Abstract
Dichotomizing afferents are individual dorsal root ganglion (DRG) neurons that innervate two distinct structures thereby providing a form of afferent convergence that may be involved in pelvic organ cross-sensitization. To determine the distribution of dichotomizing afferents supplying the distal colon and bladder of the Sprague-Dawley rat and the C57Bl/6 mouse, we performed concurrent retrograde labeling of urinary bladder and distal colon afferents using cholera toxin subunit B (CTB) fluorescent conjugates. Animals were perfused 4–5 days after sub-serosal organ injections, and the T10-S2 DRG were removed, sectioned, and analyzed using confocal microscopy. In the rat, CTB-positive afferents retrogradely labeled from the bladder were nearly 3 times more numerous than those labeled from the distal colon, while in the mouse, each organ was equally represented. In both species, the majority of colon and bladder afferents projected from lumbosacral (LS) ganglia and secondarily from thoracolumbar (TL) ganglia. In the rat, 17% of the total CTB-positive neurons were retrogradely labeled from both organs with 11% localized in TL, 6% in LS, and 0.8% in thoracic (TH) ganglia. In the mouse, 21% of the total CTB-positive neurons were dually-labeled with 12% localized in LS, 4% in TH, and 4% in TL ganglia. These findings support the existence of dichotomizing pelvic afferents, which provide a pre-existing neuronal substrate for possible immediate and maintained pelvic organ cross-sensitization and ultimately may play a role in the overlap of pelvic pain disorders.
Keywords: Cholera toxin B, Alexa Fluor, Retrograde Labeling, Convergence, Doral Root Ganglia
INTRODUCTION
Chronic pelvic pain (CPP) can develop following acute or chronic irritation of individual pelvic visceral organs, their associated striated sphincters, other striated muscular structures of the pelvic floor, or striated and cutaneous components of the pelvic abdominal wall and/or perineum (Doggweiler-Wiygul et al. 2001). CPP affects 15% of both men and women, and commonly involves the pelvic cavity, as in irritable bowel syndrome (IBS) and interstitial cystitis (IC), and/or the pelvic floor (Berger et al. 1998; Clemens et al. 2005; Mathias et al. 1996; Moldwin 2002; Wesselmann 2001; Zondervan et al. 2001). Considering that the colorectum and urinary bladder, exclusive from other pelvic organs, function as an integral part of daily, physiological pelvic activity, it is not surprising that IBS and IC, analogous disorders of pelvic visceral pain and urgency, account for half of all cases of CPP (Zondervan et al. 1999).
Neural “cross-talk” within the pelvis is necessary for the normal regulation of sexual, bladder, and bowel function and is likely mediated by the convergence of sensory pathways in the spinal cord (de Groat and Booth 1993; de Groat et al. 1993a; de Groat et al. 1981; de Groat et al. 1993b; de Groat and Steers 1981; Janig and Koltzenburg 1990). We recently provided evidence of pre-existing neural pathways in the development of pelvic organ cross-sensitization by demonstrating that colonic hypersensitivity develops following the induction of acute cystitis and vice versa (Pezzone et al. 2005). Follow-up studies in our laboratory employing single unit C-fiber bladder afferent recording revealed that acute colonic irritation is capable of sensitizing urinary bladder afferents to mechanical and chemical stimuli, and interruption of the neural input to the bladder can ameliorate this effect, suggesting a direct afferent pathway from the colon (Ustinova et al. 2006).
Although these pre-existing afferent pathways have not been extensively studied in the setting of CPP, viscero-visceral sensitization in the pelvis and the development of overlapping CPP disorders could originate peripherally via antidromic axon reflexes from a single dichotomizing primary afferent supplying two structures (pre-spinal convergence). Dichotomizing neurons within the dorsal root ganglia (DRG) were first proposed by Sinclair (Sinclair et al. 1948), and have been shown in several species to range from 0.5–15% of all afferents (McNeill and Burden 1986). It has been reported that there are 2.3 times as many peripheral nerve fibers as there are cell bodies in corresponding sacral DRG, which was interpreted as playing a role in referred pain via dichotomizing sacral afferents (Langford and Coggeshall 1981). By means of such a pathway, irritation of one pelvic organ may lead to referred pain and/or neurogenic inflammation in another organ via backfiring of afferent terminals emanating from the same DRG neuron.
To determine whether dichotomizing DRG neurons supplying the pelvic viscera may contribute to our model of cross-organ sensitization, we performed concomitant retrograde labeling of both urinary bladder and distal colon afferents in Sprague-Dawley rats and C57Bl/6 mice rats using fluorescent cholera toxin B conjugates.
MATERIALS and METHODS
Animals
Experiments were performed on male Sprague-Dawley rats, 200–250 g in weight (Hilltop Lab Animals, Inc.; Scottsdale, PA), and six-week old male C57Bl/6 mice (Charles River, Wilmington, MA). Animals were housed in standard polypropylene cages with ad libitum access to food and water in the Department of Laboratory Animal Resources at the University of Pittsburgh. All studies were approved by the University of Pittsburgh’s Institutional Animal Care & Use Committee and were found to meet the standards for humane animal care and use as set by the Animal Welfare Act and the NIH Guide for the Care and Use of Laboratory Animals.
Fluorescent Retrograde Tracers
Cholera toxin subunit B (recombinant) conjugates (Alexa Fluor 488 and 647) were purchased from Molecular Probes (Eugene, OR) and used at concentrations of 2 mg/ml in phosphate-buffered saline. Absorption and fluorescent emission maxima of the Alexa Fluor 488 conjugate was 495 and 519 nm, respectively, while that of the Alexa Fluor 647 conjugate was 650 and 668 nm, respectively.
Animal Surgery
All surgical procedures were performed under sterile conditions in a designated animal surgery area. Sprague-Dawley rats (n=5) were anesthetized using 4% inhaled isoflurane, and via a midline laparotomy and under the guidance of a Leica MZ6 dissecting stereomicroscope (Leica Microsystems, Inc., Wetzlar, Germany), 10μl of Alexa Fluor 647-conjugated cholera toxin subunit B (CTB) was injected circumferentially and subserosally into the distal colon (2–4 cm from the anal verge) in 2–3μl increments using dye-dedicated, 25μL Hamilton syringes fitted with 30 gauge needles. Prior to tracer injection, the needle tip was tunneled 4 mm subserosally to prevent back flow from the injection site into the peritoneum upon needle withdrawal. In addition, each puncture site was swabbed with fresh cotton-tipped applicators to absorb any potentially leaking tracer. Similarly, 10μl of Alexa Fluor 488-conjugated CTB was injected into the urinary bladder body bilaterally. Prior to closing, the peritoneal cavity was lavaged with warm saline. Animals were prophylactically treated with ampicillin, 100 mg/kg (Fort Dodge, Fort Dodge, IA)
Mice (n=5) were anesthetized by inhaled isoflurane, followed by intraperitoneal injection of 2.5% avertin in saline (2,2,2-tribromoethanol and tert-amyl alcohol diluted in 0.9% saline; 20μl/g body weight). A laparotomy was made to expose the pelvic viscera. Using a Hamilton microsyringe (33 gauge needle), a single 5μL injection of Alexa Fluor 647-conjugated CTB was made beneath the serosal layer in the distal region of the colon, 1 cm proximal to the anus. A second single 5 μL injection of Alexa Fluor 488-conjugated CTB was made beneath the serosal layer in the urinary bladder body. As above, injection sites were swabbed to remove any excess of tracer and the wounds were sutured shut. Previously we have applied 5μL of CTB onto the serosal surface of the distal colon (and adjacent tissues) to determine the extent of retrograde labeling that might arise from dye leakage (n=3). On average, this resulted in only 2 CTB-positive cells per section in the L6 ganglia (Christianson et al. 2006b).
In some rats, the urinary bladder was completely denervated (n=4) prior to tracer injection. This involved blunt dissection and cutting of visible post-ganglionic fibers projecting to the bladder.
Tissue Processing
Five days following laparotomy, rats were anesthetized with pentobarbital (100 mg/kg) and transcardially perfused with ice-cold 4% paraformaldehyde in 0.1M phosphate buffer (PB), pH 7.4. Mice were anesthetized as described above and similarly perfused four days following surgery. Paired T10-S2 dorsal root ganglia (DRG) were dissected and embedded into 10% gelatin. The embedded tissue was post-fixed for 1 hour at 4°C and cryoprotected in 25% sucrose at 4°C overnight. Mouse DRGs were sectioned at 35μm and rat DRGs were sectioned at 50μm on a sliding microtome and stored at 4°C in 0.1M PB. Every other section was mounted in 0.1% gelatin onto Superfrost microscope slides (Fisher Scientific, Pittsburgh, PA).
Confocal Microscopy
Sections were viewed using a Leica confocal microscope (Leica Microsystems; Wetzlar, Germany). Ganglia pairs were embedded and processed together, however only the ganglion with the most complete series of sections was analyzed for quantification of CTB-positive neurons. To visualize CTB-positive neurons, 16μm thick optical sections were captured for every other tissue section throughout each ganglion using a 20X objective. Each optical section was further divided into four transverse sections and the number of CTB-positive neurons from the topmost and bottommost section was counted and averaged together. Any CTB-positive neurons present in both sections were not counted to ensure that large cells were not disproportionately represented as recommended by the stereological technique contained in Pakkenberg and Gundersen (1988). The total number of CTB-positive neurons was calculated for each ganglion by multiplying the number of CTB-positive cells counted in each section by two. Adobe Photoshop 7.0 (Adobe Systems Incorporated, San Jose, CA) was used to adjust photomicrographs for brightness/contrast and to construct all figures.
Statistical Analysis
The average number of CTB-labeled cells was calculated for each DRG level and doubled to represent the entire ganglion. Comparisons were made using one-way analysis of variance (ANOVA) and Fisher’s post-hoc test for multiple comparisons (PLSD; StatView, SAS Institute Inc, Cary, NC). All data are expressed as mean ± SEM.
RESULTS
Four-to-five days following urinary bladder and distal colon injections of Alexa Fluor 488- and 647-conjugated cholera toxin B (CTB), respectively, retrogradely-labeled cell bodies in rat and mouse T10-S2 DRG were identified using fluorescent confocal microscopy. CTB binds with high affinity to the neuronal GM1 monosialoganglioside and undergoes fast retrograde axonal transport (102 mm/day) (Wu et al. 1999). While CTB has most commonly been used to retrogradely label large, myelinated somatic afferents (Robertson and Arvidsson 1985), CTB has also been shown to be more efficient at labeling unmyelinated visceral afferents than either wheat germ agglutinin or isolectin B4 (Wang et al. 1998). CTB-positive bladder afferents contained only Alexa Fluor 488 (green), colon afferents contained only Alexa Fluor 647 (red), and dichotomizing or dually-projecting afferents contained both Alexa Fluor dyes (yellow; Fig. 1 and Fig. 2). Nearly all CTB-positive cells were small-to-medium in size and displayed dense cytoplasmic staining. Ganglia were grouped according to spinal level and relative distribution of CTB-positive neurons. In the rat, ganglia were grouped into thoracic (TH, T10–T12), thoracolumbar (TL, T13-L2) and lumbosacral (LS, L6-S1), while in the mouse, ganglia were grouped into TH (T10–T12), TL (T13-L1), and LS (L5-S1). The L2–L4 and S2 ganglia in the mouse and the L3–L5 and S2 ganglia in the rat did not contain appreciable numbers of CTB-positive afferents (< 2 cells/ganglion) and were therefore excluded from comparisons.
Figure 1.
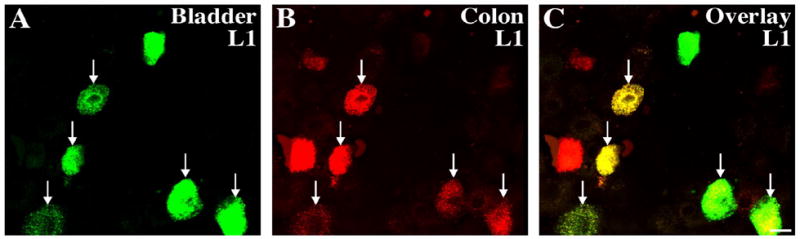
Retrogradely labeled cell bodies in the rat L1 DRG 5 days following urinary bladder and distal colon injections of Alexa Fluor 488- and 647-conjugated cholera toxin B (CTB), respectively, as visualized under confocal microscopy. CTB-positive bladder afferents appear green (A), CTB-positive distal colon afferents appear red (B), and dually-labeled cells appear yellow (C). (Bar represents 20 um)
Figure 2.

Retrogradely labeled cell bodies in the mouse L6 DRG 5 days following urinary bladder and distal colon injections of Alexa Fluor 488- and 647-conjugated cholera toxin B (CTB), respectively, as visualized under confocal microscopy. CTB-positive bladder afferents appear green (A), CTB-positive distal colon afferents appear red (B), and dually-labeled cells appear yellow (C). (Bar represents 20 um)
Rat
Injection of CTB into the urinary bladder and distal colon of the rat resulted in 987 ± 46 CTB-positive DRG neurons per animal (Table 1). CTB-positive afferents retrogradely labeled from the urinary bladder were approximately 2.8 times more numerous than those labeled from the distal colon (597 ± 28 vs. 216 ± 17, P < 0.0001) and 3.5 times more numerous than those labeled from both organs (174 ± 28, P < 0.0001; Table 1, Fig. 3A). Specifically, 60% ± 1 of the CTB-positive neurons were exclusively labeled from the bladder, 22% ± 2 were exclusively labeled from the distal colon (P < 0.0001), and 17% ± 2 were labeled from both organs (P < 0.0001; Fig. 3B).
Table 1.
Quantitation of CTB-positive DRG neurons in rats and mice. See Results for specific TH, TL, and LS designations for each species.
| Rat | ||||
|---|---|---|---|---|
| TH | TL | LS | All | |
| Bladder | 17.0 ± 10.4** | 181. 6 ± 34.2* | 398.2 ± 32.6 | 596.8 ± 28.1 |
| Colon | 1.8 ± 0.97** | 62.0 ± 14.3**# | 152.4 ± 10.8## | 216.2 ± 16.7## |
| Dual | 8.0 ± 3.1* | 108.8 ± 19.7* | 56.8 ± 14.9## | 173.6 ± 28.5## |
| Total | 26.8 ± 10.7** | 352.4 ± 39.7* | 607.4 ± 38.6 | 986.6 ± 46.1 |
| Mouse | ||||
| TH | TL | LS | All | |
| Bladder | 29.6 ± 20.0** | 57.4 ± 11.8**+ | 225.8 ± 48.6+ | 312.8 ± 75.2+ |
| Colon | 24.2 ± 11.5** | 47.6 ± 17.5* | 256.6 ± 55.3 | 328.4 ± 37.4+ |
| Dual | 35.8 ± 18.4* | 27.2 ± 4.7*+ | 94.2 ± 25.3 | 157.2 ± 46.2 |
| Total | 89.6 ± 20.7*+ | 132.2 ± 22.0*+ | 576.6 ± 104.2 | 798.4 ± 123.2 |
P < 0.05,
P < 0.0001 vs. LS;
P < 0.05 vs. bladder;
P < 0.05 vs. rat.
Figure 3.
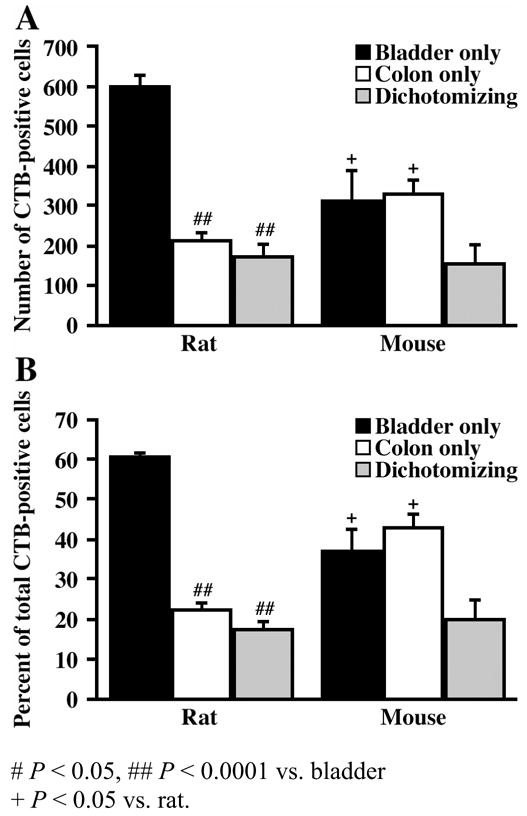
Bar graphs illustrating the number (A) and percentage (B) of bladder-specific, distal colon-specific, and dually-labeled cells across all DRG levels for both the rat and mouse.
The LS ganglia of the rat contained a significantly greater percentage of CTB-positive afferents (62% ± 4) than either the TH (2.7% ± 1) or TL ganglia (35% ± 3, P < 0.0001) (Table 1). With respect to organ-specific projection patterns, bladder and distal colon afferents were similarly more prevalent in the LS ganglia (Table 1, Fig. 4A). Bladder-specific afferents in the LS ganglia comprised 41% ± 3 of all CTB-positive afferents, whereas bladder-specific afferents in the TH and TL ganglia comprised only 1.7% ± 1 and 18% ± 3 of the total population, respectively (P < 0.0001 vs. LS; Fig. 4A). Colon-specific afferents had a similar distribution comprising 16% ± 1 (LS), 6.5% ± 1 (TL) and 0.2% ± 0.1 (TH) of the total population of CTB-positive afferents (P < 0.05 vs. LS; Fig. 4A). In contrast to bladder- and colon-specific afferents, afferents labeled from both organs were preferentially observed in the TL ganglia. Dually-labeled afferents in the TL ganglia comprised 11% ± 1 of the total CTB-positive afferents, compared to only 0.8% ± 0.3 and 6% ± 1 in the TH and LS ganglia, respectively (P < 0.05; Fig. 4A). Of the total CTB-positive afferents observed, bladder afferents in the TL and LS ganglia were significantly more prevalent than either colon or dually-projecting afferents (P < 0.05), however no significant difference was observed between the three afferent types in the TH ganglia (P > 0.05; Table 1, Fig. 4A).
Figure 4.
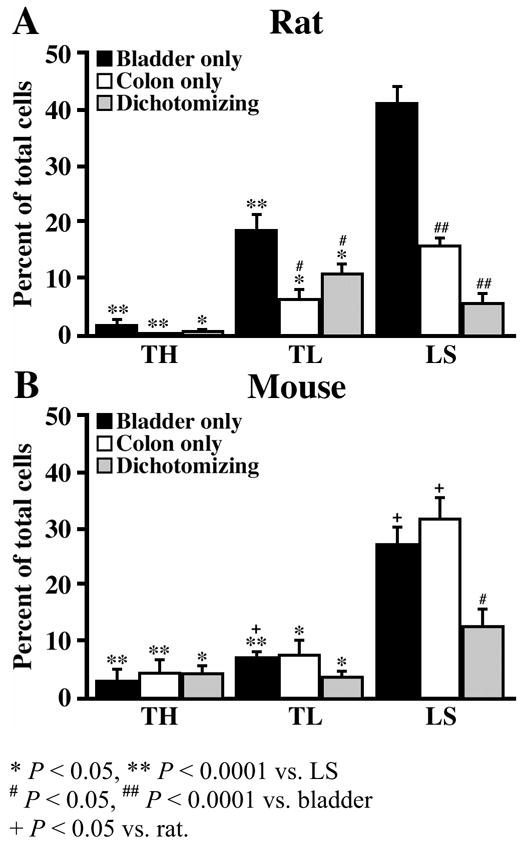
Bar graphs illustrating the percentage of bladder-specific, distal colon-specific, and dually-labeled cells with respect to all labeled cells (T10-S1) and stratified by TH, TL, and LS DRG levels for the rat (A) and mouse (B).
Analysis of the composition of CTB-positive afferents within each spinal level revealed that dually-labeled afferents were more prevalent within the TH and TL ganglia (Fig. 5A). Dually-projecting afferents comprised 38% and 31% of all CTB-positive afferents within the TH and TL ganglia, respectively, compared to only 9% within the LS ganglia (P < 0.05 vs. TH; Fig. 5A). Furthermore, as depicted in Figure 5A, in the more rostral ganglia, colon afferents were more likely to be represented by the dually-projecting population than the colon-specific population (37.7% vs 7.7%; TH) (30.9% vs. 18.1%; TL). This trend was not observed among bladder afferents.
Figure 5.
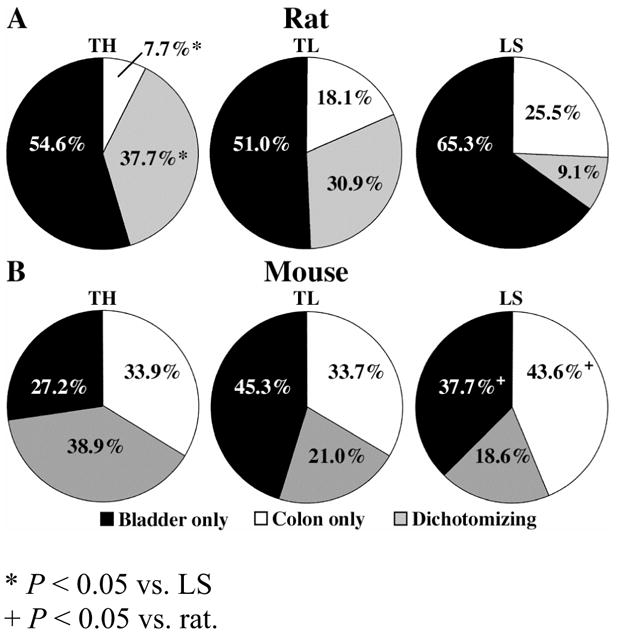
Pie graphs illustrating the percentage of TH, TL, and LS bladder-specific, distal colon-specific, and dually-labeled cells with respect to the total number of labeled cells at each respective DRG level for the rat (A) and mouse (B).
Mouse
Injection of CTB into the urinary bladder and distal colon of the mouse resulted in an average total of 798 ± 123 CTB-positive DRG neurons per animal (Table 1). In contrast to the rat, equivalent numbers of CTB-labeled DRG cells were retrogradely labeled from the urinary bladder (313 ± 75) and the distal colon (328 ± 37, P > 0.05; Table 1, Fig. 3A). Specifically, 37% ± 5 of the CTB-positive afferents were exclusively labeled from the bladder, 43% ± 3 were exclusively labeled from the distal colon, and 21% ± 5 were labeled from both organs (P < 0.05 vs. bladder and colon afferents; Fig. 3B).
Similar to the findings in the rat, a significantly larger percentage of CTB-labeled bladder afferents was observed within the LS ganglia (71% ± 4) than in either the TH (11% ± 2) or TL ganglia (18% ± 3, P < 0.0001) (Table 1). The LS ganglia in the mouse contained the largest percentage of CTB-positive afferents projecting exclusively to the bladder or the colon alone as well as those projecting to both organs (Fig. 4B). This is in contrast to the rat, where the largest population of dually-projecting afferents was in the TL ganglia (Figs. 4A and 4B). Bladder-specific afferents made up 3% ± 2 (TH), 7% ± 1 (TL), and 27% ± 3 (LS, P < 0.0001) of all CTB-positive afferents, while colon-specific afferents made up 4% ± 2 (TH), 7% ± 3 (TL), and 31% ±4 (LS, P < 0.05; Fig. 4B) of all CTB-positive afferents. Dually-projecting afferents made up 4% ± 2 (TH), 4% ± 1 (TL) and 12% ± 3 (LS, P < 0.05) of all CTB-positive afferents (Fig. 4B). Only within the LS ganglia was there a significant difference in the prevalence of the three afferent types, in that the percentage of dually-projecting afferents was significantly lower than that of either bladder or colon afferents (P < 0.05; Fig. 4B).
Analysis of the composition of CTB-positive afferents within each spinal level of the mouse also revealed that dually-projecting afferents were most prevalent among TH afferents (Fig. 5B). Dually-projecting afferents comprised 39% ± 12 of all CTB-positive afferents within the TH ganglia, whereas they comprised only 21% ± 3 and 19% ± 6 of the CTB-positive afferents within the TL and LS afferents, respectively (Fig. 5B). While this decrease in dually-projecting afferents was not significant, it did suggest a trend similar to that observed in the rat where the highest occurrence of dichotomizing afferents was observed within the more rostral ganglia, despite the more caudal ganglia containing a significantly higher percentage of total CTB-positive neurons.
Bladder Denervation
Surgical denervation of the urinary bladder was performed in 4 rats to determine whether CTB leakage might result in false-positive dually-projecting afferents. Only TL and LS ganglia were examined due to the very small number of CTB-positive afferents present in the TH ganglia (< 3% of the total). Following denervation of the bladder (DB) and tracer injection into both the bladder and the distal colon, the total number of labeled CTB-positive afferents decreased from 973 ± 48 in control rats to 322 ± 31 in DB rats (P < 0.0001). As expected, the total number of bladder-specific CTB-positive afferents was significantly decreased from 580 ± 24 in control rats to 56 ± 10 in DB rats (P < 0.0001; Fig. 6A). This decrease was observed in both the TL and LS ganglia, where the number of bladder-specific afferents significantly decreased from 182 ± 34 in the control TL ganglia to 51 ± 15 in the DB TL ganglia (P < 0.05; Fig. 6B) and 398 ± 33 in the control LS ganglia to 5 ± 4 in the DB LS ganglia (P < 0.05; Fig. 6C). The total number of dually-projecting CTB-positive afferents was also significantly decreased from 166 ± 28 in control rats to 41 ± 18 in DB rats (P < 0.05; Fig. 6A). The number of dually-projecting afferents in the TL ganglia was significantly decreased from 109 ± 20 in the control group to 22 ± 15 in the DB group (P < 0.05; Fig. 6B). Dually-projecting afferents in the LS ganglia were also decreased in number between the control (59 ± 15) and DB rats (19± 16), however the difference was not significant (P > 0.05; Fig. 6C), due presumably to the low number of dichotomizing afferents normally seen at this level and the high variability associated with the number of afferents arising from LS ganglia. As expected, the number of CTB-positive afferents projecting to the colon was not significantly different between control and DB rats either overall or within each spinal level (P > 0.05; Fig. 6A).
Figure 6.
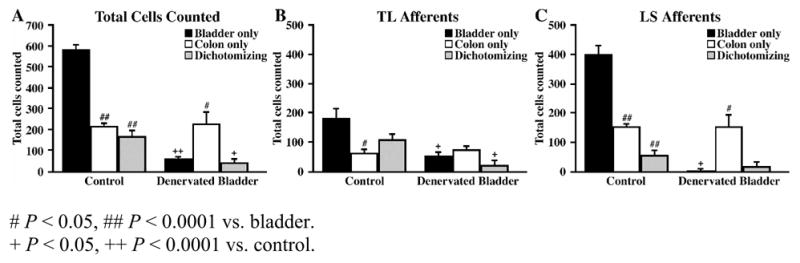
Bar graphs illustrating the number of bladder-specific, distal colon-specific, and dually-labeled cells in control and bladder denervated rats with respect to all labeled cells (T10-S1) (A), TL afferents (B), and LS afferents (C).
DISCUSSION
Using fluorescent conjugates of cholera toxin subunit B (CTB), we have successfully identified individually- and dually-labeled pelvic afferents supplying the distal colon and urinary bladder in Sprague-Dawley rats and C57Bl/6 mice. Antidromic axon reflexes, a form of pre-spinal convergence whereby a single dichotomizing primary afferent supplies two pelvic structures (i.e. the distal colon and urinary bladder), could contribute to the common clinical overlap of IBS, IC, and other CPP disorders (Alagiri et al. 1997; Prior et al. 1989; Whorwell et al. 1986). As we have recently shown, cross-organ pelvic reflexes, as well as, cross-organ alterations in physiological functioning and sensation following irritation (bladder-to-bowel and vice versa), suggested the involvement of pre-existing dichotomous afferent pathways in the pelvis (Pezzone et al. 2005). This putative role of afferent pathways was further verified by single unit recordings of C-fiber bladder afferents within the pelvic nerve that exhibited sensitized mechanical and chemogenic response properties following acute colonic irritation with trinitrobenzenesulfonic acid (TNBS) (Ustinova et al. 2006). Moreover, interruption of extrinsic neural input to the bladder ameliorated this effect, further confirming a neurogenic process originating in the colon. Thus, it was concluded that bowel-to-bladder, cross-organ afferent sensitization may occur peripherally, via antidromic axon reflexes from a single dichotomizing primary afferent supplying both the bowel and bladder as well as centrally via spinal or supra-spinal circuits (Ustinova et al. 2006).
Similar to previous studies, we found the fluorescent conjugates of CTB to be very efficient in labeling visceral afferents of the pelvis in the rat and mouse (Christianson et al. 2006a; Christianson et al. 2006b; Wang et al. 1998). As expected and in agreement with existing literature (Jancso and Maggi 1987; Robinson et al. 2004; Wang et al. 1998; Zhong et al. 2003), most bladder afferents projected from the L6-S1 DRG in both rodents, while a smaller, but still notable, population was present in TL DRG. In our studies, the distribution of retrogradely-labeled distal colon afferents also paralleled that of the bladder and, in general, was in agreement with the bimodal LS and TL peaks as noted in the Wistar rat (Vizzard et al. 2000) and C57Bl/6 mice (Christianson et al. 2006b; Robinson et al. 2004). In support of the specificity of our labeling studies, dually-labeled cells were identified only at DRG levels that have previously been shown to contain both distal colon and urinary bladder afferents (Christianson et al. 2006b; de Groat et al. 1987; Keast and De Groat 1992; Robinson et al. 2004). Further supporting the specificity of our findings, bladder denervation substantially reduced labeling of bladder afferents and consequentially the number of dually-labeled cells. For example in LS ganglia, bladder afferent labeling decreased 99% (from 398 ± 33 to 5 ± 4) while colonic afferent labeling was unchanged. The lack of complete absence of bladder and/or dual labeling in some animals following bladder denervation can be ascribed to the oft incomplete effects of mechanical surgical denervation.
In the rat, the number of CTB-positive afferents retrogradely labeled from the urinary bladder was, on average, 2.8 times greater than from the colon. Similar findings were noted in the LS DRG by Keast and De Groat (1992) who studied afferent innervation of the urogenital organs and colon of the Wistar rat. In the C57Bl/6 mouse, we found equivalent numbers of CTB-positive colon and bladder afferents in all ganglia examined. Presumably, species and strain differences in lower urinary tract intrinsic pacemakers - intramural ganglia that function like that of the intrinsic nervous system of the gut (i.e. interstitial cells of Cajal) - and/or degrees of supraspinal input could likewise account for differences in bladder afferent distribution patterns and hence bladder-to-colon afferent projection ratios as we have observed (Pezzone et al. 2003).
We found that 17% and 21% of the total CTB-positive neurons were dually-labeled in the rat and mouse, respectively, suggesting that a single afferent is capable of supplying both the bladder and bowel. These dually-labeled neurons were further categorized by spinal level, with TH having the greatest proportion of dichotomizing afferents and LS having the smallest proportion in both species. Such a relatively high proportion of dual-labeled cells is not without precedent, as DRG neurons with dichotomizing axons were first proposed by Sinclair (1948) and have been reported to represent 0.5–15% of all afferents as studied across several different species (McNeill and Burden 1986). Additionally, previous studies have observed that sacral DRG have 2.3 times as many fibers in peripheral nerves as there are cell bodies, further supporting the notion of dichotomous afferent pathways in the pelvis (Langford and Coggeshall 1981). Thus, an anatomical substrate is present which would allow irritation of one pelvic organ to induce referred pain and/or neurogenic inflammation in another organ via these dichotomizing afferent pathways,.
Dichotomizing afferents have been identified in the pelvic viscera by both dual retrograde labeling studies and/or electrophysiological recordings (Bahns et al. 1987; de Groat et al. 1987; Keast and De Groat 1992). Using retrograde tracers, de Groat found that 3–6% of afferents innervating the colon and urogenital organs in both the Wistar rat and the cat were dually-labeled (de Groat et al. 1987; Keast and De Groat 1992). Physiological existence of dichotomizing afferents was also observed by Bahns et al., (1987) who found single units that were excited by stimulation of two separate pelvic organs. Similar findings were also noted by Berkley et al., (1990), and most recently, by Malykhina et al., (2004) who have also begun physiologically characterizing dually-labeled DRG neurons projecting to both the bladder and colon. Sengupta and Gebhart (1994) reported no evidence of individual sacral dorsal root afferents responding to distension of both the colon and bladder, however it is possible that afferents innervating more than one organ are either not responsive to mechanical stretch (e.g. they are mucosal or serosal afferents (Brierley et al. 2004)) or that they are “silent” nociceptors, which become mechanically-sensitive following inflammation or other insult.
The distinct and differential localization of the dually-labeled afferents also supports the specificity of our labeling and may also provide insight into the physiological role of these dichotomizing afferents. In the rat, the largest number of dually-labeled afferents was observed in the TL ganglia, despite the majority of colon- and bladder-specific afferents originating from LS. In both species, the more rostral ganglia contained a higher percentage of dually-labeled afferents than the more caudal ganglia. Previous studies have shown that while LS afferents in the pelvic nerve respond to acute, mechanical stimuli in the pelvic viscera, the TL afferents in the hypogastric nerve do not respond to acute stimuli, but rather mediate inflammatory pain and become mechanically sensitive following injury or inflammation (Berkley et al. 1993a; Berkley et al. 1993b; Lin and Al-Chaer 2003; Mitsui et al. 2001; Sandner-Kiesling et al. 2002; Traub 2000). Therefore, in our model of acute pelvic organ irritation, activation of the dually-projecting TL afferents may underlie the cross-organ sensitization observed in our studies (Pezzone et al. 2005).
In conclusion, we have successfully identified individually- and dually-labeled pelvic afferents supplying the distal colon and urinary bladder in Sprague-Dawley rats and C57Bl/6 mice using fluorescent conjugates of CTB. The identification and localization of dually-labeled cells support the existence of dichotomizing pelvic afferents, which may provide a pre-existing neuronal substrate for immediate and maintained pelvic organ cross-sensitization and also play a major role in the overlap of pelvic pain disorders. Further studies are underway to better comprehensively characterize these labeled dichotomizing afferents both before and after pelvic inflammation.
Acknowledgments
This work was supported by NIH grants DK066658 (MAP), NS050758 (BMD) and NS051021 (JAC). Preliminary results from these studies were presented as an oral presentation at Digestive Disease Week at the 105th Annual Meeting of the American Gastroenterological Association, New Orleans, LA, 2004, and in abstract form at the annual meeting of the Society for Neuroscience, San Diego, CA, 2004.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology. 1997;49:52–7. doi: 10.1016/s0090-4295(99)80332-x. [DOI] [PubMed] [Google Scholar]
- Bahns E, Halsband U, Janig W. Responses of sacral visceral afferents from the lower urinary tract, colon and anus to mechanical stimulation. Pflugers Arch. 1987;410:296–303. doi: 10.1007/BF00580280. [DOI] [PubMed] [Google Scholar]
- Berger RE, Miller JE, Rothman I, Krieger JN, Muller CH. Bladder petechiae after cystoscopy and hydrodistension in men diagnosed with prostate pain. J Urol. 1998;159:83–5. doi: 10.1016/s0022-5347(01)64018-7. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Hotta H, Robbins A, Sato Y. Functional properties of afferent fibers supplying reproductive and other pelvic organs in pelvic nerve of female rat. J Neurophysiol. 1990;63:256–72. doi: 10.1152/jn.1990.63.2.256. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Hubscher CH, Wall PD. Neuronal responses to stimulation of the cervix, uterus, colon, and skin in the rat spinal cord. J Neurophysiol. 1993a;69:545–56. doi: 10.1152/jn.1993.69.2.545. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Robbins A, Sato Y. Functional differences between afferent fibers in the hypogastric and pelvic nerves innervating female reproductive organs in the rat. J Neurophysiol. 1993b;69:533–44. doi: 10.1152/jn.1993.69.2.533. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–78. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience. 2006a doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Traub RJ, Davis BM. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol. 2006b;494:246–59. doi: 10.1002/cne.20816. [DOI] [PubMed] [Google Scholar]
- Clemens JQ, Meenan RT, Rosetti MC, Gao SY, Calhoun EA. Prevalence and incidence of interstitial cystitis in a managed care population. J Urol. 2005;173:98–102. doi: 10.1097/01.ju.0000146114.53828.82. discussion 102. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Booth AM. Neural control of penile erection. In: Maggi CA, editor. Nervous Control of the Urogenital System. Harwood; London: 1993. pp. 467–524. [Google Scholar]
- de Groat WC, Booth AM, Oshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. Nervous Control of the Urogenital System. Harwood; London: 1993a. pp. 227–290. [Google Scholar]
- de Groat WC, Kawatani M, Houston MB, Rutigliiano M, Erdman S. Identification of neuropeptides in afferent pathways to the pelvic viscera of the cat. In: Ciriello J, Calaresu F, Renaud L, Polosa C, Liss AR, editors. Organization of the Autonomic Nervous System: Central and Peripheral Mechanisms, Neurology and Neurobiology. New York: 1987. pp. 81–90. [Google Scholar]
- de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135–60. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Roppolo JR, Yoshimura N, Sugaya K. Neural control of the urinary bladder and colon. Proc 2nd Int Symp. Brain-Gut Interactions; Boca Raton, VL. 1993b. [Google Scholar]
- de Groat WC, Steers WD. Neuroanatomy and neurophysiology of penile erection. In: Tanagho EA, Lue TF, McClure RD, editors. Contemporary Management of Impotence and Infertility. Baltimore: Williams & Wilkins, Baltimore; 1981. pp. 3–27. [Google Scholar]
- Doggweiler-Wiygul R, Blankenship J, MacDiarmid SA. Review on chronic pelvic pain from a urological point of view. World J Urol. 2001;19:160–5. doi: 10.1007/s003450100198. [DOI] [PubMed] [Google Scholar]
- Jancso G, Maggi CA. Distribution of capsaicin-sensitive urinary bladder afferents in the rat spinal cord. Brain Res. 1987;418:371–6. doi: 10.1016/0006-8993(87)90106-5. [DOI] [PubMed] [Google Scholar]
- Janig W, Koltzenburg M. On the function of spinal primary afferent fibres supplying colon and urinary bladder. J Auton Nerv Syst. 1990;30(Suppl):S89–96. doi: 10.1016/0165-1838(90)90108-u. [DOI] [PubMed] [Google Scholar]
- Keast JR, De Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615–23. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- Langford LA, Coggeshall RE. Branching of sensory axons in the peripheral nerve of the rat. J Comp Neurol. 1981;203:745–50. doi: 10.1002/cne.902030411. [DOI] [PubMed] [Google Scholar]
- Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971:73–82. doi: 10.1016/s0006-8993(03)02358-8. [DOI] [PubMed] [Google Scholar]
- Malykhina AP, Qin C, Foreman RD, Akbarali HI. Colonic inflammation increases Na+ currents in bladder sensory neurons. Neuroreport. 2004;15:2601–5. doi: 10.1097/00001756-200412030-00008. [DOI] [PubMed] [Google Scholar]
- Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321–7. doi: 10.1016/0029-7844(95)00458-0. [DOI] [PubMed] [Google Scholar]
- McNeill DL, Burden HW. Convergence of sensory processes from the heart and left ulnar nerve onto a single afferent perikaryon: a neuroanatomical study in the rat employing fluorescent tracers. Anat Rec. 1986;214:441–4. 396–7. doi: 10.1002/ar.1092140416. [DOI] [PubMed] [Google Scholar]
- Mitsui T, Kakizaki H, Matsuura S, Ameda K, Yoshioka M, Koyanagi T. Afferent fibers of the hypogastric nerves are involved in the facilitating effects of chemical bladder irritation in rats. J Neurophysiol. 2001;86:2276–84. doi: 10.1152/jn.2001.86.5.2276. [DOI] [PubMed] [Google Scholar]
- Moldwin RM. Similarities between interstitial cystitis and male chronic pelvic pain syndrome. Curr Urol Rep. 2002;3:313–8. doi: 10.1007/s11934-002-0056-x. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Total number of neurons and glial cells in human brain nuclei estimated by the disector and the fractionator. J Microsc. 1988;150(Pt 1):1–20. doi: 10.1111/j.1365-2818.1988.tb04582.x. [DOI] [PubMed] [Google Scholar]
- Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953–64. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Pezzone MA, Watkins SC, Alber SM, King WE, de Groat WC, Chancellor MB, Fraser MO. Identification of c-kit-positive cells in the mouse ureter: the interstitial cells of Cajal of the urinary tract. Am J Physiol Renal Physiol. 2003;284:F925–9. doi: 10.1152/ajprenal.00138.2002. [DOI] [PubMed] [Google Scholar]
- Prior A, Wilson K, Whorwell PJ, Faragher EB. Irritable bowel syndrome in the gynecological clinic. Survey of 798 new referrals. Dig Dis Sci. 1989;34:1820–4. doi: 10.1007/BF01536698. [DOI] [PubMed] [Google Scholar]
- Robertson B, Arvidsson J. Transganglionic transport of wheat germ agglutinin-HRP and choleragenoid-HRP in rat trigeminal primary sensory neurons. Brain Res. 1985;348:44–51. doi: 10.1016/0006-8993(85)90357-9. [DOI] [PubMed] [Google Scholar]
- Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–24. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Sandner-Kiesling A, Pan HL, Chen SR, James RL, DeHaven-Hudkins DL, Dewan DM, Eisenach JC. Effect of kappa opioid agonists on visceral nociception induced by uterine cervical distension in rats. Pain. 2002;96:13–22. doi: 10.1016/s0304-3959(01)00398-0. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1994;72:2420–30. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- Sinclair DC, Weddell G, Feindel WH. Referred pain and associated phenomena. Brain Res. 1948;71:184–211. doi: 10.1093/brain/71.2.184. [DOI] [PubMed] [Google Scholar]
- Traub RJ. Evidence for thoracolumbar spinal cord processing of inflammatory, but not acute colonic pain. Neuroreport. 2000;11:2113–6. doi: 10.1097/00001756-200007140-00011. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Fraser MO, Pezzone MA. Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: an afferent origin of pelvic organ cross-sensitization. Am J Physiol Renal Physiol. 2006;290:F1478–87. doi: 10.1152/ajprenal.00395.2005. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Brisson M, de Groat WC. Transneuronal labeling of neurons in the adult rat central nervous system following inoculation of pseudorabies virus into the colon. Cell Tissue Res. 2000;299:9–26. doi: 10.1007/s004419900128. [DOI] [PubMed] [Google Scholar]
- Wang HF, Shortland P, Park MJ, Grant G. Retrograde and transganglionic transport of horseradish peroxidase-conjugated cholera toxin B subunit, wheatgerm agglutinin and isolectin B4 from Griffonia simplicifolia I in primary afferent neurons innervating the rat urinary bladder. Neuroscience. 1998;87:275–88. doi: 10.1016/s0306-4522(98)00061-x. [DOI] [PubMed] [Google Scholar]
- Wesselmann U. Neurogenic inflammation and chronic pelvic pain. World J Urol. 2001;19:180–5. doi: 10.1007/s003450100201. [DOI] [PubMed] [Google Scholar]
- Whorwell PJ, McCallum M, Creed FH, Roberts CT. Non-colonic features of irritable bowel syndrome. Gut. 1986;27:37–40. doi: 10.1136/gut.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Russell RM, Karten HJ. The transport rate of cholera toxin B subunit in the retinofugal pathways of the chick. Neuroscience. 1999;92:665–76. doi: 10.1016/s0306-4522(99)00018-4. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Banning AS, Cockayne DA, Ford AP, Burnstock G, McMahon SB. Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience. 2003;120:667–75. doi: 10.1016/s0306-4522(03)00243-4. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Yudkin PL, Vessey MP, Dawes MG, Barlow DH, Kennedy SH. Prevalence and incidence of chronic pelvic pain in primary care: evidence from a national general practice database. Br J Obstet Gynaecol. 1999;106:1149–55. doi: 10.1111/j.1471-0528.1999.tb08140.x. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Yudkin PL, Vessey MP, Jenkinson CP, Dawes MG, Barlow DH, Kennedy SH. The community prevalence of chronic pelvic pain in women and associated illness behaviour. Br J Gen Pract. 2001;51:541–7. [PMC free article] [PubMed] [Google Scholar]


