Abstract
Increased activation of the transcription factor NFκB in the neutrophils has been associated with the pathogenesis of sepsis, acute lung injury (ALI), bronchopulmonary dysplasia (BPD), and other neutrophil-mediated inflammatory disorders. Despite recent progress in analyzing early NFκB activation in human neutrophils, activation of NFκB in persistently stimulated neutrophils has not been previously studied. Because it is the persistent NFκB activation that is thought to be involved in the host response to sepsis and the pathogenesis of ALI and BPD, we hypothesized that continuously stimulated human neutrophils may exhibit a late phase of NFκB activity. The goal of this study was to analyze the NFκB activation and expression of IκB and NFκB proteins during neutrophil stimulation with inflammatory signals for prolonged times. We demonstrate that neutrophil stimulation with lipopolysaccharide (LPS) and tumor necrosis factor-α (TNFα) induces, in addition to the early activation at 30–60 min, a previously unrecognized late phase of NFκB activation. In LPS-stimulated neutrophils, this NFκB activity typically had a biphasic character, whereas TNFα-stimulated neutrophils exhibited a continuous NFκB activity peaking around 9 h after stimulation. In contrast to the early NFκB activation that inversely correlates to the nuclear levels of IκBα, however, in continuously stimulated neutrophils, NFκB is persistently activated despite considerable levels of IκBα present in the nucleus. Our data suggest that NFκB is persistently activated in human neutrophils during neutrophil-mediated inflammatory disorders, and this persistent NFκB activity may represent one of the underlying mechanisms for the continuous production of proinflammatory mediators.
INTRODUCTION
Neutrophils (polymorphonuclear leukocytes, or PMN) are a crucial part of the innate immune system and play a vital role in the inflammatory response that characterizes sepsis, acute lung injury (ALI), and bronchopulmonary dysplasia (BPD) (1–4). In addition to their phagocytic and killing properties, neutrophils synthesize numerous proinflammatory cytokines and chemokines that may amplify the inflammatory process (5–9). Furthermore, recent studies have shown that neutrophil apoptosis is delayed in patients with sepsis, ALI, and BPD (10–12). Expression of many of these proinflammatory and antiapoptotic genes is regulated at the level of transcription by the transcription factor NFκB (13–22).
In most resting cells other than neutrophils, NFκB is present in the cytoplasm bound to the inhibitory protein IκBα (13,16,23). Cell stimulation with inflammatory signals leads to phosphorylation of the cytoplasmic IκBα, followed by its rapid ubiquitination and degradation by the proteasome. This process releases the NFκB proteins from the inhibitory complex, and they then translocate to the nucleus and bind to the NFκB-responsive promoters (13–17). Thus, in this classic model of NFκB regulation, NFκB activity is regulated by the cytoplasmic degradation of IκBα, and by the nuclear translocation of NFκB subunits.
Despite remarkable progress in understanding the NFκB regulation in other human cells as well as in animal models (13–17), much remains unknown about the mechanisms regulating NFκB activity in human neutrophils. Previous studies from our laboratory have demonstrated that human neutrophils differ from monocytic and other cells, in that they contain predominant amounts of IκBα in the nucleus of resting cells (24). Our results have shown that neutrophil stimulation with inflammatory signals such as LPS and TNFα result in the degradation of both cytoplasmic and nuclear IκBα, and that the NFκB activity induced by LPS and TNFα stimulation for 30–60 min inversely correlates with the nuclear levels of IκBα (25,26). In addition, we have shown that the increased nuclear level of IκBα in human neutrophils is associated with the inhibition of NFκB activity and increased neutrophil apoptosis (25). A recent study has provided evidence for the nuclear IκBα degradation in neutrophils by the constitutively expressed nuclear IκB kinase complex (27). Thus, nuclear IκBα is crucial for the regulation of NFκB activity and neutrophil apoptosis (24–26). However, the exact mechanisms by which nuclear IκBα regulates the NFκB-dependent transcription remain unknown.
Increased activation of NFκB in neutrophils has been associated with the pathogenesis of ALI, BPD, sepsis, and other inflammatory diseases (28–33). Whereas the acute NFκB activation induced by neutrophil stimulation for 30–60 min has been well documented (34–41), NFκB activation during the prolonged neutrophil stimulation that is likely to occur in inflammatory disorders has not been previously studied. In this study, we describe a previously unrecognized persistent NFκB activation in human neutrophils stimulated with TNFα and LPS for up to 12 h. Whereas the early activation of NFκB is regulated by the nuclear levels of IκBα, the newly synthesized nuclear IκBα induced by continuous neutrophil stimulation appears to be no longer sufficient to inhibit the persistent NFκB activity. These data suggest that the NFκB activity persistently increased during neutrophil-mediated inflammatory disorders is regulated by a new, IκBα-independent mechanism.
MATERIALS AND METHODS
Materials
Ficoll-Paque PLUS, dextran T-500, T4 polynucleotide kinase, poly(dI-dC), and Sephadex G25 spin columns were purchased from Pharmacia (Piscataway, NJ, USA). Hanks balanced salt solution, RPMI 1640, and endotoxin-tested, heat-inactivated fetal calf serum were obtained from Life Technologies (Grand Island, NY, USA). E. coli–expressed purified recombinant human TNFα was purchased from R&D Systems (Minneapolis, MN, USA). [32P]γ -ATP was purchased from Perkin Elmer (Boston, MA, USA). Purified polyclonal antibodies against human IκBα (sc-371), IκBβ (C-20; sc-945), IκBβ (N-20; sc-969), IκBɛ (sc-7156), p50 NFκB (sc-7178), c-Rel NFκB (sc-70X), histone H2A (sc-10807), lamin B (sc-6216), p21CIP1 (sc-469), and IκBα-agarose conjugate (sc-203AC) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Purified polyclonal antibody against p65 NFκB (SA-171) was obtained from Biomol (Plymouth Meeting, PA, USA), and lactate dehydrogenase (LDH) antibody (20-LG22) from Fitzgerald Industries International (Concord, MA, USA). Horseradish peroxidase (HRP)-conjugated anti-rabbit and anti-goat secondary antibodies were from Amersham (Arlington Heights, IL, USA). All other reagents were molecular biology grade and were purchased from Sigma (St Louis, MO, USA). All reagents and plasticware used throughout the experiments were pyrogen-free.
Neutrophil Isolation and Culture
Fresh blood was obtained from healthy adult human volunteers and collected in heparinized preservative-free tubes as described (24,36). The study was approved by the Institutional Review Board of the North Shore–Long Island Jewish Health System, and informed written consent was obtained from all subjects. Neutrophils were purified under endotoxin-free conditions using Ficoll-Paque centrifugation, followed by dextran sedimentation and hypotonic lysis of residual erythrocytes as described (24,36). This neutrophil isolation procedure takes approximately 3 h and yields neutrophils that are 95% to 98% pure and 98% to 99% viable (24,36). Purified neutrophils were resuspended in RPMI 1640 supplemented with 10% low-endotoxin fetal calf serum at a final concentration of 5 × 106 cells/mL and incubated in 48-well cell culture plates at 37° C in a 5% CO2 humidified atmosphere.
Electrophoretic Mobility Shift Assay (EMSA)
The nuclear extracts were prepared, and EMSA assays were performed as described (24,42).
Western Blot Analysis
Cytoplasmic extracts (CE) and nuclear extracts (NE) were prepared as described (24–26). Denatured proteins were separated on 12% denaturing polyacrylamide gels, and immunoblotting analysis was performed as described (24–26). The images were analyzed by densitometry by using image analysis software (UN-SCAN-IT gel v. 5.1 from Silk Scientific, Orem, UT, USA) as described (36).
Immunoprecipitation
Nuclear extracts prepared as described (24,25) were immunoprecipitated (4 h, 4°C) on anti–IκBα-agarose. The immune complexes were washed 4 times with PBS buffer, and the resulting immunoprecipitated proteins were resolved on 10% SDS gel and detected with IκBα and p65 antibodies as described (25).
Indirect Immunofluorescence Microscopy
Neutrophils were fixed with 2% paraformaldehyde. After washing, the cells were resuspended in PBS, cytospun onto slides, and permeabilized with 0.5% Triton X-100 for 10 min. After blocking (1 h at room temperature and overnight at 4°C) in PBS containing 10% bovine serum, the cells were incubated (2 h at room temperature) with anti-IκBα antibody diluted 1:20, anti-p65 antibody diluted 1:100, or anti-p50 antibody diluted 1:20 in PBS containing 2.5% bovine serum and 0.01% Tween-20. The cells were washed and incubated (1 h at room temperature) with FITC-conjugated secondary anti-rabbit IgG antibody diluted (1:100) in PBS containing 2.5% bovine serum and 0.01% Tween-20. The cells were washed, incubated (10 min) with 4′6′-diamidino-2-phenylindole (DAPI) to visualize DNA, and after washing, mounted onto coverslips. The slides were observed using a Nikon Eclipse 800 microscope, and only experimental series that showed no signal in the absence of primary antibodies were analyzed.
Confocal Microscopy
Neutrophils were fixed and permeabilized as described above. After blocking, the cells were incubated (2 h at room temperature) with anti-IκBα antibody diluted 1:10 in PBS containing 2.5% bovine serum. The cells were washed and incubated (1 h at room temperature) with FITC-conjugated secondary anti-rabbit IgG antibody diluted 1:50 in PBS containing 2.5% bovine serum. The cells were washed, incubated (10 min) with propidium iodide (PI) to visualize DNA, and mounted onto coverslips. The slides were observed using a Leica TCS SL confocal microscope.
Subcellular Fractionation
The subcellular fractions were prepared by the procedure of He et al. (43). Briefly, the cells were lysed in cytoskeleton (CSK) buffer (10 mM PIPES, pH 6.8, 0.1 M NaCl, 300 mM sucrose, 3 mM MgCl2, 1 mM EGTA, 0.5% Triton X-100, 2 mM DTT) containing the protease and phosphatase inhibitors as described (24) and centrifuged (3 min, 5000 rpm =1700 g). The supernatants were collected and labeled as soluble proteins. The nuclear pellets were washed with CSK buffer; the chromatin associated proteins were extracted by incubation with DNase I (15 min, 37°C), followed by incubation with 0.25 M ammonium sulfate (5 min, 4°C). The samples were centrifuged (5 min, 5000 rpm =1700 g), and the supernatants were collected and labeled as a chromatin fraction. The pellets were washed with CSK buffer containing 2M NaCl; the supernatants were collected and labeled as a chromatin wash. Supernatants from each extraction step and the final nuclear matrix pellets were boiled in SDS sample buffer and analyzed by SDS-electrophoresis and immunoblotting.
ELISA
TNFα and IL-8 release was measured in cell culture supernatants using commercially available ELISA kits (R&D) as described (26).
Statistical Analysis
The results represent at least three independent experiments. Numerical results are presented as means ± SE. Data were analyzed using an InStat software package (GraphPAD, San Diego, CA, USA). Statistical significance was evaluated using Mann-Whitney U test with Bonferroni correction for multiple comparisons, and P < 0.05 was considered significant.
RESULTS
Prolonged Stimulation of Human Neutrophils with LPS or TNFα Induces Persistent Activation of NFκB That Is Independent of the Newly Synthesized IκBα
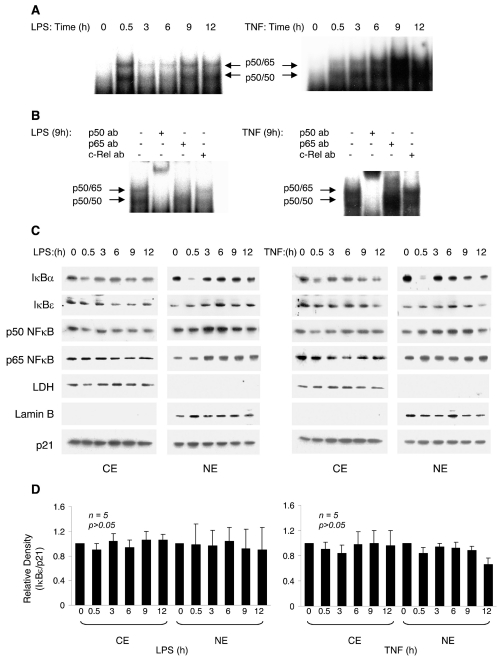
Because it is the persistent NFκB activation that is associated with the pathogenesis of sepsis and ALI (28–33,44), we hypothesized that human neutrophils stimulated with proinflammatory signals for prolonged times may exhibit persistent or intermittent activation of NFκB. To measure the NFκB activity in persistently stimulated human neutrophils, neutrophils were stimulated with LPS (100 ng/mL) or TNFα (10 ng/mL) for up to 12 h, and NFκB DNA binding activity was measured in nuclear extracts by EMSA. As illustrated in Figure 1A (left panel), neutrophil stimulation with LPS induced NFκB activity that typically had a biphasic character: the previously observed NFκB activity peaking around 30 min (24,25), and a second phase of NFκB activation peaking around 9 h after stimulation. Neutrophil stimulation with TNFα induced, in addition to the early NFκB activation at 30–60 min (34–41), a strong and sustained activation of NFκB, which in terms of magnitude of activation, far exceeded the early phase of NFκB activation induced by neutrophil stimulation for 30 min. This persistent NFκB activity reached the maximum around 9 h after stimulation, and lasted up to 12 h (Figure 1A, right panel).
Figure 1.
NFκB activity and cytoplasmic and nuclear levels of IκB and NFκB proteins during persistent neutrophil stimulation with LPS and TNFα. (A) Neutrophils were stimulated with LPS (100 ng/mL) or TNFα (10 ng/mL) for 0, 0.5, 3, 6, 9, and 12 h, and NFκB activity was measured in nuclear extracts by EMSA. (B) Supershift analysis of NFκB complexes induced in human neutrophils after 9-h stimulation with LPS and TNFα. Nuclear extracts prepared from neutrophils stimulated with LPS (100 ng/mL) and TNFα (10 ng/mL) for 9 h were incubated with NFκB antibodies specific against p50, p65, and c-Rel subunits. (C) Neutrophils were stimulated with LPS (100 ng/mL) or TNFα (10 ng/mL) for 0, 0.5, 3, 6, 9, and 12 h, and the cytoplasmic (CE) and nuclear (NE) levels of IκBα, IκBɛ, and p50 and p65 NFκB proteins were analyzed by immunoblotting. The presence of cytoplasmic proteins in the nuclear fraction was monitored using lactate dehydrogenase (LDH) antibody. Nuclear contamination in the cytoplasmic fraction was assessed using lamin B–specific antibody. Each lane contains approximately 5 × 105 cells. (D) Densitometric evaluation of IκBɛ levels in stimulated human neutrophils. The IκBɛ bands were scanned and the densities were normalized to densities of p21 used a loading control. The values at T = 0 for CE and NE were arbitrarily set to 1, and the other values are presented relative to those values. The data represent the means of five independent experiments ± SE.
To determine whether the persistent NFκB activity was composed of the p50 and p65 NFκB subunits like the NFκB activity induced at 30 min (36), we performed a supershift analysis of the NFκB complexes induced by LPS and TNFα stimulation for 9 h. Figure 1B shows that the NFκB activity induced by neutrophil stimulation with LPS (left panel) and TNFα (right panel) for 9 h consisted of subunits similar to those of the 30-min induced NFκB activity (36): p50/50 homodimers and p50/65 heterodimers.
We have previously shown that the immediate NFκB activation induced by neutrophil stimulation for 30 min is mediated by degradation of nuclear IκBα, which is later resynthesized and translocates again to the nucleus (25). Figure 1C illustrates the cytoplasmic and nuclear levels of IκB and NFκB proteins during neutrophil stimulation with LPS (left panels) and TNFα (right panels) for up to 12 h. As shown in Figure 1C, after the cytoplasmic and nuclear IκBα was degraded within 30 min after neutrophil stimulation, it was followed by reappearance of IκBα in both cytoplasm and nucleus. Although IκBα levels were reduced during the later time points (6–12 h), there were still considerable amounts of IκBα present in the nucleus at the time of maximal NFκB activity 9 h after stimulation. The cytoplasmic and nuclear levels of NFκB p50 and p65 proteins during neutrophil stimulation with LPS and TNFα did not correlate with the NFκB activity (Figure 1).
To determine whether NFκB activity in the neutrophils might be regulated by other IκB proteins, we analyzed the intracellular levels of IκBβ and IκBɛ. The IκBβ expression was analyzed by two antibodies raised against the C-terminus (C-20) and N-terminus of IκBβ (N-20). Although these antibodies previously identified IκBβ in other human cells (42), no reacting protein was detected in the neutrophils, indicating that human neutrophils do not express the IκBβ protein (data not shown). Our data showed that IκBɛ is expressed in human neutrophils and is localized in both cytoplasm and nucleus (Figure 1C). Because the IκBɛ protein has not been previously described in human neutrophils, we analyzed the intracellular levels of IκBɛ in LPS and TNFα-stimulated neutrophils by densitometry (Figure 1D). The changes in the IκBɛ cytoplasmic or nuclear levels were not statistically significant (P > 0.05), and the IκBɛ levels did not correlate with the NFκB activity, suggesting that IκBɛ degradation does not play an important role in the regulation of transcriptional NFκB activity in human neutrophils.
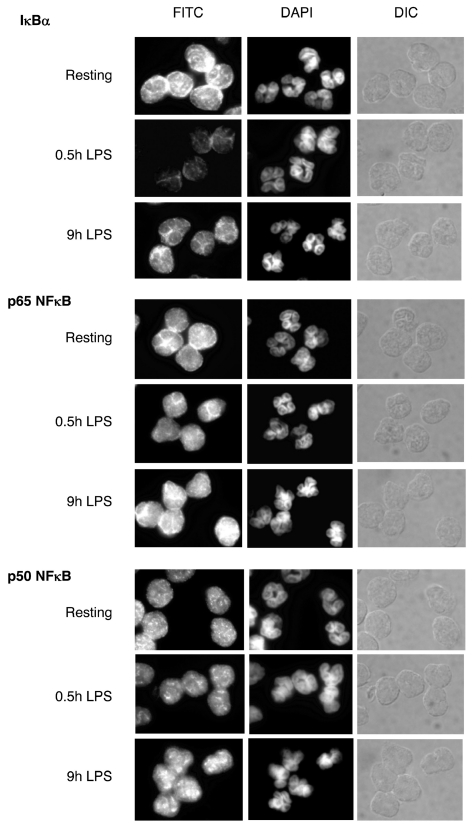
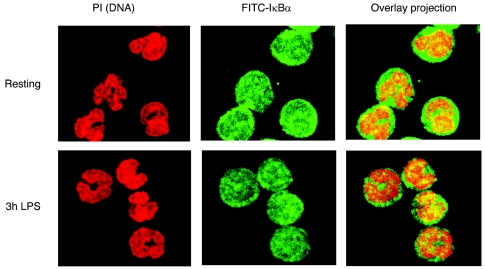
To analyze the cellular levels of IκBα and NFκB proteins in resting and stimulated neutrophils at the level of individual cells, we performed indirect immunofluorescence microscopy. As shown in Figure 2, in the resting neutrophils, IκBα, p50, and p65 NFκB proteins displayed both cytoplasmic and nuclear staining. After neutrophil stimulation with LPS for 30 min, IκBα was degraded and exhibited reduced cellular staining. No substantial differences were observed in the immunofluorescence staining of the NFκB p50 and p65 subunits. These results, and the immunoblotting data shown in Figure 1, indicate that in human neutrophils stimulated with inflammatory signals for 30 min, the cellular levels of IκBα, and not the NFκB subunits, serve as a better marker for NFκB activity. When neutrophils were stimulated with LPS for 9 h, the newly synthesized IκBα was no longer degraded, and its cellular levels were comparable to those in the resting neutrophils. Similarly, the nuclear and cytoplasmic levels of NFκB p50 and p65 subunits at 9 h after stimulation were essentially unchanged from the resting neutrophils (Figure 2). Similar results were observed when neutrophils were stimulated for 30 min and 9 h with TNFα instead of LPS (data not shown). Figure 3 illustrates the intracellular localization of IκBα analyzed by confocal immunofluorescence microscopy in resting neutrophils and neutrophils stimulated with LPS for 3 h. Consistent with the indirect immunofluorescence microscopy (Figure 2), in both resting and stimulated neutrophils, IκBα was localized both in the cytoplasm and in the nucleus; however, the punctuated staining of IκBα in the nucleus indicated that it might be binding to intranuclear components or structures.
Figure 2.
Indirect immunofluorescence microscopy of IκBα and NFκB p50 and p65 proteins during neutrophil stimulation with LPS (100 ng/mL). The left panels show FITC staining of IκBα and NFκB p50 and p65 proteins. The middle panels illustrate DNA nuclear staining with 4′6&o\prime;-diamidino-2-phenylindole (DAPI). The right panels show differential interference contrast (DIC) microscopic images of the cells.
Figure 3.
Confocal immunofluorescence microscopy of IκBα in LPS-stimulated neutrophils. Resting neutrophils and neutrophils stimulated for 3 h with LPS (100 ng/mL) were fixed and analyzed by confocal laser scanning microscopy using anti-IκBα antibody. Left panels show DNA staining with propidium iodide (PI). The middle panels illustrate IκBα localization by using FITC staining. The right panels show overlap of the DNA and FITC staining.
IκBα and NFκB p50 and p65 Proteins Associate with the Nuclear Matrix in Human Neutrophils
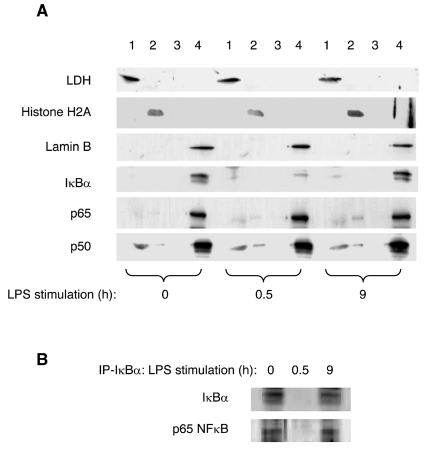
To gain insight into the subnuclear localization of IκBα and NFκB proteins during neutrophil stimulation, we isolated soluble chromatin and nuclear matrix fractions by the sequential extraction procedure of He et al. (43). In the first extraction step, soluble (cytoplasmic and nuclear) proteins are removed by extraction with Triton X-100. Chromatin-bound proteins are then released by DNase digestion and extraction with ammonium sulfate. After washing with 2 M NaCl, the last fraction is composed of structural nuclear proteins and the nuclear matrix–associated proteins. Supernatants from each extraction step and the final nuclear matrix pellets were analyzed by SDS-PAGE and immunoblotting. The purity of the cellular fractions was monitored by using lamin B (nuclear matrix marker), histone H2A (chromatin marker), and lactate dehydrogenase (LDH; soluble cytoplasmic protein). Surprisingly, as shown in Figure 4A, in the resting neutrophils, the vast majority of IκBα was associated with the nuclear matrix, also containing the NFκB p65 and p50 subunits. Neutrophil stimulation with LPS for 30 min induced degradation of IκBα; however, the intracellular levels and localization of NFκB p50 and 65 proteins remained essentially unchanged. Neutrophil stimulation with LPS for 9 h induced resynthesis of IκBα, which was again localized in the nuclear matrix. As in the neutrophils stimulated with LPS for 30 min, 9-h stimulation did not have any significant effect on the nuclear levels of NFκB p50 and p65 subunits (Figure 4A). Very similar results were observed when neutrophils were stimulated with TNFα (data not shown).
Figure 4.
IκBα associates with NFκB in the nuclear matrix of human neutrophils. (A) Neutrophils were stimulated with LPS (100 ng/mL) for 0, 0.5, and 9 h and fractionated to prepare soluble (1), chromatin (2), chromatin wash (3), and nuclear matrix (4) fractions. The fractions were analyzed by Western blotting using LDH, histone H2A, lamin B, IκBα, and p50 and p65 NFκB antibodies. Each lane contains approximately 106 cells. (B) Neutrophils were stimulated with LPS (100 ng/mL) for 0, 0.5, and 9 h, and the nuclear extracts were immunoprecipitated on anti–IκBα-agarose. The immunoprecipitated proteins were detected by immunoblotting using IκBα and p65 NFκB antibodies.
The above data suggested that the nuclear IκBα may associate with NFκB proteins in the nucleus of human neutrophils. To test this hypothesis, we immunoprecipitated IκBα from the nuclear extracts of resting and stimulated neutrophils and analyzed the associated p65 NFκB by immunoblotting. As shown in Figure 4B, in the resting neutrophils, p65 NFκB coimmunoprecipitated with the nuclear IκBα. In neutrophils stimulated with LPS for 30 min, the nuclear IκBα was degraded, and thus neither IκBα nor p65 NFκB was recovered on anti–IκBα-agarose. In neutrophils stimulated with LPS for 9 h, the newly synthesized IκBα was again immunoprecipitated on anti–IκBα-agarose. Interestingly, p65 NFκB coimmunoprecipitated with the nuclear IκBα from the nuclear extracts of neutrophils stimulated for 9 h, indicating that the p65 NFκB binding by the newly synthesized nuclear IκBα is not sufficient to prevent the persistent NFκB activation in continuously stimulated neutrophils.
Release of NFκB-Dependent Mediators During Continuous Neutrophil Stimulation
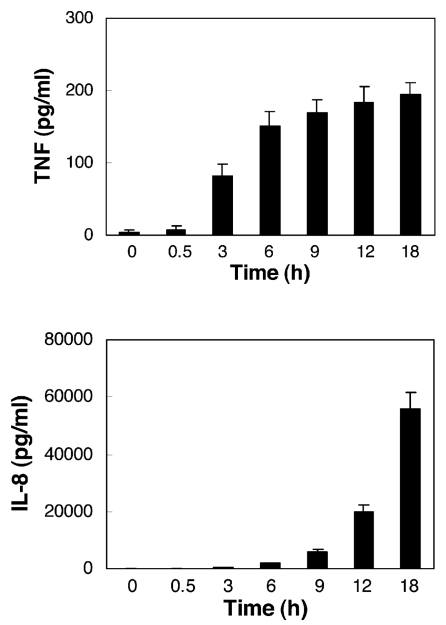
To investigate whether the prolonged NFκB activation correlates with the synthesis of NFκB-dependent mediators, we measured release of IL-8 and TNFα from LPS-stimulated neutrophils. As illustrated in Figure 5, whereas the TNFα release was induced early (around 3 h after stimulation), after the early activation of NFκB in LPS-stimulated neutrophils, IL-8 was released after 9 h of stimulation, after the second wave of NFκB activation. Neutrophils cultured for 18 h in the absence of LPS released only 0.4% of IL-8 compared with LPS-stimulated PMN (200 ± 74 pg/mL compared with 55,533 ± 6,043 pg/mL, n = 3), and 6% of TNFα compared with LPS-stimulated PMN (11 ± 2 pg/mL compared with 194 ± 21 pg/mL, n = 3).
Figure 5.
TNFα and IL-8 release from LPS-stimulated neutrophils. TNFα and IL-8 release from LPS (100 ng/mL)-stimulated neutrophils (5 × 106/mL) was assayed from cell culture media. Data for both panels are expressed as mean values of three independent experiments ± SE.
DISCUSSION
In this study, we demonstrate that TNFα- and LPS-stimulated neutrophils exhibit a previously unrecognized NFκB activation that lasts up to 12 h. In contrast to the early activation of NFκB that is regulated by the nuclear levels of IκBα (24–26), this delayed NFκB activity in 9 h stimulated neutrophils is increased despite the presence of IκBα in the nucleus. Based on this and previous studies from our laboratory (25,26), we propose a new model of NFκB regulation during persistent neutrophil stimulation with inflammatory signals. In the resting neutrophils, IκBα is localized in the nuclear matrix that also contains NFκB p50 and p65 subunits, and by binding to p65 NFκB it inhibits NFκB activity. After neutrophil stimulation with proinflammatory signals, both the nuclear and cytoplasmic IκBα are degraded within 30 min, thus releasing p65 NFκB from the inhibitory complex to bind to NFκB-dependent promoters (25). Our results indicate that during persistent neutrophil stimulation, IκBα is again synthesized and translocates to the nucleus (Figure 1C). This is supported by our previous study demonstrating that cycloheximide treatment prevents the reappearance of both nuclear and cytoplasmic IκBα after 2-h neutrophil stimulation with LPS or TNFα (25). Thus, it seems that the newly synthesized nuclear IκBα that associates with p65 NFκB in the nuclear matrix (Figure 4), is no longer sufficient to inhibit the persistent NFκB activity. The reduced ability of the nuclear IκBα to inhibit NFκB DNA binding at 9 h after stimulation suggests that the newly synthesized nuclear IκBα may be posttranslationally modified, and its binding to p65 NFκB does not inhibit, or may even stimulate, the NFκB DNA binding. Alternatively, the concentration of the newly synthesized IκBα in the nucleus might be lower than the concentrations of NFκB proteins. In addition, it cannot be completely ruled out at present that even though IκBɛ is not degraded after neutrophil stimulation, it can still regulate the transcriptional activity of NFκB. Studies are in progress to discriminate between these models, and to identify the exact mechanisms by which the nuclear IκB proteins regulate the NFκB-dependent transcription in stimulated human neutrophils.
The nuclear matrix is a specialized proteinaceous nuclear structure that serves as a scaffold for chromatin loops. Studies have suggested that the nuclear matrix is tightly associated with transcriptionally active, but not inactive, DNA (45,46). To our knowledge, this study provides the first demonstration that IκBα associates with the nuclear matrix. Our results are consistent with the morphological study of Trubiani et al. (47) showing that in stimulated epithelial cells, the subnuclear localization of NFκB p50 and p65 proteins is the nuclear matrix. We suggest that the nuclear localization of IκBα and attachment to nuclear matrix represent one of the underlying mechanisms for the decreased activation of NFκB in human neutrophils (34,48) and for the increased apoptosis of these cells.
The subunit composition of NFκB complexes activated in LPS- and TNFα-stimulated neutrophils at 9 h (Figure 1B) is the same as the NFκB subunit composition at 30 min (36): p50/50 homodimers and p50/65 heterodimers. In addition, Western blotting (Figures 1 and 4) as well as immunofluorescence microscopy (Figure 2) revealed that the p50 and p65 NFκB nuclear protein levels are not substantially changed during neutrophil stimulation with TNFα or LPS, indicating that the persistent NFκB activity in stimulated human neutrophils is not regulated primarily by the nuclear translocation of NFκB subunits. Thus, when assessing NFκB activity during neutrophil-mediated inflammatory disorders, it is important to analyze the extent of NFκB activity by EMSA, and not only the nuclear levels or translocation of NFκB subunits.
There is an urgent need for early detection and safer and more specific therapies that minimize tissue injury for ALI, sepsis, and BPD. Neutrophil apoptosis, regulated by NFκB, plays a critical role in the resolution of inflammation associated with these disorders (28–32,49). Although studies have shown that NFκB is activated in patients with ALI, sepsis, and BPD (4,30–33,44), the stage of NFκB activation in these patients is not known. It seems plausible that it is the persistent NFκB activity, and not the early NFκB activation induced by neutrophil stimulation for 30 min, that is associated with the tissue injury in these inflammatory disorders. Whereas TNFα, one of the first mediators of sepsis (50,51), is released early during neutrophil stimulation, IL-8 is released during later time points (Figure 5). We hypothesize that different genes may be activated by NFκB during different times of neutrophil stimulation. In this scenario, the TNFα transcription would be expected to be induced by the early NFκB activation in LPS-stimulated neutrophils, and the IL-8 transcription during the second wave. It might be possible that the second wave of NFκB activation in LPS-stimulated neutrophils is caused by the release of TNFα or other NFκB-regulated mediator. However, this does not seem very likely, because the second wave of NFκB activation in LPS-stimulated neutrophils is not associated with the degradation of cytoplasmic or nuclear IκBα (Figure 1). A detailed analysis by chromatin immunoprecipitation of the nuclear IκB and/or NFκB complexes that are recruited to NFκB promoters during neutrophil stimulation will be essential to identify the specific NFκB-regulated proinflammatory and anti-apoptotic genes synthesized during different stages of neutrophil activation.
Previous studies have shown that the early activation of NFκB is regulated by PKCδ, IκB kinase, and p38 MAP kinase and by PP1/PP2A phosphatases (24,27, 41,26); however, the mechanisms that regulate the persistent NFκB activity during continuous neutrophil stimulation are unknown. In addition, inhibitors of NFκB, such as dexamethasone or cur-cumin, inhibit production of NFκB-dependent proinflammatory cytokines and induce neutrophil apoptosis (52,53). It will therefore be important to identify the upstream signaling mechanisms that regulate the persistent NFκB activity and to determine whether this delayed NFκB activity in continuously stimulated human neutrophils can be inhibited by antiinflammatory or proapoptotic drugs like dexamethasone and curcumin.
Previous work from our laboratory has demonstrated that there are significant differences in the NFκB regulation between human neutrophils and other cells, such as monocytic cells (24–26). The early stage of NFκB activation in human neutrophils is regulated predominantly by the nuclear levels of IκBα (24–26). This study shows that NFκB is persistently activated in human neutrophils stimulated with LPS or TNFα, but this persistent NFκB activation is independent of the nuclear levels of IκBα. A better understanding of the mechanisms regulating the initiation and persistence of NFκB activation in human neutrophils may provide new information for the development of early detection and safer therapies for BPD, ALI, sepsis, and other neutrophil-mediated inflammatory disorders.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health Grant HD39643 and by the St. John’s University Faculty Research Award to IV.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–9. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 2.Tracey KJ, Lowry SF, Cerami A. Ca-chetin/TNF-alpha in septic shock and septic adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:1377–9. doi: 10.1164/ajrccm/138.6.1377. [DOI] [PubMed] [Google Scholar]
- 3.Worthen SG, Henson PM. Mechanism of acute lung injury. Clin Lab Med. 1983;3:601–17. [PubMed] [Google Scholar]
- 4.Ye RD. Leukocyte inflammatory mediators and lung pathophysiology: an update. Am J Physiol Lung Cell Mol Physiol. 2004;286:L461–2. doi: 10.1152/ajplung.00391.2003. [DOI] [PubMed] [Google Scholar]
- 5.Cassatella MA, Gasperini S, Russo MP. Cytokine expression and release by neutrophils. Ann N Y Acad Sci. 1997;832:233–42. doi: 10.1111/j.1749-6632.1997.tb46251.x. [DOI] [PubMed] [Google Scholar]
- 6.Dubravec DB, Spriggs DR, Mannick JA, Rodrick ML. Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor α. Proc Natl Acad Sci U S A. 1990;87:6758–61. doi: 10.1073/pnas.87.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol. 2002;14:123–32. doi: 10.1006/smim.2001.0349. [DOI] [PubMed] [Google Scholar]
- 8.Strieter RM, Kunkel SL, Keane MP, Standiford TJ. Chemokines in lung injury: Thomas A. Neff Lecture. Chest. 1999;116:103S–10. doi: 10.1378/chest.116.suppl_1.103s. [DOI] [PubMed] [Google Scholar]
- 9.Abraham E. NFκB and its role in sepsis-associated organ failure. J Infect Dis. 2003;187:S364–9. doi: 10.1086/374750. [DOI] [PubMed] [Google Scholar]
- 10.Ayala A, Chung CS, Grutkoski PS, Song GY. Mechanisms of immune resolution. Crit Care Med. 2003;31:S558–71. doi: 10.1097/01.CCM.0000081438.04801.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotecha S, Mildner RJ, Prince LR, Vyas JR, Currie AE, Lawson RA, Whyte MK. The role of neutrophil apoptosis in the resolution of acute lung injury in newborn infants. Thorax. 2003;58:961–7. doi: 10.1136/thorax.58.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin TR, Nakamura M, Matute-Bello G. The role of apoptosis in acute lung injury. Crit Care Med. 2003;31:S184–8. doi: 10.1097/01.CCM.0000057841.33876.B1. [DOI] [PubMed] [Google Scholar]
- 13.Baeuerle PA, Baltimore D. NFκB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin AS. The NFκB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 15.Fan J, Ye RD, Malik AB. Transcriptional mechanisms of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1037–50. doi: 10.1152/ajplung.2001.281.5.L1037. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh S, Karin M. Missing pieces in the NFκB puzzle. Cell. 2002;109:S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Takada Y, Boriek AM, Aggarwal BB. NF-κB: its role in health and disease. J Mol Med. 2004;82:434–48. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 18.Liu SF, Ye X, Malik AB. Inhibition of NFκB activation by pyrrolidine dithiocarban prevents in vivo expression of proinflammatory genes. Circulation. 1999;100:1330–7. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- 19.Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 20.Park GY, Christman JW. Nuclear factor kappa B is a promising therapeutic target in inflammatory lung disease. Curr Drug Targets. 2006;7:661–8. doi: 10.2174/138945006777435317. [DOI] [PubMed] [Google Scholar]
- 21.Christman JW, Lancaster LH, Blackwell TS. Nuclear factor κB: a pivotal role in the systemic inflammatory response syndrome and new target for therapy. Intensive Care Med. 1998;24:1131–8. doi: 10.1007/s001340050735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lentsch AB, Ward PA. Activation and regulation of NFκB during acute inflammation. Clin Chem Lab Med. 1999;37:205–8. doi: 10.1515/CCLM.1999.038. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Verma IM. NFκB regulation in the immune system. Nature Rev Immunol. 2002;2:725–34. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 24.Vancurova I, Miskolci V, Davidson NF-κB activation in TNF-stimulated neutrophils is mediated by protein kinase C-δ: correlation to nuclear IκBα. J Biol Chem. 2001;276:19746–52. doi: 10.1074/jbc.M100234200. [DOI] [PubMed] [Google Scholar]
- 25.Castro-Alcaraz S, Miskolci V, Kalasapudi B, Davidson D, Vancurova I. NFκB regulation in human neutrophils by nuclear IκBα: correlation to apoptosis. J Immunol. 2002;169:3947–53. doi: 10.4049/jimmunol.169.7.3947. [DOI] [PubMed] [Google Scholar]
- 26.Miskolci V, Castro-Alcaraz S, Nguyen P, Vancura A, Davidson D, Vancurova I. Okadaic acid induces sustained activation of NFκB and degradation of the nuclear IκBα in human neutrophils. Arch Biochem Biophys. 2003;417:44–52. doi: 10.1016/s0003-9861(03)00336-9. [DOI] [PubMed] [Google Scholar]
- 27.Ear T, Cloutier A, McDonald PP. Constitutive nuclear expression of the IκB kinase complex and its activation in human neutrophils. J Immunol. 2005;175:1834–42. doi: 10.4049/jimmunol.175.3.1834. [DOI] [PubMed] [Google Scholar]
- 28.Hofman P. Molecular regulation of neutrophil apoptosis and potential targets for therapeutic strategy against the inflammatory process. Curr Drug Targets Inflamm Allergy. 2004;3:1–9. doi: 10.2174/1568010043483935. [DOI] [PubMed] [Google Scholar]
- 29.Nolan B, Collette H, Baker S, Duffy A, De M, Miller C, Bankey P. Inhibition of neutrophil apoptosis after severe trauma is NFκB dependent. J Trauma. 2000;48:599–604. doi: 10.1097/00005373-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Wright JG, Christman JW. The role of NFκB in the pathogenesis of pulmonary diseases: implications for therapy. Am J Respir Med. 2003;2:211–9. doi: 10.1007/BF03256650. [DOI] [PubMed] [Google Scholar]
- 31.Yang KY, Arcaroli JJ, Abraham E. Early alterations in neutrophil activation are associated with outcome in acute lung injury. Am J Respir Crit Care Med. 2003;167:1567–74. doi: 10.1164/rccm.200207-664OC. [DOI] [PubMed] [Google Scholar]
- 32.Zingarelli B, Sheehan M, Wong HR. NFκB as a therapeutic target in critical care medicine. Crit Care Med. 2003;31:S105–11. doi: 10.1097/00003246-200301001-00015. [DOI] [PubMed] [Google Scholar]
- 33.Cheah FC, Hampton MB, Darlow BA, Winterbourn CC, Vissers MC. Detection of apoptosis by caspase-3 activation in tracheal aspirate neutrophils from premature infants: relationship with NF-kappaB activation. J Leukoc Biol. 2005;77:432–7. doi: 10.1189/jlb.0904520. [DOI] [PubMed] [Google Scholar]
- 34.McDonald PP, Bald A, Cassatella MA. Activation of NFκB pathway by inflammatory stimuli in human neutrophils. Blood. 1997;89:3421–33. [PubMed] [Google Scholar]
- 35.Ward C, et al. NF–κB activation is a critical regulator of human granulocyte apoptosis in vitro. J Biol Chem. 1999;274:4309–18. doi: 10.1074/jbc.274.7.4309. [DOI] [PubMed] [Google Scholar]
- 36.Vancurova I, Bellani P, Davidson D. Activation of NFκB and its suppression by dexamethasone in polymorphonuclear leukocytes: newborn versus adult. Pediatr Res. 2001;49:257–62. doi: 10.1203/00006450-200102000-00021. [DOI] [PubMed] [Google Scholar]
- 37.Carballo M, et al. Characterization of calcineurin in human neutrophils: inhibitor effect of hydrogen peroxide on its enzyme activity and on NFκB DNA binding. J Biol Chem. 1999;274:93–100. doi: 10.1074/jbc.274.1.93. [DOI] [PubMed] [Google Scholar]
- 38.Choi M, Rolle S, Wellner M, Cardoso MC, Scheidereit C, Luft FC, Kettritz R. Inhibition of NFκB by a TAT-NEMO-binding domain peptide accelerates constitutive apoptosis and abrogates LPS-delayed neutrophil apoptosis. Blood. 2003;102:2259–67. doi: 10.1182/blood-2002-09-2960. [DOI] [PubMed] [Google Scholar]
- 39.Kettritz R, Choi M, Rolle S, Wellner M, Luft FC. Integrins and cytokines activate nuclear transcription factor-κB in human neutrophils. J Biol Chem. 2004;279:2657–65. doi: 10.1074/jbc.M309778200. [DOI] [PubMed] [Google Scholar]
- 40.Kilpatrick LE, Lee JY, Haines K, Campbell DE, Sullivan KE, Korchak HH. A role for PKC-δ and PI 3-kinase in TNF-α-mediated anti-apoptotic signaling in the human neutrophil. Am J Physiol Cell Physiol. 2002;283:C48–57. doi: 10.1152/ajpcell.00385.2001. [DOI] [PubMed] [Google Scholar]
- 41.Nick JA, et al. Selective activation and functional significance of p38alpha mitogen-activated protein kinase in lipopolysaccharide-stimulated neutrophils. J Clin Invest. 1999;103:851–8. doi: 10.1172/JCI5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vancurova I, Wu R, Miskolci V, Sun S. Increased p50/50 NFκB activation in human papillomavirus type-6 or type 11 induced laryngeal papilloma tissue. J Virol. 2002;76:1533–6. doi: 10.1128/JVI.76.3.1533-1536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He DC, Nickerson JA, Penman S. Core filaments of the nuclear matrix. J Cell Biol. 1990;110:569–80. doi: 10.1083/jcb.110.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abraham E. Alterations in cell signaling in sepsis. Clin Infect Dis Suppl. 2005;7:S459–64. doi: 10.1086/431997. [DOI] [PubMed] [Google Scholar]
- 45.Jackson DA, Hassan AB, Errington RJ, Cook PR. Visualization of focal sites of transcription within human nuclei. EMBO J. 1993;12:1059–65. doi: 10.1002/j.1460-2075.1993.tb05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng C, et al. Identification of a nuclear matrix targeting signal in the leukemia and bone-related AML/CBF-α transcription factors. Proc Natl Acad Sci U S A. 1997;94:6746–51. doi: 10.1073/pnas.94.13.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trubiani O, De Fazio P, Pieri C, Mazzanti L, Di Primio R. Nuclear matrix provides linkage sites for translated NFκB: morphological evidence. Histochem Cell Biol. 2000;113:369–77. doi: 10.1007/s004180000145. [DOI] [PubMed] [Google Scholar]
- 48.Browning DD, Pan ZK, Prossnitz ER, Ye RD. Cell type- and developmental stage-specific activation of NFκB by fMet-Leu-Phe in myeloid cells. J Biol Chem. 1997;272:7995–8001. doi: 10.1074/jbc.272.12.7995. [DOI] [PubMed] [Google Scholar]
- 49.Hu M, Lin X, Du Q, Miller EJ, Wang P, Simms HH. Regulation of polymorphonuclear leukocyte apoptosis: role of lung endothelium-epithelium bilayer transmigration. Am J Physiol Lung Cell Mol Physiol. 2005;288:L266–74. doi: 10.1152/ajplung.00209.2004. [DOI] [PubMed] [Google Scholar]
- 50.Tracey KJ, Cerami A. Cachectin/tumor necrosis factor and other cytokines in infectious disease. Curr Opin Immunol. 1989;1:454–61. doi: 10.1016/0952-7915(88)90026-x. [DOI] [PubMed] [Google Scholar]
- 51.Czura CJ, Yang H, Tracey KJ. High mobility group box-1 as a therapeutic target downstream of tumor necrosis factor. J Infect Dis. 2003;187:S391–6. doi: 10.1086/374753. [DOI] [PubMed] [Google Scholar]
- 52.Irakam A, Miskolci V, Vancurova I, Davidson D. Dose-related inhibition of pro-inflammatory cytokine release from neutrophil of the newborn by dexamethasone, betamethasone, and hydrocortisone. Biol Neonate. 2002;82:89–95. doi: 10.1159/000063094. [DOI] [PubMed] [Google Scholar]
- 53.Hu M, Du Q, Vancurova I, Lin X, Miller EJ, Simms HH, Wang P. Proapoptotic effect of curcumin on human neutrophils: activation of the p38 mitogen-activated protein kinase pathway. Crit Care Med. 2005;33:2571–8. doi: 10.1097/01.ccm.0000186760.20502.c7. [DOI] [PubMed] [Google Scholar]