Abstract
The specific dechlorination pathways for Aroclor 1260 were determined in Baltimore Harbor sediment microcosms developed with the 11 most predominant congeners from this commercial mixture and their resulting dechlorination intermediates. Most of the polychlorinated biphenyl (PCB) congeners were dechlorinated in the meta position, and the major products were tetrachlorobiphenyls with unflanked chlorines. Using PCR primers specific for the 16S rRNA genes of known PCB-dehalogenating bacteria, we detected three phylotypes within the microbial community that had the capability to dechlorinate PCB congeners present in Aroclor 1260 and identified their selective activities. Phylotype DEH10, which has a high level of sequence identity to Dehalococcoides spp., removed the double-flanked chlorine in 234-substituted congeners and exhibited a preference for para-flanked meta-chlorines when no double-flanked chlorines were available. Phylotype SF1 had similarity to the o-17/DF-1 group of PCB-dechlorinating bacteria. Phylotype SF1 dechlorinated all of the 2345-substituted congeners, mostly in the double-flanked meta position and 2356-, 236-, and 235-substituted congeners in the ortho-flanked meta position, with a few exceptions. A phylotype with 100% sequence identity to PCB-dechlorinating bacterium o-17 was responsible for an ortho and a double-flanked meta dechlorination reaction. Most of the dechlorination pathways supported the growth of all three phylotypes based on competitive PCR enumeration assays, which indicates that PCB-impacted environments have the potential to sustain populations of these PCB-dechlorinating microorganisms. The results demonstrate that the variation in dechlorination patterns of congener mixtures typically observed at different PCB impacted sites can potentially be mediated by the synergistic activities of relatively few dechlorinating species.
Polychlorinated biphenyls (PCBs) were widely used between 1929 and the late 1970s for industrial applications requiring chemical stability, low flammability, and high vaporization temperature. The stable properties of these compounds led to their widespread accumulation in the environment, first documented in the 1960s, and to growing concerns about the effects of these environmental contaminants on the health of humans and wildlife (48). Although the manufacture of PCBs stopped in most countries by the late 1970s, they remain ubiquitous contaminants transported globally in the air, water, and in suspended sediment (32, 34). As a result of these concerns, PCBs are listed as priority organic pollutants by the EPA (http://nlquery.epa.gov).
Historically, their use as dielectric fluid of liquid-filled transformers represented the second largest usage of PCBs (approximately 30%), of which the predominant commercial form between 1930 and 1971 was Aroclor 1260 (53). Aroclor 1260 is a mixture of highly chlorinated PCB congeners and is less susceptible to loss by volatilization and transformation by microbial activity than less-chlorinated Aroclor mixtures (4, 44). This might be due to a combination of factors, including (i) lower bioavailability caused by greater hydrophobicity, (ii) greater toxicity of individual more highly chlorinated congeners, and (iii) the lack of less-chlorinated PCB congeners associated with the stimulation of microbial transformation (44). However, despite this lower susceptibility to biotransformation, microbial transformation of Aroclor 1260 by anaerobic reductive dechlorination was reported as early as 1987 (14, 15), and several investigators since then have shown reductive dechlorination of Aroclor 1260 in sediment, as well as laboratory microcosms (4, 6, 8, 11, 33, 44, 45, 51, 52, 61, 62). Brown and coworkers (15) proposed that microorganisms could use PCBs as electron acceptors for respiration, enabling them to occupy a unique niche in anaerobic environments where other electron acceptors are limiting.
Aroclor 1260 is reductively dechlorinated through diverse patterns of congener transformations, depending on the contaminated sediment source and, presumably, the community of PCB-dechlorinating bacteria present (4, 8, 10, 14, 15, 44). Several investigators have attempted to isolate or identify microorganisms responsible for the reductive dechlorination of PCBs (36, 39, 47, 64), and although earlier studies suggested that dechlorination supported microbial growth (18, 31, 61), identification of the microbial catalysts by enrichment and isolation remained elusive. The first PCB-reducing bacteria were identified when the microbial communities in two sediment-free cultures with different dechlorination specificities were characterized by comparative sequence analysis of PCR-amplified 16S rRNA genes (21, 43, 56, 58). The dehalogenating microorganism o-17, which selectively dechlorinates PCB congeners with flanked ortho- and flanked meta-chlorines (22), and DF-1, which selectively dechlorinates congeners with double-flanked chlorines (59), were shown to belong to a deep branch of the phylum Chloroflexi, with their 16S rRNA gene sequences approximately 90% identical to that of the chloroethene-dechlorinating microorganism Dehalococcoides ethenogenes (38). Species within the Dehalococcoides group have been shown to reductively dechlorinate a number of chlorinated compounds (3, 16, 20, 27, 28). Indeed, Dehalococcoides ethenogenes 195 has since been shown to dechlorinate 2,3,4,5,6-pentachlorobiphenyl and other aromatic organochlorines when grown with tetrachloroethene (25); however, the authors did not investigate whether PCB alone could support growth of D. ethenogenes 195. Using 16S rRNA gene primers, Bedard and coworkers (6) identified phylotypes similar to Dehalococcoides spp. in a sediment-free culture dechlorinating Aroclor 1260, further suggesting that Dehalococcoides spp. and related microorganisms within the phylum Chloroflexi are the likely catalysts for the reductive dehalogenation of PCBs in the environment. However, there are currently no reports on how many different microorganisms are required to reductively dechlorinate a commercial PCB mixture such as Aroclor 1260 into less-chlorinated congeners containing unflanked chlorines, which are the terminal products of the dechlorination process.
All prior reports on the selective activities of PCB-dechlorinating bacteria have been conducted with individual PCB congeners. Previously, we showed (24) that two phylotypes, DEH10 and SF1, with high sequence similarity to Dehalococcoides spp. sequentially dechlorinated 2,2′3,3′,4,6′-hexachlorobiphenyl (abbreviated as 234-236-CB or PCB 132) to PCB 91 (236-24-CB) and to PCB 51 (24-26-CB). This was the first report to demonstrate the sequential dechlorination of a PCB congener by the synergistic activities of two PCB-dechlorinating microorganisms. Here, we report that these two phylotypes, in addition to a third phylotype, SF2, which is similar to o-17, are selectively enriched during the reductive dechlorination of Aroclor 1260 in Baltimore Harbor (BH) sediment microcosms. Using sediment microcosms containing the 11 most predominant individual PCB congeners of this Aroclor, we show that most steps in these processes are linked to the growth of these phylotypes, which suggests that PCB-impacted environments can sustain these PCB-dechlorinating organisms. Individual PCB dechlorination pathways, terminal end products, and the microorganisms responsible for each step in the pathways are also reported for the first time. Interestingly, these three phylotypes were responsible for the anaerobic dechlorination of Aroclor 1260 to congeners containing unflanked chlorine congeners, which could be subsequently oxidized by aerobic PCB-degrading bacteria (1). The characterization of the selective and synergistic activities of Aroclor 1260-dechlorinating microorganisms reported here is an essential development for designing effective in situ treatment strategies of PCB-impacted sites.
MATERIALS AND METHODS
Anaerobic enrichment cultures.
Sediment from BH, an estuarine tributary in the Chesapeake Bay, was used to prepare microcosms as previously described (24) with a defined, low-sulfate (<0.3 mM), estuarine salts medium (E-Cl) and a fatty acid mixture (acetate, propionate, and butyrate at 2.5 mM each) as the electron donor and carbon source (12). Aroclor 1260 and individual PCB congeners were each added to a final concentration of 50 ppm (mg/liter) in acetone (24). Controls with solvent only and sterile controls were also prepared as described previously (24). Aroclor 1260 and 12 individual PCB microcosms (PCBs 194, 187, 183, 180, 174, 170, 153, 151, 149, 138, 132, and 101) (defined in Table 1) were transferred four times after dechlorination activity had been detected (except microcosms with PCB 194, which never showed dechlorination activity). To confirm the PCB dechlorination pathways, individual microcosms were then subcultured with intermediate PCB congeners (PCBs 147, 154, 146, 135, 90, 130, 137, 102, 99, 95, 92, and 91) detected after incubation with the parent PCB congener. All cultures were inoculated (1 ml) in triplicate and incubated at 30°C in the dark. The results reported here, including the Aroclor 1260 cultures, represent microcosms assayed after four sequential transfers, with the exception of microcosms dechlorinating PCBs 174, 153, 151 (meta activity), 135, and 95, which were all assayed after three sequential transfers.
TABLE 1.
Pathways and rates of reductive dechlorination for individual PCB congeners
| Parent | Product | Activity | Chlorine removal rate (10−3)a,b | End mol%b,c |
|---|---|---|---|---|
| 187 (2356-245) | 149 (236-245) | ortho-flanked meta | 1.3 (0.3) | 51 (1.5) |
| 183 (2346-245) | 154 (245-246) | Double-flanked meta | 3.1 (0.3) | 21 (3.8) |
| 180 (2345-245) | 153 (245-245) | Double-flanked meta | 2.5 (0.2) | 29 (4.8) |
| 180 (2345-245) | 146 (235-245) | Double-flanked para | 2.4 (0.2) | 29 (4.8) |
| 174 (2345-236) | 149 (236-245) | Double-flanked meta | 3.2 (1.9) | 44 (3.3) |
| 170 (2345-234)d | 138 (234-245) | Double-flanked meta | 1.5 (0.1) | 47 (2.6) |
| 137 (2345-24) | Double-flanked meta | |||
| 130 (234-235) | Double-flanked para | |||
| 154 (245-246) | 100 (246-24) | para-flanked meta | 0.7 (0.2) | 66 (4.0) |
| 153 (245-245) | 99 (245-24) | para-flanked meta | 3.3 (0.5) | 12 (4.0) |
| 151 (2356-25) | 95 (236-25) | ortho-flanked meta | 5.5 (2.0) | 24 (17) |
| 151 (2356-25) | 92 (235-25) | Flanked ortho | 2.2 | 45 |
| 149 (236-245) | 102 (245-26) | ortho-flanked meta | 6.7 (0.7) | 30 (3.0) |
| 147 (2356-24) | 91 (236-24) | ortho-flanked meta | 0.8 (0.2) | 70 (3.5) |
| 146 (235-245) | 90 (235-24) | para-flanked meta | 2.9 (0.5) | 36 (0.9) |
| 138 (234-245) | 99 (245-24) | Double-flanked meta | 6.1 (0.3) | 60 (6.6) |
| 137 (2345-24) | 99 (245-24) | Double-flanked meta | 7.3 (1.2) | 4 (0.7) |
| 137 (2345-24) | 90 (235-24) | Double-flanked para | 4.5 (1.7) | 4 (0.7) |
| 135 (235-236) | 94 (235-26) | ortho-flanked meta | 4.5 (0.5) | 30 (8.9) |
| 132 (234-236) | 91 (236-24) | Double-flanked meta | 6.2 (1.3) | 61 (5.7) |
| 130 (234-235) | 90 (235-24) | Double-flanked meta | 2.0 (0.6) | 41 (5.7) |
| 102 (245-26) | 51 (24-26) | para-flanked meta | 10 (0.6) | 14 (2.3) |
| 101 (245-25) | 49 (24-25) | para-flanked meta | 15 (2.4) | 13 (9.3) |
| 99 (245-24) | 47 (24-24) | para-flanked meta | 9.5 (1.3) | 19 (8.8) |
| 95 (236-25) | 53 (25-26) | ortho-flanked meta | 8.2 (1.8) | 32 (9.1) |
| 92 (235-25) | 52 (25-25) | ortho-flanked meta | 3.0 (0.4) | 13 (5.7) |
| 92 (235-25) | 72 (25-35) | Flanked ortho | 0.9 (0.1) | 13 (5.7) |
| 91 (236-24) | 51 (24-26) | ortho-flanked meta | 17 (1.5) | 12 (7.7) |
| 90 (235-24) | 49 (24-25) | ortho-flanked meta | 1.6 (0.7) | 19 (2.4) |
| 90 (235-24) | 68 (24-35) | Flanked ortho | 1.7 (0.5) | 19 (2.4) |
Number of chlorines removed per biphenyl molecule per day.
Standard deviations are shown in parentheses.
After 150 to 400 days of incubation.
Multiple intermediates detected.
Analytical techniques.
Microcosms were sampled for PCB dechlorination every 50 days by vortexing the culture to suspend the sediment and removing 0.5 ml of culture with a 1-ml glass pipette. PCBs were extracted with hexane, passed through a copper-Florisil (1:4) column, and analyzed using a gas chromatograph equipped with a Ni63 electron capture detector as described previously (12). PCB congeners were identified and quantified as described by Fagervold et al. (24). Dechlorination curves for all of the PCB congeners were based upon mol%. The total amount of PCBs was determined in each replicate, and the mol% was calculated for each congener in the sample. The average mol% and the standard deviation for each congener were determined from triplicate cultures.
Calculation of dechlorination rates.
Dechlorination curves were made for all PCB congeners in 50-day intervals over the course of incubation. Dechlorination rates for each congener were determined by calculating number of moles dechlorinated over time within the linear slope of the dechlorination curve. In instances where a congener was dechlorinated in several positions, the dechlorination rate was calculated from the total increase in concentration of each of the daughter products. The dechlorination rate was calculated by dividing the mole amount dechlorinated by the total number of moles present in the culture and the time elapsed in days. The average rate and the standard deviation were calculated from triplicate cultures (Table 1).
Bacterial community 16S rRNA gene analyses.
DNA from pooled samples (0.5 ml from each culture replicate) was extracted every 50 days using a Fast DNA SPIN for soil kit (MP Biochemicals, Solon, OH) or UltraClean soil DNA kit (Mo Bio, Carlsbad, CA) according to manufacturers' protocols. The concentration was determined using a DU 650 spectrophotometer (Beckman, Fullerton, CA), and DNA extracts were diluted with Tris-EDTA (TE) buffer to 10 μg/ml. Diluted DNA (1 μl) was used in all subsequent PCRs for a total of 1 ng of DNA.
Microbial community DNA from Aroclor 1260 and individual PCB congener microcosms was amplified by PCR with either universal 16S rRNA gene primers (40), which resulted in PCR fragments of approximately 160 bp, or primers specific for 16S rRNA genes of a monophyletic group within the phylum Chloroflexi, Chl348FGC and Dehal884R, as described previously (24), which resulted in PCB products of approximately 470 bp. Denaturing gradient gel electrophoresis (DGGE) was performed as described by Watts et al. (56) using the D-Code universal mutation detection system (Bio-Rad, Hercules, CA.). Briefly, 6% (wt/vol) polyacrylamide gels (Sigma, St. Louis, MO) containing a 30 to 70% denaturing gradient and fragments were separated by electrophoresis for 18 h at 75 V. The gels were stained with SYBR green 1 DNA stain (Molecular Probes, Eugene, OR) and visualized using a Storm PhosphorImager (GE Healthcare, Piscataway, NJ). DGGE bands of interest were excised and DNA was eluted by incubation in 30 μl TE buffer overnight at 4°C. PCR and DGGE were repeated until purity was confirmed for DNA fragments in each eluted band.
DNA sequencing and analysis.
PCR products from excised bands were used as templates for dye terminator cycle sequencing using the Big Dye 3.1 kit (Applied Biosystems, Foster City, CA) and an AB3100 genetic analyzer (Applied Biosystems). Sequences were examined for errors and assembled using the software Pregap4 and Gap4 of the Staden software package (http://sourceforge.net/projects/staden). Chimera formation was examined using Chimera Check (19). Sequences similarities were analyzed using the Basic Local Alignment Search Tool (BLAST) (5). In order to taxonomically classify sequences, we used the “classify” program by the Joint Genomic Institute (JGI) Greengenes server (http://greengenes.lbl.gov) after sequences were aligned using the align tool from the Greengenes NAST server (23).
Enumeration of PCB-dechlorinating phylotypes.
Putative dehalogenating members of the phylum Chloroflexi were enumerated by a competitive PCR (cPCR) assay (B. Kjellerup, X. Sun, U. Ghosh, H. May and K. Sowers, submitted for publication). A competitor was constructed based on the primers 348F/884R by using the DNA template supplied in the competitive DNA construction kit (RR017; TaKaRa Bio, Inc., Japan). Briefly, the numbers of 16S rRNA gene copies per μl of normalized DNA sample (1 ng DNA per μl) were determined according to the manufacturer's instructions (TaKaRa Bio). DNA samples (10 μg/ml) were amplified as described above for 35 PCR cycles with 1/10 dilutions of a competitor template with a known concentration. The ratio of the target PCR product to the competitor PCR product (T/C) measured by densitometry was determined using the image analysis software Quantity One (Bio-Rad, Hercules, CA) and log T/C was plotted against the log of copies of the competitor. The regression equation was solved for log C/T = 0 (i.e., equal amounts of target and competitor).
Nucleotide sequence accession numbers.
The 16SrRNA gene sequences for DGGE fragments have been submitted to GenBank under accession no. EF150839 to EF150845.
RESULTS
Dechlorination of Aroclor 1260.
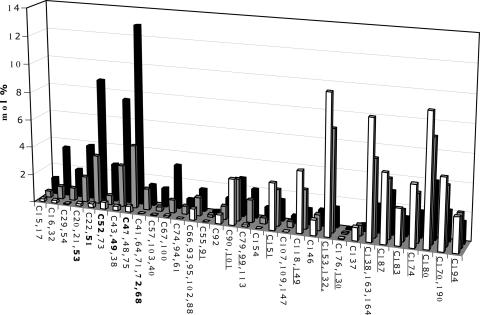
Sediment from BH was used to enrich for Aroclor 1260-dehalogenating microorganisms because it contains historically high concentrations of this PCB mixture in sediments that are anaerobic within 2 cm of the sediment-water interface as a result of high organic loading. BH microcosms incubated with Aroclor 1260 showed significant dechlorination activity within 100 days that continued through 400 days. Generally, the lag time decreased with each transfer (data not shown). Figure 1 shows the mol% distribution of each of the 12 most predominant congeners in Aroclor 1260, the intermediate congeners, and products at days 0, 100, and 400 in the fifth sequential transfer.
FIG. 1.
PCB congener distribution of the 12 most predominant congeners in Aroclor 1260 microcosms. Bars represent congener distributions at days 0 (white bars), 100 (gray bars), and 400 (black bars). Congeners that represent less than 0.05 wt% in Aroclor 1260 are not included (26). The 12 most dominant congeners are underlined, and the major congener end products are shown in bold text.
All of the 12 most predominant PCB congeners in the Aroclor 1260 mixture were dechlorinated, but the extent of dechlorination varied among congeners. The highly chlorinated congener PCB 194 (2345-2345) decreased by 1 mol% from day 0 to day 400, which is equivalent to 40% total dechlorination of this congener. In contrast, some of the less-chlorinated congeners were dechlorinated to a greater extent. For example, the combined decrease of PCB 153 (245-245) and PCB 132 (234-236), which coelute, was from 10 mol% ± 0.5 mol% to 1.4 mol% ± 1.3 mol%, constituting 86% total dechlorination. The predominant dechlorination products of Aroclor 1260 after 400 days of incubation were PCB 47 (24-24) at 12.4 mol% ± 2.1 mol%, PCB 49 (24-25) at 7.5 mol% ± 1.0 mol%, and PCB 51 (24-26) at 8.74 mol% ± 1.68 mol%. Dechlorination was not detected in sterile controls.
Dechlorination of individual Aroclor 1260 congeners.
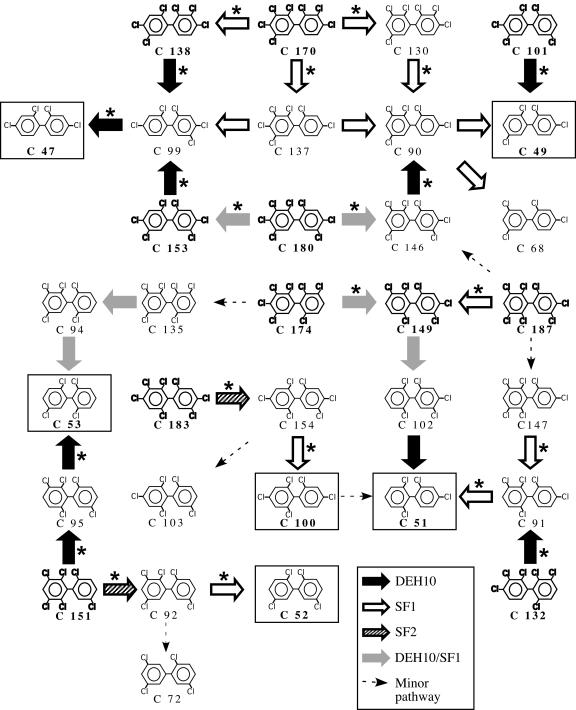
BH sediment microcosms incubated with each of the 12 most predominant PCB congeners in Aroclor 1260 lagged between 3 and 6 months before dechlorination was detected in the initial microcosms, but the lag time generally decreased to less than 50 days by the fourth transfer (data not shown). The PCB congeners used in this experiment are listed in Table 1, except PCB 194 and PCB 94, which were not dechlorinated 500 days and 200 days, respectively, after the initial enrichment inoculation. Figure 2 shows the dechlorination pathways from each of the starting congeners to the final products. Table 1 describes the positions of the target chlorines, the rate of each reaction, and the end mol% for each of the starting congeners. The dechlorination rates of the parent compounds were lower than the dechlorination rates of the daughter compounds, with one exception: PCB 183 (2346-245) was dechlorinated more rapidly than PCB 154 (245-246). Also, the products in the single-congener experiments were the same products observed in the Aroclor 1260 mixture (Fig. 1 and 2), confirming that dechlorination pathways with the individual congeners were representative of the activities observed with the Aroclor.
FIG. 2.
PCB dechlorination pathways of the predominant PCB congeners in Aroclor 1260. Parent congeners are shown in bold text. The pathways are shown with large arrows that indicate different phylotypes: black solid arrows, DEH10; open arrows, SF1; hatched arrows, SF2; gray solid arrows, both DEH10 and SF1. Small arrows indicate minor pathways. The predominant end products are boxed. Reactions in which there was at least a twofold increase in the number of dechlorinating phylotypes relative to the no-PCB control are indicated with asterisks.
Identification of dechlorinating phylotypes in Aroclor 1260 microcosms.
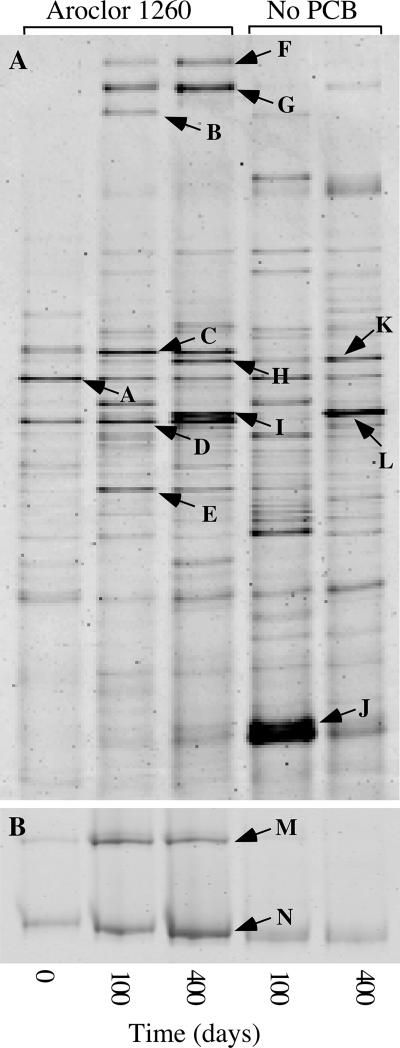
DGGE analysis of 16S rRNA genes amplified with universal primers from the dechlorinating microcosms as well as the no-PCB control at days 0, 100, and 400 are shown in Fig. 3A. DNA fragments identified by DGGE of the Aroclor 1260 microcosm (Fig. 3) were excised, purified, and sequenced (Table 2). The sequence from fragment A was 97% identical to several clones identified in PCB- and dioxin-dechlorinating microcosms (63, 65). However, since the DGGE fragment corresponding to this sequence was present with PCB and in the no-PCB controls, this phylotype is likely not growing by reductive dechlorination of the added PCB. Comparative sequence analyses of fragments B and J indicate they are chimeras, and DNA fragments F and G represent a single sequence with high similarity to Spirochaetes. Fragment H was 100% identical to Thermotogales found in a fosmid library constructed from Baltimore Harbor sediments (41). We could detect phylotypes for two putative dehalogenators: fragment D was 100% identical to phylotype SF1, and fragment C was 100% identical to phylotype DEH10. Both phylotypes were described previously from BH sediment microcosms enriched with PCBs 101 (245-25-CB) and 132 (2346-23-CB) (24). Neither of these phylotypes was detected in the no-PCB control, indicating that they were enriched by the Aroclor.
FIG. 3.
DGGE of Aroclor 1260-dechlorinating microcosms and no-PCB controls. Panel A is total DNA PCR amplified with universal primers (40), and panel B is total DNA PCR amplified with primers specific for PCB-dechlorinating phylotypes (24). DNA fragments labeled A to N were sequenced, and comparative sequence analyses are shown in Table 2.
TABLE 2.
Phylogenetic assignment of microorganisms in Aroclor 1260 microcosms
| Banda | Closest relative (accession no.) | % Identity | Phylogenetic groupb |
|---|---|---|---|
| A | Dechlorination associated phylotypesc | 97 | Thermotogae (Firmicutes) |
| Be | Uncultured bacterium clone (EF031090) | 86 | Spirochaetes (Proteobacteria) |
| C | Dehalococcoides spp. (DEH10) | 100 | Chloroflexi |
| D | SF1 (DQ021870) | 100 | Chloroflexi |
| E | Uncultured Bacteriodetes (DQ167087) | 96 | Bacteriodetes |
| F | Uncultured bacterium (AJ853575) | 96 | Spirochaetesd |
| G | Uncultured bacterium (AJ853575) | 96 | Spirochaetesd |
| H | Uncultured Thermotogales (AM184116) | 100 | Thermotogae |
| I | Uncultured bacterium (AB177206) | 89 | SAR406 marine group A (Proteobacteria) |
| Je | Paper mill wastewater bacterium (AY426469.1) | 82 | Spirochaetesd |
| K | Uncultured Thermotogales (AM184116) | 100 | Thermotogae |
| L | Uranium mining waste pile clone (AJ532716.1) | 86 | Chloroflexi (Proteobacteria) |
| M | Dehalococcoides spp. (DEH10) | 100 | Chloroflexi |
| N | SF1 (DQ021870) | 100 | Chloroflexi |
Bands correspond to the DNA fragments in Fig. 3.
Classified according to Hugenholtz with the NCBI classification in parentheses obtained using Greengenes (23). When these two classifications agree, there is no entry in parentheses.
The sequence from fragment A was 97% identical to several clones identified in PCB- and dioxin-dechlorinating microcosms (63, 65).
NCBI could not classify.
Possible chimera.
To increase the detection sensitivity for potential PCB-dechlorinating microorganisms, we conducted DGGE analysis of the Aroclor1260 microcosm with PCR primers that have high specificity for a monophyletic group within the phylum Chloroflexi (Fig. 3B). The results revealed the same two phylotypes detected with universal primers. DNA sequence of fragment M was 100% identical to that of Dehalococcoides spp. phylotype DEH10, and fragment N was 100% identical to phylotype SF1.
Enumeration of Aroclor 1260-dechlorinating phylotypes.
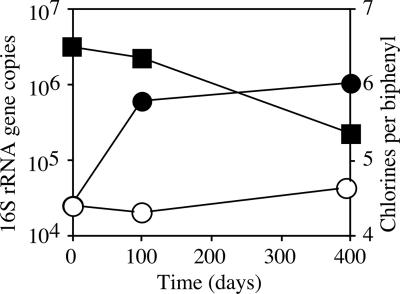
To determine whether dechlorination of Aroclor 1260 supported microbial growth, microorganisms were enumerated in microcosms by cPCR using a selective primer specific set for PCB-dechlorinating phylotypes. The number of 16S rRNA gene copies of putative dechlorinators per μl of normalized DNA from microcosms dechlorinating Aroclor 1260 shows putative dechlorinators increase in numbers as Aroclor 1260 is dechlorinated (Fig. 4). In contrast, the control culture incubated without added Aroclor 1260 showed only a slight increase over 400 days [(2.46 ± 0.18) × 104 to (4.47 ± 0.27) × 104], which could be accounted for by PCB carried over in the transfer from the Aroclor 1260 microcosm. After 400 days of incubation, the number of PCB-dechlorinating phylotypes increased 25-fold in microcosms with Aroclor 1260 added compared to the no-PCB control.
FIG. 4.
Enumeration of PCB-dechlorinating phylotypes in Aroclor 1260 microcosms. Numbers of 16S rRNA gene copies per μl of normalized DNA of putative dechlorinators are shown from Aroclor 1260 microcosms (•) and no-PCB controls (○). Dechlorination activity is shown as the number of chlorines per biphenyl in Aroclor 1260 microcosms (▪). Error bars (not shown) were smaller than the symbols.
Specific dechlorination pathways catalyzed by individual phylotypes.
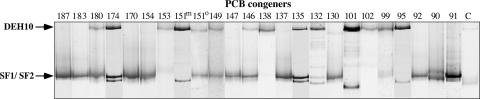
The phylotypes responsible for individual dechlorination reactions were identified in microcosms using 11 of the predominant Aroclor 1260 congeners and subenrichment microcosms with the intermediate products of the congeners. Figure 5 shows the results from each PCB congener microcosm in a composite DGGE gel of DNA amplified with primers specific for dechlorinating phylotypes within the phylum Chloroflexi (24). Sequencing of all DNA fragments revealed three phylotypes. All of the uppermost DNA fragments were 100% identical to phylotype DEH10, and most lower DNA fragments for each congener microcosm were 100% identical to phylotype SF1, which were both detected in the Aroclor enrichment microcosms described above. However, there were two exceptions. In microcosms that dechlorinated PCB 151 in the ortho position (labeled “151°” in Fig. 5) and PCB 183, the lower fragments were 100% identical to PCB-dechlorinating bacterium o-17 (22), which we here call SF2. Figure 2 shows the pathways associated with each phylotype. Additional DNA fragments observed in some lanes (e.g., PCB 132 and PCB 101, lower band) appeared to be PCR artifacts, as repeated attempts to sequence them were unsuccessful (24).
FIG. 5.
Composite DGGE of cultures dechlorinating individual PCB congeners. The primers used are specific for a monophylogenetic group within the phylum Chloroflexi that includes known PCB-dehalogenating microorganisms (24). Numbers indicate PCB congeners. 151m, meta dechlorination of PCB 151; 151°, meta and ortho dechlorination of PCB 151. The control microcosm (C) was incubated without PCB congener.
We performed cPCR enumeration assays on the same microcosms to investigate whether the specific reactions supported the growth of the PCB-dechlorinating phylotypes (Table 3). Reactions that supported at least a twofold increase in the number of putative dechlorinators, compared with the no-PCB control, are indicated with asterisks in Fig. 2. The extent of growth contributed by individual dechlorination reactions could not be distinguished in multistep pathways. For example, PCB congener 138 (234-245) was dechlorinated in two steps, with PCB 99 (245-24) as an intermediate. While it is clear that there was an 8.5-fold increase in the number of putative dechlorinators (Table 3) in the microcosms dechlorinating PCB 99, we could determine the specific increase associated with the reaction step from PCB 138 to PCB 99, since dechlorination of PCB 99 occurs simultaneously in this microcosm. Generally, the extent of growth varied with different congeners. The highest increase was a 110-fold increase during ortho-flanked meta dechlorination of PCB 91 (236-24), while the dechlorination of PCB 102 (245-26) yielded no detectable growth. However, there were no significant differences between the maximum growth ranges of SF1 and DEH10.
TABLE 3.
Enumeration of dechlorinating phylotypes by cPCR
| Congener | No. of 16S rRNA gene copies (103)/μl of normalized DNAa
|
Change (fold) ± PCB | ||
|---|---|---|---|---|
| Day 0 | PCB | No PCB | ||
| 187 | 74 (15) | 410 (46) | 65 (4.2) | 6.2 |
| 183 | 0.3 (0.1) | 370 (16) | 33 (6.3) | 11 |
| 180 | 9.8 (5.7) | 880 (52) | 58 (8.8) | 15 |
| 174 | 19 (1.8) | 150 (9.2) | 25 (5.1) | 5.9 |
| 170 | 14 (1.1) | 350 (42) | 35 (6.3) | 9.9 |
| 154 | 45 (6.6) | 360 (12) | 94 (3.8) | 3.8 |
| 153 | 3.1 (1.0) | 26 (4.9) | 2.3 (0.3) | 11 |
| 151b | NDc | 320 (20) | 19 (1.4) | 17 |
| 151 | ND | 110 (4.6) | 12 (2.1) | 8.8 |
| 149 | ND | 57 (12) | 52 (3.7) | 1.1 |
| 147 | 52 (4.2) | 580 (21) | 43 (6.7) | 13 |
| 146 | 18 (3.0) | 32 (6.3) | 3.1 (0.4) | 10 |
| 138 | 1.9 (0.1) | 490 (44) | 37 (5.5) | 13 |
| 137 | 15 (2.9) | 59 (6.6) | 24 (3.8) | 2.4 |
| 135 | 87 (26) | 140 (17) | 41 (1.2) | 3.3 |
| 132d | 7.5 | 2100 | 150 | 14 |
| 130 | 12 (1.7) | 1300 (140) | 34 (1.9) | 38 |
| 102 | ND | 2.6 (0.1) | 16 (1.3) | 0.2 |
| 101d | 23 | 240 | 23 | 10 |
| 99 | 1.6 (0.2) | 460 (45) | 53 (6.6) | 8.5 |
| 95 | ND | 160 (12) | 9.9 (3.0) | 16 |
| 92 | 0.5 (0.3) | 110 (11) | 6.3 (1.4) | 17 |
| 91b | 9.3 | 460 | 4.3 | 110 |
| 90 | 8.8 (1.4) | 530 (70) | 430 (170) | 1.2 |
Values in parentheses represent standard deviation.
Only meta dechlorination of PCB 151 was observed.
ND, not detected.
Gene copies were calculated from the study by Fagervold et al. (24).
Congener specificity of the PCB-dechlorinating phylotypes.
BH microcosms preferentially dechlorinated double-flanked chlorines, and most of the double-flanked dechlorination was catalyzed by SF1. SF1 dechlorinated all 2345-substituted chlorophenyl rings preferentially in the meta position, although some para dechlorination was observed, such as the dechlorination of PCB 137 (2345-24) to PCB 90 (235-24) (Fig. 2). In addition, SF1 dechlorinated the 234-substituted chlorophenyl ring in PCB 130 (234-235) in the double-flanked meta position. SF1 also dechlorinated 2356-, 236-, and 235-substituted chlorophenyl rings in the ortho-flanked meta position when the other ring contained 245- or 24-substitutions (i.e., PCBs 187, 147, 91, and 90). SF1 dechlorinated PCB 92 (235-25), which contained a 25-substitution on the other ring, in the ortho-flanked meta position. Phylotype SF2 dechlorinated the only 2346-substituted chlorophenyl ring tested (PCB 183) in the double-flanked meta position and the 2356-substituted chlorophenyl ring of PCB 151 in the ortho position.
DEH10 dechlorinated the double-flanked chlorine in 234-substituted chlorophenyl rings, except with PCB 130. DEH10 showed a preference for para-flanked meta-chlorines when no double-flanked chlorines were available and thus dechlorinated 245-substituted chlorophenyl rings in the meta position, with one exception: PCB 154 (245-246), which contains a 246-substitution on the other ring. DEH10 also dechlorinated in the ortho-flanked meta position (PCBs 151 and 95), when the other ring contained 25-substitutions.
DISCUSSION
Patterns of dechlorination of Aroclor 1260 congeners by BH microcosms.
The pattern of Aroclor 1260 dechlorination in BH sediment microcosms resembles “process N,” first identified in Aroclor 1260 microcosms from Silver Lake sediments (44). Process N was described as exclusive dechlorination in the meta position, with a characteristically high accumulation of PCB 47 (24-24). This pattern has been subsequently observed in sediment microcosms from several PCB-impacted freshwater sources, including Woods Pond (8) and the Hudson River (46), and from sediment-free microcosms developed from the Housatonic River (6). However, the patterns observed in sediments from BH differ from the exclusive meta dechlorination pattern reported in freshwater sources by also showing dechlorination of Aroclor 1260 and individual PCB congeners in the ortho position, which is a rarely observed activity in the environment (12, 21, 62). In the present study, the major dechlorination products in the Aroclor 1260 and single-congener experiments were PCB 100 (246-24), PCB 53 (25-26), PCB 52 (25-25), PCB 49 (24-25), and PCB 47 (24-24), all containing unflanked chlorines. Aroclor 1260 microcosms also yielded trace amounts of dichlorobiphenyls and trichlorobiphenyls with unflanked chlorines after 400 days of incubation.
All 12 major parent congeners, which account for over 50 wt% of Aroclor 1260, were dechlorinated in Aroclor 1260 microcosms, including PCB 194, which was not dechlorinated when incubated as an individual congener in BH sediment microcosms. The dechlorination of PCB 194, as well as the accumulation of tri- and dichlorobiphenyls in Aroclor 1260 microcosms, may be due to the presence of multiple congeners that have been shown to have both stimulatory and inhibitory effects on congener specificity by PCB-dehalogenating microorganisms (37, 44). In the present study, the stimulatory effect of specific or multiple congeners in the Aroclor 1260-dechlorinating microcosm likely promoted the reductive dechlorination of PCB 194.
Several investigators have used single PCB congeners to infer PCB dechlorination pathways (2, 12, 13, 24, 42, 46, 49, 52, 57, 60). However, this is the first comprehensive report on the dechlorination of all major PCB congeners present in Aroclor 1260 by single-congener experiments. Several of the pathways in Fig. 2 have been proposed previously from inference of Aroclor 1260 dechlorination products. For example, a previous report with BH Aroclor 1260 microcosms (62) predicted the ortho dechlorination pathway of PCB 151 (2356-25) to PCB 72 (25-35). However, the previously predicted dechlorination pathway from PCB 170 (2345-234) to PCB 68 (24-35) was only partly consistent with our results (Fig. 2), as most of PCB 170 was dechlorinated to either 26 mol% PCB 47 (24-24) or 13 mol% PCB 49 (24-25) at day 400. Several observations were consistent with the dechlorination pathways for PCBs 101, 132, 138, 153, 170, and 180 in “process N,” proposed in a comprehensive review by Bedard and Quensen (10). We determined the dechlorination pathways for PCB 183, which is, to our knowledge, the first report of this pathway. In addition, we defined the pathways of PCB 174 and PCB 151, which were ambiguous in previous reports (6, 7).
In a previous report on dechlorination of Aroclor 1260 in Housatonic River microcosms (6), several proposed dechlorination pathways for 2345-substituted congeners were based upon the assumption that 60% of these congeners were dechlorinated in the double-flanked para position and 40% in the double-flanked meta position. Woods Pond sediments have also been shown to have para dechlorination activity (7, 8). Although we observed some examples of double-flanked para dechlorination, our results showed that dechlorination in BH microcosms more often occurred in the double-flanked meta position. This was especially true for PCB 174 (2345-236), where all of the dechlorination occurred in the double-flanked meta position and for PCB 180 (2345-245), where 67% occurred in the double-flanked meta position and 33% of the dechlorination occurred in the double-flanked para position. On the other hand, PCB 137 (2345-24) was dechlorinated equally in the double-flanked meta and para positions.
Congeners with 2356-substitutions (i.e., PCBs 149, 151, and 187) were dechlorinated in the ortho-flanked meta position. However, we also observed some minor ortho dechlorination of PCB 151 (2356-25), PCB 90 (235-24), and PCB 92 (235-25), which is consistent with prior reports of ortho dechlorination activity of both Aroclor 1260 and single congeners in BH microcosms (12, 21, 22, 37, 62). Based on observations with Aroclor 1260 and the individual congeners, the general sequence of dechlorination in BH microcosms is as follows: double-flanked meta or para position of 2345-substituted chlorophenyl rings, double-flanked meta position of 234- or 2346-substituted chlorophenyl rings, ortho-flanked meta position of 2356-substituted chlorophenyl rings, para-flanked meta position of 245-substituted chlorophenyl rings, ortho-flanked meta position of 236-substituted chlorophenyl rings, ortho-flanked meta position of 235-substituted chlorophenyl rings, and flanked ortho position of 2356-substituted chlorophenyl rings.
Effect of congener characteristics on dehalogenation.
An analysis of (i) the differences in the estimated Gibbs free energy of formation between parent and daughter congeners (29), (ii) the differences in relative retention time (17), (iii) the aqueous solubilities (29), and(iv) the number of total chlorines and the number of ortho-, meta-, and para-chlorines on the ring subjected to dechlorination, as well as on the opposite ring, showed that there were no significant relationships (P < 0.05) between these parameters and the dechlorination rates, end mol%, or the number of putative dechlorinators in BH microcosms. However, we observed weak relationships (with low R2 values) between the dechlorination rate and the aqueous solubility of the PCB congener (r2 = 0.25), the total amount of chlorines (r2 = 0.22), as well as the number of meta-chlorines (r2 = 0.28). Our results indicate that dechlorination rates increased in microcosms with more-soluble PCB congeners and decreased with both the number of total chlorines and the number of meta-chlorines.
Diversity of Aroclor 1260-dehalogenating phylotypes.
PCB-dehalogenating microorganisms have been previously identified as belonging to either Dehalococcoides spp. or the o-17/DF-1 clade within a deep branch of the Chloroflexi (6, 22, 24, 25, 43, 55, 58, 59, 63), and these phylotypes have been detected in microcosms dechlorinating Aroclor 1260 (6). Although the microbial community in the Aroclor 1260 microcosms was diverse (Fig. 3), several lines of evidence indicate that only two phylotypes, DEH10 and SF1, were the predominant biocatalysts of Aroclor 1260 dechlorination: (i) they were only detected in Aroclor 1260 and individual congener microcosms and not detected using universal PCR primers with the no-PCB controls, (ii) they increased in numbers only during active dechlorination of Aroclor 1260 and individual congeners, and (iii) the phylotypes have high sequence similarity to phylotypes and isolates previously shown to reductively dechlorinate PCBs (24, 25, 55). Phylotypes belonging to Bacteriodetes and Spirochaetes (Fig. 3, DNA fragments B and E) were also only present in the dechlorinating cultures, and although similar phylotypes have been previously detected in BH microcosms (43), to date, there is no evidence they reductively dechlorinate PCBs.
When using PCR-based assays of microbial communities, microorganisms with more than one 16S rRNA gene copy (54) and the inherent biases with PCR (50) can influence the results. In our experiments, we compared the diversity and the growth of putative dehalogenators between cultures with and without PCB to detect microbial phylotypes within the microbial community that are specifically associated with active dechlorination of PCBs. Also, since we directly compare the presence of the same phylotypes between dechlorinating microcosms and no-PCB controls, any bias due to multiple 16S rRNA copies or PCR would be the same in both treatments. In some cases, we detected putative dehalogenators in the no-PCB controls (Fig. 3 and Table 3), but this is likely due to enrichment resulting from traces of PCB cotransferred into no-PCB controls during inoculation from active cultures. Dehalococcoides species with very similar or even identical 16S rRNA sequences can have different dechlorination activities (30). Although the 16S rRNA gene sequence of phylotypes DEH10, SF1, and SF2 detected in this study are 100% identical to the phylotypes detected previously in BH sediment microcosms, we cannot confirm that they are each the same species or strain since they came from different microcosms. However, the high identity combined with the fact they each came from the same source and each have the same selective dechlorination activities as previously described phylotypes indicates a high likelihood that they are the same microorganisms.
SF1 and DEH10 exhibited specific activities towards the PCB congeners we tested. The combined activities of SF1 were different from those previously reported for either o-17 (flanked ortho and ortho-flanked meta-chlorines) or DF-1 (double-flanked chlorines). Since phylotype SF2 was only unequivocally associated with two pathways in the present study, this does not represent an adequately comprehensive overview of its specific dechlorinating activities. BH sediment microcosms have been shown previously (62) and here (data not shown) to dechlorinate ortho-chlorines in Aroclor 1260 mixtures. However, our inability to detect SF2 in Aroclor 1260 microcosms suggests that it may have a relatively minor role in the dechlorination process.
When grown with the single congeners or Aroclor 1260, the populations of PCB-dechlorinating microorganisms increased only 1 to 2 orders of magnitude during the course of reductive dehalogenation. These results are consistent with prior reports that showed a similar range of increases for congener mixtures by other methods, including most probable number enumeration and most probable number PCR of 16S rRNA gene copies (18, 24, 31). However, we show unequivocally that phylotypes within Dehalococcoides spp. and the DF-1/o-17-group of the dehalogenating members of the phylum Chloroflexi are directly responsible for the reductive dechlorination of an Aroclor. The relative growth of the individual dechlorinating phylotypes varied among different PCB congeners, increasing up to 2 orders of magnitude, with an average 13.5-fold increase for the 24 congeners tested. Although this supports a conclusion by Kim et al. (31) that the size of the dechlorinating population might be an indicator for PCB dechlorination potential in a site, the results also suggest that other factors, including the types of congeners and the indigenous dechlorinating phylotypes, will have an impact on the size of the population.
Prior reports have shown that Aroclor 1260 is reductively dechlorinated in sediment microcosms, and PCB-dechlorinating microorganisms have been identified from individual congener enrichments. The results of this study show for the first time that the synergistic activities of only three Chloroflexi phylotypes (SF1, SF2, and DEH10) reductively dechlorinated the 11 major PCB congeners in Aroclor 1260 to unflanked tetra- and trichlorobiphenyls in our microcosms. Two of the phylotypes (SF1 and DEH10) were also the predominant phylotypes in Aroclor 1260 enrichments, indicating they likely have a significant role in the dechlorination of other Aroclor 1260 congeners. Demonstration that dechlorination of Aroclor 1260, as well as most of the individual congeners, supports growth of SF1, SF2, and DEH10 suggests that PCB-impacted environments can sustain populations of these PCB-dechlorinating organisms. This is particularly relevant for the development of biostimulation or bioaugmentation strategies for the bioremediation of PCBs. The final products of Aroclor 1260 dechlorination by these three phylotypes (unflanked tetra- and trichlorobiphenyls) can potentially be further transformed by bioaugmentation with microcosms that have been shown to dechlorinate unflanked congeners (9) or could serve directly as substrates for aerobic mineralization by PCB-degrading bacteria (35). Further characterization of the selective and synergistic activities of PCB-dechlorinating microorganisms with different Aroclor mixtures and sediment microcosms is essential for generating models to predict the dechlorination potential at specific PCB-impacted sites and design effective in situ treatment strategies.
Acknowledgments
This work was supported by the Office of Naval Research, U.S. Department of Defense (grant N000014-03-1-0035 to K.R.S. and grant N000014-03-1-0034 to H.D.M.).
We thank Kimberly Anderson, Marcelino T. Suzuki, and Joy E. M. Watts for helpful comments.
Footnotes
Published ahead of print on 9 March 2007.
REFERENCES
- 1.Abramowicz, D. A. 1990. Aerobic and anaerobic biodegradation of PCBs: a review. Crit. Rev. Biotechnol. 10:241-251. [Google Scholar]
- 2.Abramowicz, D. A., M. J. Brennan, H. M. Van Dort, and E. L. Gallagher. 1993. Factors influencing the rate of polychlorinated biphenyl dechlorination in Hudson River sediments. Environ. Sci. Technol. 27:1125-1131. [Google Scholar]
- 3.Adrian, L., U. Szewzyk, J. Wecke, and J. Görisch. 2000. Bacterial dehalorespiration with chlorinated benzenes. Nature 408:580-583. [DOI] [PubMed] [Google Scholar]
- 4.Alder, A. C., M. M. Häggblom, S. R. Oppenheimer, and L. Y. Young. 1993. Reductive dechlorination of polychlorinated biphenyls in anaerobic sediments. Environ. Sci. Technol. 27:530-538. [Google Scholar]
- 5.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 6.Bedard, D. L., J. J. Bailey, B. L. Reiss, and G. Van Slyke Jerzak. 2006. Development and characterization of stable sediment-free anaerobic bacterial enrichment cultures that dechlorinate Aroclor 1260. Appl. Environ. Microbiol. 72:2460-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedard, D. L., S. C. Bunnell, and L. A. Smullen. 1996. Stimulation of microbial para-dechlorination of polychlorinated biphenyls that have persisted in Housatonic River sediment for decades. Environ. Sci. Technol. 30:687-694. [Google Scholar]
- 8.Bedard, D. L., and R. J. May. 1996. Characterization of the polychlorinated biphenyls in the sediments of Woods Pond: evidence for microbial dechlorination of Aroclor 1260 in situ. Environ. Sci. Technol. 30:237-245. [Google Scholar]
- 9.Bedard, D. L., E. A. Pohl, J. J. Bailey, and A. Murphy. 2005. Characterization of the PCB substrate range of microbial dechlorination process LP. Environ. Sci. Technol. 39:6831-6838. [DOI] [PubMed] [Google Scholar]
- 10.Bedard, D. L., and J. F. Quensen. 1995. Microbial reductive dechlorination of polychlorinated biphenyls, p. 127-216. In L. Y. Young and C. E. Cerniglia (ed.), Microbial transformation and degradation of toxic organic chemicals. John Wiley & Sons, New York, NY.
- 11.Bedard, D. L., H. M. VanDort, R. J. May, and L. A. Smullen. 1997. Enrichment of microorganisms that sequentially meta, para-dechlorinate the residue of Aroclor 1260 in Housatonic River sediment. Environ. Sci. Technol. 31:3308-3313. [Google Scholar]
- 12.Berkaw, M., K. R. Sowers, and H. D. May. 1996. Anaerobic ortho dechlorination of polychlorinated biphenyls by estuarine sediments from Baltimore Harbor. Appl. Environ. Microbiol. 62:2534-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyle, A. W., C. K. Blake, W. A. Price III, and H. D. May. 1993. Effects of polychlorinated biphenyl congener concentration and sediment supplementation on rates of methanogenesis and 2,3,6-trichlorobiphenyl dechlorination in an anaerobic enrichment. Appl. Environ. Microbiol. 59:3027-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown, J. F., Jr., D. L. Bedard, M. J. Brennan, J. C. Carnahan, H. Feng, and R. E. Wagner. 1987. Polychlorinated biphenyl dechlorination in aquatic sediments. Science 236:709-712. [DOI] [PubMed] [Google Scholar]
- 15.Brown, J. F., Jr., R. E. Wagner, H. Feng, D. L. Bedard, M. J. Brennan, J. C. Carnahan, and R. J. May. 1987. Environmental dechlorination of PCBs. Environ. Toxicol. Chem. 6:579-593. [Google Scholar]
- 16.Bunge, M., L. Adrian, A. Kraus, M. Opel, W. G. Lorenz, J. R. Andreesen, H. Görisch, and U. Lechner. 2003. Reductive dehalogenation of chlorinated dioxins by an anaerobic bacterium. Nature 421:357-360. [DOI] [PubMed] [Google Scholar]
- 17.Chen, I. M., F. C. Chang, and Y. S. Wang. 2001. Correlation of gas chromatic properties of chlorobenzenes and polychlorinated biphenyls with the occurrence of reductive dechlorination by untamed microorganisms. Chemosphere 45:223-229. [DOI] [PubMed] [Google Scholar]
- 18.Cho, Y. C., E. B. Ostrofsky, R. C. Sokol, R. C. Frohnhoefer, and G. Y. Rhee. 2002. Enhancement of microbial PCB dechlorination by chlorobenzoates, chlorophenols and chlorobenzenes. FEMS Microbiol. Ecol. 42:51-58. [DOI] [PubMed] [Google Scholar]
- 19.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cupples, A. M., A. M. Spormann, and P. L. McCarty. 2003. Growth of Dehalococcoides-like microorganism on vinyl chloride and cis-dichloroethene as electron acceptors as determined by competitive PCR. Appl. Environ. Microbiol. 69:953-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cutter, L., K. R. Sowers, and H. D. May. 1998. Microbial dechlorination of 2,3,5,6-tetrachlorobiphenyl under anaerobic conditions in the absence of soil or sediment. Appl. Environ. Microbiol. 64:2966-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cutter, L. A., J. E. M. Watts, K. R. Sowers, and H. D. May. 2001. Identification of a microorganism that links its growth to the reductive dechlorination of 2,3,5,6-chlorobiphenyl. Environ. Microbiol. 3:699-709. [DOI] [PubMed] [Google Scholar]
- 23.DeSantis, T. Z., P. Hugenholtz, K. Keller, E. L. Brodie, N. Larsen, Y. M. Piceno, R. Phan, and G. L. Andersen. 2006. NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res. 34:W394-W399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fagervold, S. K., J. E. M. Watts, H. D. May, and K. R. Sowers. 2005. Sequential reductive dechlorination of meta-chlorinated polychlorinated biphenyl congeners in sediment microcosms by two different Chloroflexi phylotypes. Appl. Environ. Microbiol. 71:8085-8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fennell, D. E., I. Nijenhuis, S. F. Wilson, S. H. Zinder, and M. M. Häggblom. 2004. Dehalococcoides ethenogenes strain 195 reductively dechlorinates diverse chlorinated aromatic pollutants. Environ. Sci. Technol. 38:2075-2081. [DOI] [PubMed] [Google Scholar]
- 26.Frame, G. M., R. E. Wagner, J. C. Carnahan, J. F. Brown, R. J. May, L. A. Smullen, and D. L. Bedard. 1996. Comprehensive, quantitative, congener-specific analyses of eight Aroclors and complete PCB congener assignments on DB-1 capillary GC columns. Chemosphere 33:603-623. [Google Scholar]
- 27.He, J., K. M. Ritalahti, K. L. Yang, S. S. Koenigsberg, and F. E. Löffler. 2003. Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 424:62-65. [DOI] [PubMed] [Google Scholar]
- 28.He, J., Y. Sung, R. Krajmalnik-Brown, K. M. Ritalahti, and F. E. Loffler. 2005. Isolation and characterization of Dehalococcoides sp. strain FL2, a trichloroethene (TCE) and 1,2-dichloroethene-respiring anaerobe. Environ. Microbiol. 7:1442-1450. [DOI] [PubMed] [Google Scholar]
- 29.Holmes, D. A., B. K. Harrison, and J. Dolfing. 1993. Estimation of Gibbs free energies of formation for polychlorinated biphenyls. Environ. Sci. Technol. 27:725-731. [Google Scholar]
- 30.Holmes, V. F., J. He, P. K. H. Lee, and L. Alvarez-Cohen. 2006. Discrimination of multiple Dehalococcoides strains in a trichloroethene enrichment by quantification of their reductive dehalogenase genes. Appl. Environ. Microbiol. 72:5877-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, J., and G.-Y. Rhee. 1997. Population dynamics of polychlorinated biphenyl-dechlorinating microorganisms in contaminated sediments. Appl. Environ. Microbiol. 63:1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ko, F. C., and J. E. Baker. 2004. Seasonal and annual loads of hydrophobic organic contaminants from the Susquehanna River basin to the Chesapeake Bay. Mar. Pollut. Bull. 48:840-851. [DOI] [PubMed] [Google Scholar]
- 33.Kuipers, B., W. R. Cullen, and W. W. Mohn. 2003. Reductive dechlorination of weathered Aroclor 1260 during anaerobic biotreatment of Arctic soils. Can. J. Microbiol. 49:9-14. [DOI] [PubMed] [Google Scholar]
- 34.Leister, D., and J. E. Baker. 1994. Atmospheric deposition of organic contaminants to the Chesapeake Bay. Atmos. Environ. 28:1499-1520. [Google Scholar]
- 35.Master, E. R., V. W. Lai, B. Kuipers, W. R. Cullen, and W. W. Mohn. 2002. Sequential anaerobic-aerobic treatment of soil contaminated with weathered Aroclor 1260. Environ. Sci. Technol. 36:100-103. [DOI] [PubMed] [Google Scholar]
- 36.May, H. D., A. W. Boyle, W. A. Price III, and C. K. Blake. 1992. Subculturing of a polychlorinated biphenyl-dechlorinating anaerobic enrichment on solid media. Appl. Environ. Microbiol. 58:4051-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.May, H. D., L. A. Cutter, G. S. Miller, C. E. Milliken, J. E. M. Watts, and K. R. Sowers. 2006. Stimulatory and inhibitory effects of organohalides on the dehalogenating activities of PCB-dechlorinating bacterium o-17. Environ. Sci. Technol. 40:5704-5709. [DOI] [PubMed] [Google Scholar]
- 38.Maymo-Gatell, X., Y.-T. Chien, J. M. Gossett, and S. H. Zinder. 1997. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568-1571. [DOI] [PubMed] [Google Scholar]
- 39.Morris, P. J., W. W. Mohn, J. F. Quensen III, J. M. Tiedje, and S. A. Boyd. 1992. Establishment of polychlorinated biphenyl-degrading enrichment culture with predominantly meta dechlorination. Appl. Environ. Microbiol. 58:3088-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muyzer, G., E. C. de Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nesbø, C. L., M. Dlutek, O. Zhaxybayeva, and W. F. Doolittle. 2006. Evidence for existence of “mesotogas,” members of the order Thermotogales adapted to low-temperature environments. Appl. Environ. Microbiol. 72:5061-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nies, L., and T. M. Vogel. 1991. Identification of the proton source for the microbial reductive dechlorination of 2,3,4,5,6-pentachlorobiphenyl. Appl. Environ. Microbiol. 57:2771-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pulliam Holoman, T. R., M. A. Elberson, L. A. Cutter, H. D. May, and K. R. Sowers. 1998. Characterization of a defined 2,3,5,6-tetrachlorobiphenyl-ortho-dechlorinating microbial community by comparative sequence analysis of genes coding for 16S rRNA. Appl. Environ. Microbiol. 64:3359-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quensen, J. F., III, S. A. Boyd, and J. M. Tiedje. 1990. Dechlorination of four commercial polychlorinated biphenyl mixtures (Aroclors) by anaerobic microorganisms from sediments. Appl. Environ. Microbiol. 56:2360-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quensen, J. F., III, J. M. Tiedje, and S. A. Boyd. 1988. Reductive dechlorination of polychlorinated biphenyls by anaerobic microorganisms from sediments. Science 242:752-754. [DOI] [PubMed] [Google Scholar]
- 46.Rhee, G.-Y., R. C. Sokol, C. M. Bethoney, and B. Bush. 1993. Dechlorination of polychlorinated biphenyls by Hudson River sediment organisms: specificity to the chlorination pattern of congeners. Environ. Sci. Technol. 27:1190-1192. [Google Scholar]
- 47.Rhee, G. Y., B. Bush, C. M. Bethoney, A. DeNucci, H. M. Oh, and R. C. Sokol. 1993. Anaerobic dechlorination of Aroclor 1242 as affected by some environmental conditions. Environ. Toxicol. Chem. 12:1033-1039. [Google Scholar]
- 48.Safe, S. H. 1994. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit. Rev. Toxicol. 24:87-149. [DOI] [PubMed] [Google Scholar]
- 49.Sokol, R. C., C. M. Bethoney, and G. Y. Rhee. 1994. Effect of hydrogen on the pathway and products of PCB dechlorination. Chemosphere 29:1735-1742. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki, M. T., and S. J. Giovannoni. 1996. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl. Environ. Microbiol. 62:625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tiedje, J. M., J. F. Quensen III, J. Chee-Sanford, J. P. Schimel, and S. A. Boyd. 1993. Microbial reductive dechlorination of PCBs. Biodegradation 4:231-240. [DOI] [PubMed] [Google Scholar]
- 52.Van Dort, H. M., and D. L. Bedard. 1991. Reductive ortho and meta dechlorination of a polychlorinated biphenyl congener by anaerobic microorganisms. Appl. Environ. Microbiol. 57:1576-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Versar, I. 1976. PCBs in the United States: industrial use and environmental distribution. EPA report 560/6-76-005. Environmental Protection Agency, Washington, DC.
- 54.Wang, Y., Z. Zhang, and N. Ramanan. 1997. The actinomycete Thermobispora bispora contains two distinct types of transcriptionally active 16S rRNA genes. J. Bacteriol. 179:3270-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watts, J. E. M., S. K. Fagervold, K. R. Sowers, and H. D. May. 2005. A PCR based specific assay reveals a population of bacteria within the Chloroflexi associated with the reductive dehalogenation of polychlorinated biphenyls. Microbiology 151:2039-2046. [DOI] [PubMed] [Google Scholar]
- 56.Watts, J. E. M., Q. Wu, S. B. Schreier, H. D. May, and K. R. Sowers. 2001. Comparative analyses of PCB dechlorinating communities in enrichment cultures using three different molecular screening techniques. Environ. Microbiol. 2:710-719. [DOI] [PubMed] [Google Scholar]
- 57.Williams, W. A. 1994. Microbial reductive dechlorination of trichlorobiphenyls in anaerobic sediment slurries. Environ. Sci. Pollut. 28:630-635. [DOI] [PubMed] [Google Scholar]
- 58.Wu, Q., K. R. Sowers, and H. D. May. 2000. Establishment of a polychlorinated biphenyl-dechlorinating microbial consortium, specific for doubly flanked chlorines, in a defined, sediment-free medium. Appl. Environ. Microbiol. 66:49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu, Q., J. E. M. Watts, K. R. Sowers, and H. D. May. 2002. Identification of a bacterium that specifically catalyzes the reductive dechlorination of polychlorinated biphenyls with doubly flanked chlorines. Appl. Environ. Microbiol. 68:807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu, Q., and J. Wiegel. 1997. Two anaerobic polychlorinated biphenyl-dehalogenating enrichments that exhibit different para-dechlorination specificities. Appl. Environ. Microbiol. 63:4826-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu, Q., D. L. Bedard, and J. Wiegel. 1999. 2,6-Dibromobiphenyl primes extensive dechlorination of Aroclor 1260 in contaminated sediment at 8-30 degrees C by stimulating growth of PCB-dehalogenating microorganisms. Environ. Sci. Technol. 33:595-602. [Google Scholar]
- 62.Wu, Q., K. R. Sowers, and H. D. May. 1998. Microbial reductive dechlorination of Aroclor 1260 in anaerobic slurries of estuarine sediments. Appl. Environ. Microbiol. 64:1052-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan, T., T. M. LaPara, and P. J. Novak. 2006. The reductive dechlorination of 2,3,4,5-tetrachlorobiphenyl in three different sediment cultures: evidence for the involvement of phylogenetically similar Dehalococcoides-like bacterial populations. FEMS Microbiol. Ecol. 55:248-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye, D., J. F. Quensen III, J. M. Tiedje, and S. A. Boyd. 1992. Anaerobic dechlorination of polychlorobiphenyls (Aroclor 1242) by pasteurized and ethanol-treated microorganisms from sediments. Appl. Environ. Microbiol. 58:1110-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshida, N., N. Takahashi, and A. Hiraishi. 2005. Phylogenetic characterization of a polychlorinated-dioxin-dechlorinating microbial community by use of microcosm studies. Appl. Environ. Microbiol. 71:4325-4334. [DOI] [PMC free article] [PubMed] [Google Scholar]