Abstract
The anxiolytic effects of benzodiazepines appear to involve opioid processes in the amygdala. In previous experiments, overexpression of enkephalin in the amygdala enhanced the anxiolytic actions of the benzodiazepine agonist diazepam in the elevated plus maze. The effects of systemically administered diazepam are also blocked by injections of naltrexone into the central nucleus of the amygdala. The current studies investigated the role of delta opioid receptors in the anxiety-related effects of diazepam. Three days following bilateral stereotaxic injections of viral vectors containing cDNA encoding proenkephalin or β-galactosidase (control vector), the delta opioid receptor antagonist naltrindole (10 mg/kg, s.c.) attenuated the enhanced anxiolytic effects of 1–2 mg/kg diazepam in rats overexpressing preproenkephalin in the amygdala. Despite this effect, naltrindole failed to attenuate the anxiolytic action of higher diazepam doses (3 mg/kg) in animals with normal amygdalar enkephalin expression. Similarly, the mu opioid receptor antagonist, β-funaltrexamine (20mg/kg, sc), had no effect on the anxiolytic effect of diazepam alone. These data support a role for delta opioid receptors in the opioid-enhanced anxiolytic effects of diazepam.
Keywords: Delta Opioid Receptors, Enkephalin, Benzodiazepines, Amygdala, Anxiety, Herpes Viral Vectors
INTRODUCTION
The anxiolytic effects of benzodiazepines may in part be mediated through modulating the endogenous opioid system, although the exact nature of this interaction and opioid receptor subtype(s) involved remain unclear. The systemic administration of nonselective opioid antagonists can block the anticonflict and anxiety-reducing effects of benzodiazepines in several animal models (Billingsley and Kubena 1978;Koob et al. 1980;Soubrie et al. 1980;Duka et al. 1982;Agmo et al. 1995;Tsuda et al. 1996), as well as in humans (Duka et al. 1982), although some studies failed to show such effects of naloxone (Britton et al. 1981). These behavioral studies show that opioid antagonists are able to block the anxiolytic effect of benzodiazepines in the elevated plus maze task or anticonflict tests (Billingsley and Kubena 1978;Koob et al. 1980;Soubrie et al. 1980;Agmo et al. 1995;Tsuda et al. 1996) and can increase shock-induced freezing in rats (Fanselow and Bolles 1979). In contrast, some studies indicate that the systemic administration of nonselective opioid antagonists actually enhance the anxiolytic actions of low doses of benzodiazepine agonists (diazepam, chlordiazepoxide), but not partial agonists (Belzung and Agmo 1997b;Frussa-Filho et al. 1999;Belzung et al. 2000). These prior studies suggest that both doses of opioids and benzodiazepines used in these studies (Agmo et al. 1995;Belzung and Agmo 1997a), as well as the novelty of the testing situation (File and Rodgers 1979a;Frussa-Filho et al. 1999) influence the ability of opioids agonists and antagonists to modify the anxiolytic influences of benzodiazepines.
The role of specific opioid receptors in mediating the anxiolytic actions of benzodiazepines is also unclear. Although systemic administration of selective mu and kappa opioid receptor antagonists can block the anticonflict effects of diazepam in mice (Tsuda et al. 1996), mu opioid receptor (MOR) knockout mice showed unaltered anxiolytic effects of diazepam in the elevated plus maze compared to control strains (LaBuda and Fuchs 2001). Delta opioid receptor (DOR) knockout mice show increased anxiety in several tests (Filliol et al. 2000), and administration of the delta receptor antagonist naltrindole intracerebroventricularly (i.c.v.) increased anxiety in the light:dark test (Narita et al. 2006a). Administration of the delta opioid receptor antagonist naltrindole, however, failed to block the anticonflict effects of diazepam (Tsuda et al. 1996).The systemic administration of kappa opioid receptor agonists can produce anxiolytic effects in the plus maze (Privette and Terrian 1995), but the administration of dynorphin i.c.v. in mice decreased time in the lit compartment in a light: dark test (Narita et al. 2006a).
These previous studies have further suggested a role of amygdalar opioids in the anxiolytic effects of benzodiazepines, although the opioid peptides and opioid receptor subtypes mediating such effects is unclear. The amygdala has both mu and delta opioid receptor sites (Mansour et al. 1995a;Wilson et al. 2002;Poulin et al. 2006), and met-ENK colocalizes with the GABA synthetic enzyme glutamate decarboxylase (GAD) in the central nucleus of the amygdala (Veinante et al. 1997). Morphine injections into the amygdala also produce partial anxiolytic effects in the social interaction test (File and Rodgers 1979a). The administration of a MOR antagonist, a DOR antagonist and dynorphin in to the amygdala all decreased time in the lit compartment of a light: dark transition test(Narita et al. 2006a). In addition to these influences on basal anxiety state, we have previously shown that herpes virus-mediated overexpression of enkephalin (ENK) in the amygdala potentiates the anxiolytic effects of the benzodiazepine agonist diazepam in the elevated plus maze test (Kang et al. 2000). These effects are reversed by systemic administration of the non-selective opioid receptor antagonist naloxone. Localized injections of the non-selective antagonist naltrexone in the central nucleus of the amygdala, but not the basolateral amygdala, attenuate the anxiolytic influences of diazepam in the elevated plus maze (Kang et al. 2000;Burghardt and Wilson 2006).
Amygdalar lesions, administration of peptides, and anxiolytic drugs can show divergent results in differing animal tests of anxiety or fear, and it has been suggested that these models assess differing aspects of fear or anxiety responses (Green 1991). The elevated plus maze takes advantage of the animals natural tendencies to avoid brightly lit, open, elevated spaces, but relies on a passive avoidance response to detect anxiety behavior (e.g., avoidance of open arms) and can be confounded by changes in activity levels. In contrast, the defensive prod burying model is less affected by locomotor changes and (more importantly) the index of anxiety involves an active behavioral response, specifically burying of a discrete object (the shock probe). Therefore, we also examined the ability of systemic administration of delta and mu opioid receptor antagonists to attenuate the anxiolytic actions of diazepam in these two distinct models, since these tests appear to be differentially influenced by opioid drugs and amygdala processes (Billingsley and Kubena 1978;Fanselow and Bolles 1979;Koob et al. 1980;Soubrie et al. 1980;Treit 1985;Grijalva et al. 1990;Kopchia et al. 1992;Treit et al. 1993;Agmo et al. 1995;Tsuda et al. 1996;de Boer and Koolhaas 2003). Several studies support the possibility that anxiolytic effects of benzodiazepines in conflict-based procedures, including the plus maze, might be inhibited by opioid antagonists (Billingsley and Kubena 1978;Koob et al. 1980;Soubrie et al. 1980;Agmo et al. 1995;Tsuda et al. 1996). The situation is less clear for prod burying, although shock-induced freezing is disrupted by naloxone (Fanselow and Bolles 1979;Treit 1985;Treit et al. 1993;de Boer and Koolhaas 2003).
The present experiments were designed to 1) confirm the role of the delta opioid receptor in mediating the influences of amygdalar ENK-overexpression on the anxiolytic effects of diazepam, and 2) assess the ability of both selective delta (naltrindole, NTI) and mu (β-funaltrexamine, FNA) opioid receptor antagonists to attenuate the anxiolytic effects of diazepam in rats. These studies utilized a new viral vector encoding rat prepro-ENK, for comparison to our previous studies using viral vectors encoding for human prepro-ENK (Kang et al. 2000). It was predicted that virally mediated over-expression of amygdalar ENK in the amygdala would potentiate the anxiolytic effects of diazepam in the elevated plus maze test, and these effects would be attenuated by the delta opioid receptor antagonist naltrindole (NTI).
METHODS
Subjects
Adult Long-Evans male rats (Harlan, Indianapolis, IN) weighing 175–200g at time of arrival were used in this experiment. In Experiment 1, rats were housed individually and in Experiment 2, rats were housed 3/cage. All rats were on a 12/12 LD cycle (lights on at 0700) with food/water available ad libitum. All procedures were approved by the University of South Carolina Institutional Animal Care and Use Committee and followed the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Virus Construction
Replication-defective, recombinant herpes viruses were created using the KOS strain of HSV-1 with both copies of ICP4 (IE3) deleted (DeLuca et al. 1985). A shuttle plasmid, pEPVG, was created to insert an expression cassette between the open reading frames of UL36 and UL37 in the HSV genome. First, an Xho I-Not I DNA fragment containing KOS DNA corresponding to HSV nucleotides 80176–81324 (GenBank X14112) was cloned into pNEB193 (New England Biolabs) modified by removal of the Hind III site and replacing the Sma I site with a second Pac I site. Next, an Ase I-Afl II fragment of pIRES2-EGFP (Clontech) was cloned blunt into the Hind III site of the HSV sequence. The latter fragment contained the following elements: human cytomeglovirus immediate early enhancer-promoter (hCMV), multiple cloning sites, internal ribosome entry site (IRES), enhanced green fluorescent protein cDNA (EGFP) and SV40 polyadenylation site. The rat prepro-enkephalin cDNA was cloned into the pEPVG multiple cloning site at Eco47 III as a blunt EcoR I-Sma I fragment from pYSEC1 (Yoshikawa et al. 1984;Yoshikawa and Sabol 1986) generating pEPGrPE . Similarly, a BamH I fragment from pCMVβ (MacGregor and Caskey 1989) containing lac Z was cloned into pEPVG cut with BamH I and Bgl II, creating pEPGZ.
For virus generation, Spe I-cut ICP4– viral DNA was transfected with Pac I-digested pEPGrPE or pEPGZ into the complementing 7B cell line (Marconi et al. 1996). Recombinant viruses were selected by 3 rounds of limiting dilution. Because Spe I is a unique site in HSV, this method gives high rates of recombination, similar to that reported when a unique Pac I site is introduced into the viral DNA (Krisky et al. 1997). Recombinant viruses were characterized by Southern blotting. Virus stocks were prepared using 7B cells, concentrated by centrifugation and were stored in MEM containing 10% cosmic calf serum (HyClone), 5 mM HEPES, pH 7.4 and 10% glycerol. A diagram of the final viral construct is illustrated in Figure 1A.
Figure 1.

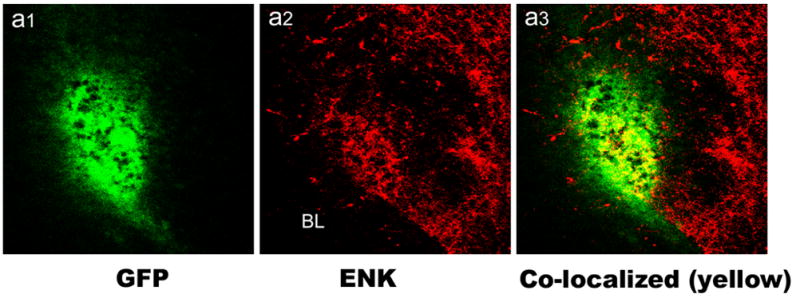

Figure 1A: Schematic diagram of the herpes simplex virus-1 constructs used in this study. Viruses encoded either rat prepro-ENK or the Lac Z reporter gene product, β-galactosidase (control virus). See Methods for construction details and abbreviations. 1B: Co-localization of ENK and Green Fluorescent Protein (EGFP) in the lateral capsular area of the central nucleus of the amygdala using immunofluorescence confocal laser scanning microscopy. “BL” indicates location of the basolateral amygdala for reference. Panel a1 shows expression of GFP (green), panel a2 shows ENK staining (red), and panel a3 shows merging of red and green channels with double-labeling seen as yellow. Amygdala was injected with viral construct DSGrPPE, which expresses both GFP and preproENK. Note the localized staining of GFP (green) demonstrating extent of viral expression, and the heightened level of ENK immunoreactivity is localized with viral expression. Scale bar represents 50 μm. 1C. Photomicrograph of the amygdala (Bregma level -3.1 of the atlas by Paxinos and Watson, 1997) with a box indicating the area shown in Fig. 1B (lateral is left). BLa, anterior subdivision of the basolateral nucleus; BLp, posterior subdivision of the basolateral nucleus; Lat, lateral nucleus; Ce, central nucleus; CP, caudatoputamen.
Herpes Simplex Virus-1 mediated gene transfer surgery
Rats in Experiment 1 were anesthetized with a combination of sodium pentobarbital (Nembutal, 25mg/kg, i.p.) and ketamine hydrochloride (40mg/kg, i.p.). Rats were placed in a stereotaxic apparatus (David Kopf, Tajunga, CA), a midline incision was made and bregma was measured. Bilateral injections of the replication deficient HSV-1 encoding either rat prepro-enkephalin (DSGrPPE, 2.0x106 pfu/ul) or lac Z (DSGZ, 2.0x106 pfu/ul) were made through two holes drilled into the rat’s skull. Injections were made with a 26-gauge Hamilton syringe and aimed at the central nucleus of the amygdala (AP –2.1, LM± 4.6, DV –8.7; (Paxinos and Watson 1997)). Injections were made at a rate of 0.2μl/min using a motorized injector (Micro 4, Microsyringe Pump Controller, World Precision Instruments) followed by a 10 min period in which the virus was allowed to diffuse prior to the needle being withdrawn. Following surgery, an injection of nalbuphine (Sigma, 1mg/kg, s.c.) was given for postoperative analgesia. Three days following viral injection, rats were tested in the elevated plus maze test. This time point is based on Kang et al. (2000) showing an increase in prepro-ENK mRNA levels three days following injections of virus encoding human prepro-ENK into the amygdala.
Localization of viral expression
Initial analysis of viral expression using these viral vectors was assessed using dual-label immunofluorescence confocal laser scanning microscopy (CLSM) as described in Mascagni and McDonald (2003). Rats were injected with HSV-1 vectors encoding prepro-enkephalin (DSGrPPE, 2.0x106 pfu/ul) into the amygdala using stereotaxic techniques. Three days later animals were anesthetized with chloral hydrate (580 mg/kg) and perfused intracardially with phosphate-buffered saline (PBS; pH 7.4 containing 1% sodium nitrite), followed by 4 % paraformaldehyde in phosphate buffer (PB, 7.4). Following perfusion, brains were removed, postfixed for 4 h and sectioned on a vibratome at a thickness of 50 μm in the coronal plane. Sections were processed for immunohistochemistry in wells of tissue culture chamber slides. All antibodies were diluted in PBS containing Triton X-100 (0.4%) and 1% normal goat serum. Sections were incubated overnight at 4°C with primary antisera to met-ENK (1:2000; ImmunoStar, Inc) and EGFP (1:2000; Sigma-Aldrich Chemical Co, monoclonal antisera to Clone GFP-20). After rinses (3 X10 min) in PBS, sections were incubated in secondary antisera conjugated with Alexa488 labeled goat anti-mouse IgG (1:400, Molecular Probes) and Alexa 568-labeled goat anti-rabbit IgG (1:400, Molecular probes) for 3–4 hours at room temperature. Following incubation in secondary antisera, sections were rinsed in PBS (3X10 min), mounted on glass slides, and coverslipped with Vectashield mounting medium (Vector Laboratories). Sections were examined with a BioRad MRC 1024 confocal laser scaning microscope equipped with an argon-krypton laser attached to a Nikon Optiphot fluorescence microscope. Fluorescence of Alexa488 (green) and Alexa568 (red) dyes was analyzed using filter configurations for sequential excitation/imaging using 488 and 568 nm channels. Merged images produced by the image analysis system represent double-labeled cells as yellow. Immunofluorescent analysis was used for qualitative assessment of the extent of virus-mediated transgene expression, and in combination with our prior studies (Kang et al. 2000), to confirm expression of enkephalin with these vectors. (See Figure 1B).
Drug treatments
In Experiment 1, three days following viral injections, each rat was injected with diazepam (DZ; either 1 or 2mg/kg, i.p.) or vehicle (VEH, 10% ethanol, 40% propylene glycol). In addition, each animal also received either naltrindole (NTI, 10mg/kg, s.c.) or vehicle (VEH, sterile saline). This dose of NTI was based on Ossipov et al.,(Ossipov et al. 1994). For each viral construct (DSGZ, DSGrPPE) there were six groups: VEH +VEH, DZ-1 mg/kg +VEH, DZ-2 mg/kg+VEH, VEH + NTI, DZ-1 mg/kg+NTI, DZ-2 mg/kg +NTI, (n=5–13/group). Injections were given 30 minutes prior to behavioral testing. No statistically significant differences were seen between effects of 1 and 2 mg/kg diazepam (also see Wilson et al., 2004), and the results from both doses have been combined for analysis.
In Experiment 2, the ability of both the delta (naltrindole, NTI) and mu (β-funaltrexamine, FNA) opioid receptor antagonists to attenuate the anxiolytic effects of a higher diazepam dose (3 mg/kg) in the absence of overexpression of ENK was tested. Since these animals had normal enkephalin tone, and we anticipated that these antagonists would attenuate benzodiazepine actions in these tasks, we used a dose of diazepam that produced robust anxiolysis in our previous studies (e.g., 3 mg/kg, i.p.; (Wilson et al. 2004)). The effects of opioid receptor antagonists in conjunction with diazepam were examined in two animal models, the elevated plus maze and the defensive prod burying tests. Injections of β-funaltrexamine (FNA, 20mg/kg, s.c.) or vehicle (VEH, sterile saline) were given 24h prior to behavioral testing, and animals received diazepam (DZ-3, 3mg/kg, i.p.) or vehicle (VEH, 10% ethanol, 40% propylene glycol) 30 min before testing. This FNA injection paradigm was based on Paul et al. (1990),Pick et al. (1991), and Negus et al., (1993) (Paul et al. 1990;Pick et al. 1991;Negus et al. 1993). For NTI studies, thirty minutes prior to behavioral testing each rat was injected with diazepam (DZ-3, 3mg/kg, i.p.) or vehicle (VEH, 10% ethanol, 40% propylene glycol) and an injection of either naltrindole (NTI, 10mg/kg, s.c.) or vehicle (VEH, sterile saline). There were a total of six groups: VEH/VEH, VEH/NTI, VEH/FNA, DZ-3/VEH, DZ-3/NTI, DZ-3/FNA, (n=7–11 rats/group).
Elevated Plus Maze Test
All behavioral testing took place between 0900 and 1300h in a room separated from the colony room. A white noise generator was used to mask extraneous noises. The plus maze apparatus had two open (56 x 10 x 1 cm) and two closed arms (56 x 10 x 40 cm) and was elevated 50cm above the floor. Rats were placed in the center of the plus maze and videotaped for 5 minutes. Rats were then removed and returned to their home cage. The maze was cleaned with a 10% chlorine bleach solution between subjects. An experimenter blind to group assignment measured time spent in open and closed arms, entries into open and closed arms and center time. Percent time spent in open arms ((time in open arm/time in open + closed arm) x100) was used as a measure of anxiety, and the number of closed arm entries was used as a measure of spontaneous locomotor activity. Rats that failed to make at least four arm entries were removed from data analysis.
Defensive Prod Burying Test
Rats were habituated to the defensive prod chamber for three days prior to receiving shock. Rats were placed in the chamber in groups of 3 (with cagemates) for 30 min between 1400 and 1500h in a room separated from the colony room. The defensive prod chamber is a Plexiglas chamber (45 x 30 x 44cm) filled up to 5.5cm with pine bedding. On testing day, the prod (5.6 x .6 cm) was placed into the chamber at a height of 7.5cm (approx 2cm above the pine bedding). The rats were individually placed into the chamber, facing away from the prod. Each rat received a single shock (5mA, Coulbourn Precision Regulated Animal Shocker) when they placed at least one paw on the prod. Failure to touch the prod within five minutes resulted in removal from the study. Following the prod shock, animals remained in the chamber for an additional 15min and were videotaped for subsequent analysis of prod-burying behaviors. Latency to touch the prod, latency to bury following shock, duration of burying following shock and the number of rears following shock were measured by an observer blind to the treatment conditions.
Histological Procedures
In Experiment 1, one day following behavioral testing, rats were anesthetized with 400mg/kg chloral hydrate and killed by transcardial perfusion of 4% paraformaldehyde/0.1M sodium phosphate buffer (pH 7.6). Brains were removed and stored in 15% sucrose/0.1M sodium phosphate buffer (pH 7.6) at 4°C until cutting. Brains were sliced in 60 μm sections through the amygdala. All brain sections were mounted on slides and then stained with 0.3% neutral red (Sigma-Aldrich). Correct placement of viral injections was determined by evaluation of needle track placement in comparison with the Rat Brain Atlas of Paxinos and Watson (1997). Animals with incorrect injection placement were removed from data analysis. A total of 5 rats were removed from Experiment 1. Histology was not conducted on rats from Experiment 2 since surgeries were not performed.
Data Analysis
In Experiment 1, planned comparisons (two-way ANOVAs) were conducted to assess the effects of NTI administration on diazepam-related anxiety effects in rats overexpressing amygdalar ENK (DSGrPPE) and control rats (DSGZ). Bonferonni post-hoc analyses for dose of diazepam were conducted. Significance levels for all measures were set at p<0.05.
In Experiment 2, two-way ANOVAs were performed for all measures. Diazepam treatment was one variable and opioid receptor antagonist was the other variable. Since there were three levels of opioid receptor antagonist (VEH, NTI, FNA), Bonferroni post-hoc analyses were conducted when a main effect of opioid receptor antagonist was found. Data from rats receiving each opioid receptor antagonist was statistically compared with data from rats receiving vehicle injections.
RESULTS
Experiment 1: Effect of a delta opioid antagonist on enkephalin-mediated enhancement of diazepam in the elevated plus maze
This experiment examined the effects of herpes virus-mediated overexpression of amygdalar proenkephalin on responses to diazepam (1–2 mg/kg) in the elevated plus maze and the ability of the DOR antagonist, naltrindole, to block these responses. Figure 1B demonstrates the localized expression of ENK induced by viral gene transfer of the rat proenkephalin cDNA in the amygdala and that of EGFP expressed from the same viral vector. While the figure shows significant co-localization of ENK and EGFP (yellow) induced by gene transfer in the CEA region, there is also ENK immunoreactivity outside the region of viral expression (see red immunofluorescence in panel 1B surrounding EGFP). These qualitative studies are similar to previous results demonstrating the ability of these vectors to enhance ENK expression in the amygdala (Kang et al. 2000).
As in our previous studies in male rats tested under these conditions, there was little distinction and no statistical difference between the effects of doses of 1 and 2mg/kg diazepam and the results from both doses have been combined for analysis (Wilson et al. 2004). In rats receiving the control virus (DSGZ), diazepam administration did not significantly alter anxiety measures in the elevated plus maze (percent time spent in open arms, Figure 2; F(1,40) = 0.3, p=0.6 for diazepam effect), however, diazepam increased locomotor activity (number of closed arm entries, Figure 3), (F(1,40) =6.9, p<0.02). These results are similar to those reported earlier (Stock et al. 2000;Kang et al. 2000;Wilson et al. 2004), in that these doses of diazepam produce marginal anxiolytic effects in male rats, with or without a lacZ (control) virus injection, under these test conditions. Naltrindole administration did not affect anxiety (F(1,40) = 0.3), or activity (F(1,40) = 0.01) measures in vehicle or diazepam-treated control (DSGZ) rats (p > 0.05).
Figure 2.

The effects of diazepam and naltrindole (NTI) administration on anxiety measures in the elevated plus maze. Rats received amygdalar injections of a control virus (DSGZ; upper panel) or a virus encoding for rat preproenkephalin (DSGrPPE; lower panel) prior to testing. Lower doses of diazepam (1–2 mg/kg) increased percent time spent in the open arms in rats overexpressing amygdalar ENK (DSGrPPE; p<0.05)), but not in control rats (DSGZ). Naltrindole attenuated these effects in rats overexpressing amygdalar ENK. Data are shown as means ± SEM, n=7–15/group.
Figure 3.
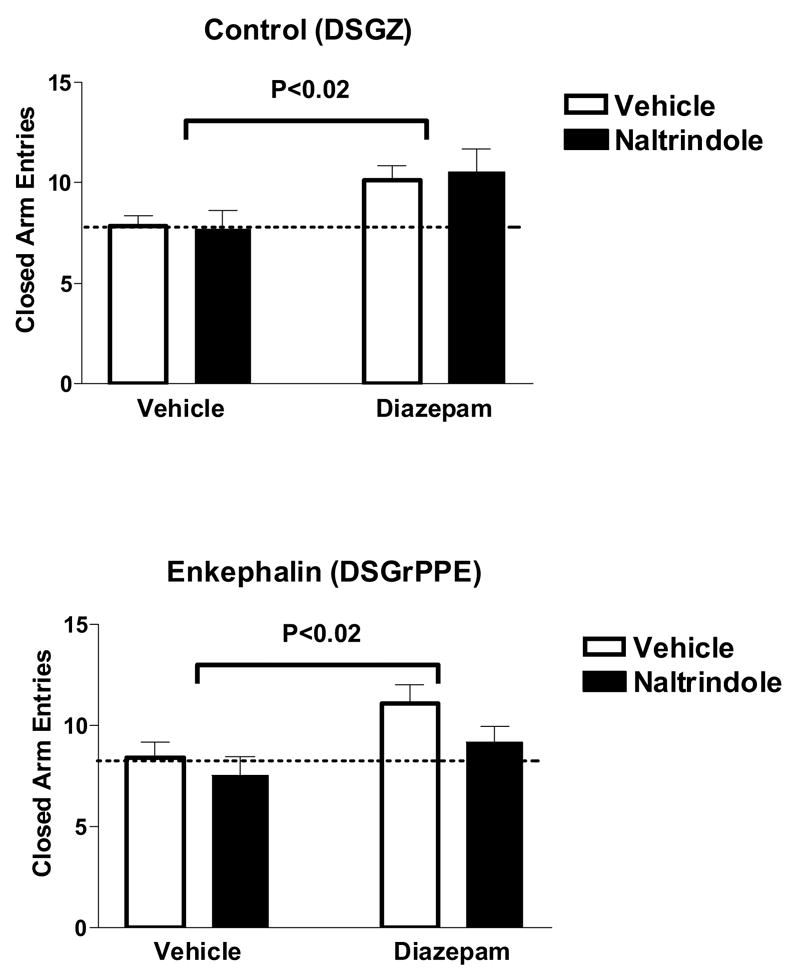
Activity measures in the elevated plus maze test following diazepam and naltrindole (NTI) administration in rats. Upper panel shows rats with injections of a control virus (DSGZ) into the amygdala and lower panel shows results with rats injected with a virus encoding for rat preproenkephalin (DSGrPPE). Diazepam increased activity (number of closed arm entries) in control rats (DSGZ) and rats overexpressing amygdalar ENK (DSGrPPE), but naltrindole did not attenuate these effects. Data are shown as means ± SEM, n=7–15/group.
As reported for over-expression of human pro-enkephalin the rat amygdala (Kang et al. 2000), in animals over-expressing rat pro-ENK (DSGrPPE) in the amygdala DZ administration increased percent time spent in the open arms of the elevated plus maze as compared to vehicle administration (F(1,49) = 13.1, p<0.001; Figure 2). Amygdalar ENK over-expression potentiated the anxiolytic effects of diazepam in this test, as seen by an increase in percent open arm time from 35 ± 5% in DSGZ rats after 1–2 mg/kg diazepam up to 48 ± 4% in DSGrPPE-treated rats receiving diazepam (Figure 2). It is important to note that baseline anxiety levels are not altered between control (DSGZ) rats and rats overexpressing amygdalar ENK (DSGrPPE) (percent open arm time (means ± SEM): 23 ± 4 % and 25 ± 4 %, respectively). At the doses given in this experiment, diazepam shows locomotor enhancing effects (rather than sedation), as indicated by slight enhancement in closed arm entries closed arm entries (F(1,49) = 6.08, p=0.02; Figure 3). This effect of DZ on activity was not potentiated by amygdalar ENK overexpression (compare upper and lower panels).
Administration of NTI attenuated the anxiolytic effects of diazepam in the elevated plus maze in rats over-expressing amygdalar ENK (DSGrPPE) (F(1,49) = 6.17, p<0.02; Figure 2). Percent open arm time was decreased from 48 ± 4% after diazepam in DSGrPPE rats to 30 ± 5% in groups receiving diazepam and NTI. Naltrindole administration did not attenuate the activity-inducing effects of diazepam in either the DSGZ (F(1,40)=0.1 p=0.9)or DSGrPPE rats (F(1,49)=2.4 p=0.13), suggesting that a separate mechanism mediates the locomotor effects of DZ.
Experiment 2: Effects of opioid antagonists on diazepam-mediated anxiolysis
This experiment investigated the effects of the selective delta and mu opioid receptor antagonists, naltrindole and β-funaltrexamine, respectively, on the anxiolytic effects of DZ in the elevated plus maze test and defensive prod burying test. A dose of 3 mg/kg was used to produce a more robust anxiolytic effect in these tests, since animals had normal expression levels of amygdalar enkephalin. In the elevated plus maze test, DZ administration increased the percent time spent in the open arms, F(1,57) = 9.82, p<.01, (see Figure 4) and increased the percent entries into the open arms, F(1,57) = 9.31, p<.01 (data not shown), compared to rats receiving vehicle. However, no main effect of opioid receptor antagonist was found for either anxiety-related measure in the plus maze. Diazepam (3mg/kg) administration also increased activity in the elevated plus maze test as measured by increased closed arm entries, F(1,57) = 4.14, p<.05 (See Figure 4). Again, opioid receptor antagonists did not have a significant effect on activity measures in this test.
Figure 4.

The elevated plus maze test was used to investigate the effects of MOR and DOR opioid receptor antagonists on the anxiety-related and activity-related effects of diazepam. Diazepam (3 mg/kg) administration increased the amount of time spent in the open arms and the number of entries into the closed arms of the elevated plus maze test, suggesting an anxiolytic and activity-inducing effect of diazepam. Neither the MOR nor the DOR opioid receptor antagonist attenuated these effects. Data are shown as means ± SEM, n=9–11/group.
In the defensive prod burying test, neither DZ nor the opioid receptor antagonists altered the latency to touch the prod. However, the number of rats removed from the experiment due to failure to touch the prod was higher in rats receiving DZ injections as opposed to VEH injections (21.9% and 3.4%, respectively). Opioid receptor antagonists significantly increased the latency to bury following shock, F(2,49) = 4.88, p<.02. Specifically, post-hoc tests revealed a significant difference between rats receiving VEH and FNA, p< .01 (See Figure 5); rats receiving VEH began burying sooner than rats receiving FNA. This difference may have been due to a difference in shock sensitivity between rats receiving VEH and FNA, however, this was not directly tested. The shock level was intentionally set at a high level to avoid this potential confound, although it cannot be completely discounted. There was no main effect of DZ on latency to bury and no interaction effect was found. On duration of burying, rats receiving VEH buried longer than rats receiving DZ, suggesting an anxiolytic effect of DZ F(1,48) = 5.86, p<.02 (See Figure 5). No effects were found for opioid receptor antagonists and no interactions were found. There were no differences between groups on the number of rears made following shock.
Figure 5.

Anxiety measures in the defensive prod burying test. MOR opioid receptor antagonist (FNA) administration significantly increased the latency to bury the prod following shock. Diazepam (3mg/kg) administration decreased the duration of burying following shock, suggesting an anxiolytic effect of diazepam. The anxiolytic influences of diazepam were not modified by either naltrindole (NTI) or FNA. Data are shown as means ± SEM, n=7–11/group
DISCUSSION
The purpose of the present experiments was to further explain the role of amygdalar opioids in the anxiolytic effects of the benzodiazepine receptor agonist, diazepam. Previous studies from this laboratory have shown that virally mediated increases in amygdalar ENK potentiate the effects of a low dose of diazepam (1 mg/kg) on anxiety-related measures in the elevated plus maze (Kang et al. 2000). In the first experiment, over-expression of amygdalar ENK was found to similarly potentiate the effects of lower DZ doses (1–2 mg/kg) compared to effects seen in control groups injected with a viral vector encoding β-galactosidase. This potentiation was seen as an increase in percent open arm time from 35 ± 5 (mean ± SEM) in control rats and to 48 ± 4 (mean ± SEM) in rats overexpressing amygdalar ENK after diazepam.
Importantly, the use of a new viral construct, encoding rat prepro-ENK (the current experiment) produced similar behavioral effects when combined with diazepam as did the viral construct which encodes human prepro-ENK (Kang et al. 2000), thereby further validating the use of viral vectors as important tools to alter behaviors in mammalian systems. As reported previously (Kang et al. 2000), ENK overexpression enhanced the anxiolytic effects of diazepam without modifying the influences on activity in the plus maze.
Notably, naltrindole was able to attenuate the enhanced anxiolytic effects of diazepam induced by overexpression of ENK in this animal model of anxiety, suggesting that the anxiolytic effects of diazepam are mediated through DOR. Naltrindole administration did not alter baseline anxiety (percent open arm time) in control (DSGZ) rats or ENK over-expressing (DSGrPPE) rats when compared to vehicle injected (non-diazepam treated) groups. Likewise, baseline locomotor activity was not altered following NTI administration or amygdalar ENK overexpression. Naltrindole failed to attenuate the locomotor effects of diazepam, suggesting separate neural mechanism for the activity-inducing and anxiety-reducing effects of diazepam. This is supported by studies demonstrating opioid agonists or antagonists influence the anxiety or anticonflict effects of benzodiazepines (Billingsley and Kubena 1978;Koob et al. 1980;Soubrie et al. 1980;Agmo et al. 1995;Tsuda et al. 1996), but not the open field or ataxic effects of these drugs (Billingsley and Kubena 1978;File and Rodgers 1979b). Non-selective antagonists (naloxone), however, can block the decreases in locomotor activity in mice after high but not low doses of chlordiazepoxide (Rodgers et al. 1985). Further, we consistently observe that various treatments (benzodiazepine administration, opioid antagonists, chronic running) have distinct effects on the anxiety and activity measures in the elevated plus maze, suggesting that separate neural substrates underlie changes in these behavioral endpoints (Kang et al. 2000;Burghardt et al. 2004;Burghardt and Wilson 2006). The present data suggest that DOR is an integral component of the relationship between amygdalar ENK and diazepam on anxiety-related behaviors, but not activity-related behaviors.
The second experiment was conducted to further investigate the relationship of diazepam and opioid receptors. Specifically, this experiment investigated the effects of systemic administration of the DOR antagonist, naltrindole, and the MOR antagonist, β-funaltrexamine, on an anxiolytic dose of DZ (3 mg/kg) in the elevated plus maze test and the defensive prod burying test. As predicted (and as previously demonstrated), DZ (3 mg/kg) produced a robust anxiolytic-like effect in the elevated plus maze test (Wilson et al. 2004). Diazepam administration also increased activity-related behaviors in this test (closed arm entries). The systemic administration of MOR and DOR receptor antagonists failed to modify the behavioral effects of DZ in this test. These results differ from previous findings demonstrating that the anxiolytic effects of DZ in the elevated plus maze test were attenuated by injections of the non-selective opioid receptor antagonist naltrexone into the central, but not the basolateral, amygdala (Kang et al. 2000;Burghardt and Wilson 2006). These differences could be due to influences of systemically administered opioid receptor antagonists on DOR and MOR in several different brain areas that might be offsetting the amygdalar effects of these drugs, and/or the use of receptor selective antagonists. Future studies will be needed to clarify this issue.
The ability of NTI to attenuate the enhanced DZ effects following virus-mediated gene transfer suggests the influences of heightened ENK expression are being mediated through the delta opioid receptor. This is in contrast to the lack of NTI effects on the anxiolytic influences of DZ in non-virally injected animals. This disparity might suggest that different doses of DZ induce distinct neuronal changes, and that lower doses preferentially involve DOR mechanisms. Alternatively, the enhanced DZ effects in ENK over-expressing animals might be specifically related to the use of virus-mediated gene transfer procedures. This is supported by the slight (but non-significant) effects of NTI in animals receiving the control vector, where NTI appeared to also blunt DZ effects. This might suggest an interactive effect between amygdala opioid processes mediating DZ actions and nonspecific neural responses associated with the viral injection procedures. Recent studies by Narita et al. (2006a) suggest that DOR receptor activity modulates levels of astrocytes in cingulate cortex, and that such alterations can modify anxiety levels in the elevated plus maze and light: dark transition tests (Narita et al. 2006b). Alternatively, virus-mediated over-expression might result in ENK release from populations of amygdalar neurons that do not normally produce opioid peptides, leading to activation of delta opioid receptors, modulation of amygdalar circuitry, and thus enhancement diazepam’s anxiolytic effects through pathways not normally activated. Current studies are addressing this possibility through the use of selected cell targeting using the prepro-enkephalin promoter in newly devised viral constructs.
The effects of amygdalar ENK over-expression on the anxiolytic effects of DZ may be mediated through various mechanisms. The amygdala has a high density of GABAA and benzodiazepine receptor sites as well as opioid receptor sites (Shiosaka et al. 1983;Mansour et al. 1995a;Veinante et al. 1997;Wilson et al. 2002;Poulin et al. 2006). The anxiolytic actions of benzodiazepines are thought to be mediated by neurons expressing the GABAA receptor containing the α2 subunit. This particular subunit is largely localized in limbic structures, including the amygdala (Low et al. 2000;Rudolph et al. 2001). PreproENK mRNA is seen in various nuclei of the amygdala including the central, basolateral and posteroventral portion of the medial nucleus, and most of the ENK afferents to the centromedial amygdala arise from intra-amygdaloid sources or the bed nucleus of the stria terminalis (BNST) (Poulin et al. 2006). Combined with our previous data (Kang et al. 2000) and the localization of reporter (GFP) expression (Figure 1b), this suggests that viral overexpression most likely changes intra-amygdalar ENK projections that subsequently modulate diazepam effects in the plus maze. One possibility is that DZ induces ENK release in these intra-amygdalar circuits that subsequently modulates GABAA receptor responses and/or GABA/BZ interactions, producing anxiolysis. Colocalization of glutamatic acid decarboxylase and met-ENK immunoreactivity has been found in the central nucleus of the amygdala (Veinante et al. 1997) providing for the possibility that viral overexpression increases release of ENK from GABAergic neurons in this nucleus. It is also possible that ENK overexpression could increase GABA release (Veinante et al. 1997), thus enhancing the effectiveness of DZ at GABAA/BZ receptor sites. Several physiological studies in amygdala and periaqueductal gray, however, indicate that opioid agonists, including met-ENK, inhibit GABAergic neurotransmission via presynaptic effects although these effects are induced via activation of MOR receptors (Sugita and North 1993;Vaughan et al. 1997;Finnegan et al. 2005;Finnegan et al. 2006). Although enkephalins interact with highest affinity at DOR, these opioid peptides do not show a large degree of discrimination for the three opioid receptor subtypes (Schoffelmeer et al. 1990;Mansour et al. 1995a;Mansour et al. 1995b;Clarke et al. 2003). Similarly, met-ENK also affected presynaptic glutamate release in the central amygdala, but these effects were mediated via MOR-dependent mechanisms and were not seen with the DOR selective agonist (Zhu and Pan 2005). Chieng et al. (2006), however, demonstrated that approximately 20% of the cells in the medial portion of central nucleus showed responses to the DOR agonist deltorphin II (Chieng et al. 2006), suggesting some populations of neurons within the amygdala are modulated by activation of DOR. Furthermore, these electrophysiological studies demonstrate that the opioid effects in this region are dependent upon characterization of the cell type in this area (Zhu and Pan 2004;Zhu and Pan 2005;Finnegan et al. 2005;Chieng et al. 2006), suggesting that opioid effects in the amygdalar circuitry might be very complex based on receptor-specific interactions with selective cell types. Although the specific mechanisms remain to be elucidated, the current data continue to suggest a critical interaction of amygdalar GABA/BZ and opioid systems in the anxiolytic actions of benzodiazepines.
Overall, the present experiments support previous findings that the anxiolytic effects of low doses of benzodiazepines appear to be mediated through the opioid system (Billingsley and Kubena 1978;Agmo et al. 1995;Kang et al. 2000), particularly the amygdalar enkephalinergic system. Our results are consistent with others (LaBuda and Fuchs 2001) indicating the anxiolytic effects of DZ appear to be mediated by DOR. However, future experiments will investigate the specific roles of DOR and MOR in anxiety-related behaviors and the anxiolytic effects of DZ and other benzodiazepine agonists.
Acknowledgments
Grants RO1 MH063344 to MAW, the USC School of Medicine Research Development Fund and the USC Office of Research Opportunity Fund. The authors thank Dr. J.C. Glorioso (University of Pittsburgh) for providing the 7B cell line, Dr. Steven Sabol (National Institutes of Health) for providing the plasmid containing the rat prepro-enkephalin cDNA and L.A. Smith for technical assistance in virus production.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Agmo A, Galvan A, Heredia A, Morales M. Naloxone blocks the antianxiety but not the motor effects of benzodiazepines and pentobarbital: experimental studies and literature review. Psychopharmacology. 1995;120:186–194. doi: 10.1007/BF02246192. [DOI] [PubMed] [Google Scholar]
- Belzung C, Agmo A. Naloxone blocks anxiolytic-like effects of benzodiazepines in Swiss but not in Balb/c mice. Psychopharmacology (Berl) 1997a;132:195–201. doi: 10.1007/s002130050336. [DOI] [PubMed] [Google Scholar]
- Belzung C, Agmo A. Naloxone potentiates the effects of subeffective doses of anxiolytic agents in mice. European Journal of Pharmacology. 1997b;323:133–136. doi: 10.1016/s0014-2999(97)00142-8. [DOI] [PubMed] [Google Scholar]
- Belzung C, Barreau S, Agmo A. Naloxone potentiates anxiolytic-like actions of diazepam, pentobarbital and meprobamate but not those of Ro19–8022 in the rat. Eur J Pharmacol. 2000;394:289–294. doi: 10.1016/s0014-2999(00)00151-5. [DOI] [PubMed] [Google Scholar]
- Billingsley ML, Kubena RK. The effects of naloxone and picrotoxin on the sedative and anticonflict effects of benzodiazepines. Life Sci. 1978;22:897–906. doi: 10.1016/0024-3205(78)90614-8. [DOI] [PubMed] [Google Scholar]
- Britton DR, Britton KT, Dalton D, Vale W. Effects of naloxone on anti-conflict and hyperphagic actions of diazepam. Life Sci. 1981;29:1297–1302. doi: 10.1016/0024-3205(81)90671-8. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 2004;1019:84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Wilson MA. Microinjection of naltrexone into the central, but not the basolateral, amygdala blocks the anxiolytic effects of diazepam in the plus maze. Neuropsychopharm. 2006;31:1227–1240. doi: 10.1038/sj.npp.1300864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng BC, Christie MJ, Osborne PB. Characterization of neurons in the rat central nucleus of the amygdala: Cellular physiology, morphology, and opioid sensitivity. J Comp Neurol. 2006;497:910–927. doi: 10.1002/cne.21025. [DOI] [PubMed] [Google Scholar]
- Clarke S, Zimmer A, Zimmer AM, Hill RG, Kitchen I. Region selective up-regulation of micro-, delta- and kappa-opioid receptors but not opioid receptor-like 1 receptors in the brains of enkephalin and dynorphin knockout mice. Neuroscience. 2003;122:479–489. doi: 10.1016/j.neuroscience.2003.07.011. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- DeLuca NA, McCarthy AM, Schaffer PA. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Millan MJ, Ulsamer B, Doenicke A. Naloxone attenuates the anxiolytic action of diazepam in man. Life Sci. 1982;31:1833–1836. doi: 10.1016/0024-3205(82)90222-3. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Bolles RC. Naloxone and shock-elicited freezing in the rat. Journal of Comparative & Physiological Psychology. 1979;93:736–744. doi: 10.1037/h0077609. [DOI] [PubMed] [Google Scholar]
- File SE, Rodgers RJ. Exploratory behaviour and aversive thresholds in rats following microinjection of morphine into central and medial nuclei of the amygdala. Br J Pharmacol. 1979a;66:145P–146P. [PMC free article] [PubMed] [Google Scholar]
- File SE, Rodgers RJ. Partial anxiolytic action of morphine sulphate following microinjection into the central nucleus of the amygdala in rats. Pharm Biochem Behav. 1979b;11:313–318. doi: 10.1016/0091-3057(79)90141-2. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chulba J, Martin M, Matthes HWD, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for δ- and μ-opioid receptors exhibit opposing alterations of emotional responses. Nature Genetics. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Finnegan TF, Chen SR, Pan HL. Effect of the {mu} opioid on excitatory and inhibitory synaptic inputs to periaqueductal gray-projecting neurons in the amygdala. J Pharmacol Exp Ther. 2005;312:441–448. doi: 10.1124/jpet.104.074633. [DOI] [PubMed] [Google Scholar]
- Finnegan TF, Chen SR, Pan HL. Mu opioid receptor activation inhibits GABAergic inputs to basolateral amygdala neurons through Kv1.1/1.2 channels. J Neurophysiol. 2006;95:2032–2041. doi: 10.1152/jn.01004.2005. [DOI] [PubMed] [Google Scholar]
- Frussa-Filho R, Barbosa-Junior H, Silva RH, Da Cunha C, Mello CF. Naloxone potentiates athe axiolytic effects of chlordiazepoxide in rats exposed to novel environments. Psychopharmacology. 1999;147:168–173. doi: 10.1007/s002130051157. [DOI] [PubMed] [Google Scholar]
- Green S. Benzodiazepines, putative anxiolytics and animal models of anxiety. TINS. 1991;14:101–104. doi: 10.1016/0166-2236(91)90070-b. [DOI] [PubMed] [Google Scholar]
- Grijalva CV, Levin ED, Morgan M, Roland B, Martin FC. Contrasting effects of centromedial nad basolateral amygdaloid lesions on stress-related responses in the rat. Physiol Behav. 1990;48:495–500. doi: 10.1016/0031-9384(90)90289-g. [DOI] [PubMed] [Google Scholar]
- Kang W, Wilson MA, Wilson SP. Overexpression of proenkephalin in the amygdala potentiates the anxiolytic effects of benzodiazepines. Neuropsychopharm. 2000;22:77–88. doi: 10.1016/S0893-133X(99)00090-1. [DOI] [PubMed] [Google Scholar]
- Koob GF, Strecker RE, Bloom FE. Effects of naloxone on the anticonflict properties of alcohol and chlordiazepoxide. Subst Alcohol Actions Misuse. 1980;1:447–457. [PubMed] [Google Scholar]
- Kopchia KL, Altman HJ, Commissaris RL. Effects of lesions of the central nucleus of the amygdala on anxiety-like behaviors in the rat. Pharm Biochem Behav. 1992;43:453–61. doi: 10.1016/0091-3057(92)90176-g. 3. [DOI] [PubMed] [Google Scholar]
- Krisky DM, Marconi PC, Oligino T, Rouse RJ, Fink DJ, Glorioso JC. Rapid method for construction of recombinant HSV gene transfer vectors. Gene Ther. 1997;4:1120–1125. doi: 10.1038/sj.gt.3300497. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN. The anxiolytic effect of acute ethanol of diazepam exposure is unaltered in mu-opioid receptor knockout mice. Brain Res Bull. 2001;55:755–766. doi: 10.1016/s0361-9230(01)00569-x. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- MacGregor GR, Caskey CT. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989;17:2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. TINS. 1995a;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Mansour A, Watson SJ, Akil H. Opioid receptors: past, present and future. Trends Neurosci. 1995b;18:69–70. [PubMed] [Google Scholar]
- Marconi P, Krisky D, Oligino T, Poliani PL, Ramakrishnan R, Goins WF, Fink DJ, Glorioso JC. Replication-defective herpes simplex virus vectors for gene transfer in vivo. Proc Natl Acad Sci U S A. 1996;93:11319–11320. doi: 10.1073/pnas.93.21.11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharm. 2006a;31:739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- Narita M, Kuzumaki N, Narita M, Kaneko C, Tamai E, Khotib J, Miyatake M, Shindo K, Nagumo Y, Tanaka S, Suzuki T. Age-related emotionality is associated with cortical delta-opioid receptor dysfunction-dependent astrogliosis. Neuroscience. 2006b;137:1359–1367. doi: 10.1016/j.neuroscience.2005.10.067. [DOI] [PubMed] [Google Scholar]
- Negus SS, Henriksen SJ, Mattox A, Pasternak GW, Portoghese PS, Takemori AE, Weinger MB, Koob GF. Effect of antagonists selective for mu, delta and kappa opioid receptors on the reinforcing effects of heroin in rats. J Pharmacol Exp Ther. 1993;265:1245–1252. [PubMed] [Google Scholar]
- Ossipov MH, Kovelowski CJ, Vanderah T, Porreca F. Naltrindole, an opioid delta antagonist, blocks the enhancement of morphine-antinociception induced by a CCKB antagonist in the rat. Neurosci Lett. 1994;94(9–2):9. doi: 10.1016/0304-3940(94)90548-7. [DOI] [PubMed] [Google Scholar]
- Paul D, Levison JA, Howard DH, Pick CG, Hahn EF, Pasternak GW. Naloxone benzoylhydrazone (NalBzoH) analgesia. J Pharmacol Exp Ther. 1990;255:769–774. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Pick CG, Paul D, Pasternak GW. Comparison of naloxonazine and beta-funaltrexamine antagonism of mu 1 and mu 2 opioid actions. Life Sci. 1991;48:2005–2011. doi: 10.1016/0024-3205(91)90155-5. [DOI] [PubMed] [Google Scholar]
- Poulin JF, Chevalier B, Laforest S, Drolet G. Enkephalinergic afferents of the centromedial amygdala in the rat. J Comp Neurol. 2006;496:859–876. doi: 10.1002/cne.20956. [DOI] [PubMed] [Google Scholar]
- Privette TH, Terrian DM. Kappa opioid agonists produce anxiolytic-like behavior on the elevated plus-maze. Psychopharmacology (Berl) 1995;118:444–450. doi: 10.1007/BF02245945. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Randall J, Kelway B. Naloxone potentiates the depressant effect of chlordiazepoxide on spontaneous activity in mice. Neurosci Lett. 1985;58:97–100. doi: 10.1016/0304-3940(85)90335-0. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Mohler H. GABAA receptor subtypes: dissecting their pharmaoclogical functions. Trends in Pharmacological Sciences. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, Yao YH, Gioannini TL, Hiller JM, Ofri D, Roques BP, Simon EJ. Cross-linking of human [125I]beta-endorphin to opioid receptors in rat striatal membranes: biochemical evidence for the existence of a mu/delta opioid receptor complex. J Pharmacol Exp Ther. 1990;253:419–426. [PubMed] [Google Scholar]
- Shiosaka S, Sakanaka M, Inagaki S, Senba E, Hara Y, Takatsuki K, Takagi H, Kawai Y, Tohyama M. Putative neurotransmitters in the amygdaloid complex with special reference to peptidergic pathways. In: Emson PC, editor. Chemical Neuroanatomy. New York: Raven Press; 1983. pp. 359–389. [Google Scholar]
- Soubrie P, Jobert A, Thiebot MH. Differential effects on naloxone against the diazepam-induced release of behavior in rats in three aversive situations. Psychopharmacology. 1980;69:101–105. doi: 10.1007/BF00426529. [DOI] [PubMed] [Google Scholar]
- Stock HS, Foradori C, Ford K, Wilson MA. A lack of tolerance to the anxiolytic effects of diazepam on the plus-maze: a comparison of male and female rats. Psychopharmacology. 2000;147:362–370. doi: 10.1007/s002130050004. [DOI] [PubMed] [Google Scholar]
- Sugita S, North RA. Opioid actions on neurons of rat lateral amygdala in vitro. Brain Res. 1993;612:151–155. doi: 10.1016/0006-8993(93)91655-c. [DOI] [PubMed] [Google Scholar]
- Treit D, Pesold C, Rotzinger S. Dissociating the anti-fear effects of septal and amygdaloid lesions using two pharmacologically validated models of rat anxiety. Behav Neurosci. 1993;107:770–779. doi: 10.1037//0735-7044.107.5.770. [DOI] [PubMed] [Google Scholar]
- Treit D. The Inhibitory Effect of Diazepam on Defensive Burying: Anxiolytic vs. Analgesic Effects. Pharm Biochem Behav. 1985;22:47–52. doi: 10.1016/0091-3057(85)90484-8. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Suzuki T, Misawa M, Nagase H. Involvement of the opioid system in the anxiolytic effect of diazepam in mice. Eur J Pharmacol. 1996;307:7–14. doi: 10.1016/0014-2999(96)00219-1. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature (Lond ) 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- Veinante P, Stoeckel ME, Freund-Mercier MJ. GABA- and peptide-immunoreactivies co-localize in the rat central extended amygdala. NeuroReport. 1997;8:2985–2989. doi: 10.1097/00001756-199709080-00035. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Burghardt PR, Ford KA, Wilkinson MB, Primeaux SD. Anxiolytic effects of diazepam and ethanol in two behavioral models: comparison of males and females. Pharmacol Biochem Behav. 2004;78:445–458. doi: 10.1016/j.pbb.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Mascagni F, McDonald AJ. Sex differences in delta opioid receptor immunoreactivity in rat medial amygdala. Neurosci Lett. 2002;328:160–164. doi: 10.1016/s0304-3940(02)00481-0. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Sabol SL. Glucocorticoids and cyclic AMP synergistically regulate the abundance of preproenkephalin messenger RNA in neuroblastoma-glioma hybrid cells. Biochem Biophys Res Commun. 1986;139:1–10. doi: 10.1016/s0006-291x(86)80071-7. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Williams C, Sabol SL. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem. 1984;259:14301–14308. [PubMed] [Google Scholar]
- Zhu W, Pan ZZ. Synaptic properties and postsynaptic opioid effects in rat central amygdala neurons. Neuroscience. 2004;127:871–879. doi: 10.1016/j.neuroscience.2004.05.043. [DOI] [PubMed] [Google Scholar]
- Zhu W, Pan ZZ. Mu-opioid-mediated inhibition of glutamate synaptic transmission in rat central amygdala neurons. Neuroscience. 2005;133:97–103. doi: 10.1016/j.neuroscience.2005.02.004. [DOI] [PubMed] [Google Scholar]


