Abstract
Lactococcus lactis QU 5 isolated from corn produces a novel bacteriocin, termed lacticin Q. By acetone precipitation, cation-exchange chromatography, and reverse-phase high-performance liquid chromatography, lacticin Q was purified from the culture supernatant of this organism, and its molecular mass was determined to be 5,926.50 Da by mass spectrometry. Subsequent analyses of amino acid and DNA sequences revealed that lacticin Q comprised 53 amino acid residues and that its N-terminal methionine residue was formylated. In contrast to most bacteriocins produced by gram-positive bacteria, lacticin Q had no N-terminal extensions such as leader or signal sequences. It showed 66% and 48% identity to AucA, a hypothetical protein from Corynebacterium jeikeium plasmid pA501, and aureocin A53, a bacteriocin from Staphylococcus aureus A53, respectively. The characteristics of lacticin Q were determined and compared to those of nisin A. Similar to nisin A, lacticin Q exhibited antibacterial activity against various gram-positive bacteria. Lacticin Q was very stable against heat treatment and changes in pH; in particular, it was stable at alkaline pH values, while nisin A was inactivated. Moreover, lacticin Q induced ATP efflux from a Listeria sp. strain in a shorter time and at a lower concentration than nisin A, indicating that the former affected indicator cells in a different manner from that of the latter. The results described here clarified the fact that lacticin Q belongs to a new family of class II bacteriocins and that it can be employed as an alternative to or in combination with nisin A.
Bacteriocins are ribosomally synthesized antimicrobial peptides or proteins that are active against closely related species (17). Those produced by lactic acid bacteria (LAB) have attracted increasing interest in terms of their safety since many LAB are generally regarded as safe (GRAS) (7). Bacteriocins produced by gram-positive bacteria, including LAB, are mainly classified into three classes (18, 26). Class I bacteriocins, the so-called lantibiotics, are heat-stable post-translationally modified peptides that contain multiple rings bridged by lanthionine or 3-methyllanthionine residues (22). Class II bacteriocins are small heat-stable nonlantibiotic peptides and are in turn divided into three subgroups (25, 26). Class IIa bacteriocins are Listeria sp.-active peptides with a consensus YGNGVXC sequence at their N termini (10). Class IIb bacteriocins comprise two peptides, both of which are required for complete antibacterial activity. Class IIc bacteriocins are the other class II bacteriocins; this subclass includes enterocin P, which is processed and secreted by the Sec pathway (4), and enterocin L50 (5), which is secreted without a leader sequence. However, some class II bacteriocins can be placed in more than one subclass, while others do not fit easily into any of the subclasses (27). Class III bacteriocins are heat-labile proteins, including millericin B, that hydrolyze specific peptide bonds in the stem and/or interpeptide bridges in the peptidoglycan layer of susceptible bacteria (1). This classification system may eventually change when new bacteriocin sequences become available, along with information regarding their characteristics. In fact, new classification criteria have recently been suggested by Cotter et al. (8).
Nisin belongs to the class I bacteriocins and is produced by Lactococcus lactis; it is the most extensively studied LAB bacteriocin. It comprises 34 amino acids, including the post-translationally modified amino acids thioether-bridged lanthionine and 3-methyllanthionine and unsaturated 2,3-didehydroalanine and 2,3-didehydrobutyrine. Nisin exhibits antibacterial activity against a wide range of gram-positive bacteria, including LAB and bacteria of the genera Listeria, Staphylococcus spp., Bacillus spp., and Clostridium spp. In addition, nisin is highly stable under conditions of acidic pH and high temperature (9). Thus far, three nisin variants, namely, nisin A (14), nisin Z (24), and nisin Q (33), have been recognized. Nisin A is the only LAB bacteriocin that has been approved as a safe food preservative by the World Health Organization and the Food and Agriculture Organization, and it is used in more than 50 countries (9). However, its low stability at neutral and alkaline pH values limits the range of its use (9), and this is also a drawback of several other LAB bacteriocins. Moreover, the continuous use of only nisin may lead to the emergence of nisin-resistant strains in the environment because at the experimental level, exposure to stepwise-increasing concentrations of nisin was found to render bacterial strains resistant (20, 23). These circumstances give rise to the need for novel bacteriocins that not only compensate for the instability of nisin but also exhibit an antibacterial activity and spectrum comparable to those of nisin.
Among the various LAB bacteriocins that have been identified to date, lactococcal bacteriocins have attracted attention particularly because of their potential use as novel biopreservatives; this is because L. lactis strains are GRAS organisms. Lactococcal bacteriocins such as lacticin 3147 have been widely studied for their potential application (15). L. lactis is a model microorganism that is extensively used in the manufacture of fermentation foods. Its importance is increasing both in terms of application and in fundamental studies wherein it serves as a representative LAB. Furthermore, novel bacteriocins produced by L. lactis are extremely important. Novel lactococcal bacteriocins are in great demand as the next generation of nisin.
In this report, we describe the structural analysis and characterization of lacticin Q, a novel bacteriocin produced by L. lactis QU 5 isolated from corn. The antibacterial activity and spectrum of lacticin Q are comparable to those of nisin. In addition, lacticin Q is highly stable and active under alkaline conditions and exhibits unusually rapid bactericidal action, in contrast to other LAB bacteriocins such as nisin. These unique characteristics and structure indicate that lacticin Q is an unusual bacteriocin belonging to a novel type of class II bacteriocins.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacteriocin-producing strain L. lactis QU 5 was isolated from fresh corn grown in Aso, Kumamoto, Japan, and it was identified by 16S rRNA gene sequencing and sugar fermentation pattern analysis. L. lactis QU 5 was stored at −80°C in MRS medium (Oxoid, Basingstoke, United Kingdom) with 15% glycerol, and before use, it was propagated in MRS medium at 30°C for 18 h. Furthermore, before use, bacterial strains that were used as indicator strains for the bacteriocin assay were propagated for 18 h at the temperature (30°C or 37°C) recommended by culture collections. MRS medium was used to culture all LAB strains, except those of Streptococcus spp. Tryptic soy broth (Difco, Sparks, MD) supplemented with 0.6% yeast extract (Nacalai Tesque, Kyoto, Japan) (TSY) was used to culture Streptococcus strains and other gram-positive indicator strains.
Bacteriocin activity assay.
The bacteriocin assay was performed using the spot-on-lawn method (21), in which 10 μl of twofold dilutions of a bacteriocin preparation was spotted onto a double layer comprising 5 ml of TSY supplemented with 1% agar that was inoculated with an overnight culture of the indicator strains at a level of ca. 107 CFU/ml overlaid on a Tryptic soy agar (Difco) plate supplemented with 0.6% yeast extract. After overnight incubation at temperatures appropriate for the indicator strains, the bacterial lawns were checked for inhibition zones. In the purification steps, the activity titer was defined as the reciprocal of the highest dilution that yielded a clear zone of growth inhibition in the indicator lawn; this value was expressed in arbitrary activity units per milliliter of bacteriocin preparation.
The MICs of the bacteriocins against the various indicator strains were determined using the above-mentioned spot-on-lawn method with purified bacteriocin solutions. The MIC was defined as the minimum bacteriocin concentration that yielded a clear zone of growth inhibition in the indicator lawn.
Bacteriocin purification.
Using a three-step procedure, lacticin Q was purified from the cell-free supernatant of a 250-ml culture of L. lactis QU 5 that was grown to the late exponential phase in MRS broth at 30°C. Proteins, including the bacteriocin, were precipitated using 750 ml cooled acetone, and the resulting precipitates were then collected by centrifugation at 10,000 × g for 10 min and dissolved in 80 ml of 50 mM sodium phosphate buffer (pH 5.6; buffer A). This solution was loaded on an SP Sepharose Fast Flow cation-exchange chromatography column (length, 100 mm, and internal diameter, 10 mm; Amersham Biosciences, Uppsala, Sweden) that was equilibrated with buffer A. After the column was washed with 100 ml buffer A, the bacteriocin was eluted with 20 ml of 0.5 M NaCl in buffer A. This fraction was applied to a 3-ml RESOURCE RPC column (Amersham Biosciences) in an LC-2000Plus high-performance liquid chromatography (HPLC) system (JASCO, Tokyo, Japan). An active fraction was eluted in the following manner with a gradient of H2O-acetonitrile containing 0.1% trifluoroacetic acid at a flow rate of 1 ml/min: 0 to 20 min, 20% to 60% (vol/vol); 20 to 25 min, 60% to 100% (vol/vol); and 25 to 30 min, 100% (vol/vol) acetonitrile. Purified lacticin Q was stored at −30°C. The antibacterial activity of the fraction obtained in each purification step was determined as described above by using Bacillus coagulans JCM 2257T as the indicator strain. The protein concentration of each fraction was estimated using a GeneQuant pro RNA/DNA calculator (Amersham Biosciences) according to the manufacturer's instructions and the following formula: protein concentration (mg/ml) = 1.55 A280 − 0.76 A260.
Nisin A was purified from a commercial nisin preparation (Sigma, St. Louis, MO) by using cation-exchange chromatography and reverse-phase HPLC, as described above.
For MIC determination and characterization of the bacteriocins, the solvents were removed from the purified fractions by using a SpeedVac concentrator (Savant, Farmingdale, NY), and the bacteriocins were dissolved at appropriate concentrations in buffer A supplemented with 0.1% Tween 80 unless mentioned otherwise. The lacticin Q and nisin A concentrations were determined by directly weighing dry matter and by the Lowry method (19), respectively.
Mass spectrometry and amino acid sequencing.
The molecular mass of purified lacticin Q was analyzed by electrospray ionization time-of-flight mass spectrometry (ESI-TOF [MS]) with a JMS-T100LC mass spectrometer (JEOL, Tokyo, Japan). The amino acid sequence was determined based on Edman degradation with a 476A gas-phase automatic sequencer (Applied Biosystems, Foster City, CA).
Cyanogen bromide treatment of lacticin Q.
Purified lacticin Q was dissolved in 70% (vol/vol) formic acid with a 100-fold molar excess of cyanogen bromide. The reaction was performed at 30°C for 24 h. The product was purified by reverse-phase HPLC, as described for the purification of bacteriocins, and it was then used for mass spectrometry and amino acid sequencing as described above.
DNA sequencing analysis.
DNA manipulations were performed according to a previously described standard protocol (30). DNA polymerases, restriction enzymes, and other reagents were used according to the manufacturers' instructions. Total DNA was extracted from L. lactis QU 5 cells treated with lysozyme (Seikagaku, Tokyo, Japan) and cetyltrimethylammonium bromide (Wako, Osaka, Japan) according to previously described procedures (3), and it was subsequently used for PCR and other procedures. The oligonucleotide primers used in this study are listed in Table 1. Degenerate primers (QU5-f1 and QU5-r1) and the L. lactis QU 5 total DNA were employed to amplify a part of the lacticin Q structure gene by using Taq DNA polymerase (Promega, Madison, WI), according to the standard protocol. The products obtained were used as templates for a second PCR using primers QU5-f2 and QU5-r2. The resulting single fragment was cloned into the pGEM-T vector (Promega) and used for DNA sequencing (Macrogen, Seoul, Korea). To amplify the upstream region of the structure gene, ligation-anchored PCR was performed as described previously with some modifications (31, 32). L. lactis QU 5 total DNA was digested with BamHI, EcoRI, HindIII, KpnI, SacI, or XbaI (Nippon Gene, Tokyo, Japan) and was ligated into dephosphorylated pUC18 cloning vector (Toyobo, Osaka, Japan) that was treated with the corresponding restriction enzyme. These six mixtures were used as templates for the PCR using a structure gene-specific primer (ST-1F) and vector-specific primers (1stMup13-f and 1stMup13-r). The second (with the primers ST-2F, Mup13-f, and Mup13-r) and third (with ST-3F, s-M13-f, and s-M13-r) PCRs were performed as described above. The fragments obtained were purified by using a QIAquick PCR purification kit (QIAGEN, Hilden, Germany) and sequenced. Similarly, the DNA sequence of the downstream region was analyzed using the specific primers ST-1R, ST-2R, and ST-3R and vector-specific primers. A single fragment containing the entire lacticin Q gene was amplified using the newly designed specific primers LnqQ-F and LnqQ-R. This reaction was catalyzed by KOD plus DNA polymerase (Toyobo), according to the standard protocol. The product was purified and directly sequenced.
TABLE 1.
Oligonucleotide primers used to amplify and analyze the structure gene of lacticin Q
| Primer name | Sequence |
|---|---|
| QU5-f1 | 5′-ATGGCNGGNTTYYTDAAAGT-3′ |
| QU5-r1 | 5′-GTYCGNTADCTRACCCANA-3′ |
| QU5-f2 | 5′-GTNCARTGGGCNTGGGC-3′ |
| QU5-r2 | 5′-TTYTADTTYGTYTAYRANCC-3′ |
| ST-1F | 5′-GCTAAATATGGTTCTAAAGCTG-3′ |
| ST-1R | 5′-CAGCTTTAGAACCATATTTAGC-3′ |
| 1stMup13-f | 5′-TTAACTATGCGGCATCAGA-3′ |
| 1stMup13-r | 5′-TAATGTGAGTTAGCTCACTC-3′ |
| ST-2F | 5′-GGCAAACAAGGGTAAGATTT-3′ |
| ST-2R | 5′-AAATCTTACCCTTGTTTGCC-3′ |
| Mup13-f | 5′-AAGGCGATTAAGTTGGGTA-3′ |
| Mup13-r | 5′-GTATGTTGTGTGGAATTGTG-3′ |
| ST-3F | 5′-TTAATGCAGGTCAGGCAATA-3′ |
| ST-3R | 5′-AATAGCCTGACCTGCATTAA-3′ |
| s-M13-f | 5′-GTAAAACGACGGCCAGT-3′ |
| s-M13-r | 5′-TTCACACAGGAAACAGG-3′ |
| LnqQ-F | 5′-TTAGCACTACCTTCACTGAA-3′ |
| LnqQ-R | 5′-AGAAAATCGATAATACTAGTGAGAT-3′ |
Computer analysis of DNA and amino acid sequences.
The obtained DNA and amino acid sequences were analyzed using GENETYX-WIN software (GENETYX, Tokyo, Japan). Database searches were performed using BLAST of the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/BLAST/). Alignment analysis of peptides was performed using ClustalW of the DNA Data Bank of Japan (DDBJ; http://clustalw.ddbj.nig.ac.jp/top-e.html).
pH and heat treatment of bacteriocins.
Bacteriocin solutions of various pH values were prepared by dissolving purified lacticin Q or nisin A at a concentration of 0.3 μM in the following buffers (50 mM): glycine-HCl (pH 2.0), sodium acetate (pH 4.0), sodium phosphate (pH 6.0), Tris-HCl (pH 8.0), and glycine-NaOH (pH 10.0).
To examine pH stability, the bacteriocin preparations at various pH values were incubated at 30°C for 3 h. Their pH was then adjusted to 6.0 by mixing with 0.5 M sodium phosphate buffer (pH 6.0), and these solutions were assayed for residual activity. To examine heat stability, the bacteriocin preparations at various pH values were treated at 30°C, 60°C, 80°C, 100°C, and 121°C for 15 min. Their pH was then adjusted to 6.0 as described above, and these solutions were assayed for residual activity. A bacteriocin activity assay was performed using the spot-on-lawn method using B. coagulans JCM 2257T as the indicator strain, as described above.
Analysis of bacteriocin-induced ATP efflux.
Bacteriocin-induced ATP efflux was measured using Lucifer HS (Kikkoman, Noda, Japan), an ATP-luciferase reaction kit, and Lumitester C (Kikkoman), a luminescence-measuring instrument, according to the manufacturer's instructions. Listeria innocua ATCC 33090T, an indicator strain, was propagated in 3 ml of TSY medium at 37°C for 15 h. The cells were harvested by centrifugation at 4°C for 10 min at 9,000 × g, washed twice with 50 mM sodium phosphate buffer (pH 6.0), and suspended in 10 ml of the same buffer. The reaction mixtures for ATP efflux analysis comprised 100 μl each of the cell suspension, purified bacteriocin solution, and the reaction solution from the kit. The bacteriocin concentrations in the reaction mixture were adjusted to 0.75, 1.5, and 15 μM. Samples were collected at the appropriate time intervals and measured for bacteriocin-induced ATP efflux, which was represented in terms of relative light units (RLU).
Nucleotide sequence accession number.
The DNA sequence described in this study has been deposited in DDBJ under the accession number AB235201.
RESULTS
Purification and amino acid sequencing of lacticin Q.
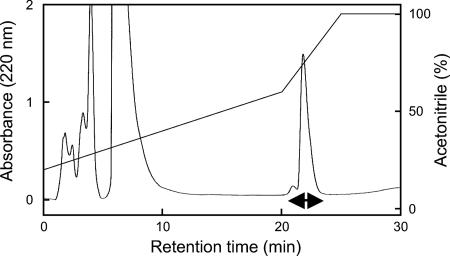
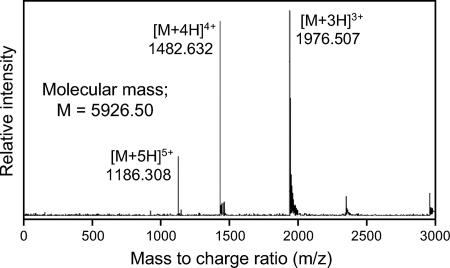
By acetone precipitation, cation-exchange chromatography, and reverse-phase HPLC, the lacticin Q produced by L. lactis QU 5 was purified from culture supernatant as an active fraction (Fig. 1). Overall, a 5,840-fold purification and a 64% yield were obtained, as summarized in Table 2. The purified lacticin Q was subjected to mass spectrometry and amino acid sequencing. ESI-TOF (MS) analysis revealed that its molecular mass was 5,926.50 Da (Fig. 2). No sequence was obtained by Edman degradation, probably because the N terminus of lacticin Q was blocked. Cyanogen bromide treatment yielded a product with a molecular mass of 5,767.70 Da, which is ca. 159 Da less than that of lacticin Q. This indicated that lacticin Q had N-formylmethionine at its N terminus because this formylation results in a 159-Da decrease in the molecular mass after cyanogen bromide treatment. The resulting product was subjected to amino acid sequencing, and the following sequence was obtained: AGFLKVVQLLAKYGSKAVQWAWANKGKILDWLNAGQAIDWVVSKIKQILG. The calculated mass of the obtained sequence was 5,525.47 Da, which is less than the observed mass, indicating that the amino acid sequencing was not complete.
FIG. 1.
Reverse-phase HPLC profile of lacticin Q produced by L. lactis QU 5. Lacticin Q was isolated using a 3-ml RESOURCE RPC column (Amersham Biosciences) at a flow rate of 1.0 ml/min. Antibacterial activity was detected in the fractions indicated by a double-headed arrow.
TABLE 2.
Purification of lacticin Q produced by L. lactis QU 5
| Step | Volume (ml) | Total activity (AU)a | Total protein (mg)b | Specific activity (AU/mg) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|---|
| Culture supernatant | 250 | 2.56 × 106 | 910 | 2.81 × 103 | 1 | 100 |
| Acetone precipitation | 80 | 2.05 × 106 | 270 | 7.51 × 103 | 2.67 | 80 |
| SP Sepharose | 20 | 2.05 × 106 | 4.16 | 4.93 × 105 | 175 | 80 |
| Reverse-phase HPLC | 2 | 1.64 × 106 | 0.1 | 1.64 × 107 | 5,840 | 64 |
Antibacterial activity (in arbitrary units [AU]) was assayed by the spot-on-lawn method using B. coagulans JCM 2257T as an indicator strain.
Protein concentration (mg/ml) was estimated by 1.55 A280 − 0.76 A260.
FIG. 2.
ESI-TOF (MS) of the purified lacticin Q. Multiple charged molecular ions were detected and are indicated.
DNA sequencing analysis for elucidation of the primary structure of lacticin Q.
Degenerate primers were designed on the basis of the amino acid sequence obtained. When PCR was performed with the total DNA from L. lactis QU 5 and these degenerate primers, a 100-bp fragment was amplified. Specific primers designed from the DNA sequence of the fragment were employed for ligation-anchored PCRs to obtain the adjacent sequences. Thus, a region of the lacticin Q structure gene, termed lnqQ, was confirmed. Putative promoter elements, a ribosome-binding site, and a terminator sequence were also identified. The DNA sequence obtained and its deduced amino acid sequence are shown in Fig. 3.
FIG. 3.
DNA sequence of a 420-bp fragment containing the structure gene of lacticin Q. The deduced amino acid sequence of lacticin Q is shown below the DNA sequence. Putative promoter elements (−35 and −10) and a ribosome binding site (RBS) are underlined, and a potential rho-independent transcriptional terminator is indicated by horizontal arrows.
Lacticin Q was found to comprise 53 amino acid residues and lacked a leader or signal sequence. The mass calculated from the amino acid sequence deduced from the DNA sequence was 5,898.00 Da, which is ca. 28 Da less than the observed mass. This suggested that the N-terminal methionine was formylated. In the BLAST search, lacticin Q showed 66% identity to AucA, a hypothetical protein encoded in Corynebacterium jeikeium plasmid pA501 (gene accession number AY266269), and it showed 48% identity to aureocin A53, a bacteriocin produced by Staphylococcus aureus A53 (gene accession number AF447813) (28) (Fig. 4). In addition, it showed 47% identity to mutacin BHT-B, a recently reported bacteriocin produced by Streptococcus rattus strain BHT (gene accession number DQ145753) (16).
FIG. 4.
Alignment of lacticin Q and related peptides. Identical (asterisks), highly conserved (double dots), and weakly conserved (single dots) residues are indicated.
Antibacterial spectra of lacticin Q and nisin A.
The MICs of lacticin Q and nisin A against various gram-positive indicator strains are listed in Table 3. In particular, lacticin Q showed relatively high activity against Bacillus spp. The antibacterial spectrum of lacticin Q was as broad as that of nisin A, and both exhibited MICs at nanomolar levels. However, their patterns were significantly different. Almost half the indicator strains were more sensitive to lacticin Q than to nisin A. The lacticin Q producer L. lactis QU 5 was not highly resistant to lacticin Q or nisin A, whereas the nisin A producer L. lactis NCDO 497 was relatively resistant to both.
TABLE 3.
Antibacterial spectra of lacticin Q and nisin A
| Indicator species | Straina | MIC (nM)
|
|
|---|---|---|---|
| Lacticin Q | Nisin A | ||
| Bacillus cereus | JCM 2152T | 5.3 | 25 |
| Bacillus circulans | JCM 2504T | 33 | 43 |
| Bacillus coagulans | JCM 2257T | 2.7 | 9.0 |
| Bacillus subtilis | JCM 1465T | 140 | 37 |
| Enterococcus faecalis | JCM 5803T | 54 | 88 |
| Enterococcus faecium | JCM 5804T | 56 | 47 |
| Enterococcus mundtii | QU 2 | 22 | 71 |
| Enterococcus hirae | ATCC 10541 | 230 | 47 |
| Lactococcus lactis | ATCC 19435T | 3.5 | 79 |
| Lactococcus lactis | NCDO 497 | 250 | 390 |
| Lactococcus lactis | QU 5 | 98 | 62 |
| Lactobacillus acidophilus | JCM 1132T | 0.24 | 4.0 |
| Lactobacillus alimentarius | JCM 1095T | 33 | 45 |
| Lactobacillus brevis | JCM 1059T | 230 | 180 |
| Lactobacillus casei | JCM 1134T | 900 | 0.20 |
| Lactobacillus coryniformis | JCM 1164T | 110 | 450 |
| Lactobacillus sakei | JCM 1157T | 30 | 15 |
| Leuconostoc mesenteroides | JCM 6124T | 56 | 19 |
| Listeria innocua | ATCC 33090T | 70 | 69 |
| Micrococcus luteus | IFO 12708 | 790 | 88 |
| Pediococcus pentosaceus | JCM 5885 | 310 | 110 |
| Staphylococcus aureus | ATCC 12600T | 1,800 | 47 |
| Streptococcus salivarius | JCM 5707T | 7,200 | 750 |
ATCC, American Type Culture Collection, Manassas, VA; IFO, Institute for Fermentation, Osaka, Japan; JCM, Japan Collection of Microorganisms, Wako, Japan; NCDO, National Collection of Dairy Organisms, Reading, United Kingdom; QU, Laboratory of Microbial Technology, Kyushu University, Fukuoka, Japan.
Stability of lacticin Q against pH and heat treatment.
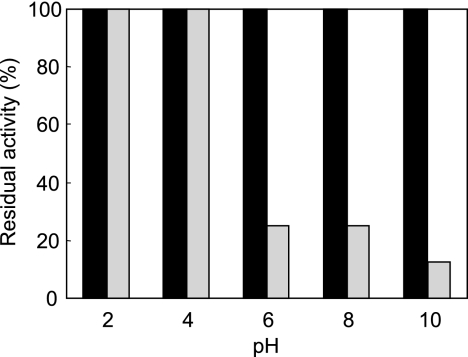
To examine pH stability, the results of lacticin Q were compared with those of nisin A. Lacticin Q was highly stable under a wide range of pH values between 2 and 10, whereas nisin A lost considerable activity at neutral and alkaline pH values (Fig. 5). Lacticin Q retained its activity even after exposure to pH 10, while nisin A completely lost activity. In addition, when each pH-adjusted preparation was directly tested without incubation and pH readjustment, the pH changes were not observed to affect the activity of lacticin Q (data not shown). Lacticin Q was also stable against heat treatment in a pH range of 2 to 10. In this pH range, it was stable against treatment at 100°C for 15 min and retained 25% of its activity even after the usual autoclaving conditions of 121°C for 15 min.
FIG. 5.
pH stability of lacticin Q and nisin A. Preparations of lacticin Q (black) and nisin A (gray) at the indicated pH values were incubated at 30°C for 3 h. Their pH level was then adjusted to 6.0, and the residual activity was assayed using B. coagulans JCM 2257T as the indicator strain. The initial activity level of each at pH 6.0 is indicated as 100%.
ATP efflux induced by lacticin Q and nisin A.
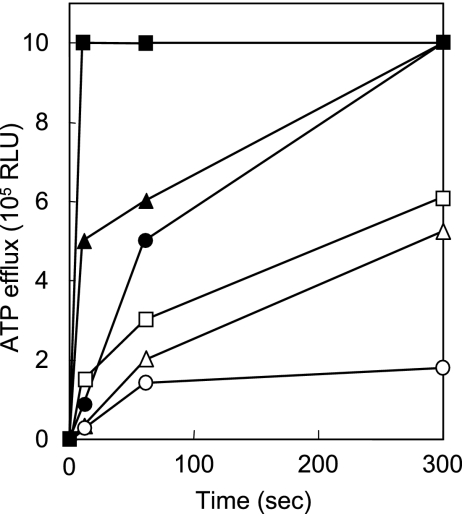
The ATP efflux induced by lacticin Q and nisin A was evaluated using L. innocua ATCC 33090T as the indicator strain; both bacteriocins showed equivalent MICs against this organism (Table 3). As depicted in Fig. 6, both lacticin Q and nisin A induced ATP efflux within 10 s at concentrations higher than 0.75 μM. However, the ATP efflux induced by lacticin Q was apparently faster than that by nisin A at all the concentrations tested. Rapid leakage of ATP due to lacticin Q was also observed at different cell concentrations of the indicator strain (data not shown). The ATP efflux induced by lacticin Q reached the detection limit (1 × 106 RLU) within 300 s, whereas that by nisin A was lower than 6 × 105 RLU after 300 s, even at a concentration of 15 μM.
FIG. 6.
ATP efflux caused by lacticin Q and nisin A. Lacticin Q (closed symbols) and nisin A (open symbols) were tested at concentrations of 0.75 (circle), 1.5 (triangle), and 15 (square) μM. The MICs of both against L. innocua ATCC 33090T that was used as an indicator strain were equivalent, as determined using the spot-on-lawn method (Table 3).
DISCUSSION
In this report, we describe the structural analysis and characterization of a novel bacteriocin, termed lacticin Q, produced by L. lactis QU 5 isolated from corn. Interestingly, we isolated various kinds of bacteriocin-producing LAB from the same corn sample. We isolated not only a nisin Z producer but also L. lactis QU 4, which produces the novel two-peptide bacteriocin lactococcin Q, which is active only against L. lactis strains (34). These three bacteriocins were active against each other's producers (data not shown), indicating that these strains compete in the environment.
By acetone precipitation, cation-exchange chromatography, and reverse-phase HPLC, lacticin Q was purified from the culture supernatant of L. lactis QU 5 at a high yield. The N-terminal sequencing of native lacticin Q was blocked, but cyanogen bromide treatment allowed complete sequencing of lacticin Q. Amino acid and DNA sequence analyses revealed that lacticin Q comprised 53 amino acid residues without a leader or signal sequence, which is present in most bacteriocins produced by gram-positive bacteria. Among the bacteriocins produced by LAB and related species, enterocin I (12), enterocins L50A and L50B (5), enterocin Q (6), aureocin A53 (28), LsbB (13), and mutacin BHT-B (16) have thus far been reported to be synthesized without a typical bacteriocin leader sequence or a sec-dependent signal peptide. To the best of our knowledge, lacticin Q is the second lactococcal bacteriocin after LsbB to be expressed without an N-terminal extension sequence. These bacteriocins have methionine at their N termini, corresponding to the initiation codon. A formylated N-terminal methionine was identified in enterocins L50A and L50B and in aureocin A53, which could be sequenced only after cyanogen bromide treatment, as in the case of lacticin Q. Formylation of the initiation methionine in mutacin BHT-B was deduced by a difference between the predicted and observed molecular masses of this bacteriocin. Such a difference was also reported for enterocin Q. Bacteriocins that lack leader or signal sequences are expected to have N-formylmethionine at their N termini.
Lacticin Q was placed in a large group of antibacterial compounds known as class II bacteriocins. It had no unusual amino acids, and this indicated that it did not belong to class I bacteriocins, which contain post-translationally modified amino acid residues such as dehydrated amino acids and lanthionine. Similar to aureocin A53, lacticin Q was not significantly homologous with hemolysins (28). In addition, purified lacticin Q as well as growing cells of the QU 5 strain exhibited no hemolytic activity on sheep and horse blood agar plates (data not shown).
Among the homologous bacteriocins described above, AucA shows the highest identity to lacticin Q but has not yet been confirmed as a bacteriocin; however, aureocin A53 is well studied and characterized (28, 29). Lacticin Q shares certain characteristics that have been reported for aureocin A53. Both bacteriocins are synthesized without a leader sequence or signal peptide and have N-formylmethionine at their N termini. They are highly cationic (net charge, 6+ for lacticin Q and 8+ for aureocin A53), and this property is important to ensure a strong electrostatic interaction between bacteriocin peptides and the negatively charged phospholipids in bacterial cell membranes. In addition, they are rich in tryptophan (4 in lacticin Q and 5 in aureocin A53), which is known as an amphiphilic amino acid and plays an important role in the interaction between antibacterial peptides and biological membranes (11). Another homolog, namely, mutacin BHT-B, is also similar in that it is a highly cationic tryptophan-rich peptide lacking a leader sequence. Considering these structural characteristics, these peptides can be categorized into a new family in class II bacteriocins of gram-positive bacteria.
Lacticin Q showed antibacterial activity comparable to that of nisin A in terms of both intensity and spectrum. At nanomolar levels, both lacticin Q and nisin A inhibited a wide range of gram-positive indicator strains. However, their antibacterial spectra differed with regard to the MIC pattern. The MICs of lacticin Q against almost half of the indicator strains were less than those of nisin A. This suggested that a combination of the two or the appropriate selection of either one enables more effective control of undesirable bacteria. In addition, the proper use of these bacteriocins could prevent the emergence of bacteriocin-resistant bacteria, which might occur due to the continuous use of only a single antimicrobial agent.
LAB bacteriocins are generally highly stable under acidic conditions, but many of them, including nisin, are easily inactivated under neutral and alkaline conditions (9). This has been an obstacle in the expansion of their application. Lacticin Q was found to be highly stable under neutral and alkaline pH conditions. Additionally, it retained its antibacterial activity after heat treatment under alkaline conditions, which completely inactivated nisin A. This suggested that lacticin Q can compensate for nisin because of its stability, especially under neutral or alkaline conditions.
Lacticin Q induced ATP efflux in a shorter time and at lower concentrations than nisin A, although both showed equivalent MICs against L. innocua ATCC 33090T, as determined by the spot-on-lawn method (Table 3). This indicated that the modes of action of these bacteriocins might be different and should be clarified in detail. In the case of nisin, lipid II in the bacterial cell wall is the primary target for it to bind cells and exhibit antibacterial activity (2). Lacticin Q may use another target or bind without the aid of any docking molecules.
The properties of lacticin Q described in this report can be used to increase the application of LAB bacteriocins. Lacticin Q can be used in conjunction with nisin A to compensate for the disadvantages of the latter, such as its poor stability under alkaline conditions. The proper use of lacticin Q with nisin A will enable more effective control of undesirable bacteria, and the use of lacticin Q would prevent the emergence of resistant variants, which might occur due to the continuous use of only nisin A. Further studies will provide valuable information for studying the atypically rapid mode of action and alkali tolerance of lacticin Q. An explanation of the mechanisms of its biosynthesis based on genetic information is also of great importance to further application of this new bacteriocin.
Acknowledgments
We thank S. Nagata of the University of Tokyo for amino acid sequence analysis and S. Iwatani of Kyushu University for experimental assistance.
This work was partially supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) and by the Urakami Foundation for Food and Food Culture, the Hokuto Foundation for Bioscience, and the Iijima Memorial Foundation for the Promotion of Food Science and Technology.
Footnotes
Published ahead of print on 9 March 2007.
REFERENCES
- 1.Beukes, M., G. Bierbaum, H.-G. Sahl, and J. W. Hastings. 2000. Purification and partial characterization of a murein hydrolase, millericin B, produced by Streptococcus milleri NMSCC 061. Appl. Environ. Microbiol. 66:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breukink, E., I. Wiedemann, C. van Kraaij, O. P. Kuipers, H.-G. Sahl, and B. de Kruijff. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361-2364. [DOI] [PubMed] [Google Scholar]
- 3.Cheng, Y.-J., W.-W. Guo, H.-L. Yi, X.-M. Pang, and X. Deng. 2003. An efficient protocol for genomic DNA extraction from Citrus species. Plant Mol. Biol. Rep. 21:177a-177g. [Google Scholar]
- 4.Cintas, L. M., P. Casaus, L. S. Håvarstein, P. E. Hernández, and I. F. Nes. 1997. Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl. Environ. Microbiol. 63:4321-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cintas, L. M., P. Casaus, H. Holo, P. E. Hernández, I. F. Nes, and L. S. Håvarstein. 1998. Enterocins L50A and L50B, two novel bacteriocins from Enterococcus faecium L50, are related to staphylococcal hemolysins. J. Bacteriol. 180:1988-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cintas, L. M., P. Casaus, C. Herranz, L. S. Håvarstein, H. Holo, P. E. Hernández, and I. F. Nes. 2000. Biochemical and genetic evidence that Enterococcus faecium L50 produces enterocins L50A and L50B, the sec-dependent enterocin P, and a novel bacteriocin secreted without an N-terminal extension termed enterocin Q. J. Bacteriol. 182:6806-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleveland, J., T. J. Montville, I. F. Nes, and M. L. Chikindas. 2001. Bacteriocins: safe, natural antimicrobial for food preservation. Int. J. Food Microbiol. 71:1-20. [DOI] [PubMed] [Google Scholar]
- 8.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 9.Delves-Broughton, J., P. Blackburn, R. J. Evans, and J. Hugenholtz. 1996. Applications of the bacteriocin, nisin. Antonie Leeuwenhoek 69:193-202. [DOI] [PubMed] [Google Scholar]
- 10.Ennahar, S., T. Sashihara, K. Sonomoto, and A. Ishizaki. 2000. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol. Rev. 24:85-106. [DOI] [PubMed] [Google Scholar]
- 11.Fimland, G., V. G. H. Eijsink, and J. Nissen-Meyer. 2002. Mutational analysis of the role of tryptophan residues in an antimicrobial peptide. Biochemistry 41:9508-9515. [DOI] [PubMed] [Google Scholar]
- 12.Floriano, B., J. L. Ruiz-Barba, and R. Jimenez-Diaz. 1998. Purification and genetic characterization of enterocin I from Enterococcus faecium 6T1a, a novel antilisterial plasmid-encoded bacteriocin which does not belong to the pediocin family of bacteriocins. Appl. Environ. Microbiol. 64:4883-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gajic, O., G. Buist, M. Kojic, L. Topisirovic, O. P. Kuipers, and J. Kok. 2003. Novel mechanism of bacteriocin secretion and immunity carried out by lactococcal multidrug resistance proteins. J. Biol. Chem. 278:34291-34298. [DOI] [PubMed] [Google Scholar]
- 14.Gross, E., and J. L. Morell. 1971. The structure of nisin. J. Am. Chem. Soc. 93:4634-4635. [DOI] [PubMed] [Google Scholar]
- 15.Guinane, C. M., P. D. Cotter, C. Hill, and R. P. Ross. 2005. Microbial solutions to microbial problems; lactococcal bacteriocins for the control of undesirable biota in food. J. Appl. Microbiol. 98:1316-1325. [DOI] [PubMed] [Google Scholar]
- 16.Hyink, O., M. Balakrishnan, and J. R. Tagg. 2005. Streptococcus rattus strain BHT produces both a class I two-component lantibiotic and a class II bacteriocin. FEMS Microbiol. Lett. 252:235-241. [DOI] [PubMed] [Google Scholar]
- 17.Jack, W., J. R. Tagg, and B. Ray. 1995. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59:171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-85. [DOI] [PubMed] [Google Scholar]
- 19.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 20.Mazzotta, A. S., A. D. Crandall, and T. J. Montville. 1997. Nisin resistance in Clostridium botulinum spores and vegetative cells. Appl. Environ. Microbiol. 63:2654-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayr-Harting, A., A. J. Hedges, and R. C. W. Berkeley. 1972. Methods for studying bacteriocins. Methods Microbiol. 7A:315-422. [Google Scholar]
- 22.McAuliffe, O., R. P. Ross, and C. Hill. 2001. Lantibiotic: structure, biosynthesis and mode of action. FEMS Microbiol. Rev. 25:285-308. [DOI] [PubMed] [Google Scholar]
- 23.Ming, X., and M. A. Daeschel. 1993. Nisin resistance of foodborne bacteria and the specific resistance responses of Listeria monocytogenes Scott A. J. Food Prot. 56:944-948. [DOI] [PubMed] [Google Scholar]
- 24.Mulders, J. W. M., I. J. Boerrigter, H. S. Rollema, R. J. Siezen, and W. M. de Vos. 1991. Identification and characterization of the lantibiotic nisin Z, a natural nisin variant. Eur. J. Biochem. 201:581-584. [DOI] [PubMed] [Google Scholar]
- 25.Nes, I. F., D. B. Diep, L. S. Håvarstein, M. B. Brurberg, V. G. H. Eijsink, and H. Holo. 1996. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek 70:113-128. [DOI] [PubMed] [Google Scholar]
- 26.Nes, I. F., and H. Holo. 2000. Class II antimicrobial peptides produced by lactic acid bacteria. Biopolymers 55:50-61. [DOI] [PubMed] [Google Scholar]
- 27.Nes, I. F., H. Holo, G. Fimland, H. H. Hauge, and J. Nissen-Meyer. 2002. Unmodified peptide-bacteriocins (class II) produced by lactic acid bacteria, p. 81-115. In G. J. Dutton, M. A. Haxell, H. A. I. McArthur, and R. G. Wax (ed.), Peptide antibiotics. Discovery, mode of action, and applications. Marcel Dekker, Inc., New York, NY.
- 28.Netz, D. J. A., R. Pohl, A. G. Beck-Sickinger, T. Selmer, A. J. Pierik, M. C. F. Bastos, and H.-G. Sahl. 2002. Biochemical characterisation and genetic analysis of aureocin A53, a new, atypical bacteriocin from Staphylococcus aureus. J. Mol. Biol. 319:745-756. [DOI] [PubMed] [Google Scholar]
- 29.Netz, D. J. A., M. C. F. Bastos, and H.-G. Sahl. 2002. Mode of action of the antimicrobial peptide aureocin A53 from Staphylococcus aureus. Appl. Environ. Microbiol. 68:5274-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Troutt, A. B., M. G. McHeyzer-Williams, B. Pulendran, and G. J. V. Nossal. 1992. Ligation-anchored PCR: a simple amplification technique with single-sided specificity. Proc. Natl. Acad. Sci. USA 89:9823-9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaughan, A., V. G. H. Eijsink, and D. van Sinderen. 2003. Functional characterization of a composite bacteriocin locus from malt isolate Lactobacillus sakei 5. Appl. Environ. Microbiol. 69:7194-7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zendo, T., M. Fukao, K. Ueda, T. Higuchi, J. Nakayama, and K. Sonomoto. 2003. Identification of the lantibiotic nisin Q, a new natural nisin variant produced by Lactococcus lactis 61-14 isolated from a river in Japan. Biosci. Biotechnol. Biochem. 67:1616-1619. [DOI] [PubMed] [Google Scholar]
- 34.Zendo, T., S. Koga, Y. Shigeri, J. Nakayama, and K. Sonomoto. 2006. Lactococcin Q, a novel two-peptide bacteriocin produced by Lactococcus lactis QU 4. Appl. Environ Microbiol. 72:3383-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]