Abstract
The interdependence between geoelectrical signatures at underground petroleum plumes and the structures of subsurface microbial communities was investigated. For sediments contaminated with light non-aqueous-phase liquids, anomalous high conductivity values have been observed. Vertical changes in the geoelectrical properties of the sediments were concomitant with significant changes in the microbial community structures as determined by the construction and evaluation of 16S rRNA gene libraries. DNA sequencing of clones from four 16S rRNA gene libraries from different depths of a contaminated field site and two libraries from an uncontaminated background site revealed spatial heterogeneity in the microbial community structures. Correspondence analysis showed that the presence of distinct microbial populations, including the various hydrocarbon-degrading, syntrophic, sulfate-reducing, and dissimilatory-iron-reducing populations, was a contributing factor to the elevated geoelectrical measurements. Thus, through their growth and metabolic activities, microbial populations that have adapted to the use of petroleum as a carbon source can strongly influence their geophysical surroundings. Since changes in the geophysical properties of contaminated sediments parallel changes in the microbial community compositions, it is suggested that geoelectrical measurements can be a cost-efficient tool to guide microbiological sampling for microbial ecology studies during the monitoring of natural or engineered bioremediation processes.
Petroleum contamination of the subsurface from accidental oil spills or leaking underground storage tanks remains a significant environmental problem. The U.S. Environmental Protection Agency reports a backlog of about 117,000 leaking underground storage tanks (23). For the clean-up of many of these sites, monitored natural attenuation is considered to be the most efficient alternative technique (24). However, for successful intrinsic or engineered bioremediation approaches, the complex relationships among pollutants, geochemical and hydrological conditions, and the microorganisms involved in contaminant degradation must be understood (28). In addition, monitoring tools are needed to observe the migration or removal of the contaminant mass, the rate of biodegradation, and the possible development of more toxic intermediates.
Geophysical subsurface imaging techniques have been successfully used in mapping subsurface lithologies, water saturation, and more recently, the extent of underground petroleum plumes (49, 53). In sediments, petroleum hydrocarbons, more specifically the light non-aqueous-phase liquids (LNAPLs), partition into free-product, dissolved, and residual phases. The free-product phase consists of pure hydrocarbons floating on top of the groundwater table, while the dissolved phase corresponds to hydrocarbons dissolved in groundwater. The residual-phase LNAPLs occur above and below the free-phase petroleum where hydrocarbons adhere to the sediments in a smear zone due to fluctuations of the water table (36, 48). Petroleum hydrocarbons naturally exhibit electrically resistive properties; however, geophysical studies have revealed electrically conductive characteristics of aged petroleum plumes (14, 48, 49).
Multiple factors are being discussed as causes of the anomalous elevated conductivity measurements. The growth and metabolic activities of microorganisms are known to alter the chemical and physical conditions of the environment. The biodegradation of hydrocarbons results in the production of bacterial metabolites, including organic acids and CO2. Carbonic acid and organic acids such as acetate and fumarate drive mineral dissolution by releasing ions from the sediment grains (18, 37, 45). Moreover, microorganisms alter their physical surroundings by colonizing sediment surfaces in the form of microcolonies or biofilms or by driving the precipitation of metal sulfides at cell or sediment grain surfaces (1, 2, 40, 57). In addition, the presence of microbial nanowires, which have recently been identified as highly conductive pili on the surfaces of iron-reducing bacteria such as Geobacter (43) and Shewanella (27) species, may also play a role in increasing subsurface conductivity levels in certain geomicrobiological environments.
Numerous publications have analyzed microbial activities and the microbial community structures at underground petroleum spills, for example, at the site of a pipeline burst in Bemidji, MN (11, 47), and at the former Wurtsmith Air Force Base in Oscoda, MI (21, 29). These studies showed that a complex array of factors influences the spatial and temporal heterogeneity of microbial communities in contaminated aquifers, including the diverse physical, chemical, geological, and climate conditions. No study so far, however, has attempted to analyze the compositions of microbial communities in relationship to anomalous high conductivity measurements at underground petroleum hydrocarbon spill sites.
In this study, we present a detailed spatial analysis of the microbial community structure in an aged underground petroleum plume at the site of a former refinery in Carson City, MI. This site has been studied extensively with geochemical and geoelectrical methods (5, 6, 8). Preliminary microbiological measurements showed that high ratios of culturable hydrocarbon-degrading microbial populations coincided with the peak of the hydrocarbon contamination and with elevated electrical conductivity levels (8). Here, we use a non-culture-based method (16S rRNA gene libraries) to describe the microbial community structure along vertical gradients. We show a possible link between the presence of certain microbial populations and the geophysical changes in the subsurface, suggesting that geophysical techniques may provide a noninvasive and cost-efficient tool to monitor natural attenuation and to guide microbiological sampling for microbial ecology studies of petroleum-contaminated aquifers.
MATERIALS AND METHODS
Site description.
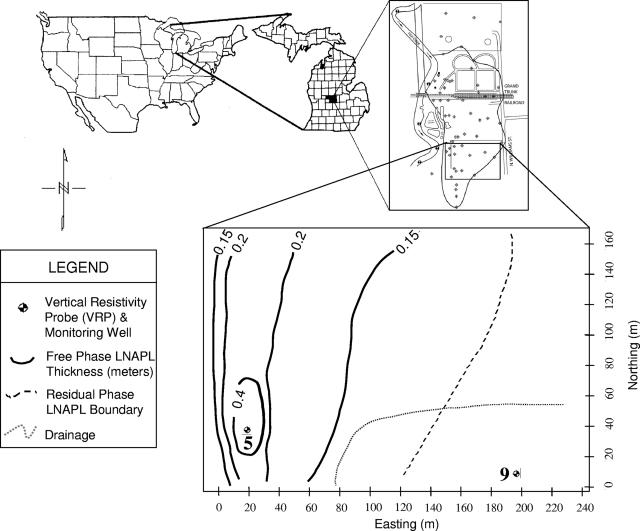
The study site was a city park containing an underground petroleum hydrocarbon plume adjacent to the former Crystal Refinery Company in Carson City, MI (Fig. 1). A continuous release of hydrocarbons (mainly crude oil, JP4 jet fuel, and diesel fuel) from underground storage facilities occurred for more than 50 years (20). The contaminated aquifer is about 2 m thick and composed of loam soils and glaciofluvial sands and gravels underlain by clay (56). The mineralogy of the aquifer is dominated by quartz, with minor inclusions of calcite, albite, anorthite, gypsum, and dolomite (20). The contaminated portion of the aquifer has hydrocarbons in the dissolved, free, and residual phases (20). The free-phase petroleum zone is up to 0.4 m thick above the water table. The residual-phase-contaminated zone averages about 1 m above and below the free-product-contaminated zone due to the annual fluctuation of the groundwater table of 0.9 m (56).
FIG. 1.
Detailed map of the field study site within the Carson City Park, Carson City, MI. The positions of the VRPs at the contaminated (5) and background (9) locations are noted. Samples for microbial analysis were collected at the same locations, adjacent to the VRPs. To the north of the sampling site is the former petroleum refinery where the leaking underground storage tanks were located. The thicknesses (in meters) of the free-phase LNAPLs over the study area are given, as well as the boundary of residual LNAPL contamination. The location of a drainage ditch running through the site is also shown.
Conductivity measurements in sediments.
The bulk electrical conductivity levels of sediments were measured using vertical resistivity probes (VRPs) installed in contaminated and uncontaminated locations. The VRPs were constructed from 3.8-cm-diameter polyvinylchloride pipe with electrical contacts (stainless steel screws) every 2.5 cm extending into the sediments. The VRPs were installed in boreholes by using push technology (Geoprobe drill rig), which ensures good probe electrode contact with the formation. The electrical measurements were obtained by using an internal slider that makes electrical contact between four of the screw contact points. By passing an electrical current in series from the first to the fourth electrode, the second and third electrodes detect the voltage potential due to the electrical properties of the surrounding media. Electrical conductivity measurements for a 5.0-cm Wenner array were collected by using a Syscal R2 resistivity meter with an automated/semiautomated switching system for switching between the electrodes (56). Further information on the VRP installation, Wenner array, and data collection can be found elsewhere (8, 56). Geoelectrical data were collected in 16 different months between March 1999 and November 2000. Mean conductivity values (± standard deviations) are based on 16 different measurements, whereas the data for conductivity profiles of the contaminated sampling site (VRP5) and the control site (VRP9) correspond to the month in which sediments were sampled for the microbiological analysis (August 2000).
Sediment sampling for microbial analysis.
The sampling for the microbial analysis was carried out on 8 August 2000. Sediment samples were collected by coring less than 1 m away from the VRPs. Sediments were collected in 95% ethanol-sanitized core liners fitted into a hand-operated direct-push soil-coring device. After coring, the liners were removed and immediately capped, secured with electrical tape, and transported to the laboratory on ice (22). Four 1-g sediment subsamples from each selected depth were collected aseptically and used for culture-dependent most-probable-number (MPN) analyses within 48 h of sampling. The recovery of the bacterial fraction was carried out according to the protocol of van Elsas and Smalla (55). Pooled subsamples of 1 g were collected aseptically from selected depths within the cores and stored at −20°C for DNA extraction.
MPN analyses.
For the MPN analyses, 1/10 tryptic soy broth (Becton Dickinson, Detroit, MI) was used for heterotrophic bacteria and Bushnell-Haas medium (Becton Dickinson, Detroit, MI) supplemented with 2.5% n-hexadecane was used for hydrocarbon degraders. The growth of heterotrophic bacteria was assessed with a microplate reader (EL800; BIO-TEK Instruments, Winooski, VT) by measuring the optical density at 600 nm. The growth of hydrocarbon-degrading bacteria was evaluated by adding p-iodonitrotetrazolium violet to the Bushnell-Haas medium, after which plates were visually examined for the appearance of red precipitates indicating growth and were scored accordingly (58). The results were entered into the most-probable-number calculator version 4.04 to determine the MPN of bacteria per gram of soil compared to published MPN charts (35).
DNA purification and construction of 16S rRNA gene libraries.
Total chromosomal DNA was extracted from sediment subsamples by using the UltraClean soil DNA kit (Mo Bio, Solana Beach, CA) and stored at −20°C. Community 16S rRNA genes were PCR amplified from the bulk DNA in 50-μl reaction mixtures containing (as final concentrations) 50 mM KCl, 1.5 mM MgCl2, 10 mM Tris-HCl (pH 8.3), 0.01% gelatin, a 0.2 mM concentration of each deoxyribonucleotide, 100 pmol of each forward and reverse primer, and 1.5 U of Red-Taq DNA polymerase (Sigma-Aldrich Corp., St. Louis, MO). PCR amplification was carried out in a Mastercycler gradient DNA thermal cycler (Eppendorf, Westbury, NY), with an initial denaturation step for 2 min at 94°C; 25 cycles at 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min for elongation; and a final elongation step at 74°C for 7 min. The 16S rRNA genes from the samples were amplified using the universal oligonucleotide forward primer 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and the universal reverse primer 1492R (5′-CCGTCAATTCMTTTRAGTTT-3′) according to the method of Hayes and Lovley (30), but the latter primer actually anneals to the 900 region of the Escherichia coli 16S rRNA gene (our unpublished data) and is similar to 907R (15). PCR products were visualized on a 1.2% agarose gel and purified with a Wizard SV gel and PCR clean-up kit (Promega, Madison, WI). The purified PCR products were cloned with the TOPO TA cloning kit, version R, in accordance with the instructions of the manufacturer (Invitrogen, Carlsbad, CA). Plasmid DNA containing inserts was purified from transformed clones by using the fast plasmid mini kit (Eppendorf, Westbury, NY) and stored at −20°C.
Library screening, DNA sequencing, and phylogenetic analysis.
16S rRNA gene inserts from recombinant clones were reamplified by PCR in 50-μl reaction mixtures as stated above. PCR amplification was carried out in a Mastercycler gradient DNA thermal cycler (Eppendorf, Westbury, NY), with an initial denaturation step for 2 min at 94°C; 33 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min for elongation; and a final elongation step at 74°C for 7 min. The 16S rRNA gene inserts were amplified using vector primers M13R (5′-CAGGAAAAGCTATGAC-3′) and T7F (5′-TAATACGACTCACTATAGGG-3′; Invitrogen, Carlsbad, CA). Aliquots (7 μl) of the PCR products were digested with restriction endonuclease MspI (New England Biolabs, Beverly, MA). Plasmid inserts from representative clones were sequenced at Cornell University's Biotechnology Resource Center (Ithaca, NY) by using the vector primer M13R. The 16S rRNA gene sequences were compared to sequences in the GenBank database by using BLAST (basic local alignment search tool) (3) and to those in the Ribosomal Database Project (17) to determine their approximate phylogenetic affiliations. Chimeric sequences were identified by using the CHECK_CHIMERA program (17). Sequence alignments for phylogenetic inference were performed with the ClustalX multiple-sequence alignment program (54) and exported for phylogenetic tree construction by using PAUP 4.0b (Sinauer Associates, Sunderland, MA). The branching orders were determined and compared using distance-based neighbor-joining algorithms with Kimura two-parameter correction.
Diversity, similarity, and multivariate analyses.
Clones with the same restriction fragment length polymorphism (RFLP) patterns as well as those with sequence similarities of greater than 97% were considered to represent the same phylotypes. Jaccard's incidence-based similarity index was calculated using SPADE (version 3.1; Anne Chao and Tsung Jen-Shen, National Tsing Hua University, Taiwan; http://chao.stat.nthu.edu.tw/softwareCE.html). Rarefaction curves were calculated using Analytic Rarefaction (version 1.3; Steven Holland, University of Georgia; http://www.uga.edu/∼strata/software/Software.html). A multivariate analysis was performed with MVSP 3.1 software (Kovach Computing Services) by using correspondence analysis.
Nucleotide sequence accession numbers.
16S rRNA gene sequences were deposited in GenBank under the accession numbers DQ663791 to DQ663860 (library 5V), DQ663861 to DQ663928 (library 5F), DQ663929 to DQ663992 (library 5C), DQ663993 to DQ664042 (library 5S), DQ664043 to DQ664107 (library 9V), and DQ664108 to DQ664179 (library 9S). After submission, sequences with the last four digits 3811, 3857, 3937, 3950, 3987, 4001, 4016, 4080, 4084, 4086, 4124, 4154, and 4168 turned out to be chimeric sequences as determined by the CHECK_CHIMERA program (17).
RESULTS
Grain size and hydrocarbon phase distribution.
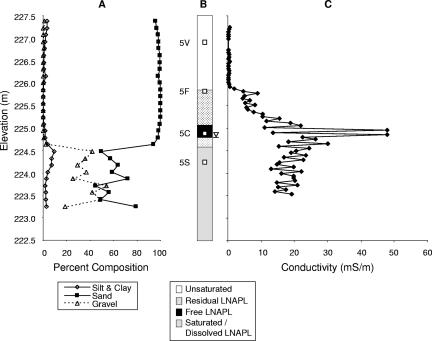
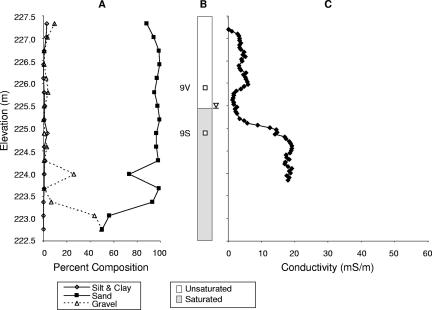
Fig. 2A and 3A show the grain size distributions at the contaminated site, VRP5, and at the uncontaminated background site, VRP9, respectively. Sand-sized grains were predominant in sediments above the water table at both the locations (>95%). Sediments with up to 50% gravel-sized grains were present at the VRP5 location below the water table, whereas at the VRP9 location, the percentage of gravel was generally lower and reached 50% only at the lowest elevation.
FIG. 2.
Soil lithology, soil conditions, and conductivity profiles of the petroleum-contaminated site (VRP5). (A) Percentages of silt and clay, sand, and gravel. (B) Residual, free, and dissolved LNAPL phases; water saturation; and sampling locations for 16S rRNA gene libraries 5V, 5F, 5C, and 5S. The inverted triangle denotes the level of the groundwater table. (C) Levels of conductivity expressed in millisiemens per meter.
FIG. 3.
Soil lithology, soil conditions, and bulk conductivity profiles of the background site (VRP9). (A) Percentages of silt and clay, sand, and gravel. (B) Water saturation and sampling locations for 16S rRNA gene libraries 9V and 9S. The inverted triangle denotes the level of the groundwater table. (C) Levels of conductivity expressed in millisiemens per meter.
VRP5 was within the center of the free-phase LNAPL-contaminated area (Fig. 1). At this location, an approximately 30-cm layer of free petroleum was found floating on top of the water table during sediment sampling. The depth distribution of the residual-phase (sediment particles contaminated by hydrocarbon staining), free-phase (sediment saturated with hydrocarbons), and dissolved-phase (water-saturated sediments with dissolved hydrocarbons) contamination is shown in Fig. 2B. Because of a fluctuating water table at the site, a zone nearly 1.5 m thick straddling the water table had been coated with free LNAPL, resulting in the development of a hydrocarbon smear zone (Fig. 2B). The background location, VRP9, was separated from the contaminated area by a drainage ditch and did not show any measurable hydrocarbon contamination (Fig. 3B).
Geoelectrical measurements.
The conductivity profiles shown in Fig. 2C and 3C are based on the electrical conductivity values measured in August 2000. Almost identical conductivity profiles for VRP5 and VRP9 were obtained during measurements in 16 different months between March 1999 and November 2000 (56). At the contaminated location, VRP5, conductivity values were low (<1 mS/m) from the surface down to an elevation of 225.9 m above sea level, where the residual petroleum contamination began (Fig. 2C). From the residual-phase-contaminated zone to the upper boundary of the free-phase petroleum-contaminated zone (225.1 m), the bulk conductivity steadily increased to 22 mS/m. In the free-phase petroleum-contaminated zone, peak conductivity values were observed at elevations between 224.8 and 225.0 m, where the conductivity reached up to 48 mS/m. In the water-saturated zone with dissolved-phase LNAPL contamination, from 224.7 to 223.5 m, the conductivity fluctuated steadily between 12 and 30 mS/m, with a mean of 19 mS/m.
No petroleum was detected at the background location, VRP9, as reflected in Fig. 3B. The conductivity measurements for sediments at the uncontaminated location showed steadily increasing values of up to 6 mS/m for the area between the surface and an elevation of 226 m, followed by a decrease to <2 mS/m towards the water table (225.5 m) (Fig. 3C). In saturated sediments below the water table, the bulk conductivity increased steadily up to 20 mS/m, with a mean of 14.5 mS/m.
Culturable microbial populations.
To obtain a first glimpse of the culturable microbial populations, the fraction of the total community of heterotrophic bacteria able to degrade hydrocarbons was determined (see Materials and Methods). The ratios of culturable hydrocarbon degraders to the total heterotrophic microbial community were generally low (≤0.05) at most of the selected depths at VRP5 and VRP9 (Table 1). The exceptions were the highest elevation at VRP5 (226.93 m), which is influenced by topsoil and plant roots, where 12% of the culturable community was able to degrade hydrocarbons, and the lowest elevation at VRP5 (224.31 m), the saturated zone containing dissolved LNAPL, where 24% of the culturable population was able to degrade hydrocarbons (Table 1).
TABLE 1.
Parameters associated with 16S rRNA gene libraries from the contaminated and control sampling sites
| Site | Library | Sampling elevation (m) | Mean conductivity (mS/m) (SD)a | Fraction of culturable hydrocarbon degradersb | Total no. of clones in library | No. of different RFLP patterns (OTUs)c |
|---|---|---|---|---|---|---|
| VRP5 (contaminated) | 5V | 226.93 | 0.09 (±0.08) | 0.12 | 102 | 70 |
| 5F | 225.85 | 1.74 (±0.54) | 0.01 | 100 | 68 | |
| 5C | 224.93 | 51.57 (±7.43) | 0.03 | 100 | 64 | |
| 5S | 224.31 | 21.90 (±2.25) | 0.24 | 97 | 50 | |
| VRP9 (control) | 9V | 225.85 | 2.78 (±1.22) | 0.04 | 87 | 65 |
| 9S | 224.93 | 9.66 (±3.11) | 0.05 | 103 | 72 |
Mean conductivity values are based on 16 different measurements between March 1999 and November 2000.
Numbers of culturable hydrocarbon degraders (MPN per gram of soil as determined in Bushnell-Haas medium with hexadecane as the sole carbon source) are expressed as fractions of the numbers of total culturable heterotrophs (MPN per gram of soil as determined in 10% TSB medium) calculated as the means of data collected in five different months in the year 2000.
OTUs, operational taxonomic units.
16S rRNA gene library analysis.
In order to determine the microbial community structures in sediments within zones of higher and lower conductivity levels, 16S rRNA gene libraries were constructed. For the VRP5 contaminated site, library 5V was constructed from DNA purified from sediments collected in the upper vadose zone, which exhibited low conductivity readings over 16 months (mean, 0.1 mS/m) (Table 1; Fig. 2B). Library 5F was constructed from DNA from sediments in the upper fringe region of residual LNAPL contamination, where the conductivity began to increase steadily (mean, 1.8 mS/m). Library 5C was constructed from DNA from the free-phase petroleum-contaminated sediments, for which the highest mean conductivity value was recorded (52 mS/m), and library 5S was constructed from DNA from sediments in the water-saturated zone with dissolved contaminant, for which a mean conductivity value of 22 mS/m was measured. For the background site, VRP9, the 16S rRNA gene library 9V was constructed from DNA from vadose sediments in the unsaturated zone, which displayed a mean conductivity of 2.8 mS/m, and library 9S was constructed from DNA from sediments in the groundwater-saturated zone exhibiting a mean conductivity of 9.7 mS/m (Table 1; Fig. 3B).
A total of 589 different clones containing partial 16S rRNA gene inserts were obtained from the DNA isolated from the sediment samples (Table 1). The 16S rRNA gene inserts were screened for their RFLP patterns by using the restriction endonuclease MspI. Library 5S contained the lowest number of clones showing unique restriction patterns (50), whereas 9S contained the highest number of unique clones (72) (Table 1).
Phylogenetic analysis.
All unique clones determined by RFLP analysis and a single representative clone from each group of clones with identical RFLP patterns were used for DNA sequencing. The program CHECK_CHIMERA (Materials and Methods) identified 13 chimeric sequences, which were omitted from the following analysis. The phylogenetic analysis of the 16S rRNA gene sequences (Table 2) revealed diverse microbial communities in sediments at each different sampling depth within the contaminated and uncontaminated locations, with the communities representing 22 of the 52 phyla presently recognized (33, 42).
TABLE 2.
Distribution of clones from 16S rRNA gene libraries from the contaminated site (5V, 5F, 5C, and 5S) and the control site (9V and 9S) among different bacterial phyla
| Phylogenetic group | % of clones in library
|
|||||
|---|---|---|---|---|---|---|
| 5V | 5F | 5C | 5S | 9V | 9S | |
| Acidobacteria | 54 | 12 | 2 | 6 | 29 | 32 |
| Green nonsulfur bacteria | 19 | 18 | 23 | 11 | 4 | |
| Alphaproteobacteria | 11 | 39 | 7 | 10 | 4 | 9 |
| Deltaproteobacteria | 5 | 22 | 33 | 1 | 2 | |
| Clostridia | 3 | 17 | 4 | 12 | 1 | |
| Gammaproteobacteria | 3 | 3 | 1 | |||
| Planctomycetes | 3 | 1 | 5 | 9 | ||
| Actinobacteria | 1 | 9 | 7 | 4 | 23 | 9 |
| OP10 | 1 | 1 | 1 | |||
| Betaproteobacteria | 34 | 3 | 1 | 5 | ||
| Bacilli | 2 | 8 | ||||
| Gemmatimonadetes | 5 | 4 | ||||
| Nitrospira | 1 | 11 | ||||
| WS3 | 3 | |||||
| WS6 | 1 | |||||
| Chlorobi | 2 | 8 | 1 | |||
| Bacteroidetes | 4 | 6 | ||||
| Spirochaetes | 11 | 8 | ||||
| OP5 | 2 | 2 | ||||
| OP8 | 3 | 1 | ||||
| OP11 | 2 | |||||
| Epsilonproteobacteria | 1 | |||||
Among the clones from the upper vadose sediments at the VRP5 contaminated site (library 5V), more than half of the 16S rRNA gene sequences (54%) showed high identity to those from several groups within the Acidobacteria phylum (Table 2), recently recognized as one of the phyla most abundant in the soil (10). Nineteen percent of the clones in the 5V library grouped with the green nonsulfur bacteria, and 11% grouped with the Alphaproteobacteria. Figure S1 in the supplemental material shows phylogenetic trees depicting the closest relatives of the sequenced clones. Among the Alphaproteobacteria group, the largest proportion of clones (7 clones) showed >98% identity to the methanotrophic species Methylocapsa acidiphila (Fig. S1C in the supplemental material). A large methanotrophic population was also found to be represented in library 5F, with 18 clones showing >98% identity to Methylocapsa acidiphila and 8 clones showing >98% identity to Hyphomicrobium methylovorum (Fig. S1C in the supplemental material). Furthermore, the most abundant sequences (21 clones) in library 5F were >98% identical to sequences from the aromatic hydrocarbon-degrading species “Brachymonas petroleovorans” of the Betaproteobacteria subdivision. In addition, 7 other clones among the 100 clones in the 5F library showed high levels of identity to sequences from the aromatic hydrocarbon-degrading species Sphingomonas aromaticivorans of the Alphaproteobacteria, indicating that known aromatic hydrocarbon-degrading populations constituted at least 28% of the entire 5F microbial community (Fig. S1C in the supplemental material). Three clones each in the 5V and 5F libraries were >95% identical to Beggiatoa alba within the Gammaproteobacteria, which is a known sulfide-oxidizing bacterium. The remainder of the 16S rRNA sequences from libraries 5V and 5F were similar to sequences from common soil microbiota from the Deltaproteobacteria, Gammaproteobacteria, Clostridia, Bacilli, Planctomycetes, Actinobacteria, and OP10 divisions (Table 2; Fig. S1C, D, and E in the supplemental material).
The most common 16S rRNA gene sequences detected in the libraries from the free-phase-contaminated zone (5C) and the dissolved-phase-contaminated zone (5S) included those grouping with sequences from species from the Deltaproteobacteria (5C, 22%; 5S, 33%), the green nonsulfur bacteria (5C, 18%; 5S, 23%), and Clostridia (5C, 17%). Twenty-one of the total 100 clones from library 5C as well as 31 of the 97 total clones from library 5S were >98% identical to the syntrophic bacterium Syntrophus sp. strain B2 of the Deltaproteobacteria subdivision (Fig. S1C in the supplemental material). In addition, 15 clones from 5C and a single clone from 5S were closely related (>98%) to the uncultured clone WCHB1-05 of the green nonsulfur bacteria identified in petroleum-contaminated sediments at the former Wurtsmith Air Force Base, Oscoda, MI (21), while 15 clones from 5S were 98% identical to the uncultured clone TA17 of the green nonsulfur bacteria isolated from a terephthalate-degrading anaerobic granular sludge system (Fig. S1B in the supplemental material). Ten clones from library 5C showed >98% identity to the Wurtsmith clone WCHB1-77, which grouped with 95% identity with the sulfate-reducing bacterium Desulfosporosinus sp. of the Clostridia phylum (Fig. S1D in the supplemental material). There were also other Clostridia populations represented in library 5C (two clones) and 5S (four clones) which were 98% identical to the syntrophic Pelotomaculum sp., while two clones from the 5C library showed high sequence identity (>98%) to the known hydrocarbon-degrading, dissimilatory-iron-reducing species Rhodoferax ferrireducens of the Betaproteobacteria (Fig. S1C in the supplemental material). There were several phyla detected exclusively in the libraries representing the contaminated zones (5C and 5S), including the Bacteroidetes, Spirochetes, OP5, and OP8 divisions (Table 2). Uniquely occurring in library 5C were clones from the OP11 and Epsilonproteobacteria phyla (Table 2).
Two 16S rRNA gene libraries from the VRP9 uncontaminated background site were also constructed, one from the unsaturated zone above the water table and one from the saturated zone below the water table (Fig. 3B). Around one-third of the clones from the 9V (29%) and the 9S (32%) libraries were distributed among the Acidobacteria phylum (Table 2; Fig. S1A in the supplemental material). Twenty-three percent of the 9V clones showed high degrees of identity to groups of Actinobacteria clones commonly detected in natural sediments (Table 2; Fig. S1E in the supplemental material), whereas 11% of library 9S clones grouped into the Nitrospira phylum. Clones from the Gemmatimonadetes and Nitrospira phyla were detected exclusively in the 9V and 9S libraries, whereas clones representing the WS3 and WS6 phyla were found exclusively in the 9S library (Table 2).
In general, no new phyla were detected at the Carson City site. Based on the distance values presently considered to be the cutoff values for bacterial phylum, family/class, genus, and species classifications (80, 90, 95, and 97%, respectively) (51), more than 61% of the clones from the 16S rRNA gene libraries showed relationships at the species level, 19% at the genus level, 19% at the family/class level, and 1% at the phylum level.
Rarefaction, similarity, and multivariate analyses.
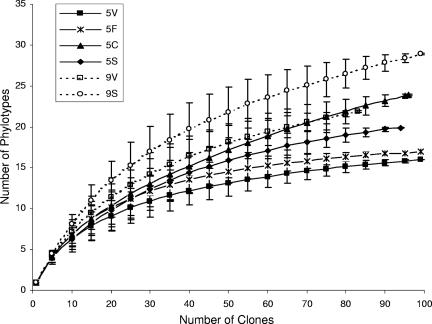
Rarefaction analysis allows for the comparison of observed levels of richness among samples (31). Rarefaction analysis was performed by plotting the number of phylotypes (groups of clones with ≥97% identity) observed against the number of clones sequenced (Fig. 4). The flattening accumulation curves indicated that a sufficient number of clones had been sequenced to represent the diversity in the different libraries. Based on the rarefaction curves, clone libraries 9S, 9V, and 5C exhibited a higher level of richness than libraries 5S, 5F, and 5V (Fig. 4).
FIG. 4.
Rarefaction curves generated for 16S rRNA gene libraries from VRP5 and VRP9 sediment samples. Error bars indicate 95% confidence intervals. Clones were grouped into phylotypes based on DNA sequence analysis, with members of phylotypes showing ≥97% sequence identity and the same RFLP patterns. Because of the omission of the chimeric clones, the total numbers of clones considered for the rarefaction analysis were 100, 100, 97, 95, 84, and 100 for the 5V, 5F, 5C, 5S, 9V, and 9S libraries, respectively.
Jaccard's incidence-based similarity index was used to estimate the similarities among the different 16S rRNA gene libraries (Table 3). Libraries 5C and 5S showed the highest similarity index, 0.26. Libraries 5V, 9V, and 9S displayed similarity indices between 0.22 and 0.25. Comparisons between the other libraries showed less similarity, with Jaccard's indices of ≤0.14. These results revealed the highest degree of similarity to be that between the two libraries from the free-phase (5C)- and the dissolved-hydrocarbon (5S)-contaminated zones. In addition, the library from the nonimpacted vadose zone at the contaminated site (5V) and the two libraries from the uncontaminated background site showed similarity to one another.
TABLE 3.
Jaccard's incidence-based similarity indices for comparison of the different 16S rRNA gene librariesa
| Library | Index for comparison with:
|
|||||
|---|---|---|---|---|---|---|
| 5V | 5F | 5C | 5S | 9V | 9S | |
| 5V | 0.138 | 0.026 | 0.029 | 0.226 | 0.250 | |
| 5F | 0.025 | 0.057 | 0.000 | 0.095 | ||
| 5C | 0.257 | 0.070 | 0.060 | |||
| 5S | 0.000 | 0.065 | ||||
| 9V | 0.220 | |||||
| 9S | ||||||
Boldface indicates the highest similarity indices.
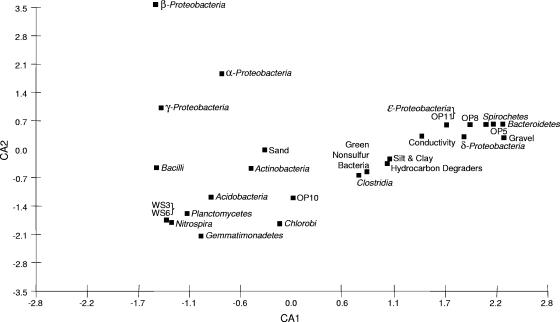
A multivariate statistical analysis was performed to determine the relationships among a sediment's conductivity, the lithology, and the microbiological results. Correspondence analysis (Fig. 5) indicated that the presence of specific microbial populations corresponded to a sediment's conductivity. The presence of Delta- and Epsilonproteobacteria, as well as the presence of the OP11 and OP8 phyla, showed the closest correlation with conductivity. The presence of silt and clay, a high ratio of hydrocarbon degraders, green nonsulfur bacteria, and Clostridia on the one hand and the presence of Spirochetes, OP5, Bacteroidetes, and gravel on the other hand further corresponded positively to the conductivity. Most of these bacterial phyla were found either exclusively or in high numbers in libraries 5C and 5S from the contaminated zones where the level of conductivity was greatest. In contrast, the presence of other phyla that were found in high proportions or exclusively in the nonimpacted zones, such as WS3, WS6, Nitrospira, Planctomycetes, Gemmatimonadetes, and Acidobacteria, corresponded negatively to the level of conductivity (Fig. 5). Thus, the correspondence analysis suggests that the observed elevated conductivity levels correspond to the presence of distinct microbial populations within these zones.
FIG. 5.
Correspondence analysis of the conductivity values, the lithologies, and the fractions of the different phylotypes observed in association with the 16S rRNA gene libraries from the different depths at the contaminated (VRP5) and uncontaminated (VRP9) sites. Numbers along the axes represent the scores as calculated by correspondence analysis based on the percentages of gravel, sand, silt, and clay present at the elevations corresponding to each library, the conductivity data and the fraction of hydrocarbon degraders as shown in Table 1, and the percentage of each bacterial group present in each library as shown in Table 2. CA1, correspondence analysis axis 1; CA2, correspondence analysis axis 2.
DISCUSSION
Although geomicrobiology is a field of emerging interest (16, 26, 34, 39, 41), few studies have investigated the biological impact on subsurface geophysical properties (46) and none to date have completed an in-depth characterization of the microbial community structures in petroleum-contaminated sediments associated with anomalously high conductivity readings. Preliminary work at the Carson City site had revealed an increase in the proportion of culturable hydrocarbon-degrading microbes in LNAPL-impacted sediments, where conductivity values showed up to a threefold increase relative to those of uncontaminated sediments (8). In addition, culture-independent ribosomal intergenic spacer analysis had demonstrated vertical changes in the microbial community structure, which paralleled changes in LNAPL contamination levels and bulk electrical conductivity (22). Here, we conducted an in-depth characterization of the microbial community structure by using 16S rRNA gene cloning and sequencing from samples collected within zones of higher and lower levels of conductivity at a petroleum-contaminated site and a background control site.
Correspondence analysis revealed that the presence of certain bacterial phyla corresponded to the higher level of conductivity in the petroleum-contaminated sediments at the Carson City site. The Deltaproteobacteria showed one of the highest degrees of correspondence to the level of conductivity and include the highest proportion of clones in the 5C and 5S libraries. In fact, 22 and 33% of the clones in libraries 5S and 5C, respectively, showed high similarity to Syntrophus species of the Deltaproteobacteria (Table 2; Fig. S1 in the supplemental material). Members of the genus Syntrophus convert the organic acid intermediates of anaerobic hydrocarbon degradation, such as propionate, acetate, and butyrate, into CO2, H2, and formate, which in turn are utilized by hydrogenotrophic organisms, such as methanogens (50). Thus, the presence of syntrophic organisms such as Syntrophus species may indicate that methanogenesis is the predominant process at VRP5. Previous geochemical findings have suggested that methanogenesis is occurring within the VRP5 contaminated zone (5). Also, the presence of green nonsulfur bacteria, Clostridia, and Bacteroidetes, as well as sulfate- and iron-reducing populations, within the free-phase-contaminant zone supports the notion that anaerobic conditions exist within the contaminated zone. Anaerobic conditions are common in aged LNAPL-contaminated sediments and are necessary for methanogenesis to occur (9). The finding of a large population of methylo- and methanotrophic bacteria (∼30%) in the 5F library from the zone above the free-phase petroleum further corroborates the occurrence of methanogenesis in the contaminated zone below. Nevertheless, in the clone libraries from VRP5, no specific methanogenic populations were detected. It can be speculated that the use of Archaea-specific primers or improved cell lysis or DNA isolation methods are necessary to detect methanogenic populations.
Further bacterial phyla corresponding to the increased conductivity levels include OP5, OP8, OP11, Epsilonproteobacteria, Spirochaetes, Bacteroidetes, Clostridia, and green nonsulfur bacteria. It is interesting that members of many of these phyla (including large syntrophic populations of the Deltaproteobacteria) were also found to be present at the former Wurtsmith Air Force Base in Oscoda, MI, which is similarly polluted with underground petroleum hydrocarbons (21). The importance of the Wurtsmith site comes from a separate geophysical study, which first reported the anomalous electrical conductivity measurements for petroleum-contaminated sediments (14).
Also corresponding to the higher level of conductivity was the higher ratio of culturable hydrocarbon degraders to total heterotrophs. Previous work analyzing the culturable fraction of the soil community at the Carson City site indicated that higher ratios of hydrocarbon-degrading microorganisms correlate with the profile of elevated conductivity levels (8). It is interesting that the correspondence analysis showed a relationship between the conductivity and the gravel content on the one side and the silt and clay content on the other side. Although generally, sediments with high gravel contents are expected to exhibit lower levels of conductivity than sediments with high silt and clay contents, the soil conductivity is influenced by many factors, among which the following three seem to be the main components: (i) the petrophysical characteristics (e.g., sediment type and size and interfacial or grain surface properties), (ii) the amount of fluid saturation of the pore space, and (iii) the fluid electrolytic (ionic strength) properties (4). At VRP5, the higher conductivity measurements below the water table coincided with a higher gravel content; however, the peak conductivity values at VRP5 were found above the water table, where sand-sized particles in sediments predominated to almost 100% (Fig. 2). Considering other VRP installations and ground-penetrating radar data from the Carson City site, no changes in fluid saturation or sediment lithology (grain size and type) were found to be coincidental with changes in the bulk conductivity observed within the petroleum-contaminated sediments (7). Therefore, the geoelectrical properties of the LNAPL-impacted sediments must be influenced by either changes in the electrolytic properties of the pore fluids or the alteration of the interfacial properties of the grain surface (surface conductance) or the combined effects of both.
Microbial activity has been shown to alter the subsurface fluid electrolytic properties in LNAPL-contaminated sediments (5). The fluid conductivity of groundwater is directly controlled by the amount of total dissolved solids (TDS; the number of ions present in the solution). Organic acids produced as metabolic by-products of hydrocarbon degradation have been shown to cause mineral dissolution resulting in the release of ions into the pore space, increasing the ionic strength and thus the conductivity of the pore fluid (19, 38). In previous work using scanning electron microscopy, highly etched sediment grains, indicative of extensive mineral weathering occurring at the Carson City site, were observed in sediments from the VRP5 contaminated zone and were not observed in the background sediments (22). Etching patterns on sediment grains have also been observed within multiple petroleum-contaminated sites and occur from the dissolution of the mineral surface due to a rapid exchange of charge-balancing cations (K+, Na+, and Ca+) for protons at the mineral surface, releasing these cations into the surrounding pore fluid (12, 13). Geochemical data on the petroleum-contaminated VRP5 pore fluid revealed a moderate increase in the amount of TDS in the sediment pore water, suggesting that microbially enhanced mineral dissolution has occurred (5). Moreover, the pH in the VRP5 well (average pH, 6.5) was lower than that in the control VRP9 well (average pH, 7.1) (5). The identification of fermentative Clostridia species in library 5C, as well as the large Syntrophus populations which metabolize organic acids such as acetate, butyrate, propionate, and fumarate, points to the groups of microorganisms potentially involved in these reactions.
Thus, these findings suggest that changes in fluid electrolytic properties due to microbially enhanced mineral dissolution may play an important role in altering the subsurface electrical conductance. Nevertheless, Atekwana et al. reported that the ionic strength (TDS levels) of the pore fluid could not completely explain the anomalously high conductivity readings in the free-phase zone (5). Furthermore, Abdel Aal et al. (1), using the induced polarization method to investigate the complex conductivity at the Carson City site, reported that changes in the interfacial properties on the grain surfaces resulting in increased surface conductance play an equally important role in altering the subsurface electrical conductivity. Alterations of the interfacial properties of the sediment grains can result from microbial colonization, exopolysaccharide production, microbial biofilm formation, and mineral precipitation of metal sulfides by microbial activity (1, 2). Williams et al. (57) demonstrated that the precipitation of iron sulfides onto the surface of sediment grains effectively alters their interfacial properties. Precipitation occurs from the abiotic reaction of microbially generated Fe(II) and H2S to produce insoluble FeS and has been shown to occur even at low sulfide concentrations (57). The presence of dissimilatory-iron-reducing bacteria, such as Rhodoferax ferrireducens, and sulfate-reducing bacteria, such as Desulfitobacterium and Desulfosporosinus, in the contaminant zone suggests that iron and sulfate reduction is occurring in the anaerobic contaminated sediments (25, 44). Furthermore, the presence of the sulfide-oxidizing Beggiatoa species in the upper sediments suggests that H2S is escaping from the lower sediments and is utilized by Beggiatoa (52).
Since this is a first report showing a correspondence between elevated conductivity levels at aged underground petroleum spills and certain microbial populations, there is still much speculation about the cause and effect of this phenomenon. For example, it remains to be elucidated whether the recently discovered electron-transferring nanowires of iron-reducing bacteria such as Geobacter and Shewanella are contributing to subsurface conductivity (27, 43). No 16S rRNA gene sequences related to Geobacter or Shewanella were found in our study; however, several clones of Pelotomaculum-related strains were identified in the 5C and 5S libraries (Fig. S1D in the supplemental material). Structures resembling nanowires have been discovered when Pelotomaculum strains were grown in coculture with a methanogen (Y. Gorbi, personal communication; 32).
In conclusion, we have demonstrated that significant changes occur vertically in the microbial community structures within the sediment layers which parallel changes in the zones of petroleum contamination and in the geoelectrical properties of those sediment zones. The low conductivity readings of the background sediments were concomitant with diverse populations of common soil microbes, such as Acidobacteria and Actinobacteria. The zone contaminated with residual hydrocarbons above the free-phase petroleum contained aromatic hydrocarbon degraders, including Sphingomonas aromaticivorans and “Brachymonas petroleovorans” (Fig. S1C in the supplemental material), and large populations of methylotrophs and methanotrophs. Conversely, the compositions of the microbial communities found in the electrically conductive petroleum-contaminated sediments were consistent with the microbial community compositions of other petroleum-contaminated locations and included significant populations of fermenters, iron and sulfur reducers, and syntrophic bacteria (21). Correspondence analysis has shown that the anomalous increase in electrical conductivity detected in the free-phase petroleum zone is attributable to the activities of these microbial populations, which possibly alter both the electrolytic properties of the pore fluid and the interfacial properties of the sediment grains under the anaerobic and carbon- and electron donor-rich environmental conditions. Thus, our study provides the first insights into the microbial community structures on vertical gradients in petroleum-contaminated sediments and suggests that if geoelectrical measurements are a reflection of microbial activity, then the changing microbial processes and resulting microbial community structures can be effectively studied using a combination of geophysical and microbiological methodologies. In future studies, geophysical methods may be useful both in monitoring bioremediation over time and in directing microbial sampling in the subsurface. Besides potentially improving the monitoring of bioremediation processes, the results of this study also form a foundation for examining how and which microbes effectively alter their environments such that changes in the physical and chemical properties of the sediments are readily detected by geophysical techniques.
Supplementary Material
Acknowledgments
This work was funded by the National Science Foundation (OCE 0433869).
The U.S. Environmental Protection Agency through its Office of Research and Development collaborated in the research described here. This report has been subjected to the agency's review and approved for publication.
Mention of trade names or commercial products does not constitute an endorsement or recommendation for use.
Footnotes
Published ahead of print on 9 March 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Abdel Aal, G. Z., E. A. Atekwana, L. D. Slater, and E. A. Atekwana. 2004. Effects of microbial processes on electrolytic and interfacial electrical properties of unconsolidated sediments. Geophys. Res. Lett. 31:L12505. [Google Scholar]
- 2.Abdel Aal, G. Z., L. D. Slater, and E. A. Atekwana. 2006. Induced-polarization measurements on unconsolidated sediments from a site of active hydrocarbon biodegradation. Geophysics 71:H13-H24. [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Archie, G. E. 1942. The electrical resistivity log as an aid in determining some reservoir characteristics. Trans. Am. Inst. Mining Metallurg. Pet. Eng. 146:54-62. [Google Scholar]
- 5.Atekwana, E. A., E. A. Atekwana, F. D. Legall, and R. V. Krishnamurthy. 2005. Biodegradation and mineral weathering controls on bulk electrical conductivity in a shallow hydrocarbon contaminated aquifer. J. Contam. Hydrol. 80:149-167. [DOI] [PubMed] [Google Scholar]
- 6.Atekwana, E. A., E. A. Atekwana, F. D. Legall, and R. V. Krishnamurthy. 2004. Field evidence for geophysical detection of subsurface zones of enhanced microbial activity. Geophys. Res. Lett. 31:L23603. [Google Scholar]
- 7.Atekwana, E. A., W. A. Sauk, and D. D. Werkema. 2000. Investigations of geoelectrical signatures at a hydrocarbon contaminated site. J. Appl. Geophys. 44:167-180. [Google Scholar]
- 8.Atekwana, E. A., D. D. Werkema, J. W. Duris, S. Rossbach, E. A. Atekwana, W. A. Sauck, D. P. Cassidy, J. Means, and F. D. Legall. 2004. In-situ apparent conductivity measurements and microbial population distribution at a hydrocarbon-contaminated site. Geophysics 69:56-63. [Google Scholar]
- 9.Atlas, R. M., and R. Bartha. 1997. Microbial ecology: fundamentals and applications, 4th ed. Benjamin/Cummings, Menlo Park, CA.
- 10.Barns, S. M., S. L. Takala, and C. R. Kuske. 1999. Wide distribution and diversity of members of the bacterial kingdom Acidobacterium in the environment. Appl. Environ. Microbiol. 65:1731-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bekins, B. A., E. M. Godsy, and E. Warren. 1999. Distribution of microbial physiologic types in an aquifer contaminated by crude oil. Microb. Ecol. 37:263-275. [DOI] [PubMed] [Google Scholar]
- 12.Bennett, P. C., F. K. Hiebert, and W. J. Choi. 1996. Microbial colonization and weathering of silicates in a petroleum-contaminated groundwater. Chem. Geol. 132:45-53. [Google Scholar]
- 13.Bennett, P. C., J. R. Rogers, W. J. Choi, and F. K. Hiebert. 2001. Silicates, silicate weathering, and microbial ecology. Geomicrobiol. J. 18:3-19. [Google Scholar]
- 14.Bermejo, J. L., W. A. Sauck, and E. A. Atekwana. 1997. Geophysical discovery of a new LNAPL plume at former Wurtsmith AFB, Oscoda, Michigan. Ground Water Monit. Remediation 17:131-137. [Google Scholar]
- 15.Buchholz-Cleven, B. E. E., B. Rattunde, and K. L. Straub. 1997. Screening for genetic diversity of isolates of anaerobic Fe(II)-oxidizing bacteria using DGGE and whole-cell hybridization. Syst. Appl. Microbiol. 20:301-309. [Google Scholar]
- 16.Chandler, D. P., F. J. Brockman, T. J. Bailey, and J. K. Fredrickson. 1998. Phylogenetic diversity of archaea and bacteria in a deep subsurface paleosol. Microb. Ecol. 36:37-50. [DOI] [PubMed] [Google Scholar]
- 17.Cole, J. R., B. Chai, R. J. Farris, Q. Wang, S. A. Kulam, D. M. McGarrell, G. M. Garrity, and J. M. Tiedje. 2005. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 33:D294-D296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cozzarelli, I. M., M. J. Baedecker, R. P. Eganhouse, and D. F. Goerlitz. 1994. The geochemical evolution of low-molecular-weight organic acids derived from the degradation of petroleum contaminants in groundwater. Geochim. Cosmochim. Acta 58:863-877. [Google Scholar]
- 19.Cozzarelli, I. M., R. P. Eganhouse, and M. J. Daedecker. 1990. Transformation of monoaromatic hydrocarbons to organic acids in anoxic groundwater environment. Environ. Geol. Water Sci. 16:135-141. [Google Scholar]
- 20.Dell Engineering. 1992. Remedial action plan for Crystal refining company, 801 North Williams Street, Carson City, MI. Report DEI no. 921660. Dell Engineering, Holland, MI.
- 21.Dojka, M. A., P. Hugenholtz, S. Haack, and N. Pace. 1998. Microbial diversity in a hydrocarbon- and chlorinated-solvent-contaminated aquifer undergoing intrinsic bioremediation. Appl. Environ. Microbiol. 64:3869-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duris, J. W. 2002. Microbial community structure in hydrocarbon impacted sediment associated with anomalous geophysical signatures. M.S. thesis. Western Michigan University, Kalamazoo, MI.
- 23.Environmental Protection Agency. 20 June 2006, posting date. FY 2006 mid-year activity report to UST/LUST regional division directors, regions 1-10. Environmental Protection Agency, Washington, DC. http://www.epa.gov/oust/cat/ca_061_2.pdf.
- 24.Environmental Protection Agency. 21 April 1999, posting date. Use of monitored natural attenuation at superfund, RCRA corrective action, and underground storage tank sites. Directive 9200.4-17P. Environmental Protection Agency, Washington, DC. http://www.epa.gov/swerust1/directiv/d9200417.pdf.
- 25.Finneran, K. T., C. V. Johnsen, and D. R. Lovley. 2003. Rhodoferax ferrireducens sp. nov., a psychrotolerant, facultatively anaerobic bacterium that oxidizes acetate with the reduction of Fe(III). Int. J. Syst. Evol. Microbiol. 53:669-673. [DOI] [PubMed] [Google Scholar]
- 26.Fredrickson, J. K., D. L. Balkwill, J. M. Zachara, S. M. Li, F. J. Brockman, and M. A. Simmons. 1991. Physiological diversity and distributions of heterotrophic bacteria in deep cretaceous sediments of the Atlantic coastal plain. Appl. Environ. Microbiol. 57:402-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorby, Y. A., S. Yanina, J. S. McLean, K. M. Rosso, D. Moyles, A. Dohnalkova, T. J. Beveridge, I. S. Chang, B. H. Kim, K. S. Kim, D. E. Culley, S. B. Reed, M. F. Romine, D. A. Saffarini, E. A. Hill, L. Shi, D. A. Elias, D. W. Kennedy, G. Pinchuk, K. Watanabe, S. Ishii, B. Logan, K. H. Nealson, and J. K. Fredrickson. 2006. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 103:11358-11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haack, S. K., and B. A. Bekins. 2000. Microbial populations in contaminant plumes. Hydrogeol. J. 8:63-76. [Google Scholar]
- 29.Haack, S. K., L. R. Fogarty, T. G. West, E. W. Alm, J. T. McGuire, D. T. Long, D. W. Hyndman, and L. J. Forney. 2004. Spatial and temporal changes in microbial community structure associated with recharge-influenced chemical gradients in a contaminated aquifer. Environ. Microbiol. 6:438-448. [DOI] [PubMed] [Google Scholar]
- 30.Hayes, L. A., and D. R. Lovley. 2002. Specific 16S rDNA sequences associated with naphthalene degradation under sulfate-reducing conditions in harbor sediments. Microb. Ecol. 43:134-145. [DOI] [PubMed] [Google Scholar]
- 31.Hughes, J. B., and J. J. Hellmann. 2005. The application of rarefaction techniques to molecular inventories of microbial diversity. Methods Enzymol. 397:292-308. [DOI] [PubMed] [Google Scholar]
- 32.Ishii, S., T. Kosaka, K. Hori, Y. Hotta, and K. Watanabe. 2005. Coaggregation facilitates interspecies hydrogen transfer between Pelotomaculum thermopropionicum and Methanothermobacter thermautotrophicus. Appl. Environ. Microbiol. 71:7838-7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janssen, P. 2006. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl. Environ. Microbiol. 72:1719-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kieft, T. L., J. K. Fredrickson, J. P. McKinley, B. N. Bjornstad, S. A. Rawson, T. J. Phelps, F. J. Brockman, and S. M. Pfiffner. 1995. Microbiological comparisons within and across contiguous lacustrine, paleosol, and fluvial subsurface sediments. Appl. Environ. Microbiol. 61:749-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klee, A. J. 1993. A computer program for the determination of most probable number and its confidence limits. J. Microbiol. Methods 18:91-98. [Google Scholar]
- 36.Lee, J. Y., J. Y. Cheon, K. K. Lee, S. Y. Lee, and M. H. Lee. 2001. Factors affecting the distribution of hydrocarbon contaminants and hydrogeochemical parameters in a shallow sand aquifer. J. Contam. Hydrol. 50:139-158. [DOI] [PubMed] [Google Scholar]
- 37.McMahon, P. B., and F. H. Chapelle. 1991. Microbial production of organic acids in aquitard sediments and its role in aquifer geochemistry. Nature 349:233-235. [Google Scholar]
- 38.McMahon, P. B., D. A. Vroblesky, P. M. Bradley, F. H. Chapelle, and C. D. Gullett. 1995. Evidence for enhanced mineral dissolution in organic acid-rich shallow ground water. Ground Water 33:207-216. [Google Scholar]
- 39.Newman, D. K., and J. F. Banfield. 2002. Geomicrobiology: how molecular-scale interactions underpin biogeochemical systems. Science 296:1071-1077. [DOI] [PubMed] [Google Scholar]
- 40.Ntarlagiannis, D., K. H. Williams, L. Slater, and S. Hubbard. 2005. On the low frequency electrical response to microbially induced sulfide precipitation. J. Geophys. Res. 110:L24402. [Google Scholar]
- 41.Onstott, T. C., D. P. Moser, S. M. Pfiffner, J. K. Fredrickson, F. J. Brockman, T. J. Phelps, D. C. White, A. Peacock, D. Balkwill, R. Hoover, L. R. Krumholz, M. Borscik, T. L. Kieft, and R. Wilson. 2003. Indigenous and contaminant microbes in ultradeep mines. Environ. Microbiol. 5:1168-1191. [DOI] [PubMed] [Google Scholar]
- 42.Rappe, M. S., and S. J. Giovannoni. 2003. The uncultured microbial majority. Annu. Rev. Microbiol. 57:369-394. [DOI] [PubMed] [Google Scholar]
- 43.Reguera, G., K. D. McCarthy, T. Mehta, J. S. Nicoll, M. T. Tuominen, and D. R. Lovley. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098-1101. [DOI] [PubMed] [Google Scholar]
- 44.Robertson, W. J., J. P. Bowman, P. D. Franzmann, and B. J. Mee. 2001. Desulfosporosinus meridiei sp. nov., a spore-forming sulfate-reducing bacterium isolated from gasolene-contaminated ground water. Int. J. Syst. Evol. Microbiol. 51:133-140. [DOI] [PubMed] [Google Scholar]
- 45.Rogers, J. R., and P. C. Bennett. 2004. Mineral stimulation of subsurface microorganisms: release of limiting nutrients from silicates. Chem. Geol. 203:91-108. [Google Scholar]
- 46.Roh, Y., S. V. Liu, G. Li, H. Huang, T. J. Phelps, and J. Zhou. 2002. Isolation and characterization of metal-reducing thermoanaerobacter strains from deep subsurface environments of the Piceance Basin, Colorado. Appl. Environ. Microbiol. 68:6013-6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rooney-Varga, J. N., R. T. Anderson, J. Fraga, D. Ringelberg, and D. R. Lovley. 1999. Microbial communities associated with anaerobic benzene degradation in a petroleum-contaminated aquifer. Appl. Environ. Microbiol. 65:3056-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sauck, W. A. 2000. A model for the resistivity structure of LNAPL plumes and their environs in sandy sediments. J. Appl. Geophys. 44:151-165. [Google Scholar]
- 49.Sauck, W. A., E. A. Atekwana, and M. S. Nash. 1998. High conductivities associated with an LNAPL plume imaged by integrated geophysical techniques. J. Environ. Eng. Geophys. 2:203-212. [Google Scholar]
- 50.Schink, B. 1997. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 61:262-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schloss, P. D., and J. Handelsman. 2004. Status of the microbial census. Microbiol. Mol. Biol. Rev. 68:686-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmidt, T. M., B. Arieli, Y. Cohen, E. Padan, and W. R. Strohl. 1987. Sulfur metabolism in Beggiatoa alba. J. Bacteriol. 169:5466-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Telford, W. M., L. P. Geldart, and R. E. Sheriff. 1990. Applied geophysics, 2nd ed. Cambridge University Press, Cambridge, United Kingdom.
- 54.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The Clustal_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Elsas, J. D., and K. Smalla. 1997. Methods for sampling soil microbes, p. 383-390. In C. J. Hurst (ed.), Manual of environmental microbiology. ASM Press, Washington, DC.
- 56.Werkema, D. D. 2002. Geoelectrical response of an aged LNAPL plume: implications for monitoring natural attenuation. Ph.D. thesis. Western Michigan University, Kalamazoo, MI.
- 57.Williams, K. H., D. Ntarlagiannis, L. Slater, A. Dohnalkova, S. S. Hubbard, and J. F. Banfield. 2005. Geophysical imaging of stimulated microbial biomineralization. Environ. Sci. Technol. 39:7592-7600. [DOI] [PubMed] [Google Scholar]
- 58.Wrenn, B. A., and A. D. Venosa. 1996. Selective enumeration of aromatic and aliphatic hydrocarbon degrading bacteria by a most-probable-number procedure. Can. J. Microbiol. 42:252-258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.