Abstract
Catabolism of sulfur-containing amino acids plays an important role in the development of cheese flavor. During ripening, cystathionine β-lyase (CBL) is believed to contribute to the formation of volatile sulfur compounds (VSCs) such as methanethiol and dimethyl disulfide. However, the role of CBL in the generation of VSCs from the catabolism of specific sulfur-containing amino acids is not well characterized. The objective of this study was to investigate the role of CBL in VSC formation by Lactobacillus helveticus CNRZ 32 using genetic variants of L. helveticus CNRZ 32 including the CBL-null mutant, complementation of the CBL-null mutant, and the CBL overexpression mutant. The formation of VSCs from methionine, cystathionine, and cysteine was determined in a model system using gas chromatography-mass spectrometry with solid-phase microextraction. With methionine as a substrate, CBL overexpression resulted in higher VSC production than that of wild-type L. helveticus CNRZ 32 or the CBL-null mutant. However, there were no differences in VSC production between the wild type and the CBL-null mutant. With cystathionine, methanethiol production was detected from the CBL overexpression variant and complementation of the CBL-null mutant, implying that CBL may be involved in the conversion of cystathionine to methanethiol. With cysteine, no differences in VSC formation were observed between the wild type and genetic variants, indicating that CBL does not contribute to the conversion of cysteine.
Lactobacillus helveticus CNRZ 32 is used as a starter and an adjunct bacterium to enhance cheese flavor development and reduce bitterness (15). Catabolism of amino acids, including sulfur-containing amino acids, by lactic acid bacteria is a major contributor to the development of flavor compounds in cheese during ripening (8). Sulfur-containing amino acids, namely, methionine, are precursors of aroma-active volatile sulfur compounds (VSCs) such as methanethiol, dimethyl disulfide, and dimethyl trisulfide (12, 16). There are two different microbial pathways potentially leading to amino acid conversion into flavor compounds: one is initiated by a transamination reaction while the other is initiated by an elimination reaction (29). The transamination pathway is catalyzed by aminotransferases, which transfer the amino acid amino group to an α-keto acid, while the elimination reaction-based pathway is catalyzed by the activity of amino acid lyases which cleave amino acid side chains (29).
Cystathionine β-lyase (CBL) (EC 4.4.1.8) is a pyridoxal-5′-phosphate (PLP)-dependent enzyme and was purified and cloned from Lactococcus lactis (1, 14). CBL is involved in the α,β-elimination of cystathionine to form homocysteine, pyruvate, and ammonia. CBL from L. lactis can also catalyze the conversion of methionine into methanethiol (1). A CBL overexpression variant of L. lactis has been shown to produce higher quantities of VSCs with methionine as a substrate compared with a wild strain (14), suggesting a possible mechanism to increase VSC production in cheese. However, this approach may be of more value when applied in a Lactobacillus strain known to be metabolically active during the cheese aging process.
The role of CBL and catabolic pathways responsible for VSC formation from sulfur amino acids/derivatives has not been well characterized in Lactobacillus spp. The objective of this study was to understand the role and mechanism of CBL in the production of VSCs from sulfur-containing amino acids/derivatives, namely, methionine, cysteine, and cystathionine, using genetic variants of L. helveticus CNRZ 32 in a model system.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacteria and plasmids used in this study are listed in Table 1. Stock cultures were maintained at −80°C in De Man, Rogosa, Sharpe (MRS) broth medium (Difco Laboratories, Detroit, MI) containing 20% (vol/vol) glycerol until needed. Working cultures were prepared from frozen stocks by transferring cultures to MRS broth medium supplemented with 2.21 mM methionine and 3.57 mM cysteine two times. L. helveticus CNRZ 32 and derivative strains were propagated at 42°C in MRS broth medium (Difco Laboratories, Detroit, MI) to early stationary phase. Escherichia coli DH5α was grown at 37°C with shaking in Luria-Bertani (LB; US Biological, Inc., Swampscott, MA) medium (21). Plasmid pTRK687 (24) was a gift from T. R. Klaenhammer of North Carolina State University, Raleigh, and plasmid pSA3 was obtained from J. J. Ferretti of the University of Oklahoma Health Sciences Center.
TABLE 1.
Bacteria and plasmids used in the study
| Bacterial strain or plasmid | Description and relevant feature(s) | Source or reference(s) |
|---|---|---|
| Strains | ||
| Lactobacillus helveticus | ||
| CNRZ 32 | Wild-type industrial strain | 3 |
| CBL+ | Wild-type strain transformed with pLHcbl; Cmr | This study |
| CNRZ 32Δcbl | CNRZ 32 cbl-null mutant | This study |
| CNRZ 32Δcbl:pLHcbl | Complementation mutant; strain CNRZ 32Δcbl transformed with pLHcbl; Cmr | This study |
| Escherichia coli DH5α | Laboratory cloning host | Invitrogen, Inc. (Carlsbad, CA) |
| Plasmids | ||
| pBluescript SK | Laboratory cloning vector; Ampr | Stratagene, Inc. (La Jolla, CA) |
| pTRK687 | Laboratory expression vector; Cmr | 24 |
| pLHcbl | 1.3-kb L. helveticus cbl wild-type gene cloned into pTRK687; Cmr | This study |
| pSA3 | E. coli-L. helveticus laboratory shuttle vector; Emr | 3, 9 |
| pJHΔcbl-1 | 1.1-kb L. helveticus cbl fragment that contained a 243-bp in-frame internal deletion, cloned into pSA3 | This study |
DNA isolation and manipulation.
Template DNA for PCR was isolated from L. helveticus CNRZ 32 strains as described previously (17). PCRs were carried out in a Techne Genius thermocycler (Krackeler Scientific, Inc., Albany, NY) with Eppendorf (Westbury, NY) Mastermix Taq DNA polymerase. The PCR conditions included a 3-min hot start at 93°C, followed by 35 cycles of denaturation at 93°C for 30 s, primer annealing at 55 or 50°C (depending on the average melt temperature of the primer set) for 30 s, and extension at 70°C for 3 min. The reactions were finished with a 5-min incubation at 70°C, and then reaction mixtures were chilled to 4°C.
Plasmid DNA from E. coli was isolated by the alkaline lysis procedure (21). Restriction enzymes were obtained from New England Biolabs Inc. (Beverly, MA) and Fermentas Inc. (Hanover, MD) and used as specified by the supplier. T4 DNA ligase was purchased from Invitrogen Life Technologies (Carlsbad, CA). Ligations were performed in 20-μl reaction mixtures at 4°C overnight and then stopped by ethanol precipitation. DNA sequencing was performed at the Utah State University Center for Integrated BioSystems by fluorescent dideoxy chain termination on an ABI Prism 3730 DNA analyzer (Applied Biosystems, Inc., Foster City, CA) using Taq FS terminator chemistry.
CBL expression mutant.
To investigate the effect of cbl expression from a multicopy plasmid on VSC production by L. helveticus CNRZ 32, the wild-type cbl gene was cloned into the expression vector pTRK687 (24). This construct was assembled by PCR amplification of the cbl gene from L. helveticus CNRZ 32 genomic DNA using the forward primer cbl-1 (5′-GTTTGTCGACCAGCTTAAAGGTTGCCAAGG-3′), which provided a SalI linker (underlined) 102 bp upstream of the predicted start codon, and reverse primer cbl-6 (5′-GTTTCTGCAGGAACTTCTAAAAGACTGTG-3′), which gave a PstI linker (underlined) 11 bp downstream of the predicted stop codon. The 1.3-kb cbl amplicon included the gene's predicted start and stop codons as well as its ribosome binding site. The amplicon was purified and then digested with SalI and PstI and ligated into SalI- and PstI-restricted pTRK687 using standard laboratory methods (21). The ligation mix was transformed into E. coli DH5α by electroporation, and transformants were identified by incubation on LB agar that contained 50 μg per ml of chloramphenicol (CHL). The presence of recombinant plasmids in E. coli lysates was verified by agarose gel electrophoresis, and the sequence integrity of the cbl clone was confirmed by nucleotide sequence analysis of the insert DNA.
One of the plasmid constructs isolated from this work, designated pLHcbl, was subsequently transformed into L. helveticus CNRZ 32 by electroporation using the procedure of Christensen and Steele (7). Transformants were identified by selection on MRS agar that contained 5 μg per ml CHL, and the presence of pLHcbl in cell lysates was confirmed by agarose gel electrophoresis and by sequence analysis of a PCR product, obtained with pTRK687-based primers (24), that spanned the insert DNA. After characterization, a representative pLHcbl transformant, designated L. helveticus CBL+, was selected for further investigation.
Construction of a null mutant.
To obtain a CBL-null mutant of L. helveticus CNRZ 32, an in-frame deletion of 81 amino acids from the CBL open reading frame was first generated by PCR amplification, and subsequent ligation, of two cbl subfragments. The region targeted for deletion included the predicted PLP binding site (as identified by homology to other cbl genes). The upstream subfragment included the 5′ 410 bp of the open reading frame and was amplified with forward PCR primer cbl-1 and reverse primer cbl-3 (5′-GTTTCCAAGTCAATTGCTTTGGTATTTGG-3′). Primer cbl-3 had a single nucleotide substitution (bold type) that produced a MunI restriction site in the amplicon (underlined). The downstream subfragment included the 3′ 531 bp of the open reading frame and was amplified by PCR with reverse primer cbl-2 (identical to primer cbl-6, except that it provided an XbaI linker instead of a PstI linker) and forward primer cbl-4 (5′-GAAACAATTGGTTACCTTCAAAATG-3′). Primer cbl-4 had a single nucleotide substitution (bold type) to produce a MunI restriction site (underlined). The two cbl subfragments were purified and then cut with MunI and ligated together. Products from that reaction were collected, cut with SalI and XbaI, and then ligated into SalI- and XbaI-restricted bacterial cloning vector pBluescript SK (Stratagene, Inc., La Jolla, CA). Ligation products were transformed into E. coli DH5α by electroporation, and transformants were identified by incubation on LB agar that contained 50 μg per ml of ampicillin. The presence of recombinant plasmid DNA in E. coli lysates was verified by agarose gel electrophoresis, and then the sequence integrity of the cbl deletion construct was confirmed by nucleotide sequence analysis of the insert DNA (3).
The recombinant plasmid pJHD-11 was purified and double digested with SalI and XbaI, and the fragments were separated in a 0.6% agarose gel. The 1.1-kb cbl construct was purified from the gel and ligated into the SalI- and XbaI-digested vector pSA3 (9). The resulting construct (designated plasmid pJHΔcbl-1) was transformed into E. coli DH5α by electroporation. Transformants were collected after incubation on LB agar that contained 50 μg per ml of CHL, and then the presence of pJHΔcbl-1 in E. coli lysates was verified by agarose gel electrophoresis and nucleotide sequence analysis of the insert fragment.
Plasmid pJHΔcbl-1 was purified from E. coli and transformed by electroporation into L. helveticus CNRZ 32. Screening for transformants was performed by incubation at 37°C on MRS agar that contained 1 μg per ml of erythromycin and 5 mM CaCl2. The presence of pJHΔcbl-1 in Emr L. helveticus CFU was established by agarose gel electrophoresis of cell lysates and by coamplification of 1.3-kb (wild-type) and 1.1-kb (internal deletion) cbl gene fragments after PCR with primers cbl-1 and cbl-2. Next, L. helveticus transformants were incubated in MRS broth that contained 1 μg erythromycin per ml at 43°C, a nonpermissive temperature for pSA3 replication (9). Derivatives in which plasmid pJHΔcbl-1 had, through a single homologous recombination event, integrated into the chromosome were identified and characterized by the method described by Christensen and Steele (7). Integrant clones were then propagated in MRS broth without antibiotic at a permissive temperature for pSA3 replication (37°C) to facilitate the excision and curing of pSA3 via a second homologous recombination event. Plasmid excision results in replacement of the native cbl gene with the deletion construct or reversion to the wild type. Erythromycin-sensitive colonies were collected and screened by PCR for cells carrying only the deleted cbl gene and not the wild-type cbl gene or the pSA3 vector. Two independent cbl-null mutants were identified, and one (designated CNRZ 32Δcbl) was selected for additional work.
Finally, a complementation mutant of L. helveticus CNRZ 32Δcbl was constructed by electroporation of this strain with pLHcbl. Transformants were selected by incubation on MRS agar that contained 5 mM CaCl2 and 5 μg CHL per ml, and isolates were screened by PCR for the presence of a chromosomal copy of the cbl deletion construct (using primers from the chromosome flanking the cbl gene) and for a copy of the wild-type gene in the expression vector (using a primer that flanked the pTRK687 cloning site and cbl-3).
Cell preparation.
Cells were grown in sterile 45-ml centrifuge tubes to early stationary phase and were harvested by centrifugation (4,500 × g for 15 min at 4°C). Cell pellets were washed at least two times with sterile 0.05 M potassium phosphate buffer (pH 6.0) and then suspended to an optical density at 600 nm of 2.0 in the same buffer. This cell suspension was used as a source of cells for gas chromatography-mass spectrometry (GC-MS) and high-pressure liquid chromatography (HPLC) work.
VSC determination.
The formation of VSCs was determined using GC (model 6890N; Agilent Technologies, Inc., Wilmington, DE) with a quadrupole mass analyzer (GC-MS; model 5973, Agilent Technologies, Inc., Wilmington, DE). Cell suspensions were mixed with 100 mM d/l-methionine, d/l-cystathionine, or l-cysteine (Sigma Chemical Co., St. Louis, MO). To this mixture were added 100 μM PLP, 1 mM furfuryl alcohol (internal standard), and 0.05 M potassium phosphate buffer (pH 6.0) in 8-ml vials with Teflon-lined septum caps, and then the mixtures were incubated at 37°C for each designated time period (48, 72, or 96 h). Negative controls were included by using cell suspensions without any substrate and by using substrate only in buffer (no cells).
Before each GC-MS assay, samples in vials were shaken well and allowed to equilibrate for 30 min in a multiblock heater at 37°C. A solid-phase microextraction (SPME) fiber (85-μm film thickness; carboxen/polydimethylsiloxane; Supelco, Inc., Bellefonte, PA) was used to extract volatile compounds. The SPME fiber was exposed to the headspace of the equilibrated (static-headspace) sample for 10 min. The volatile compounds adsorbed onto the SPME fiber were desorbed into the GC inlet at 250°C. The SPME fiber was held in the injection port over the complete program cycle (17 min) to ensure complete desorption. The VSCs were separated with an RTX-Wax column (30-m length × 0.25-mm inside diameter, 0.5-μm film thickness; Restek, Inc., Bellefonte, PA). The GC temperature was initially held at 35°C for 2 min, increased at 15°C/min to 150°C, increased at 30°C/min to 250°C, and finally held at 250°C for 4 min. The quantitative assessments of VSCs were done in the selected ion monitoring mode (m/z 48 for methanethiol, m/z 94 for dimethyl disulfide, m/z 126 for dimethyl trisulfide, m/z 34 for hydrogen sulfide, m/z 64 for dimethyl sulfide, and m/z 62 for sulfur dioxide). Standards for calibration studies were prepared on the day of analysis. The concentration of VSCs was calculated by generating calibration curves using authentic standards (Sigma Chemical Co., St. Louis, MO) over the range of 1 to 30 μM; furfuryl alcohol was used as an internal injection standard. Gaseous H2S calibration standards were generated using volumetric glassware. All calibration work was conducted in the same aqueous buffer, as described above, at pH 6.0.
VSC precursor confirmation.
The catabolic pathways were investigated using reverse-phase HPLC according to the modified method of Or-Rashid et al. (20). HPLC consisted of a tertiary gradient pump (model L-6200A; Hitachi Ltd., Tokyo, Japan) with photodiode array detection (model L-4500A; Hitachi Ltd., Tokyo, Japan). Cell suspensions incubated for 96 h at 37°C were derivatized with 9-fluorenylmethyl chloroformate and analyzed using a reverse-phase column (Synergi Hydro C18 reverse phase, 150 × 4.6 mm, 4-μm particle size, 80 Å; Phenomenex, Torrance, CA) with a guard column (4.0 × 3.0 mm). Absorbance was measured at 265 nm, and the flow rate was maintained at 1.0 ml/min. The column was kept at room temperature. Cysteine and methionine formed in the catabolic pathway of cystathionine were identified by comparing the retention times with those of known standards (Sigma Chemical Co., St. Louis, MO).
Statistical analysis.
Analysis of variance with three replicates was performed using a statistical analysis system (SAS, version 8.2; SAS Institute, Inc., Cary, NC) using a general linear model. Least square mean tests were conducted to analyze statistical differences between mean values at P < 0.05.
RESULTS
Catabolism of methionine.
All strains without added substrate produced no detectable levels of VSCs at any sampling time period. The negative-control sample containing solely methionine produced dimethyl disulfide (Fig. 1), indicating that methionine itself chemically converted to dimethyl disulfide. When methionine was provided as a substrate to the experimental cells, methanethiol, dimethyl disulfide, and dimethyl trisulfide were the major VSCs detected (Fig. 1). The concentration level of dimethyl disulfide was higher than those of methanethiol and dimethyl trisulfide (Fig. 1), reflective of the typical equilibrium of these spontaneously interconverted VSC species. Compared with the wild-type L. helveticus CNRZ 32 and L. helveticus CNRZ 32Δcbl, L. helveticus CBL+ and the complementation of CNRZ 32Δcbl produced significantly (P < 0.05) higher VSC levels over the incubation periods. There were no significant (P < 0.05) differences in VSC levels between the wild-type L. helveticus CNRZ 32 and L. helveticus CNRZ 32Δcbl. As the incubation time increased from 48 to 96 h, the concentrations of VSCs of L. helveticus CBL+ and the complementation of CNRZ 32Δcbl increased, indicating that CBL remains active over incubation periods (Fig. 1). The concentrations of methanethiol and dimethyl trisulfide from the wild-type L. helveticus CNRZ 32 and L. helveticus CNRZ 32Δcbl remained relatively constant over the incubation periods (Fig. 1A and C), while dimethyl disulfide concentrations increased over time (Fig. 1B).
FIG. 1.
Formation of VSCs (methanethiol [A], dimethyl disulfide [B], and dimethyl trisulfide [C]) by wild-type L. helveticus CNRZ 32 (white bars), L. helveticus CNRZ 32Δcbl (diagonally hatched bars), complementation of CNRZ 32Δcbl (gray bars), and L. helveticus CBL+ (horizontally hatched bars) and methionine control sample (black bars) when methionine was used as a substrate. Cells were incubated at 37°C. Each VSC of cells was determined after 48, 72, and 98 h of incubation. Results are the means of three replicates with error bars showing the standard errors of the means. Different lowercase letters in the figure indicate significantly different values (P < 0.05).
Catabolism of cystathionine.
Formation of dimethyl sulfide was observed in the negative-control sample containing cystathionine (Fig. 2), indicating that cystathionine was chemically degraded to dimethyl sulfide. When cystathionine was used as a substrate for the experimental cells, the major VSCs detected were methanethiol and dimethyl sulfide (Fig. 2). L. helveticus CBL+ and the complementation of CNRZ 32Δcbl produced methanethiol while no methanethiol was detected in the wild-type L. helveticus CNRZ 32 and L. helveticus CNRZ 32Δcbl (Fig. 2A). No significant (P < 0.05) differences in dimethyl sulfide formation were observed between the wild type and genetic variants of L. helveticus CNRZ 32 over incubation periods (Fig. 2B).
FIG. 2.
Formation of VSCs (methanethiol [A] and dimethyl sulfide [B]) by wild-type L. helveticus CNRZ 32 (white bars), L. helveticus CNRZ 32Δcbl (diagonally hatched bars), complementation of CNRZ 32Δcbl (gray bars), and L. helveticus CBL+ (horizontally hatched bars) and cystathionine control sample (black bars) when cystathionine was used as a substrate. Cells were incubated at 37°C. Each VSC of cells was determined after 48, 72, and 98 h of incubation. Results are the means of three replicates with error bars showing the standard errors of the means. Different lowercase letters in the figure are significantly different (P < 0.05).
The formation of cysteine and methionine was predominantly detected by HPLC in the wild-type L. helveticus CNRZ 32 and genetic variants when cystathionine was used as a substrate. There were no significant (P < 0.05) differences in the production of cysteine between the wild-type L. helveticus CNRZ 32 and the genetic variants (Fig. 3). The complementation of CNRZ 32Δcbl produced significantly (P < 0.05) higher methionine than did L. helveticus CNRZ 32Δcbl (Fig. 3). No significant (P < 0.05) differences were observed in methionine formation between the wild-type L. helveticus CNRZ 32, L. helveticus CNRZ 32Δcbl, and L. helveticus CBL+ (Fig. 3).
FIG. 3.
Formation of cysteine (bars with circles) and methionine (bars with grid lines) by wild-type L. helveticus CNRZ 32 (WT), L. helveticus CNRZ 32Δcbl (DEL), complementation of CNRZ 32Δcbl (COMP), and L. helveticus CBL+ (OE) when cystathionine was used as a substrate. Cells were incubated at 37°C, and cysteine and methionine were determined after 98 h of incubation. Results are the means of three replicates with error bars showing the standard errors of the means. Different lowercase letters in the figure are significantly different (P < 0.05).
Catabolism of cysteine.
At each designated time period (48, 72, or 96 h), the negative control produced hydrogen sulfide while methanethiol was not detected. Methanethiol and hydrogen sulfide were the major VSCs detected when cysteine was used as a substrate for the experimental cells. There were no significant (P < 0.05) differences in the relative concentrations of methanethiol and hydrogen sulfide between the wild type and genetic variants of L. helveticus CNRZ 32, and these values were constant over the 96-h time period (data not shown).
DISCUSSION
VSCs important in cheese flavor include methanethiol, dimethyl sulfide, dimethyl disulfide, dimethyl trisulfide, and hydrogen sulfide (22). In this study, three substrates, methionine, cystathionine, and cysteine, were used as VSC precursors in a metabolic study of the enzyme CBL. In a cheese matrix, there is 0.3 to 3% free methionine present (6, 28). With methionine, a significantly (P < 0.05) higher formation of VSCs, such as methanethiol, dimethyl disulfide, and dimethyl trisulfide, was observed in the L. helveticus CBL+ and the complementation of CNRZ 32Δcbl treatments (Fig. 1). This finding indicates that CBL from Lactobacillus spp. may be responsible for the conversion of methionine into VSCs. Although a major function of CBL is to catalyze the conversion of cystathionine to homocysteine, pyruvate, and ammonia and CBL has higher substrate specificity for cystathionine than methionine (1, 8), it appears that CBL contributes to the formation of VSCs from methionine through the elimination reaction mechanism. It has been known that dimethyl disulfide and dimethyl trisulfide result from the oxidation of methanethiol under aerobic conditions (4, 19). Fernandez et al. (14) reported that a higher dimethyl disulfide concentration was observed in a CBL overexpression variant of L. lactis than in the wild type. However, no significant (P < 0.05) differences in the formation of VSCs with methionine were observed between the wild-type L. helveticus CNRZ 32 and L. helveticus CNRZ 32Δcbl (Fig. 1). As Fernandez et al. (14) mentioned, this result implies that CBL activity may not be a primary route for VSC production and that enzymes other than CBL may also contribute to the conversion of methionine to VSCs. Methionine γ-lyase, a PLP-dependent enzyme, is able to catalyze the α,γ-elimination of methionine, producing methanethiol (2, 13). However, methionine γ-lyase has not been identified and characterized in Lactobacillus. Aminotransferase catalyzes the formation of 4-methylthio-2-oxobutyric acid, which is then converted to methanethiol in lactococci (16). Dias and Weimer (12) reported that methionine aminotransferase activity was detected in L. helveticus CNRZ 32. Recently, Wolle et al. (27) reported that when PLP alone was incubated with methionine, methanethiol was generated. Therefore, it is likely that either the aminotransferase-initiated pathway or a PLP-mediated nonenzymatic reaction is the primary route for the formation of methanethiol from methionine in wild-type L. helveticus CNRZ 32 and its Δcbl mutant.
When cystathionine was used as a substrate in L. lactis, the CBL overexpression and complemented deletion strains had higher CBL activity than that of the wild type (14). In this study, the formation of methanethiol was detected only in L. helveticus CBL+ and the complementation of CNRZ 32Δcbl with cystathionine (Fig. 2), indicating that CBL can catalyze the conversion of cystathionine to methanethiol. However, CBL may not contribute to the dimethyl sulfide formation since no significant (P < 0.05) differences in the production of dimethyl sulfide were observed between the wild type and genetic variants (Fig. 2). Some cystathionine was chemically converted to dimethyl sulfide as seen in the control sample (Fig. 2). Since dimethyl sulfide is also spontaneously degraded from S-methyl methionine (23), it is possible that the cystathionine standard used in this assay contained trace amounts of S-methyl contaminant. Geotrichum candidum produced higher levels of dimethyl sulfide than did G. candidum supplemented with methionine, indicating a possible inhibitory effect of methionine (11). In this study, wild-type L. helveticus CNRZ 32 and genetic variants supplemented with cystathionine produced dimethyl sulfide (Fig. 2B) while no dimethyl sulfide was detected in the wild-type L. helveticus CNRZ 32 and genetic variants supplemented with methionine (Fig. 1), suggesting that a similar methionine-dependent inhibitory effect may be manifest in this study.
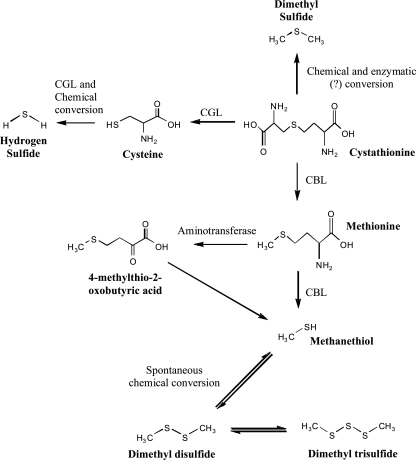
When cysteine was used as a substrate, methanethiol and hydrogen sulfide were detected. Methanethiol and hydrogen sulfide may be considered important in cheese flavor (18, 26). No significant (P < 0.05) differences in the formation of methanethiol and hydrogen sulfide were observed between the wild type and genetic variants, indicating that CBL may not be involved in the conversion of cysteine. In this study, the formation of hydrogen sulfide was most likely due to chemical degradation of cysteine as evidenced in the control sample or by the presence of another enzyme. Bruinenberg et al. (5) reported that l-cysteine is converted to hydrogen sulfide probably by the α,β-elimination reaction of cystathionine γ-lyase (CGL) in Lactococcus lactis subsp. cremoris SK11. CGL was purified and characterized in L. reuteri DSM 20016 (10). It has been reported that CGL, which is another PLP-dependent enzyme, catalyzes the conversion of cystathionine to cysteine via an α,γ-elimination mechanism (8). The formation of cysteine (Fig. 3) may indicate that CGL activity is present in wild-type L. helveticus CNRZ 32 and hence in the genetic variants. CGL may be responsible for the formation of hydrogen sulfide from cysteine. Based on these results, we propose catabolic pathways of methionine, cystathionine, and cysteine for the formation of VSCs (Fig. 4).
FIG. 4.
Proposed catabolic pathways of methionine, cystathionine, and cysteine.
In conclusion, the results of the present study demonstrate that, although the primary function of CBL is to catalyze the conversion of cystathionine to homocysteine, CBL may contribute to the production of VSCs from methionine and cystathionine in L. helveticus. This bacterium has been well studied and utilized as an adjunct culture capable of surviving in aged cheeses. These results suggest that a CBL overexpression mutant may provide a promising application for intensifying cheese flavor caused by the presence of VSCs.
Acknowledgments
This study was funded by Dairy Management, Inc., and Chr. Hansen, Inc.
We thank Mateo F. Budinich for technical assistance with the HPLC and Dennis L. Welker for technical assistance with CBL constructs.
Footnotes
Published ahead of print on 2 March 2007.
REFERENCES
- 1.Alting, A. C., W. J. M. Engels, S. van Schalkwijk, and F. A. Exterkate. 1995. Purification and characterization of cystathionine β-lyase from Lactococcus lactis subsp. cremoris B78 and its possible role in flavor development in cheese. Appl. Environ. Microbiol. 61:4037-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amarita, F., M. Yvon, M. Nardi, E. Chambellon, J. Delettre, and P. Bonnarme. 2004. Identification and functional analysis of the gene encoding methionine-γ-lyase in Brevibacterium linens. Appl. Environ. Microbiol. 70:7348-7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhowmik, T., and J. L. Steele. 1993. Development of an electroporation procedure for gene disruption in Lactobacillus helveticus CNRZ-32. J. Gen. Microbiol. 139:1433-1439. [Google Scholar]
- 4.Bonnarme, P., L. Psoni, and H. E. Spinnler. 2000. Diversity of l-methionine catabolism pathways in cheese-ripening bacteria. Appl. Environ. Microbiol. 66:5514-5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruinenberg, P. G., G. de Roo, and G. K. Y. Limsowtin. 1997. Purification and characterization of cystathionine γ-lyase from Lactococcus lactis subsp. cremoris SK11: possible role in flavor compound formation during cheese maturation. Appl. Environ. Microbiol. 63:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen, J. E., M. E. Johnson, and J. L. Steele. 1995. Production of Cheddar cheese using a Lactococcus lactis ssp. cremoris SK11 derivative with enhanced aminopeptidase activity. Int. Dairy J. 5:367-379. [Google Scholar]
- 7.Christensen, J. E., and J. L. Steele. 2003. Impaired growth rates in milk of Lactococcus helveticus peptidase mutants can be overcome by use of amino acid supplements. J. Bacteriol. 185:3297-3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutin, A. C., and P. L. H. McSweeney. 2004. Catabolism of amino acids in cheese during ripening, p. 435-454. In P. F. Fox (ed.), Cheese: chemistry, physics and microbiology, vol. 1. Elsevier Ltd., London, England. [Google Scholar]
- 9.Dao, M. L., and J. J. Ferretti. 1985. Streptococcus-Escherichia coli shuttle vector pSA3 and its use in the cloning of streptococcal genes. Appl. Environ. Microbiol. 49:115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Angelis, M., A. C. Curtin, P. L. H. McSweeney, M. Faccia, and M. Gobbetti. 2002. Lactobacillus reuteri DSM 20016: purification and characterization of a cystathionine γ-lyase and use as adjunct starter in cheesemaking. J. Dairy Res. 69:255-267. [DOI] [PubMed] [Google Scholar]
- 11.Demarigny, Y., C. Berger, N. Desmasures, M. Gueguen, and H. E. Spinnler. 2000. Flavour sulphides are produced from methionine by two different pathways by Geotrichum candidum. J. Dairy Res. 67:371-380. [DOI] [PubMed] [Google Scholar]
- 12.Dias, B., and B. Weimer. 1998. Conversion of methionine to thiols by lactococci, lactobacilli, and brevibacteria. Appl. Environ. Microbiol. 64:3320-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dias, B., and B. Weimer. 1998. Purification and characterization of l-methionine γ-lyase from Brevibacterium linens BL2. Appl. Environ. Microbiol. 64:3327-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez, M., W. van Doesburg, G. A. M. Rutten, J. D. Marugg, A. C. Alting, R. van Kranenburg, and O. P. Kuipers. 2000. Molecular and functional analyses of the metC gene of Lactococcus lactis, encoding cystathionine β-lyase. Appl. Environ. Microbiol. 66:42-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox, P. F., P. L. H. McSweeney, and C. M. Lynch. 1998. Significance of non-starter lactic acid bacteria in Cheddar cheese. Aust. J. Dairy Technol. 53:83-89. [Google Scholar]
- 16.Gao, S., E. S. Mooberry, and J. L. Steele. 1998. Use of t13C nuclear magnetic resonance and gas chromatography to examine methionine catabolism by lactococci. Appl. Environ. Microbiol. 64:4670-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Low, D., J. Ahlgren, D. Horne, D. J. McMahon, C. J. Oberg, and J. R. Broadbent. 1998. Role of Streptococcus thermophilus MR-1C capsular exopolysaccharide in cheese moisture retention. Appl. Environ. Microbiol. 64:2147-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manning, D. J. 1979. Chemical production of essential flavor compounds. J. Dairy Res. 46:531-537. [Google Scholar]
- 19.McSweeney, P. L. H., and M. J. Sousa. 2000. Biochemical pathways for the production of flavour compounds in cheeses during ripening: a review. Lait 80:293-324. [Google Scholar]
- 20.Or-Rashid, M. M., R. Onodera, S. Wadud, and N. Mohammed. 2000. Convenient method of threonine, methionine and their related amino compounds by high-performance liquid chromatography and its application to rumen fluid. J. Chromatogr. B Biomed. Sci. Appl. 741:279-287. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook, J., E. F. Fritsch, and T. S. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 22.Seefeldt, K. E., and B. C. Weimer. 2000. Diversity of sulfur compound production in lactic acid bacteria. J. Dairy Sci. 83:2740-2746. [DOI] [PubMed] [Google Scholar]
- 23.Spinnler, H. F., C. Berger, C. Lapadatescu, and P. Bonnarme. 2001. Production of sulfur compounds by several yeasts of technological interest for cheese ripening. Int. Dairy J. 11:245-252. [Google Scholar]
- 24.Sturino, J. M., and T. R. Klaenhammer. 2002. Expression of antisense RNA targeted against Streptococcus thermophilus bacteriophages. Appl. Environ. Microbiol. 68:588-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reference deleted.
- 26.Urbach, G. 1995. Contribution of lactic acid bacteria to flavor compound formation in dairy products. Int. Dairy J. 5:877-903. [Google Scholar]
- 27.Wolle, D. D., D. S. Banavara, and S. A. Rankin. 2006. Empirical and mechanistic evidence for the role of pyroxidal-5′-phosphate in the generation of methanethiol from methionine. J. Dairy Sci. 89:4545-4550. [DOI] [PubMed] [Google Scholar]
- 28.Wood, A. F., J. W. Aston, and G. K. Douglas. 1985. The determination of free amino acids in cheese by capillary column gas liquid chromatography. Aust. J. Dairy Technol. 40:166-169. [Google Scholar]
- 29.Yvon, M., and L. Rijnen. 2001. Cheese flavour formation by amino acid catabolism. Int. Dairy J. 11:185-201. [Google Scholar]