Abstract
Cleaning and disinfection of open surfaces in food industry premises leave some microorganisms behind; these microorganisms build up a resident flora on the surfaces. Our goal was to explore the phenomena involved in the establishment of this biofilm. Ceramic coupons were contaminated, once only, with Pseudomonas fluorescens suspended in meat exudate incubated at 10°C. The mean adhering population after 1 day was 102 CFU·cm−2 and 103 total cells·cm−2, i.e., the total number of cells stained by DAPI (4′,6′-diamidino-2-phenylindole). The coupons were subjected daily to a cleaning product, a disinfectant, and a further soiling with exudate. The result was a striking difference between the numbers of CFU, which reached 104 CFU·cm−2, and the numbers of total cells, which reached 2 × 106 cells·cm−2 in 10 days. By using hypotheses all leading to an overestimation of the number of dead cells, we showed that the quantity of nonculturable cells (DAPI-positive cells minus CFU) observed cannot be accounted for as an accumulation of dead cells. Some nonculturable cells are therefore dividing on the surface, although cell division is unable to continue to the stage of macrocolony formation on agar. The same phenomenon was observed when only a chlorinated alkaline product was used and the number of cells capable of reducing 5-cyano-2,3-ditolyl tetrazolium chloride was close to the number of total cells, confirming that most nonculturable cells are viable but nonculturable. Furthermore, the daily shock applied to the cells does not prompt them to enter a new lag phase. Since a single application of microorganisms is sufficient to produce this accumulation of cells, it appears that the phenomenon is inevitable on open surfaces in food industry premises.
Research reported previously by Mettler and Carpentier (25) showed that on new materials introduced into food industry premises, a resident flora, i.e., a flora that is not eliminated by cleaning operations, becomes established within a few weeks. In that study, using a smooth, pressed ceramic stoneware floor tile similar to that used in the present study, the resident flora reached densities of about 103 CFU·cm−2 in bakery premises and 3 × 105 CFU·cm−2 in cheese-making premises (25). In environments where animal-based foods are processed, the predominant genera in the resident flora are Pseudomonas and Staphylococcus (19, 25). Pseudomonas strains are highly undesirable because they spoil food, because they are able to grow at low temperatures, and because they have a vigorous proteolytic activity. Furthermore, some strains of Pseudomonas and Staphylococcus can increase the colonization of inert surfaces by Listeria monocytogenes (13) and/or protect this pathogenic bacterium from disinfectants (22).
Alliot (1), cited by Carpentier and Cerf (12), previously showed that on open surfaces in cheese-making premises, there is a flora that is not culturable on laboratory agar media (i.e., does not form visible macrocolonies) and that this flora is far more abundant than culturable flora. The culturable flora varies between 1.6 × 101 and 3.2 × 107 CFU·cm−2, while the nonculturable flora (total cells minus CFU) varies between 7 × 106 and 2.5 × 108 cells·cm−2 and includes a variable proportion (0.01% to 79%) of cells displaying respiratory activity as shown by the reduction of 5-cyano-2,3-ditolyl tetrazolium chloride (CTC). That research has been corroborated by other studies. Mettler and Carpentier (24) previously showed huge differences ranging from 3.7 to 6.7 logs between acridine-orange cell counts (orange and green cells, the sum of which is considered to be total cells) and CFU detached from gaskets of a milk pasteurization line. Peneau and Carpentier (unpublished results) observed up to 8 × 106 nonculturable cells per cm2 on the floor of catering premises after cleaning operations. High proportions of nonculturable cells have been observed on many occasions in a range of natural environments such as freshwater, seawater, and soil (for a review, see reference 2). It is now strongly suspected that a majority of the nonculturable cells are active cells, and there are various methods for revealing metabolic activity, depending on its degree of intensity (33). However, a distinction should be made between microbial species that have never been cultured in the laboratory (for a review, see reference 2) and cells that have lost their aptitude for culturing because of chemical shock or some other stresses (23) such as starvation (14). These are known as viable but nonculturable (VBNC) cells (for a review, see reference 29) or active but nonculturable cells (36). Given that the microorganisms in food industry premises are subjected to multiple stresses (chemical shock, dehydration, etc.), it is likely that at least some of the nonculturable cells observed in the field are VBNC.
The research reported here was designed to model, in the laboratory, the gradual accumulation of culturable and nonculturable flora on a surface in order to explore the phenomena that lead to the establishment of such resident flora in spite of daily cleaning and disinfection. We decided to work with a pure culture of Pseudomonas fluorescens at 10°C, a temperature found in meat processing premises. The cleaning and disinfection products used in the present study are representative of those used in the meat industry: two chlorinated alkaline cleaning products and a disinfectant based on a quaternary ammonium compound and glutaraldehyde. In some experiments, the CTC-positive (CTC+) flora was also counted to test the hypothesis that cells that are nonculturable (CTC+ cells minus CFU), because of the chemical shocks that they had suffered, are VBNC. Although several viability stains exist, CTC was chosen because it was proven to be useful by many researchers in association with DAPI (4′,6′-diamidino-2-phenylindole) to simultaneously count VBNC and total cells (5, 10, 20, 37, 39, 40).
MATERIALS AND METHODS
Substratum preparation.
Pressed ceramic stoneware tiles (catalog number 1050/12; Höganas, Thouaré sur Loire, France), a type used for floors in food and drink industries, were used here as substrata. The tiles were new, smooth (Ra0.8 [2a]: mean, 2.3 μm; standard deviation [SD], 0.2; n = 10), and 12 mm thick and had been cut into 4- by 2.5-cm coupons. A Dow Corning silicon joint sealant (Arrow Electronique, Rungis, France) was applied along the edges of a coupon to obtain an area of 4.5 cm2 that could be kept flooded.
Prior to use, the coupons were washed with RBS35 chlorinated alkaline cleaner (René Borghgraef Société, Chemical Products R. Borghgraef SA, Frelinghien, France) at a concentration of 2% (wt/vol) in water heated to 50°C (initial temperature) for 10 min, with stirring. They were then rinsed with water heated to 50°C (initial temperature) for 25 min, with stirring. They were then rinsed five times in ultrapure water for 1 min each time, with stirring. After autoclaving for 20 min, the coupons were immersed in sterilized ultrapure water for a minimum of 20 h. If this moistening process is not carried out, the cells may die from desiccation because the liquid is absorbed deep in the coupon. However, it was checked that bacterial cells were not able to penetrate the tiles because pore diameters were shown to be smaller than 0.0060 μm by using an Hg-porosimeter (Micromeritics 9310) (measurements were made by the Société Française de Céramiques, Paris, France). Lastly, the coupons were placed, with the help of forceps, into sterilized boxes measuring 26 by 15 cm containing 50 ml sterilized ultrapure water and sterilized paper. The paper prevented the coupons from sliding in the box, and the added water kept the air in the box at 100% relative humidity. For the remainder of the paper, these boxes are referred to as “incubation boxes.”
Growing biofilms on ceramic coupons.
To produce conditions close to those found in meat industry premises, the culture medium used was meat exudate. It was prepared according to a method described previously by Midelet and Carpentier (26). Briefly, after defrosting pieces of shoulder of beef about 5 by 5 by 5 cm in size, the exudate was recovered and kept at −20°C. Prior to use, it was defrosted again and centrifuged (Avanti 30; Beckman Coulter, Roissy, France) at 2,100 × g for 10 min at 20°C. The supernatant was recovered and sterilized by filtration on a Stericup filter (Millipore, Saint Quentin, Yvelines, France) fitted with a filter membrane with pores of 0.22 μm upon which we placed a prefilter retention membrane with a pore diameter of 1 μm (Millipore).
The strain used was P. fluorescens CCL 167. It was isolated earlier after having been established on materials kept at 10°C for several weeks and alternately soiled with naturally contaminated meat exudate and cleaned and disinfected by a procedure simulating cleaning and disinfection in meat processing premises (our unpublished results). Prior to use, it was seeded onto tryptone soy agar (TSA) (AES Laboratoire, Combourg, France) on a slant and incubated at 30°C for 24 h. The culture was washed by resuspending it in 9 ml of physiological saline and then centrifuged twice at 2,100 × g for 10 min at 20°C. The suspension was then diluted with physiological saline to obtain an optical density at 600 nm of about 0.150 (±0.005) in tubes with a 1.5-cm diameter. At this stage, the suspension was close to 108 CFU·ml−1. We then produced 1/10 dilutions in sterilized physiological saline and finally in exudate to obtain a suspension of 103 CFU·ml−1 or 105 CFU·ml−1. We then deposited 700 μl of this inoculum onto each coupon and incubated the coupons in incubation boxes at 10°C for periods of time that varied according to the purpose of the experiment.
In the growth-monitoring experiments, the coupons were incubated for periods of 13 to 19 days. In the experiments in which the coupons were subjected to a daily chemical treatment (cleaner or cleaner and disinfectant), as described below, they were incubated between two chemical treatments for 1 day in the presence of exudate. The total duration of the experiments ranged from 8 to 17 days. One of the experiments was conducted without adding microbial cells but under identical conditions in all other respects. This was to ensure that organic matter fluorescing after coloring with DAPI could not be a source of error in the microbial cell counts.
Repeated soiling and chemical treatment of the tile coupons.
The coupons were first rinsed to eliminate nonadhering cells. This was done by pouring on 50 ml of sterilized ultrapure water. The coupons were then treated with either one or two chemical products, as described below. Immediately after the chemical treatment, 700 μl of exudate, sterilized by filtration, was deposited onto the coupon, which was then put back into the incubation box at 10°C.
Application of two products.
One milliliter of Galorox JH (Penngar, Vaas, France), a chlorinated alkaline cleaner, at a concentration of 1% (wt/vol) was deposited on the surface of each coupon and left for 5 min. This solution had a pH of 11 and contained 0.03 g·liter−1 of free chlorine. Although chlorine is a powerful disinfectant, it was applied here on soiled surfaces and was, at least partially, consumed by proteins. For this reason, it is here considered to be a cleaning agent. The coupon was then rinsed again by pouring on 50 ml of sterilized ultrapure water. One milliliter of Galox Azur (Penngar), a disinfectant, at a concentration of 0.5% (wt/vol) was then deposited onto the coupon. The active compounds of this disinfectant were glutaraldehyde and lauryl dimethyl benzyl ammonium chloride, both at a 0.025% (wt/vol) concentration in the 5% (wt/vol) solution. After 5 min, the Galox Azur was removed by turning the coupon over. This treatment was carried out daily. The concentrations of these products recommended for use in the food industry are 2 to 5% for Galorox JH and 1 to 2% for Galox Azur. Lower concentrations were chosen here in order to have some CFU left on the surface after the first chemical treatment.
Application of one cleaning product.
One milliliter of P3-topax-M95 (Ecolab, Issy les Moulineaux, France), a chlorinated alkaline product, at a concentration of 2% (wt/vol) was deposited on the surface of each coupon, left for 5 min, and then removed by turning the coupon over. This solution had a pH of 12 and contained 0.04 g·liter−1 of free chlorine. The coupon was then rinsed by pouring on 50 ml of sterilized ultrapure water. This treatment was applied every day except the 3rd, 4th, 10th, and 11th days of each experiment. The recommended concentration for use in industry is 2 to 3%.
Swabbing.
When a determination of bacterial counts of adhering cells was need, a swabbing was performed on one coupon that was not used in the rest of the experiments. Prior to swabbing, the coupons were rinsed by pouring on 50 ml of sterilized neutralizer of the disinfectant (soy lecithin [30 g·liter−1] and l-histidine [3 g·liter−1]) when Galox Azur was used and sterilized ultrapure water when P3-topax-M95 was used. A cotton swab (VWR, Fontenay-sous-Bois, France) was wiped over the surface of a coupon in three different directions: left to right, top to bottom, and diagonal. The swab was then soaked in an Eppendorf tube (Treff Lab, Poly Labo, Strasbourg, France) containing 1.5 ml of peptone water (1 g·liter−1 peptone; AES Laboratoire, Combourg, France), and the tip was broken into the tube. The tube and swab were then vortexed together, and the swab was removed.
Bacterial counts.
The CFU were counted after spreading a maximum of 300 μl of the suspensions and appropriate dilutions in peptone water onto TSA plates and incubating plates at 30°C for 24 h. We checked several times that with cells that had been subjected to the cleaning and disinfection products, the number of CFU was no greater when the incubation period was 72 h. As nonculturability is sometime assumed to be due to the lack of appropriate culture media, we counted CFU on an agar medium containing 6% (vol/vol) meat exudate, and the counts were not significantly different from those obtained for CFU on TSA whether the incubation temperature was 10°C for 6 days or 30°C for 1 day.
Two coloring methods were used. One was done with DAPI (Sigma-Aldrich Chimie S.a.r.l., Lyon, France), a DNA marker that makes it possible to count total cells. The other used both DAPI and CTC (Interchim, Montluçon, France). CTC is a redox dye that produces red fluorescent formazan when reduced. It reveals cells that are presenting respiratory activity and, in other words, are viable. The two products were dissolved in ultrapure water, sterilized, and filtered with a Stericup filter (Millipore, Saint Quentin, Yvelines, France) fitted with a filter membrane with 0.22-μm pores.
Before staining, the suspensions to be analyzed were diluted in sterilized ultrapure water that had been filtered as described above. Organic matter removed by swabbing is fluorescent after staining with DAPI and may interfere with the cell count. To minimize this problem, the solutions were diluted 1/10. For the highest cell concentrations, a 1/100 or even a 1/1,000 dilution is necessary.
Total cell numbers by DAPI staining.
DAPI was added to part of the suspension (between 450 and 900 μl) to give a final concentration of 5 μg·ml−1. The final volume did not exceed 1 ml. After 15 min in darkness, the suspension was filtered using a 13-mm-diameter Swinnex filter holder (Millipore, Saint Quentin, Yvelines, France) and a black polycarbonate membrane with a 1.1-cm net diameter and a 0.2-μm pore size (Dutscher, Brumath, France). We placed the membrane onto a glass slide, left it to dry for about 5 min, and covered it with a coverglass. We observed it with the ×100 immersion lens of a microscope (Axioscop; Zeiss, Lepecq, France) equipped with an epifluorescence system. To observe the DAPI-positive (DAPI+) cells, a set of filters was used to produce excitation at a wavelength of 365 nm and emission at a wavelength of 397 nm.
Active and total cell numbers by CTC-DAPI double staining.
CTC and DAPI stainings were applied using a slightly modified version of a method described previously by Rodriguez et al. (31). First, a volume of tryptone soy broth (AES Laboratoire, Combourg, France) at a one-fifth dilution was added to a volume of the suspension to be analyzed. This one-fifth dilution was performed in order to obtain a glucose concentration of 0.5 g·liter−1, a value similar to that of liquid R2A medium, the medium recommended previously by Rodriguez et al. (31). CTC was added to obtain a final concentration of 5 mmol. The mixture was incubated at 28°C for 4 h in darkness, stirring slowly (100 rpm). Next, DAPI was added to give a final concentration of 5 μg·ml−1. The final volume was close to 1 ml. After 15 min in darkness, the suspension was filtered as described above. We then observed the staining with the ×100 immersion lens of a microscope equipped with an epifluorescence system using two sets of filters. The first set, for observing the DAPI+ cells, is described above. The second set, for observing the CTC+ cells, provided excitation between 450 and 490 nm and emission from 520 nm.
The aim of observations using the microscope was to count at least 200 cells in all as well as to detect DAPI+ and CTC+ cells. However, it was difficult to detect cells the first days because one can detect only between 0 and 2 cells per field, and 20 fields at most were counted. Consequently, the results obtained for the first days are less precise than those for subsequent days.
Direct epifluorescence microscopy.
Staining of attached cells was performed by the deposition of 900 μl of a 5-μg·ml−1 DAPI solution onto a rinsed ceramic coupon. After 15 min in darkness, the coupon was rinsed by pouring 10 ml filtered ultrapure water. Observations were done in the wet state by the epifluorescence microscopic system used for total cell counts.
Data processing.
Paired-set comparisons of data were performed using Microsoft Excel software, version 2000. As the numbers of values were not always the same in the experiments performed, and in order to give the same weight to each one of the experiments, all the means calculated were grand means (means of the means of each one of the independent experiments). The Baranyi growth model (6) was fitted to the growth data using MicroFit (version 1.0) (Institute of Food Research, Norwich, United Kingdom) to obtain growth rates and lag times.
RESULTS
Except where otherwise stated, the values given below are the decimal logarithms of the surface concentrations [log(CFU·cm−2)].
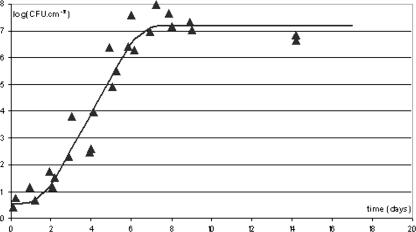
Figure 1 shows a typical example of a curve describing the increase in the number of adhering cells in a P. fluorescens population incubated at 10°C in the presence of meat exudate. Under these growth conditions, the median maximum population reached was 7.0, with a median growth rate of 3.1 day−1 and a median lag phase of 1.5 days (Table 1). During the two independent additional experiments of 13 days and 8 days, CFU and DAPI+ cells (total cells) were counted at different stages of growth (results not shown). A paired-set comparison of the 22 pairs of data shows that the total cells were significantly (P = 0.003) more numerous than the CFU but that the mean difference between total cells and CFU was small: 0.3.
FIG. 1.
Example of growth curve of the number of adhering cells of a P. fluorescens population incubated at 10°C in the presence of meat exudate. The inoculum was 103 CFU·ml−1. The ▴ symbols represent the experimental data. The curve has been fitted to the experimental data using the Microfit software package.
TABLE 1.
Growth parameters of adhering cells of a P. fluorescens population incubated at 10°C in the presence of meat exudatea
| Expt | Nmax [log(CFU·cm−2)] | μmax (day−1) | Lag (days) |
|---|---|---|---|
| 1 | 7.18 | 3.14 | 1.52 |
| 2 | 6.79 | 3.44 | 1.37 |
| 3 | 7.05 | 2.69 | 2.57 |
The inoculum was 103 CFU/ml. Nmax, final maximum cell population; μmax, maximum growth rate.
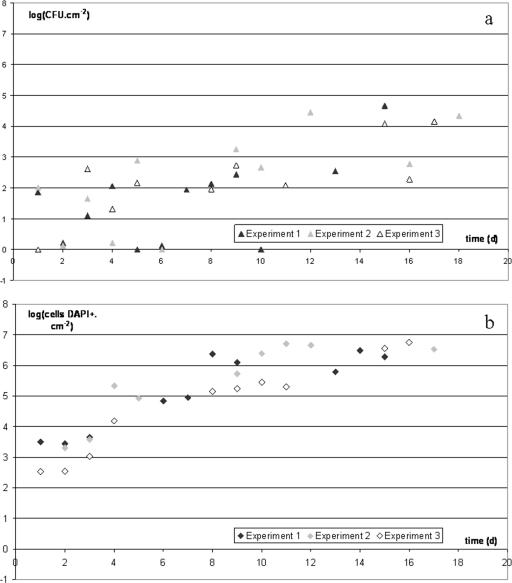
Figure 2 shows the time trend in the microbial load of material contaminated by P. fluorescens at day 0 (d0) and then subjected to alternation between a long, 1-day phase, during which the material was placed at 10°C and covered with a film of meat exudate, and a short phase when the material was rinsed, covered with a solution of a chlorinated alkaline (5 min), rinsed again, and then covered with a disinfectant solution (5 min). For greater clarity, Fig. 2 shows only the figures obtained just before cleaning and disinfection for 3 of the 6 experiments. The mean adhering culturable population after a 1-day incubation was 2.1 (zero to one value per experiment, with four values [SD = 0.43]). The increase in the number of CFU (Fig. 2a) was slow and chaotic, with a maximum of 4.7 reached once on the 15th day of one of the six experiments. The mean adhering total cell population after a 1-day incubation was 3.0 (one value per experiment, with six values [SD = 0.40]). The number of total cells (Fig. 2b) increased sharply over time, with a mean “steady state” of 6.0 reached from the 10th day (between 2 and 4 values per experiment, with 18 values [SD = 0.3]), whereas the mean of the CFU values obtained from the 10th day of the six experiments was 3.3 (between 3 and 4 values per experiment, with 19 values [SD = 0.6]).
FIG. 2.
Accumulation of CFU (a) and total cells stained by DAPI (b) of P. fluorescens on ceramic tile coupons. The coupons were incubated at 10°C in the presence of meat exudate and subjected to daily cleaning and disinfection (5 min of treatment with Galorox JH at a concentration of 1% and then 5 min of treatment with Galox Azur at a concentration of 0.5%). Meat exudate was deposited onto the coupons after each chemical treatment. The inoculum deposited onto the coupons on d0 was 105 CFU/ml. Only the cells counted before the daily chemical treatment are shown here. The detection threshold for CFU was −0.4 log(CFU·cm−2).
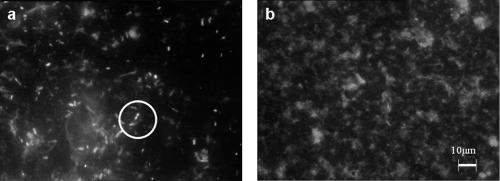
Figure 3 shows two epifluorescence microscopic views of a filter used to trap whatever is detached by swabbing and marked with DAPI. Figure 3a shows the filtrate of the liquid obtained from a coupon that had been seeded with P. fluorescens and subjected to nine daily soilings, each followed by a chemical treatment. Eighty cells and some fluorescent organic matter can be seen. Some cells are in pairs, suggesting that cell division may have just taken place. Figure 3b shows the filtrate of the liquid obtained from a coupon that had not been seeded with P. fluorescens but that had been subjected to the same treatment as the coupon seeded with P. fluorescens. Fluorescent organic matter can be seen, but no apparent cells were seen.
FIG. 3.
Epifluorescence microscopic observation of a 1/10 dilution of the filtrate of the liquid recovering what was detached from a coupon by swabbing and marked with DAPI. (a) Filtrate obtained after cleaning and disinfection on the 10th day of experiment 6 (Table 3). Two cells that appear to have divided are circled. (b) Filtrate obtained after cleaning and disinfection on the eighth day of an experiment conducted without bacterial seeding, with all other conditions being identical to those of the experiments shown in Fig. 2.
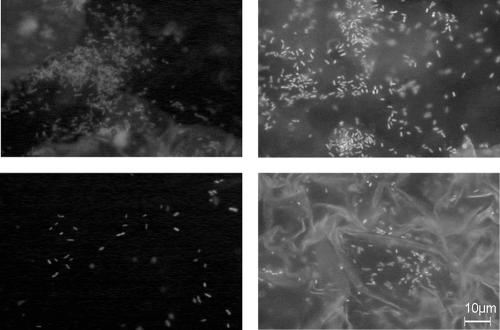
Figure 4 shows four randomly chosen epifluorescence microscopic views of cells attached to a ceramic coupon on the 17th day of an experiment before the daily cleaning and disinfection (application of two products). Although it is impossible to accurately count attached cells because of the fluorescence of the exudate and because of the biofilm heterogeneity, this figure shows a high number of cells, in agreement with the numbers of total cells detached by swabbing.
FIG. 4.
Epifluorescence microscopic observation of DAPI-stained adhering P. fluorescens cells on randomly chosen places of a same ceramic coupon that had been subjected to repeated chemical treatments (Galorox JH and Galox Azur) and soiling with meat exudate for 16 days. The photographs were obtained on the 17th day before the daily chemical treatment.
By estimating the microbial load before (Fig. 2) and after the chemical treatment, decimal reduction (DR) numbers can be calculated. The DR number showed no variation over time (results not shown). The means for the six experiments were 1.2 for CFU (between 8 and 12 values per experiment, with 55 values [SD = 0.2]) and 0.9 for total cells (4 to 10 values per experiment, with 49 values [SD = 0.4]).
When the viability marker CTC was used in two similar but independent experiments, during which only one cleaning product, the chlorinated alkaline cleaner P3-topax-M95, was used, the numbers of total cells were greater than the numbers of CTC+ cells but still very close. A paired-set comparison shows a significant difference (P = 0.04) between the means, although it is still a small difference, 0.07 (14 data pairs were compared), showing that 85% of total cells were CTC+.
DISCUSSION
Since the number of total cells was far greater than the number of CFU, one may consider that the number of nonculturable cells (DAPI+ cells minus CFU) is correctly estimated by the number of DAPI+ cells.
The presence of a large number of total cells could result from an accumulation of CFU killed by the daily chemical treatment. Our reasoning to test this hypothesis is as follows. Based on this hypothesis, all cells on the surface are either dead or culturable (CFU). Let us analyze what happens on the surface after the chemical treatment. Some of the cells that have adhered to the material are detached and eliminated along with the liquids used, some are killed where they are, and some remain attached and in a culturable state. We do not know the proportion of attached dead cells, so to simplify the reasoning, let us consider that all the adhering culturable cells are killed by the cleaning and disinfection and remain where they are. This is obviously an overestimation of the number of dead adhering cells. If the cells are dead, their number will not vary between two chemical treatment, so the number of dead cells observed on day n (dn) should be equal to the number observed on d1, i.e., just before the first chemical treatment, or total cells at d1 (TCd1) (the number of total cells at d1 is in fact greater than the number of CFU) plus the sum of CFU observed before the chemical treatment on each of the days preceding dn. In what follows, we shall call this total Sdn. Thus, for example, at d4, Sd4 = TCd1 + CFUd2 + CFUd3. We calculated these Sdn amounts for each of the experiments performed. Where a CFU value was missing, it was replaced by the mean of the values obtained from the 10th day (when the steady state was reached) to the end of the experiments [(2.00 × 103) × CFU·cm−2 in the six experiments where two chemical products were applied and (4.03 × 103) × CFU·cm−2 in the two experiments where one product was applied]. In this case also, an error would be an overestimation when considering the missing values within the first days of the experiments. The Sdn calculated for one of the experiments is shown by way of an example in Table 2, and the logarithms of the values obtained at the end of each experiment are shown in Table 3.
TABLE 2.
Number of total cells stained by DAPI observed before the daily chemical treatment and Sdn from an experiment in which P. fluorescens on a ceramic tile was incubated in the presence of meat exudate and subjected to a daily chemical treatmenta
| Time (days) | Total cells·cm−2 | Sdn |
|---|---|---|
| 1 | 3.11 × 103 | |
| 2 | 2.74 × 103 | 3.18 × 103 |
| 3 | 4.41 × 103 | 3.18 × 103 |
| 4 | ND | 3.20 × 103 |
| 5 | ND | 3.31 × 103 |
| 6 | 6.92 × 104 | 3.31 × 103 |
| 7 | 8.87 × 104 | 3.31 × 103 |
| 8 | 2.37 × 106 | 3.40 × 103 |
| 9 | 1.26 × 106 | 3.54 × 103 |
| 10 | ND | 3.82 × 103 |
| 11 | ND | 3.82 × 103 |
| 12 | ND | 5.81 × 103b |
| 13 | 6.15 × 105 | 7.81 × 103b |
| 14 | 3.07 × 106 | 8.16 × 103 |
| 15 | 1.89 × 106 | 1.02 × 104b |
Treatment consisted of 5 min of Galorox JH treatment at a concentration of 1% and 5 min of Galox Azur treatment at a concentration of 0.5%. Meat exudate was deposited onto the coupons after each chemical treatment. The inoculum of the suspension deposited on the coupons at d0 was 105 CFU/ml. ND, not determined.
The CFU count was missing and was replaced by 2.00 × 103 CFU·cm−2, which is the grand mean of the values obtained from the 10th day (when the steady state was reached) to the end of the experiments.
TABLE 3.
Logarithms of numbers of total cells stained by DAPI obtained at the end of the experiment and of Sdf of P. fluorescens on ceramic tile incubated at 10°C in the presence of meat exudate and subjected to a daily chemical treatmenta
| Chemical product | Expt | Last day of expt | Log (TCdf·cm−2) | Log(Sdf) |
|---|---|---|---|---|
| Galorox JH (cleaner) and | 1 | 15 | 6.28 | 4.01 |
| Galox Azur (disinfectant) | 2 | 17 | 6.53 | 4.66 |
| 3 | 16 | 6.75 | 4.44 | |
| 4 | 14 | 5.96 | 4.10 | |
| 5 | 13 | 6.01 | 4.49 | |
| 6 | 11 | 6.10 | 4.45 | |
| P3-topax-M95 (cleaner) | 1 | 8 | 6.63 | 4.67 |
| 2 | 12 | 6.58 | 4.51 |
The inoculum deposited onto the coupons on d0 was 105 CFU/ml. TCdf, numbers of total cells (TC) stained by DAPI obtained at the end of the experiment (df); Sdf, number of total cells at d1 plus sum of CFU counts from before chemical treatment on each of the days prior to df.
It turns out that the number of total cells is generally greater than the Sdn (9 exceptions out of 55, all before d7). Although the options that we chose for our reasoning all lead to overestimations of the number of dead adhering cells, the Sdn at the end of the experiment is always about 2 log less than the number of total cells at the end of the experiment. However, it is well known that swabbing does not detach all microbial cells from a surface. The removal rate, which includes the retention of cells in the cotton of the swab, depends on the swab material; cotton swabs, which were used here, were shown to be more efficient than polyester and rayon swabs (32). Swabbing in a moistened condition, as processed here, is more efficient than dry swabbing (32). The nature of the substratum on which bacterial cells adhere and the bacterial strain also have an influence on the removal rates obtained by swabbing (26). Such removal rates were shown to be lower after cleaning (30). To our knowledge, the lowest reported removal rate was 2% and was obtained from a chlorine-treated P. fluorescens biofilm grown on a polyvinyl chloride conveyor belt material (4). The removal rate here is, unfortunately, unknown. As the mean numbers of DR obtained by the daily chemical treatment on total cells (0.9 DR due to removal of cells) and CFU (1.2 DR due to removal and killing) are quite similar, it is possible that swabbing has a similar removal efficiency for both kinds of cells. In case the removal rates were the same for total and culturable cells, the same differences between the real total cell count and Sdn would be obtained, and our conclusion would be unchanged. Nevertheless, in case the removal rates were not the same for the culturable and the nonculturable populations, let us consider a worst-case scenario leading to a smaller difference between total cells counts and Sdn, i.e., a high removal rate for the total cells of 90% (the log value would be increased by 0.05) and a low one of 2% for CFU (the log value would be increased by 1.70). When considering the values obtained from the ninth day (from which differences are the greatest) of the six experiments (25 values), the difference between total cell counts and Sdn is still significant (P = 0.03). This difference added to all the overestimations made to calculate the Sdn allowed us to conclude that the large number of total cells cannot be due solely to the accumulation of dead cells. Some of the nonculturable cells can divide on the coupon, even though they cannot continue dividing until colonies are formed on TSA plates. Therefore, it is not surprising that when CTC+ counts were performed during a similar experiment, 85% of the DAPI+ cells were also CTC+. Again, since the number of CTC+ cells were far greater than the number of CFU, one may consider that the number of nonculturable cells with respiratory activity (CTC+cells minus CFU) is correctly estimated by the number of CTC+ cells. These cells, which respire and divide, are indeed VBNC cells.
These divisions on coupons continued until a maximum population of about 2 × 106 total cells·cm−2 was reached. This population counts single cells and small microcolonies. As mainly single cells could be seen on coupons just after the daily chemical treatment (data not shown), it is likely that the microcolonies are formed during the 1-day incubation separating the two chemical treatments.
In fact, the ability of VBNC cells to divide is a property that has long been exploited. A review by Roszak and Colwell (34) reported 14 references published from 1932 to 1982 that described microscopic methods for the detection of microcolonies. More recent publications (8, 28) reported on such methods to show that there are cells capable of forming microcolonies but not able to form macrocolonies on agar that are visible to the naked eye. It is this property that is used in the famous “direct-viable-count” technique (21). This technique consists of preventing the separation of daughter cells by blocking DNA gyrase; as a result, what ought to have been daughter cells form only one filament. As written by Barer (7), “VBNC is a state of measurable or even high activity that cannot be considered as dormancy that is a state of low metabolic activity from which cells can emerge and become culturable,” i.e., form macrocolonies on an agar nonselective medium that are visible by the naked eye. It is often written that VBNC cells are not able to divide (7), but to our knowledge, the expression “VNBC” was created by the team of Colwell in 1986 (16) to designate cells counted by the direct-viable-count method and thus cells that are able to divide. Division of VBNC cells in the conditions that prevail in food industry premises has never been demonstrated before and likely not in other environments. The VBNC state is usually studied using nonculturable cells obtained after long-term starvation of cells that were initially culturable (11, 14, 18, 27, 35). Some of the CFU enter the VBNC state (or lose culturability) after starvation. In such experiments, the division of VNBC cells is not possible because there are no nutrients available.
The numbers of adhering total populations obtained in the laboratory are of the same order of magnitude as those observed in different food processing premises (around 106 to 107 cells·cm−2) by three different researchers: Alliot (1), in a cheese-making environment, with total cells counted with a Thomas chamber; Mettler (24) on gaskets from a milk pasteurization line, with total cells counted by acridine-orange staining (green and orange cells); Mettler (unpublished results), in a cheese-making environment, with total cells counted by DAPI and CTC staining; and Peneau (unpublished results), in a catering kitchen, with total cells counted with a Malassez chamber.
It should be noted that cells accumulate without any additional input of microbial cells. We may therefore conclude that the phenomenon observed is virtually inevitable, because all it takes for the flora to get established is a single microbial input. In this case, the microbial input was 700 μl of a suspension containing 105 CFU·ml−1 applied to a coupon surface of 4.5 cm2. It would be interesting to look for a threshold below which cell accumulation cannot take place. In the experiments conducted with a cleaner and a disinfectant, the chemical products were used at half the levels of minimum recommended use. It is likely that with the recommended concentrations, bacterial cells would have been killed after the first cleaning and disinfection. However, because of the presence of water on the surfaces, or because of the inaccessibility of some places, the real concentration in contact with food industry surfaces may be lower than what is expected and close to what is used here.
Another fact revealed by these experiments, which was observable whether we consider the CFU or the total cells, is that after the third day of experiments, the daily shock applied to the cells does not prompt the cells to enter a new lag phase, even though the chemical treatment causes nearly 1 DR in the cell population. The lag time observed when cells in stationary phase are suspended in meat exudates and deposited on the ceramic tile is about 1.5 days. If the cells returned to a lag phase after the chemical shock, one would expect a lag time to be at least as long as that which follows the starvation that occurs during a culture's stationary phase. If this were the case, there would be no increase in the microbial load because cleaning and disinfection are performed daily. So these cells are behaving very differently compared to cells in the exponential growth phase cultured in broth, which, when subjected to stress, enter a new lag phase. The lag times observed in such cases by Guillier et al. (17), after cells were shocked by a quaternary ammonium compound, were markedly increased: the mean of the individual detection times (the detection time is the time needed to reach a given optical density of the growth medium) was multiplied by 1.7 because of the chemical stress that had caused a 1.5 DR of the CFU. It is well known that some cells in adhering cell populations resist antimicrobials (9, 15). In fact, the survival curves are sharply biphasic, with a first phase in which the population shrinks rapidly, as for planktonic cells, and a second phase in which it declines slowly. This phenomenon can be seen either with aggregated cells forming a thick biofilm or with single adhering cells (15). The present study, which showed no lag phase after the chemical treatment, is another demonstration that at least parts of adhering cells are phenotypically quite different from their planktonic counterparts.
We will be taking these investigations further to better describe the phenomena observed and construct a mathematical model to describe the evolution of the culturable and nonculturable microbial loads. The model would also be based on microbial populations obtained after cleaning and disinfection. In this way, we will be able to test different scenarios to determine the effects of different factors (size of inoculum, workshop temperature, frequency and intensity of cleaning operations, etc.) on the microbial load on the surfaces.
Two major questions remain unanswered. Can the phenomena observed take place with pathogenic bacteria? This is likely, because the VBNC state has been shown for many pathogens, including Salmonella enterica and L. monocytogenes (29). The return to the culturable state has been observed in embryonated eggs (11) or after a preparatory phase in mice (3, 38), but is the return to culturability in a foodstuff or in the human body possible, and is it dangerous? Until we know better the danger that VBNC forms of pathogenic bacteria represent, the large-scale release of such bacteria into the environment should be prevented. Cleaning and disinfection can eliminate small quantities of cells, but where there are large quantities (threshold to be defined), cleaning and disinfection probably cannot completely eliminate them.
Acknowledgments
We are grateful to Marie Cornu and Olivier Cerf for helpful suggestions in writing the manuscript, to Lone Gram and Joanna Veran for suggesting an additional experiment, to Harriet Coleman for the English translation, and to Anne-Marie Leconte for organizational assistance.
This work forms part of S. Peneau's Ph.D. thesis research at the University of Bourgogne, Dijon, France.
Footnotes
Published ahead of print on 2 March 2007.
REFERENCES
- 1.Alliot, M. 1999. Ecologie microbienne des sites industriels fromagers: identification, caractérisation des flores isolées et étude des facteurs régissant leur implantation. Ph.D. thesis. Institut National Agronomique de Paris-Grignon, Paris, France.
- 2.Amann, R. I., W. Ludwig, and K.-H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Anonymous. 1997. Geometrical product specification—surface texture: profile method—terms, definitions and surface texture parameters. ISO 4287. International Organization for Standardization, Geneva, Switzerland.
- 3.Asakura, H., S. Makino, T. Takagi, A. Kuri, T. Kurazono, M. Watarai, and T. Shirahata. 2002. Passage in mice causes a change in the ability of Salmonella enterica serovar Oranienburg to survive NaCl osmotic stress: resuscitation from the viable but non-culturable state. FEMS Microbiol. Lett. 212:87-93. [DOI] [PubMed] [Google Scholar]
- 4.Asséré, A., N. Oulahal-Lagsir, and B. Carpentier. Comparative evaluation of methods for counting surviving biofilm cells adhering to a polyvinyl chloride surface exposed to chlorine or dehydration. J. Appl. Microbiol., in press. [DOI] [PubMed]
- 5.Baffone, W., B. Citterio, E. Vittoria, A. Casaroli, R. Campana, L. Falzano, and G. Donelli. 2003. Retention of virulence in viable but non-culturable halophilic Vibrio spp. Int. J. Food Microbiol. 89:31-39. [DOI] [PubMed] [Google Scholar]
- 6.Baranyi, J., and T. A. Roberts. 1994. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 23:277-294. [DOI] [PubMed] [Google Scholar]
- 7.Barer, M. R. 1997. Viable but non-culturable and dormant bacteria: time to resolve an oxymoron and a misnomer? J. Med. Microbiol. 46:629-631. [DOI] [PubMed] [Google Scholar]
- 8.Binnerup, S. J., D. F. Jensen, H. Thordal-Christensen, and J. Sorensen. 1993. Detection of viable but non-culturable Pseudomonas fluorescens DF57 in soil using a microcolony epifluorescence technique. FEMS Microbiol. Ecol. 12:97-105. [Google Scholar]
- 9.Brooun, A., S. Liu, and K. Lewis. 2000. A dose-response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 44:640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappelier, J. M., V. Besnard, S. Roche, N. Garrec, E. Zundel, P. Velge, and M. Federighi. 2005. Avirulence of viable but non-culturable Listeria monocytogenes cells demonstrated by in vitro and in vivo models. Vet. Res. 36:589-599. [DOI] [PubMed] [Google Scholar]
- 11.Cappelier, J. M., J. Minet, C. Magras, R. R. Colwell, and M. Federighi. 1999. Recovery in embryonated eggs of viable but nonculturable Campylobacter jejuni cells and maintenance of ability to adhere to HeLa cells after resuscitation. Appl. Environ. Microbiol. 65:5154-5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpentier, B., and O. Cerf. 1999. Biofilms, p. 252-259. In R. K. Robinson, C. A. Batt, and P. D. Patel (ed.), Encyclopedia of food microbiology, vol. 1. Academic Press, London, United Kingdom. [Google Scholar]
- 13.Carpentier, B., and D. Chassaing. 2004. Interactions in biofilms between Listeria monocytogenes and resident microorganisms from food industry premises. Int. J. Food Microbiol. 97:111-122. [DOI] [PubMed] [Google Scholar]
- 14.Chmielewski, R. A., and J. F. Frank. 1995. Formation of viable but nonculturable Salmonella during starvation in chemically defined solutions. Lett. Appl. Microbiol. 20:380-384. [DOI] [PubMed] [Google Scholar]
- 15.Frank, J. F., and R. A. Koffi. 1990. Surface-adherent growth of Listeria monocytogenes is associated with increased resistance to surfactant sanitizers and heat. J. Food Prot. 53:550-554. [DOI] [PubMed] [Google Scholar]
- 16.Grimes, D. J., and R. R. Colwell. 1986. Viability and virulence of Escherichia coli suspended by membrane chamber in semitropical ocean water. FEMS Microbiol. Lett. 34:161-165. [Google Scholar]
- 17.Guillier, L., P. Pardon, and J. Augustin. 2005. Influence of stress on individual lag time distributions of Listeria monocytogenes. Appl. Environ. Microbiol. 71:2940-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupte, A. R., C. L. De Rezende, and S. W. Joseph. 2003. Induction and resuscitation of viable but nonculturable Salmonella enterica serovar Typhimurium DT104. Appl. Environ. Microbiol. 69:6669-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holah, J. T., J. H. Taylor, D. J. Dawson, and K. E. Hall. 2002. Biocide use in the food industry and the disinfectant resistance of persistent strains of Listeria monocytogenes and Escherichia coli. J. Appl. Microbiol. 92(Suppl. 1):111S—120S. [PubMed] [Google Scholar]
- 20.Huang, C. T., F. P. Yu, G. A. McFeters, and P. S. Stewart. 1995. Nonuniform spatial patterns of respiratory activity within biofilms during disinfection. Appl. Environ. Microbiol. 61:2252-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kogure, K., U. Simidu, and N. Taga. 1979. A tentative direct microscopic method for counting living marine bacteria. Can. J. Microbiol. 25:415-420. [DOI] [PubMed] [Google Scholar]
- 22.Leriche, V. 1999. Minimisation de l'implantation de Listeria monocytogenes sur les surfaces des ateliers alimentaires par la création et l'entretien de biofilms. Ph.D. thesis. Université de Bourgogne, Dijon, France.
- 23.Leriche, V., and B. Carpentier. 1995. Viable but non-culturable Salmonella typhimurium within single and binary species biofilms in response to chlorine treatment. J. Food Prot. 58:1186-1191. [DOI] [PubMed] [Google Scholar]
- 24.Mettler, E., and B. Carpentier. 1997. Localisation, dénombrement et identification de la contamination microbienne après nettoyage de joints en EPDM d'un circuit de pasteurisation de I'industrie laitière. Lait 77:489-503. [Google Scholar]
- 25.Mettler, E., and B. Carpentier. 1998. Variations over time of microbial load and physicochemical properties of floor materials after cleaning in food industry premises. J. Food Prot. 61:57-65. [DOI] [PubMed] [Google Scholar]
- 26.Midelet, G., and B. Carpentier. 2002. Transfer of microorganisms, including Listeria monocytogenes, from various materials to beef. Appl. Environ. Microbiol. 68:4015-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nayak, B. B., E. Kamiya, T. Nishino, M. Wada, M. Nishimura, and K. Kogure. 2005. Separation of active and inactive fractions from starved culture of Vibrio parahaemolyticus by density dependent cell sorting. FEMS Microbiol. Ecol. 51:179-186. [DOI] [PubMed] [Google Scholar]
- 28.Normander, B., N. B. Hendriksen, and O. Nybroe. 1999. Green fluorescent protein-marked Pseudomonas fluorescens: localization, viability, and activity in the natural barley rhizosphere. Appl. Environ. Microbiol. 65:4646-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver, J. D. 2005. The viable but nonculturable state in bacteria. J. Microbiol. 43:93-100. [PubMed] [Google Scholar]
- 30.Richard, J. 1980. Observations on the value of a swab technique for determining the bacteriological state of milking equipment surfaces. J. Appl. Bacteriol. 49:19-27. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez, G. G., D. Phipps, K. Ishiguro, and H. F. Ridgway. 1992. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl. Environ. Microbiol. 58:1801-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose, L., B. Jensen, A. Peterson, S. N. Banerjee, and M. J. Srduino. 2004. Swab materials and Bacillus anthracis spore recovery from nonporous surfaces. Emerg. Infect. Dis. 10:1023-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roszak, D. B., and R. R. Colwell. 1987. Metabolic activity of bacterial cells enumerated by direct viable count. Appl. Environ. Microbiol. 53:2889-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roszak, D. B., and R. R. Colwell. 1987. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 51:365-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roszak, D. B., D. J. Grimes, and R. R. Colwell. 1983. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can. J. Microbiol. 30:334-338. [DOI] [PubMed] [Google Scholar]
- 36.Sachidanandham, R., K. Y. Gin, and C. L. Poh. 2005. Monitoring of active but non-culturable bacterial cells by flow cytometry. Biotechnol. Bioeng. 89:24-31. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz, T., S. Hoffmann, and U. Obst. 2003. Formation of natural biofilms during chlorine dioxide and u.v. disinfection in a public drinking water distribution system. J. Appl. Microbiol. 95:591-601. [DOI] [PubMed] [Google Scholar]
- 38.Singh, A., R. Yeager, and G. A. McFeters. 1986. Assessment of in vivo revival, growth, and pathogenicity of Escherichia coli strains after copper- and chlorine-induced injury. Appl. Environ. Microbiol. 832-837. [DOI] [PMC free article] [PubMed]
- 39.Wirtanen, G., S. Salo, I. M. Helander, and T. Mattila-Sandholm. 2001. Microbiological methods for testing disinfectant efficiency on Pseudomonas biofilm. Colloids Surf. B Biointerfaces 20:37-50. [DOI] [PubMed] [Google Scholar]
- 40.Yu, F. P., and G. A. McFeters. 1994. Rapid in situ assessment of physiological activities in bacterial biofilms using fluorescent probes. J. Microbiol. Methods 20:1-10. [DOI] [PubMed] [Google Scholar]