylcholine released following vagus nerve stimulation significantly inhibits cytokine release via a mechanism that requires the expression of the α7 subunit of the nicotinic acetylcholine receptor (4). Activation of the cholinergic anti-inflammatory pathway with either vagus nerve stimulation or α7 agonists is efficacious in animal models of endotoxemia, sepsis, subcutaneous inflammation, and experimental arthritis. Vagotomy enhances the cytokine response to invasive stimuli, indicating that the vagus nerve is hardwired to tonically regulate the magnitude of the cytokine response under basal and stimulated conditions. The cholinergic anti-inflammatory pathway is the efferent arm of an “Inflammatory Reflex,” which can be activated by inflammatory mediators in peripheral tissues that activate firing of afferent signals in the vagus nerve which, in effect, “notify” the central nervous system about the presence of inflammation in the body. This, in turn, activates an opposing, efferent response via the cholinergic anti-inflammatory pathway, which serves to inhibit inflammation and prevent damage (5,6). A growing body of evidence has implicated excessive inflammation in the pathogenesis of atherosclerosis. Atherosclerotic plaques are infiltrated by inflammatory cells that mediate the progression of damage to the vessel wall. Serum levels of CRP and IL-6, two circulating markers of inflammation, are correlated with the progression of atherosclerosis. Accordingly, we tested the hypothesis that indices of vagus nerve activity derived from RR interval variability (RRV) are inversely related to levels of IL-6 and CRP using data from 757 participants in the CARDIA study of the evolution of risk factors in young adults.
METHODS
Study Population
The Coronary Artery Risk Development in Young Adults (CARDIA) study is a biethnic, prospective, multicenter epidemiological study of the evolution of cardiovascular risk development in young adulthood. In 1985–1986, 5155 black and white men and women, aged 18 to 30 years, were recruited at Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA, to achieve a balance at each site by race (black, white), gender, education (high school degree or less, more than high school), and age (18–24 years, 25–30 years) (7). Participants were examined at study entry and years 2, 5, 7, 10, and 15 with re-examination rates among surviving cohort members of 90.5%, 85.7%, 80.6%, 78.5%, and 73.5%, respectively. Comparisons of CARDIA subjects who participated in the year 15 exam with those who did not indicated that the latter participants were more likely to be African-American, younger, less educated and smokers (data not shown). Site institutional review committee approval and informed consent were obtained for each examination.
At the year 15 exam, subjects seen at the Oakland, CA and Chicago, IL sites (and living within 50 miles of the clinic; N = 721 and 615 respectively) were asked to participate in the RRV substudy of socioeconomic status and development of biological risk, including assessments of RRV. Of the 1,336 subjects who were eligible for the substudy, 789 (59%) agreed to participate in the RRV sub-study. Comparisons of those who did and did not participate in the substudy revealed that participants tended to be of somewhat lower education and income and had somewhat higher BMI, diastolic and systolic blood pressure.
Data Collection
Sub-Study Assessments
Participants arrived at the clinic having eaten a light breakfast but abstaining from caffeinated beverages that morning. Study protocols were explained and written consent was obtained. The RRV protocol was explained and ECG electrodes and a single respiration-monitoring band were attached. Subjects rested quietly in the seated position for a 2 min period after which data were collected for 10 min. Subjects were asked to sit quietly without moving or talking.
Measurement of RR Interval Variability
Analog ECG signals were collected for 10 min while subjects were resting quietly in the seated position. Signals were digitized at 500 Hz by a National Instruments A/D board and stored on a microcomputer. The ECG waveform was submitted to an R-wave detection routine, resulting in a time series of RR intervals (RRI). In cases in which 5 min epochs of data were compromised by electronic artifact, subject movement, or ectopic beat, identification of all R waves was impossible. RR intervals associated with these artifacts were fixed using established procedures if possible (8). If not, the epoch was excluded from spectral analysis.
Mean heart rate (HR) and the standard deviation of all RRIs (SDRR) were computed for all subjects. Spectral power in the low (0.04–0.15 Hz (LF)) and high (0.15–0.50 Hz (HF)) frequency bands was computed based on 300-second epochs using an interval method for computing Fourier transforms similar to that described by DeBoer, Karemaker, and Strackee (9). Prior to computing Fourier transforms, the mean of the RR interval series was subtracted from each value in the series and the residual series then was filtered using a Hanning window (10) and the spectral power, i.e., variance (in msec2), over the LF and HF bands was summed. Estimates of spectral power were adjusted to account for attenuation produced by this filter (10).
Measurement of Inflammatory Markers
C-reactive protein (CRP) was measured using the BNII nephelometer from Dade Behring utilizing a particle enhanced immunonepholometric assay. The assay range is 0.175–1100 mg/L. Expected values for CRP in normal, healthy individuals are < 3 mg/L. Intra-assay CVs range from 2.3–4.4% and inter-assay CVs range from 2.1–5.7%. IL-6 was measured by ultra-sensitive ELISA (R&D Systems, Minneapolis, MN). The lower detection limit is < 0.10 pg/mL, with a detection range of 0.156–10.0 pg/mL and a routine CV in the lab of 6.3%.
Covariates
Measures of systolic blood pressure (SBP), body-mass index (BMI), physical activity, and smoking from the year 15 exam were examined. Selection of these covariates was based on their known associations with both SES and RRV. SBP was measured during seated rest, the average of three measurements with a random zero sphygmomanometer. Physical activity was measured as self-reported participation in heavy and moderate intensity activities and quantified as previously described (11). Smoking was measured as self-reported current smoking (non-smoker, ex-smoker, and current smoker). Analyses examined these factors as potential mediators of the relationship between SES and RRV.
Participants were defined as having diabetes if glucose > 126 mg/dL or if they reported taking diabetes medication. Similarly, hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg or if they reported taking anti-hypertensive medications. Participants also were asked if they took medication for asthma or high cholesterol.
Prior to analyses, CRP, IL-6, HF, and LF were log-transformed to approximate a normal distribution. To control for the influence of respiratory rate on HF power, we regressed HF power on respiratory rate and used the residual instead of unadjusted HF in all subsequent analyses. Linear regression was used to assess the relationship of the 3 RRV parameters (HF, LF, and SDRR) and HR, to each of the inflammatory markers, first alone (Table 2) and then with covariates added (Table 3): ethnicity (African-American), gender (female), age, education (more than high school), smoking status (current and former separately), physical activity score, and body-mass index. Finally, self-reported medical condition and medication usage were added to the model (Table 4). Standardized parameters were calculated using SAS version 8.02, PROC REG.
Table 2.
Univariate Relationships between Inflammatory Markers and RRV
| HF (msec2)
|
LF (msec2)
|
SD (msec)
|
HR (bpm)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Inflammatory Markers | b | p | b | p | b | p | b | p |
| Ln (CRP mg/L) | −0.143 | < 0.0001 | −0.2646 | < 0.0001 | −0.009 | < 0.0001 | 0.022 | < 0.0001 |
| Ln (IL6 pg/mL) | −0.0764 | 0.0003 | −0.1755 | < 0.0001 | −0.0058 | < 0.0001 | 0.0121 | < 0.0001 |
Table 3.
Multivariable Models of RRV Predictors for Inflammatory Outcomes Including Covariates
| Inflammatory markers
|
||||
|---|---|---|---|---|
| In (CRP mg/L)
|
In (IL6 pg/mL)
|
|||
| N in Models | 734 | 678 | ||
| Measures of RRV | Coeffa | p | Coeffa | P |
| HF (msec2) | −0.0889 | 0.0055 | −0.0686 | 0.0302 |
| Black | 0.0249 | 0.4655 | 0.0842 | 0.0128 |
| Female | 0.0827 | 0.0107 | 0.0101 | 0.7526 |
| Age (years) | −0.0369 | 0.2406 | 0.0079 | 0.8002 |
| > HS | −0.0441 | 0.1726 | −0.0728 | 0.0222 |
| Current smoker | 0.1192 | 0.0003 | 0.0793 | 0.0149 |
| Ex-smoker | 0.0215 | 0.4997 | 0.0136 | 0.668 |
| Physical activity score | −0.0309 | 0.3387 | −0.0631 | 0.0504 |
| SBP (mmHg) | −0.0067 | 0.8407 | 0.0495 | 0.1352 |
| BMI (kg/m2) | 0.5115 | < 0.0001 | 0.5254 | < 0.0001 |
| LF (msec2) | −0.0954 | 0.0043 | −0.126 | 0.0001 |
| Black | 0.0076 | 0.8238 | 0.0642 | 0.0558 |
| Female | 0.0554 | 0.0933 | −0.0222 | 0.4976 |
| Age (years) | −0.0361 | 0.2498 | 0.0007 | 0.9826 |
| > HS | −0.0449 | 0.165 | −0.0685 | 0.03 |
| Current smoker | 0.1221 | 0.0002 | 0.077 | 0.017 |
| Ex-smoker | 0.0244 | 0.4429 | 0.014 | 0.6559 |
| Physical activity score | −0.0303 | 0.3483 | −0.0585 | 0.0678 |
| SBP (mmHg) | −0.0083 | 0.805 | 0.0442 | 0.1789 |
| BMI (kg/m2) | 0.5076 | < 0.0001 | 0.5162 | < 0.0001 |
| SD (msec) | −0.0729 | 0.0238 | −0.0868 | 0.0065 |
| Black | 0.0146 | 0.6668 | 0.0761 | 0.0237 |
| Female | 0.0682 | 0.0359 | −0.0036 | 0.9119 |
| Age (years) | −0.0365 | 0.2482 | 0.003 | 0.9241 |
| > HS | −0.0474 | 0.1425 | −0.0733 | 0.0207 |
| Current smoker | 0.1217 | 0.0002 | 0.0785 | 0.0156 |
| Ex-smoker | 0.0223 | 0.4847 | 0.013 | 0.6801 |
| Physical activity score | −0.0325 | 0.3155 | −0.0609 | 0.0587 |
| SBP (mmHg) | −0.0061 | 0.8547 | 0.0479 | 0.1476 |
| BMI (kg/m2) | 0.5152 | < 0.0001 | 0.5262 | < 0.0001 |
| HR (bpm) | 0.0852 | 0.0085 | 0.0708 | 0.0272 |
| Black | 0.018 | 0.5966 | 0.0796 | 0.0182 |
| Female | 0.0689 | 0.0336 | 0.0005 | 0.9877 |
| Age (years) | −0.0198 | 0.5428 | 0.022 | 0.4784 |
| > HS | −0.0497 | 0.1232 | −0.0762 | 0.0164 |
| Current smoker | 0.1161 | 0.0005 | 0.0775 | 0.0177 |
| Ex-smoker | 0.0232 | 0.4657 | 0.0145 | 0.6454 |
| Physical activity score | −0.028 | 0.3896 | −0.0585 | 0.0712 |
| SBP (mmHg) | −0.0125 | 0.7109 | 0.0451 | 0.176 |
| BMI (kg/m2) | 0.5112 | < 0.0001 | 0.524 | < 0.0001 |
coeff: Standardized regression coefficients
Table 4.
Multivariable Models of RRV Predictors for Inflammatory Markers, Including Covariates and Current Medication Use, Diabetes, and Hypertension
| Inflammatory markers
|
||||
|---|---|---|---|---|
| In (CRP mg/L)
|
In (IL6 pg/mL)
|
|||
| N in Models | 734 | 678 | ||
| Measures of RRV | Coeffa | p | Coeffa | P |
| HF adjusted for respiratory rateb | −0.0866 | 0.0118 | −0.0401 | 0.2353 |
| Black | 0.0248 | 0.5027 | 0.0975 | 0.0076 |
| Female | 0.0886 | 0.0116 | −0.0073 | 0.8321 |
| Age (years) | −0.0428 | 0.2089 | 0.0241 | 0.4734 |
| > HS | −0.0521 | 0.1348 | −0.0884 | 0.0096 |
| Current smoker | 0.1278 | 0.0004 | 0.0558 | 0.1118 |
| Ex-smoker | 0.0317 | 0.3571 | −0.0063 | 0.8524 |
| Physical activity score | −0.0307 | 0.3781 | −0.0643 | 0.0628 |
| SBP (mmHg) | −0.0272 | 0.4915 | 0.0614 | 0.1119 |
| BMI (kg/m2) | 0.4804 | < 0.0001 | 0.5111 | < 0.0001 |
| Have chronic health conditionc | 0.0680 | 0.0686 | 0.0001 | 0.9997 |
| LF (msec2) | −0.0926 | 0.0055 | −0.1260 | 0.0001 |
| Black | 0.0084 | 0.8050 | 0.0643 | 0.0560 |
| Female | 0.0549 | 0.0956 | −0.0223 | 0.4973 |
| Age (years) | −0.0391 | 0.2131 | 0.0006 | 0.9855 |
| > HS | −0.0436 | 0.1767 | −0.0685 | 0.0303 |
| Current smoker | 0.1219 | 0.0002 | 0.0769 | 0.0173 |
| Ex-smoker | 0.0245 | 0.4419 | 0.0139 | 0.6568 |
| Physical activity score | −0.0290 | 0.3689 | −0.0584 | 0.0683 |
| SBP (mmHg) | −0.0267 | 0.4533 | 0.0435 | 0.2148 |
| BMI (kg/m2) | 0.5007 | < 0.0001 | 0.5160 | < 0.0001 |
| Have chronic health condition | 0.0525 | 0.1224 | 0.0023 | 0.9461 |
| SD (msec) | −0.0723 | 0.0247 | −0.0868 | 0.0065 |
| Black | 0.0153 | 0.6526 | 0.0761 | 0.0238 |
| Female | 0.0671 | 0.0387 | −0.0036 | 0.9099 |
| Age (years) | −0.0401 | 0.2058 | 0.0028 | 0.9301 |
| > HS | −0.0459 | 0.1558 | −0.0732 | 0.0211 |
| Current smoker | 0.1212 | 0.0003 | 0.0783 | 0.0160 |
| Ex-smoker | 0.0223 | 0.4832 | 0.0130 | 0.6817 |
| Physical activity score | −0.0309 | 0.3403 | −0.0608 | 0.0594 |
| SBP (mmHg) | −0.0263 | 0.4603 | 0.0463 | 0.1883 |
| BMI (kg/m2) | 0.5074 | < 0.0001 | 0.5257 | < 0.0001 |
| Have chronic health condition | 0.0570 | 0.0940 | 0.0044 | 0.8952 |
| HR (bpm) | 0.0825 | 0.0108 | 0.0708 | 0.0276 |
| Black | 0.0185 | 0.5859 | 0.0796 | 0.0183 |
| Female | 0.0680 | 0.0356 | 0.0005 | 0.9882 |
| Age (years) | −0.0234 | 0.4546 | 0.0219 | 0.4810 |
| > HS | −0.0483 | 0.1339 | −0.0762 | 0.0166 |
| Current smoker | 0.1160 | 0.0005 | 0.0775 | 0.0179 |
| Ex-smoker | 0.0233 | 0.4639 | −0.0145 | 0.6460 |
| Physical activity score | −0.0267 | 0.4103 | −0.0585 | 0.0716 |
| SBP (mmHg) | −0.0310 | 0.3863 | 0.0447 | 0.2074 |
| BMI (kg/m2) | 0.5042 | < 0.0001 | 0.5239 | < 0.0001 |
| Have chronic health condition | −0.0531 | 0.1184 | 0.0014 | 0.9672 |
coeff: Standardized regression coefficients
HF power regressed on respiratory rate and residual used in analyses
Self-reported hypertension or diabetes or cardioactive medication usage
RESULTS
Of the 789 subjects who participated in the study, 757 subjects (96%) had technically adequate data for RRV analysis. Of these, 734 and 678 had acceptable measurement of IL-6 and CRP respectively. 44% were White and 42% were men. Table 1 presents general characteristics of the cohort.
Table 1.
Characteristics of the Cohort
| Subjects with RRV data
|
|||
|---|---|---|---|
| Characteristics | N | Mean or % | SD |
| Calculated age at year 15 | 757 | 40.0 | 3.7 |
| Sex, % of femalea | 757 | 57.6 | |
| Ethnicity, % of Caucasian | 757 | 44.4 | |
| Sex/ethnicity breakdown | |||
| % of male, Caucasian | 156 | 20.6 | |
| % of male, African American | 165 | 21.8 | |
| % of female, Caucasian | 180 | 23.8 | |
| % of female, African American | 256 | 33.8 | |
| SES | |||
| Education, years | 757 | 14.9 | 2.5 |
| ≤ HS | 164 | 21.7 | |
| > HS to college | 448 | 59.2 | |
| Post-college | 145 | 19.2 | |
| Annual Income in $ | 746 | ||
| < $42.5K | 152 | 20.4 | |
| $42.5K to < $87.5K | 279 | 37.4 | |
| $87.5K | 315 | 42.2 | |
| BMI (kg/m2) | 754 | 29.3 | 7.3 |
| Diastolic blood pressure (mmHg) | 757 | 75.5 | 10.6 |
| Systolic blood pressure (mmHg) | 757 | 114.1 | 14.1 |
| RR Interval Variability | |||
| Heart Rate (HR) (bpm) | 757 | 72.6 | 11.6 |
| Low Frequency (LF) (msec2) | 757 | 826.7 | 1106.7 |
| High Frequency (HF) (msec2) | 757 | 771.7 | 1272.9 |
| SDRR (msec) | 757 | 46.3 | 21.8 |
| Diabetes mellitus | 45 | 6.0 | |
| Hypertension | 133 | 17.6 | |
| Smoking status | 756 | ||
| Non-smoker | 486 | 64.3 | |
| Ex-smoker | 128 | 16.9 | |
| Current smoker | 142 | 18.8 | |
Two subjects had a sex change operation after year 10, and their sex is coded as 3 at year 15. Thus, they were not included in this table.
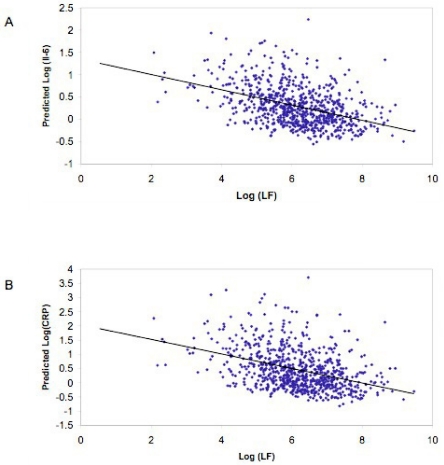
Univariate analysis revealed that all indices of RRV were strongly and inversely correlated to IL-6 and CRP levels (Table 2). In the multivariate model, the relationship between RRV and inflammatory markers remained significant although slightly attenuated (Table 3). Figure 1 displays these data.
Figure 1.
(A) Scatterplot of LF RR interval power (in msec2) vs. in IL-6 based on the regression model; fitted regression line is also plotted. (B) Scatterplot of LF RR interval power (in msec2) vs. in CPR based on the regression model; fitted regression line is also plotted.
Previous studies have suggested that RRV is reduced in patients with hypertension and diabetes (12–14). We therefore added self-reported presence of these diseases or medication use for these two conditions and asthma or high cholesterol to the multivariate model. As shown in Table 4, the inverse relationships between RRV and IL-6 and CRP remained statistically significant with the exception of the HF power-IL-6 relationship. Considered together, these results are consistent with an inverse relationship between vagus nerve activity and serum levels of inflammatory markers in healthy subjects.
DISCUSSION
The results indicate that measures of vagus nerve activity, HF and LF power, and SDRR, are inversely related to serum levels of two proinflammatory mediators, IL-6 and CRP. HR was positively related to these inflammatory markers. As expected, after controlling for important covariates and disease in multivariate analyses, these relationships were attenuated, but with a single exception they remained statistically significant. To our knowledge, these are the first results demonstrating inverse relationships between inflammatory markers and indices of cardiac autonomic regulation in a large sample of healthy young adults. These findings are consistent with evidence from animal studies indicating that the cholinergic anti-inflammatory pathway counter-regulates inflammation.
Relationships between RRV and inflammation have been reported in several studies, although in most, clinical samples have been studied. In 121 women 17 months after a myocardial infarction or revascularization, Jansky et al. reported inverse relationships between IL-6 and SDNN, very low frequency, and low frequency RRV derived from 24-h ECG recordings (15). In 64 patients with decompensated congestive heart failure, IL-6 but not TNF-α was inversely related with SDNN, total spectral power, and ultra low frequency power also derived from 24-h ECG recordings (16). Another study of CHF patients, however, showed that TNF-α was inversely related not only to SDNN but also to LF and HF power from 24-h recordings (17). A case control study of the metabolic syndrome reported that IL-6 was inversely related to RRV derived from 5-min supine recordings although the RRV indices were not identified (18). In 133 healthy 35-year-old men and women, VLF was significantly and inversely related to leukocyte count (19). Finally, in a community study employing 24-h ECG recording, CRP and leukocyte count were inversely related to SDNN but not to pNN50, an index of high frequency RRV (20).
With one exception, all of the previous studies to examine relationships between RRV and inflammatory markers found only relationships with measures of RRV < 0.15 Hz or their time domain equivalent, leading to suggestions of sympathetic nervous system activation of inflammation. However, this suggestion is inconsistent with the regularly reported inverse association between RRV and inflammation and thus raises questions about the physiological meaning of LF power.
While there is little question that high frequency RRV reflects cardiac parasympathetic modulation, especially after correction for respiratory rate, the physiological significance of LF power is less clear. The best evidence suggests that it reflects both parasympathetic and sympathetic contributions with the latter varying depending upon several factors including posture. In the supine position, atropine eliminates virtually all LF power, indicating that in this position, LF power also principally reflects parasympathetic activity (21,22). In the upright position, however, atropine alone and propranolol alone eliminated approximately 70% of LF power, suggesting that in this position, LF power also may reflect a contribution from the sympathetic system (21).
We measured RRV in the seated position and some data suggest that autonomic differences between the seated and supine positions are relatively small, especially compared with the upright position. Tulen et al. found no difference between the supine and seated positions in HF power but did not measure LF power in the 0.04–0.15 Hz frequency band (23). Taylor et al. demonstrated that atropine eliminated LF power in the 40° tilted position, intermediate between the supine and standing positions, but that atenolol had no effect (22). They also showed that change from the supine to the 40° upright position did not lead to an increase in LF power. Finally, Vybiral et al. demonstrated that administration of the vagomimetic trans-dermal scopolamine led to a significant increase in LF power in supine subjects (24). These data suggest a substantial degree of similarity in the autonomic profile of the seated and supine positions and that, in both, it appears that LF power principally reflects cardiac parasympathetic modulation.
Establishing causality is impossible in cross-sectional studies, but prior studies of vagus nerve activity and inflammation examined clinical samples in patients with disease (for example, CHF or MI). Because these clinical states promote both inflammation and reduced RRV, it is plausible that reduced RRV is the product of inflammation. However, the same inverse relationships between RRV and inflammation also appear in our data and the other community study of healthy subjects, consistent with the view that low levels of RRV are antecedent to inflammation, although the presence of a third factor responsible for both cannot be excluded. It now appears that in our data from the CARDIA study of heart disease in young adults there is an inverse relationship between low frequency RR interval variability and the inflammatory markers IL-6 and CRP, even after control of relevant covariates and cardioactive medications or hypertension or diabetes, which is consistent with the hypothesis of a cholinergic anti-inflammatory pathway that regulates inflammation in humans.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Atzeni F, Turiel M, Capsoni F, Doria A, Meroni P, Sarzi-Puttini P. Autoimmunity and anti-TNF-alpha agents. Ann N Y Acad Sci. 2005;1051:559–69. doi: 10.1196/annals.1361.100. [DOI] [PubMed] [Google Scholar]
- 2.Blumenauer B, et al. Infliximab for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev. 2002:CD003785. doi: 10.1002/14651858.CD003785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen NJ, Stein CM. New drugs for rheumatoid arthritis. N Engl J Med. 2004;350:2167–79. doi: 10.1056/NEJMra032906. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 5.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 6.Tracey KJ. Physiology and immunology of the cholinergic anti-inflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutter GR, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12:1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 8.Berntson GG, Quigley KS, Jang JF, Boysen ST. An approach to artifact identification: application to heart period data. Psychophysiology. 1990;27:586–98. doi: 10.1111/j.1469-8986.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 9.deBoer RW, Karemaker JM, Strackee J. Comparing spectra of a series of point events, particularly for heart rate variability spectra. IEEE Trans Biomed Eng. 1984;31:384–7. doi: 10.1109/TBME.1984.325351. [DOI] [PubMed] [Google Scholar]
- 10.Harris FJ. On the use of windows for harmonic analysis with the discrete Fourier transform. Proceedings of the IEEE. 1978;66:51–83. [Google Scholar]
- 11.Jacobs DR, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J of Cardiopulm Rehabil. 1989;9:448–59. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh J, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32:293–7. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder EB, et al. Hypertension, blood pressure, and heart rate variability: the atherosclerosis risk in communities (ARIC) study. Hypertension. 2003;42:1106–11. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- 14.Liao D, Carnethon M, Evans GW, Cascio WE, Heiss G. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes: the atherosclerosis risk in communities (ARIC) study. Diabetes. 2002;51:3524–31. doi: 10.2337/diabetes.51.12.3524. [DOI] [PubMed] [Google Scholar]
- 15.Janszky I, et al. Inflammatory markers and heart rate variability in women with coronary heart disease. J Intern Med. 2004;256:421–8. doi: 10.1111/j.1365-2796.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 16.Aronson D, Mittleman MA, Burger AJ. Interleukin-6 levels are inversely correlated with heart rate variability in patients with decompensated heart failure. J Cardiovasc Electrophysiol. 2001;12:294–300. doi: 10.1046/j.1540-8167.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- 17.Malave HA, Taylor AA, Nattama J, Deswal A, Mann DL. Circulating levels of tumor necrosis factor correlate with indexes of depressed heart rate variability: a study in patients with mild-to-moderate heart failure. Chest. 2003;123:716–24. doi: 10.1378/chest.123.3.716. [DOI] [PubMed] [Google Scholar]
- 18.Brunner EJ, et al. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002;106:2659–65. doi: 10.1161/01.cir.0000038364.26310.bd. [DOI] [PubMed] [Google Scholar]
- 19.Jensen-Urstad M, Jensen-Urstad K, Ericson M, Johansson J. Heart rate variability is related to leucocyte count in men and to blood lipoproteins in women in a healthy population of 35-year-old subjects. J Intern Med. 1998;243:33–40. [PubMed] [Google Scholar]
- 20.Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25:363–70. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Pomeranz B, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:H151–3. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 22.Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–55. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- 23.Tulen JHM, Boomsma F, Man AJ. Cardiovascular control and plasma catecholamines during rest and mental stress: effects of posture. Clinical Science. 1999;96:567–76. [PubMed] [Google Scholar]
- 24.Vybiral T, et al. Effects of transdermal scopolamine on heart rate variability in normal subjects. Am J Cardiol. 1990;65:604–8. doi: 10.1016/0002-9149(90)91038-8. [DOI] [PubMed] [Google Scholar]