Abstract
Bacteria belonging to the genus Dehalococcoides play a key role in the complete detoxification of chloroethenes as these organisms are the only microbes known to be capable of dechlorination beyond dichloroethenes to vinyl chloride (VC) and ethene. However, Dehalococcoides strains usually grow slowly with a doubling time of 1 to 2 days and have complex nutritional requirements. Here we describe the growth of Dehalococcoides ethenogenes 195 in a defined mineral salts medium, improved growth of strain 195 when the medium was amended with high concentrations of vitamin B12, and a strategy for maintaining Dehalococcoides strains on lactate by growing them in consortia. Although strain 195 could grow in defined medium spiked with ∼0.5 mM trichloroethene (TCE) and 0.001 mg/liter vitamin B12, the TCE dechlorination and cellular growth rates doubled when the vitamin B12 concentration was increased 25-fold to 0.025 mg/liter. In addition, the final ratios of ethene to VC increased when the higher vitamin concentration was used, which reflected the key role that cobalamin plays in dechlorination reactions. No further improvement in dechlorination or growth was observed when the vitamin B12 concentration was increased to more than 0.025 mg/liter. In defined consortia containing strain 195 along with Desulfovibrio desulfuricans and/or Acetobacterium woodii and containing lactate as the electron donor, tetrachloroethene (∼0.4 mM) was completely dechlorinated to VC and ethene and there was concomitant growth of Dehalococcoides cells. In the cultures that also contained D. desulfuricans and/or A. woodii, strain 195 cells grew to densities that were 1.5 times greater than the densities obtained when the isolate was grown alone. The ratio of ethene to VC was highest in the presence of A. woodii, an organism that generates cobalamin de novo during metabolism. These findings demonstrate that the growth of D. ethenogenes strain 195 in defined medium can be optimized by providing high concentrations of vitamin B12 and that this strain can be grown to higher densities in cocultures with fermenters that convert lactate to generate the required hydrogen and acetate and that may enhance the availability of vitamin B12.
Chlorinated organic compounds constitute one of the largest groups of environmental chemicals utilized over the last century (1, 3). They have applications as solvents, biocides, plasticizers, and intermediates for chemical synthesis (34). Tetrachloroethene (PCE) and trichlorethene (TCE) are the most prevalent chlorinated organic compounds due to their widespread uses in dry-cleaning, equipment maintenance, and metal degreasing (8, 30). Because of improper handling and disposal, these two compounds have become common groundwater contaminants, and PCE- and TCE-contaminated sites represent more than one-half of the sites on the U.S. Environmental Protection Agency Superfund list (www.atsdr.cdc.gov/tfacts70.html). PCE and TCE are suspected human carcinogens, and their degradation products dichloroethene (DCE) and vinyl chloride (VC) are also toxic; VC has been identified as a known human carcinogen (18).
While traditional pump-and-treat methods have proven to be effective for hydrologic containment of these contaminants, the low solubility and high density of the compounds commonly preclude such methods from achieving remediation to desired site closure levels (31). An alternative technology for cleaning up PCE and TCE is biological dechlorination of these compounds into the relatively harmless compound ethene by bacteria. While most anaerobic dechlorinating bacteria that have been isolated (e.g., Desulfuromonas, Sulfurospirillum multivorans, and Dehalobacter) reductively dechlorinate PCE and TCE to the toxic intermediate cis-DCE (19, 22, 24, 26, 35, 37, 39), complete detoxification past cis-DCE to the benign final product ethene can be achieved via reductive dechlorination by members of the genus Dehalococcoides (9, 11, 15, 17, 28, 38). Strains of Dehalococcoides also dehalogenate a variety of other substrates, such as chlorobenzenes, polychlorinated dibenzodioxins, and polybrominated diphenyl ethers (2, 13, 16).
Dehalococcoides cells are commonly found in mixed microbial communities that contain fermenters, such as Desulfovibrio, Eubacterium, Acetobacterium, Citrobacter, and Clostridium (32, 33). These organisms ferment a wide variety of organic compounds (e.g., hexoses, lactate, pyruvate, and butyrate) into H2 and acetate to generate energy in the absence of exogenous terminal electron acceptors (4, 5). For example, Acetobacterium woodii is capable of growth by fermentation of fructose, as well as autotrophic growth using CO2 and H2 to produce acetate (4). Dehalococcoides-containing communities commonly include species that generate high levels of corrinoids (35), which are compounds that Dehalococcoides species require for growth but are unable to synthesize de novo (29). In Dehalococcoides-containing enrichment cultures, the hydrogen, acetate, and cobalamins generated by other members in the community are utilized by Dehalococcoides as an electron donor, a carbon source, and enzymatic cofactors, while halogenated compounds are used as electron acceptors. The syntrophic growth and relatively low hydrogen threshold (<0.4 ppm by volume) of Dehalococcoides species in microbial communities make them able to successfully compete for limited hydrogen supplies with microbes that also utilize hydrogen as an electron donor, such as methanogens and sulfate reducers (12, 25, 43).
Dehalococcoides ethenogenes strain 195, the first strain of the genus Dehalococcoides identified, was isolated from an anaerobic sewage digester using H2 as an electron donor and PCE as an electron acceptor to support growth. In addition to acetate and vitamins, addition of filtered-sterilized sludge supernatant was essential for sustained growth of the pure culture, suggesting that some unidentified growth factors were required (28). In contrast, other isolated Dehalococcoides strains have been reported to grow in defined medium (2, 15, 17, 38), although the growth and dechlorination activity of Dehalococcoides species in pure cultures are not as robust as when these species are present in microbial communities (10, 14, 15, 28).
This is the first report of a method for growing strain 195 in a fully defined medium, as well as in defined consortia with lactate provided as sole electron donor and carbon source. Studying the activity and growth of strain 195 in defined cocultures with one or two other organisms and with lactate can be useful for elucidating potential interactions between strain 195 and other organisms in microbial communities and the ecological significance of these interactions.
MATERIALS AND METHODS
Chemicals.
Chloroethenes, including PCE, TCE, DCEs, and VC, were purchased from Sigma-Aldrich-Fluka (St. Louis, MO) or Supelco (Bellefonte, PA). Ethene was obtained from Alltech Associates, Inc. (Deerfield, IL). Gases (air, nitrogen, helium, hydrogen, and hydrogen-CO2 mixture) were supplied by Praxair, Inc.
Culture sources and growth conditions.
Pure cultures of D. ethenogenes strain 195 and Dehalococcoides sp. strain BAV1 were kindly provided by Stephen H. Zinder of Cornell University (Ithaca, NY) and Frank E. Löffler of the Georgia Institute of Technology (Atlanta, GA), respectively. Strain 195 grown in anaerobic medium containing an activated sludge extract with an undefined composition (28) was transferred (2%, vol/vol) to defined mineral salts medium amended with 5 mM acetate and with a H2-CO2 (80:20, vol/vol) headspace as described previously (14, 15). The mineral salts medium was modified from the medium described by Cole et al. (7) and contained (per liter) 1 g of NaCl, 0.5 g of MgCl2·6H2O, 0.2 g of KH2PO4, 0.3 g of NH4Cl, 0.3 g of KCl, 0.015 g of CaCl2·2H2O, and 0.2 g of MgSO4·7H2O. In addition, 1 ml of a trace element solution (41), 1 ml of an Na2SeO3-Na2WO4 solution (6), and 10 mg of resazurin were added per liter of medium. After the medium was boiled and cooled to room temperature under N2, the reductants Na2S, l-cysteine, and dl-dithiothreitol were added to final concentrations of 0.2, 0.2, and 0.5 mM, respectively (14). Subsequently, NaHCO3 (30 mM) was added to the medium, and the pH was adjusted to 7.2 to 7.3. After 100-ml portions were dispensed into 160-ml bottles and the bottles were sealed with butyl stoppers, autoclaved for 45 min, and cooled to room temperature, 0.5 ml of a vitamin solution (42) and TCE (∼70 μmol) were added to each bottle. Finally, inocula (2%, vol/vol) were added to bottles and incubated in the dark at 34°C without shaking. Active aliquots of strain 195 were transferred consecutively in triplicate, and TCE dechlorination activity was measured for five generations before degradation activity and growth became observably stable. All experiments described in this study were conducted with subsequent generations of the culture. Dehalococcoides sp. strain BAV1 was grown on 80 μmol of VC at 30°C utilizing the same defined medium.
Desulfovibrio desulfuricans ATCC 7757, Eubacterium limosum ATCC 51976, Clostridium propionicum ATCC 25522, Citrobacter freundii ATCC 8090, and Acetobacterium woodii ATCC 29683 were purchased from the American Type Culture Collection and were grown in media recommended by the American Type Culture Collection. The Acetobacterium isolate was grown at 25°C, while the other three isolates were grown at 34°C. To establish defined consortia, active inocula (1 or 0.1%, vol/vol) of the cultures were transferred into the medium described above for strain 195 along with actively growing Dehalococcoides cultures (5%, vol/vol); the resulting cultures were amended with lactate instead of acetate, had an N2-CO2 headspace instead of an H2-CO2 headspace, and were fed ∼40 μmol of PCE.
Analytical methods.
Chloroethene and ethene contents were determined with a Hewlett-Packard model 5890 gas chromatograph equipped with a flame ionization detector. Compounds in the headspace samples (100 μl) were separated on a 30-m J&W capillary column with a 0.32-mm inside diameter. The gas chromatography program and standard preparation used have been described previously (32).
Organic acids, including lactate and acetate, were analyzed with a high-pressure liquid chromatograph equipped with a UVD 170S UV detector (set to 210 nm) and an autosampler (injection volume, 50 μl). The eluent was 5 mM aqueous H2SO4, which was pumped at a flow rate of 0.5 ml min−1 through an Aminex HPX-87H ion exclusion organic acid analysis column (300 by 7.8 mm; Bio-Rad, Hercules, CA). Sample preparation and construction of calibration curves were performed as described previously (14).
Molecular analysis.
Actively growing cells were harvested by centrifugation (14,000 × g, 10 min). Ultra Clean microbial DNA kits (MO BIO Laboratories, Inc., Carlsbad, CA) were used to extract DNA from the cell pellets according to the manufacturer's recommendations. Quantitative real-time PCR (qPCR) with Dehalococcoides tceA gene-targeted primers and probes (forward primer 5′-ATCCAGATTATGACCCTGGTGAA-3′, probe 6-carboxyfluorescein-TGGGCTATGGCGACCGCAGG-6-carboxytetramethylrhodamine, and reverse primer 5′-GCGGCATATATTAGGGCATCTT-3′) and with Dehalococcoides 16S rRNA gene-targeted primers and probe (forward primer 5′-GGTAATACGTAGGGAAGCAAGCG-3′, probe 5′-VIC-ACATCCAACTTGAAAGACCACCTACGCTCACT-6-carboxytetramethylrhodamine-3′, and reverse primer 5′-CCGGTTAAGCCGGGAAATT-3′) was carried out as described by Johnson et al. (21) and Holmes et al. (20). Double-stranded sequence analysis of a partial 16S rRNA gene was performed by using primers 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) (44) and 1392R (5′-ACGGGCGGTGTGT-3′) (23) with a CEQ 8000 genetic analysis system (Beckman Coulter, Fullerton, CA).
RESULTS
Growth of D. ethenogenes strain 195 in defined medium.
The first generation of a D. ethenogenes strain 195 culture grown in defined medium dechlorinated the 50 μmol of TCE added initially within 6 weeks with a lag time of 2 weeks in triplicate bottles. The second generation differed from the first generation in two important respects. First, the initial lag time was 3 weeks longer. Second, the dechlorination profiles of the triplicate samples differed; while one subculture completely dechlorinated TCE (∼50 μmol) to VC and ethene in 7 weeks, the second subculture dechlorinated only one-half of the TCE to VC within 10 weeks and the third bottle showed no dechlorination activity at all over 3 months.
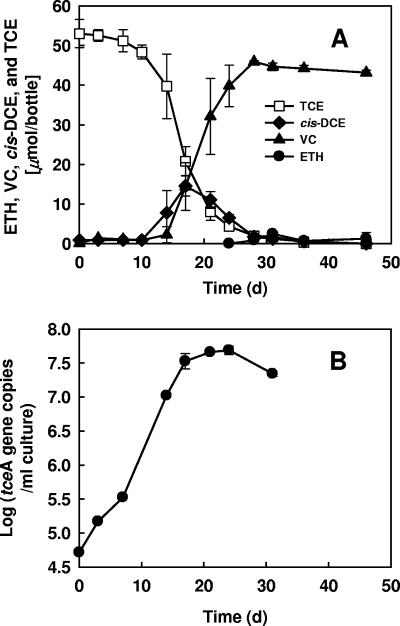
Subsequently, when the first subculture of the second generation was transferred to fresh defined medium in triplicate, all third-generation bottles demonstrated complete dechlorination of 50 μmol TCE to VC and ethene by ∼40 days with lag times of ∼14 days. After the fifth generation, growth of strain 195 in the new medium became stable and reproducible. As shown in Fig. 1A, after 2% (vol/vol) active strain 195 cells was transferred from the fifth-generation bottles to fresh medium amended with 0.001 mg/liter vitamin B12, ∼50 μmol of TCE was completely dechlorinated to VC and ethene with less than 15 μmol of cis-DCE as the intermediate within 35 days. Negligible amounts of trans- and 1,1-DCE appeared during the dechlorination process. After 95% of the TCE disappeared, ethene started to form slowly, and only 3 μmol was produced within 35 days. qPCR demonstrated that the number of copies of the tceA gene, a proxy for the number of strain 195 cells (36), increased ca. 50-fold to a final value of 5.0 × 107 copies/ml (Fig. 1B). A specific activity of 0.16 × 10−9 nmol chloride released per min per cell was determined for strain 195 growing on PCE in the defined medium. Strain 195 has been maintained reliably for more than 3 years by routine transfers in this defined medium with 0.001 mg/liter vitamin B12. The purity of the culture has been confirmed both by qPCR measurement of equal copy numbers in the culture's genomic DNA of the tceA gene and the 16S rRNA gene (measured with Dehalococcoides 16S rRNA gene-targeted primers and probe) and by the presence of a single 16S rRNA gene sequence (amplified by targeting the 16S rRNA genes with universal primers 8F and 1392R) matching the strain 195 sequence in gene products amplified from the culture's genomic DNA.
FIG. 1.
(A) Dechlorination of TCE to cis-DCE, VC, and ethene (ETH) by D. ethenogenes strain 195 in defined medium amended with 0.001 mg/liter vitamin B12. (B) Increase in the number of tceA gene copies during complete reductive dechlorination of TCE to VC and ethene by D. ethenogenes strain 195. The symbols indicate averages based on triplicate determinations. The error bars indicate standard deviations; error bars are not shown if they are smaller than the symbols.
Effect of cobalamin on the growth of D. ethenogenes strain 195.
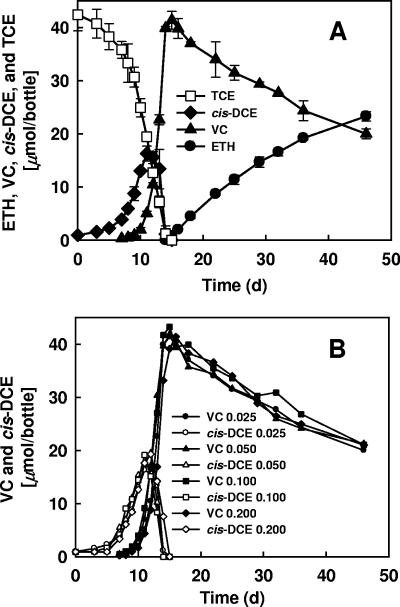
To investigate the effect of vitamin B12 (cobalamin) concentrations on the growth and density of D. ethenogenes strain 195, a range of concentrations of vitamin B12 (0.001 to 0.2 mg/liter) was tested. Cultures containing 0.001 mg/liter of vitamin B12 had an average TCE dechlorination rate of 14 μmol/liter/day, consistent with the values obtained for previous generations, as shown in Fig. 1A. However, when the vitamin B12 concentration was increased 25-fold to 0.025 mg/liter, the TCE dechlorination rate doubled and the final ethene-to-VC ratio increased (Fig. 2A), demonstrating that vitamin B12 plays an important role in the metabolism of this isolate. For cultures grown with vitamin B12 concentrations greater than 0.025 mg/liter, the profiles for dechlorination of TCE and formation of cis-DCE, VC, and ethene were almost identical (cis-DCE and VC generation curves are shown in Fig. 2B), demonstrating that higher concentrations of vitamin B12 provided no additional benefit.
FIG. 2.
TCE dechlorination by D. ethenogenes strain 195 grown with different amounts of vitamin B12. (A) Dechlorination of TCE with 0.025 mg/liter vitamin B12. (B) Profiles of VC and cis-DCE generation obtained with vitamin B12 concentrations of 0.025, 0.050, 0.100, and 0.200 mg/liter (TCE data were omitted for clarity). The symbols indicate averages based on triplicate determinations. The error bars indicate standard deviations; in panel A error bars are not shown if they are smaller than the symbols, and in panel B the standard deviations are <5% of the average values and error bars are not shown for clarity. ETH, ethene.
qPCR measurement of the number of tceA gene copies of strain 195 confirmed that the cell density was twice as high (9.46 × 107 ± 0.33 × 107 cells/ml culture) for cultures given 0.025 mg/liter vitamin B12 as for cultures given 0.001 mg/liter, the concentration used in the original defined medium (Table 1). Furthermore, qPCR results demonstrated that no further improvement in growth was obtained when the concentration of vitamin B12 was higher than 0.025 mg/liter (Table 1), echoing the dechlorination profiles described above.
TABLE 1.
Growth and specific activity of D. ethenogenes strain 195 grown with various vitamin B12 concentrations
| Vitamin B12 concn (mg/liter) | Amt of TCE consumed (μmol) | D. ethenogenes 195 density (107tceA gene copies/ml culture)a | D. ethenogenes 195 sp act (nmol Cl− released/min/109 cells) |
|---|---|---|---|
| 0.001 | 41 | 4.86 ± 0.01 | 0.39 |
| 0.025 | 42 | 9.46 ± 0.33 | 0.40 |
| 0.05 | 42 | 9.94 ± 0.13 | 0.39 |
| 0.10 | 41 | 9.56 ± 0.11 | 0.39 |
| 0.20 | 41 | 9.27 ± 0.43 | 0.41 |
The number of tceA gene copies equals the number of cells (36). The values are averages ± standard deviations based on triplicate determinations.
Effects of cobalamin on the growth of Dehalococcoides sp. strain BAV1.
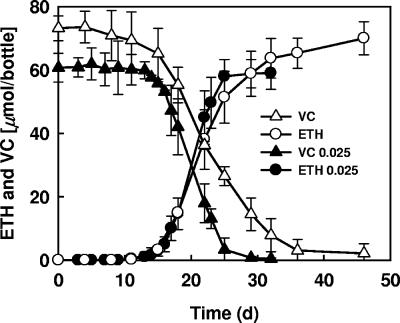
Experiments conducted to evaluate the effects of the cobalamin concentration (0.001 and 0.025 mg/liter) on the growth of Dehalococcoides sp. strain BAV1 demonstrated that the VC dechlorination rates were increased only slightly with the higher vitamin B12 concentration, as reflected by average rates of 20 and 16 μmol/liter/day (Fig. 3). qPCR analysis of the BAV1 16S rRNA gene showed that this organism grew to densities of 10.0 × 107 ± 0.25 × 107 cells/ml when 0.025 mg/liter B12 was present in cultures that consumed 60 μmol of VC, compared to densities of 4.7 × 107 ± 0.04 × 107 cells/ml when 0.001 mg/liter B12 was present in cultures that consumed 73 μmol of VC. No observable improvements in the dechlorination rate and growth of strain BAV1 were detected with vitamin B12 concentrations greater than 0.025 mg/liter.
FIG. 3.
VC dechlorination to ethene (ETH) by Dehalococcoides sp. strain BAV1 with 0.001 mg/liter (open symbols) and 0.025 mg/liter (solid symbols) of vitamin B12, respectively. The symbols indicate averages based on triplicate determinations. The error bars indicate standard deviations; error bars are not shown if they are smaller than the symbols.
Noticeably, when the vitamin B12 concentration was low (0.001 mg/liter), the VC dechlorination activity declined continuously over time and almost 3% of the total VC (∼2 μmol) still remained after 6 weeks (Fig. 3), whereas when the vitamin B12 concentration was high (0.025 mg/liter), the VC concentration dropped to a level below the detection limit after only 30 days.
Chloroethene dechlorination in established consortia.
In order to understand interactions that may occur in complex dechlorinating communities and to determine whether Dehalococcoides strains could be grown and maintained in defined consortia using simple organic compounds, such as lactate, cocultures were established with D. ethenogenes 195 and other species (e.g., Desulfovibrio, Clostridium, Eubacterium, Acetobacterium, and Citrobacter species) that have been found to be present in dechlorinating enrichment cultures (32, 33).
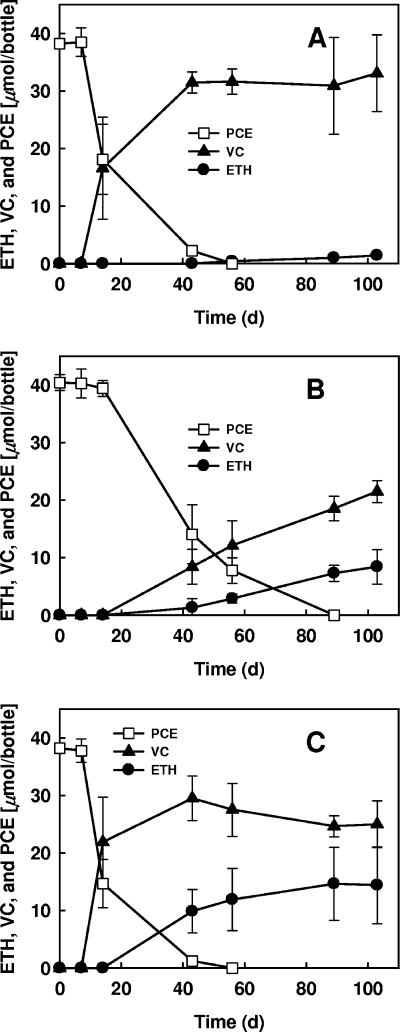
PCE dechlorination patterns showed that cocultures of D. ethenogenes 195 with D. desulfuricans or A. woodii amended with lactate dechlorinated compounds more rapidly than a culture of strain 195 alone amended with hydrogen and acetate (data not shown). Addition of fermenters such as E. limosum, C. propionicum, or C. freundii to the cocultures resulted in no improvement in the dechlorination rates and apparently undermined the beneficial effects of Desulfovibrio or Acetobacter sp., perhaps by competing for carbon sources (data not shown). In the defined cultures, PCE (40 μmol) was completely dechlorinated to VC and ethene with negligible generation of the intermediates TCE and DCE by strain 195 alone, by a coculture containing strain 195 and D. desulfuricans, and by a coculture containing strain 195, D. desulfuricans, and A. woodii (Fig. 4). Interestingly, the ratio of ethene to VC was highest in the presence of A. woodii (Fig. 4C), an organism that generates cobalamin in its metabolic processes (40). Also, more ethene was formed by the coculture containing D. desulfuricans plus strain 195 than by strain 195 alone (Fig. 4B). High-performance liquid chromatography analysis showed that the lactate added was fermented primarily to acetate and H2, which serve as a carbon source and an electron donor, respectively, for strain 195. The lactate added was consumed within 2 weeks, and subsequently acetate utilization by strain 195 continued.
FIG. 4.
PCE dechlorination by D. ethenogenes strain 195 and by established cocultures containing D. ethenogenes strain 195 and D. desulfuricans or containing D. ethenogenes strain 195, D. desulfuricans, and A. woodii. (A) Dechlorination of PCE by D. ethenogenes strain 195 in defined medium with H2 as the electron donor and acetate as the carbon source. (B) Dechlorination of PCE by a coculture containing D. ethenogenes strain 195 and D. desulfuricans with lactate as the electron donor and carbon source. (C) Dechlorination of PCE by a coculture containing D. ethenogenes strain 195, D. desulfuricans, and A. woodii with lactate as the electron donor and carbon source. All the cultures contained 0.001 mg/liter vitamin B12. The symbols indicate averages based on triplicate determinations. The error bars indicate standard deviations; error bars are not shown if they are smaller than the symbols. ETH, ethene.
In addition to complete dechlorination of PCE to VC and ethene, growth of Dehalococcoides cells was also observed in the coculture containing strain 195 and D. desulfuricans and in the coculture containing strain 195, D. desulfuricans, and A. woodii. qPCR showed that a coculture containing strain 195 and D. desulfuricans that had consumed 40 μmol of PCE contained 1.8 × 108 cells of strain 195/ml, while a coculture containing strain 195, D. desulfuricans, and A. woodii contained 1.3 × 108 cells of strain 195/ml (as measured by using the number of tceA gene copies) after it had dechlorinated the same amount of PCE to VC and ethene. Overall, the numbers of strain 195 cells were 1.5 times greater in the coculture containing strain 195 and D. desulfuricans and in the coculture containing, D. desulfuricans, and A. woodii than in the culture containing strain 195 alone (0.9 × 108 cells/ml of culture) after consumption of the same amount of PCE (Table 2).
TABLE 2.
Growth of D. ethenogenes strain 195 in pure cultures and in cocultures with PCE and 0.001 mg/liter vitamin B12
| Culture | Amt of solvent consumed (μmol) | No. of tceA gene copies produced (108/ml culture)a |
|---|---|---|
| D. ethenogenes strain 195 | 39 | 0.9 ± 0.12 |
| D. ethenogenes strain 195 + D. desulfuricans | 40 | 1.8 ± 0.32 |
| D. ethenogenes strain 195 + D. desulfuricans + A. woodii | 39 | 1.3 ± 0.18 |
The number of tceA gene copies equals the number of cells (36). The values are averages ± standard deviations based on triplicate determinations.
DISCUSSION
Improving the reductive dechlorination of chloroethenes by Dehalococcoides species is necessary in order to develop improved strategies for the bioremedation of PCE and TCE in contaminated environments. Researchers have made enormous efforts to understand the physiology of Dehalococcoides strains, to identify their functional reductases, and to sequence their complete genomes (13, 27, 28, 36; http://www.tigr.org; http://www.jgi.doe.gov). Annotation of the genome of each described Dehalococcoides sp. has suggested that these organisms lack several fundamental biosynthetic pathways and thus have complex nutrient requirements, corroborating the observation that growth is more robust in consortia than in pure cultures (10, 36). Although D. ethenogenes strain 195 has been demonstrated to be especially fastidious in terms of its growth requirements (28, 36), in this study we describe the growth of strain 195 in defined medium.
D. ethenogenes 195 and the other described Dehalococcoides strains require the corrinoid vitamin B12 for growth, as demonstrated by the incomplete biosynthesis pathways of the corrinoid cofactors in the annotated genomes (36; http://www.tigr.org; http://www.jgi.doe.gov). In this study we confirmed that D. ethenogenes 195 grows more rapidly when additional cobalamin is provided. Increasing the vitamin B12 concentration from 0.001 to 0.025 mg/liter resulted in a twofold increase in the cell density and doubled the TCE dechlorination rate for strain 195. Because these values increased in tandem, the increase in the rate reflected the increase in cell density rather than improved performance of individual cells, as shown in Table 1. No further increases in either cell growth or the dechlorination rate were observed when the vitamin B12 concentration was increased to more than 0.025 mg/liter, which is in agreement with previous results obtained with mixed cultures containing strain 195 (29). Limited improvements in the growth and activity of Dehalococcoides sp. strain BAV1 were also observed when a higher vitamin B12 concentration (0.025 mg/liter) was used, confirming that cobalamin limitation plays a role in the growth of Dehalococcoides species.
In nature, vitamin B12 is produced by a wide variety of bacteria (e.g., A. woodii) during their metabolic processes, potentially providing this essential nutrient to other microbes that are unable to biosynthesize it (e.g., Dehalococcoides). This phenomenon may have been responsible for the increased growth of D. ethenogenes 195 and more complete dechlorination to ethene in the coculture containing strain 195, D. desulfuricans, and A. woodii. For pure cultures of D. ethenogenes 195 that dechlorinated ∼40 μmol of PCE to VC and ethene, qPCR analysis showed that cultures growing in defined medium contained ∼0.9 × 108 cells/ml (Table 2), which is twofold lower than the number obtained by epifluorescence microscope quantification of strain 195 grown in complex medium (28). The specific activities calculated for strain 195 were 0.16 × 10−9 and 0.19 × 10−9 nmol Cl−/min/cell in the defined and complex media, respectively (28). The difference could be attributed to the availability of more nutrients (e.g., vitamins and other growth factors) in the sludge extracts or to the use of different quantification methods (e.g., qPCR versus microscopy). The 2.5-fold-higher specific activity when strain 195 was grown with TCE than when strain 195 was grown with PCE (∼0.39 × 10−9 versus 0.16 × 10−9 nmol Cl− released/min/cell) could have been due to different reductive dehalogenases (tceA versus pceA genes) involved in the stepwise dechlorination processes.
In summary, the growth of D. ethenogenes 195 in defined medium paves the way for further physiological and genetic studies of this important genus of microorganisms, which so far are the only microbes known to completely detoxify chloroethenes. The enhanced growth both with high vitamin B12 concentrations (>0.025 mg/liter) and in established consortia supports observations that there is robust growth of Dehalococcoides species in mixed communities, suggesting that these species can perform efficiently when they interact with other species in natural environments.
Acknowledgments
We are grateful to Stephen H. Zinder of Cornell University for providing D. ethenogenes 195 and to Frank E. Löffler of Georgia Institute of Technology and the Georgia Tech Research Corporation for providing Dehalococcoides strain BAV1 for this project.
This work was supported by the National Science Foundation (grant 0504244) and by the Superfund Basic Research Project (NIEHS grant ES04705).
Footnotes
Published ahead of print on 2 March 2007.
REFERENCES
- 1.Abelson, P. H. 1990. Inefficient remediation of ground-water pollution. Science 250:733. [DOI] [PubMed] [Google Scholar]
- 2.Adrian, L., U. Szewzyk, J. Wecke, and H. Görisch. 2000. Bacterial dehalorespiration with chlorinated benzenes. Nature 408:580-583. [DOI] [PubMed] [Google Scholar]
- 3.Ashford, N. A., and C. S. Miller. 1991. Chemical exposures—low levels and high stakes. Van Nostrand Reinhold, New York, NY.
- 4.Balch, W. E., S. Schoberth, R. S. Tanner, and R. S. Wolfe. 1977. Acetobacterium, a new genus of hydrogen-oxidizing, carbon dioxide-reducing, anaerobic bacteria. Int. J. Syst. Bacteriol. 27:355-361. [Google Scholar]
- 5.Barker, H. A., and V. Haas. 1944. Butyribacterium, a new genus of gram-positive, non-sporulating anaerobic bacteria of intestinal origin. J. Bacteriol. 47:301-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brysch, K., C. Schneider, G. Fuchs, and F. Widdel. 1987. Lithoautotrophic growth of sulfate-reducing bacteria, and description of Desulfobacterium autotrophicum gen. nov., sp. nov. Arch. Microbiol. 148:262-274. [Google Scholar]
- 7.Cole, J. R., A. L. Cascarelli, W. W. Mohn, and J. M. Tiedje. 1994. Isolation and characterization of a novel bacterium growing via reductive dehalogenation of 2-chlorophenol. Appl. Environ. Microbiol. 60:3536-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crooks, W. H. 1993. Wastewater from dry cleaners is anything but clean. Water Environ. Technol. 5:30-32. [Google Scholar]
- 9.Cupples, A. M., A. M. Spormann, and P. L. McCarty. 2004. Comparative evaluation of chloroethene dechlorination to ethene by Dehalococcoides-like microorganisms. Environ. Sci. Technol. 38:4768-4774. [DOI] [PubMed] [Google Scholar]
- 10.DiStefano, T. D., J. M. Gossett, and S. H. Zinder. 1992. Hydrogen as an electron donor for dechlorination of tetrachloroethene by an anaerobic mixed culture. Appl. Environ. Microbiol. 58:3622-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duhamel, M., K. Mo, and E. A. Edwards. 2004. Characterization of a highly enriched Dehalococcoides-containing culture that grows on vinyl chloride and trichloroethene. Appl. Environ. Microbiol. 70:5538-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fennell, D. E., J. M. Gossett, and S. H. Zinder. 1997. Comparison of butyric acid, ethanol, lactic acid, and propionic acid as hydrogen donors for the reductive dechlorination of tetrachloroethene. Environ. Sci. Technol. 31:918-926. [Google Scholar]
- 13.Fennell, D. E., I. Nijenhuis, S. F. Wilson, S. H. Zinder, and M. M. Haggblom. 2004. Dehalococcoides ethenogenes strain 195 reductively dechlorinates diverse chlorinated aromatic pollutants. Environ. Sci. Technol. 38:2075-2081. [DOI] [PubMed] [Google Scholar]
- 14.He, J., K. M. Ritalahti, M. R. Aiello, and F. E. Löffler. 2003. Complete detoxification of vinyl chloride (VC) by an anaerobic enrichment culture and identification of the reductively dechlorinating population as a Dehalococcoides species. Appl. Environ. Microbiol. 69:996-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He, J., K. M. Ritalahti, K.-L. Yang, S. S. Koenigsberg, and F. E. Löffler. 2003. Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 424:62-65. [DOI] [PubMed] [Google Scholar]
- 16.He, J., K. R. Robrock, and L. Alvarez-Cohen. 2006. Microbial reductive debromination of polybrominated diphenyl ethers (PBDEs). Environ. Sci. Technol. 40:4429-4434. [DOI] [PubMed] [Google Scholar]
- 17.He, J., Y. Sung, R. Krajmalnik-Brown, K. M. Ritalahti, and F. E. Löffler. 2005. Isolation and characterization of Dehalococcoides sp. strain FL2, a trichloroethene (TCE)- and 1,2-dichloroethene-respiring anaerobe. Environ. Microbiol. 7:1442-1450. [DOI] [PubMed] [Google Scholar]
- 18.Henschler, D. 1994. Toxicity of chlorinated organic compounds: effects of the introduction of chlorine in organic molecules. Angew. Chem. Int. Ed. Engl. 33:1920-1935. [Google Scholar]
- 19.Holliger, C., G. Schraa, A. J. M. Stams, and A. J. B. Zehnder. 1993. A highly purified enrichment culture couples the reductive dechlorination of tetrachloroethene to growth. Appl. Environ. Microbiol. 59:2991-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmes, V. F., J. He, P. K. H. Lee, and L. Alvarez-Cohen. 2006. Discrimination of multiple Dehalococcoides strains in a TCE enrichment by quantification of their reductase genes. Appl. Environ. Microbiol. 72:5877-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, D. R., P. K. H. Lee, V. F. Holmes, and L. Alvarez-Cohen. 2005. An internal reference technique for accurately quantifying specific mRNAs by real-time PCR with application to the tceA reductive dehalogenase gene. Appl. Environ. Microbiol. 71:3866-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krumholz, L. R. 1997. Desulfuromonas chloroethenica sp. nov. uses tetrachloroethylene and trichloroethylene as electron acceptors. Int. J. Syst. Bacteriol. 47:1262-1263. [Google Scholar]
- 23.Lane, D. J., N. Pace, G. J. Olsen, D. A. Stahl, and M. L. Sogin. 1985. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA 82:6955-6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Löffler, F. E., J. R. Cole, K. M. Ritalahti, and J. M. Tiedje. 2003. Diversity of dechlorinating bacteria, p. 53-87. In M. M. Häggblom and I. D. Bossert (ed.), Dehalogenation: microbial processes and environmental applications. Kluwer Academic Press, New York, NY.
- 25.Löffler, F. E., J. M. Tiedje, and R. A. Sanford. 1999. Fraction of electrons consumed in electron acceptor reduction and hydrogen threshold as indicators of halorespiratory physiology. Appl. Environ. Microbiol. 65:4049-4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luijten, M. L. G. C., J. deWeert, H. Smidt, H. T. S. Boschker, W. M. deVos, G. Schraa, and A. J. M. Stams. 2003. Description of Sulfurospirillum halorespirans sp. nov., an anaerobic, tetrachloroethene-respiring bacterium, and transfer of Dehalospirillum multivorans to the genus Sulfurospirillum as Sulfurospirillum multivorans comb. nov. Int. J. Syst. Evol. Microbiol. 53:787-793. [DOI] [PubMed] [Google Scholar]
- 27.Magnuson, J. K., M. F. Romine, D. R. Burris, and M. T. Kingsley. 2000. Trichloroethene reductive dehalogenase from Dehalococcoides ethenogenes: sequence of tceA and substrate range characterization. Appl. Environ. Microbiol. 66:5141-5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maymó-Gatell, X., Y.-T. Chien, J. M. Gossett, and S. H. Zinder. 1997. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568-1571. [DOI] [PubMed] [Google Scholar]
- 29.Maymó-Gatell, X., V. Tandoi, J. M. Gossett, and S. H. Zinder. 1995. Characterization of an H2-utilizing enrichment culture that reductively dechlorinates tetrachloroethene to vinyl chloride and ethene in the absence of methanogenesis and acetogenesis. Appl. Environ. Microbiol. 61:3928-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohn, W. W., and J. M. Tiedje. 1992. Microbial reductive dehalogenation. Microbiol. Rev. 56:482-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Research Council Committee on Source Removal of Contaminants in the Subsurface. 2004. Contaminants in the subsurface: source zone assessment and remediation. National Academy Press, Washington, DC.
- 32.Richardson, R. E., V. K. Bhupathiraju, D. L. Song, T. A. Goulet, and L. Alvarez-Cohen. 2002. Phylogenetic characterization of microbial communities that reductively dechlorinate TCE based upon a combination of molecular techniques. Environ. Sci. Technol. 36:2652-2662. [DOI] [PubMed] [Google Scholar]
- 33.Ritalahti, K. M., and F. E. Löffler. 2004. Populations implicated in the anaerobic reductive dechlorination of 1,2-dichloropropane in highly enriched bacterial communities. Appl. Environ. Microbiol. 70:4088-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossberg, M., W. Lendle, A. Tögel, E.-L. Dreher, E. Langer, H. Rassaerts, P. Kleinschmidt, H. Strack, U. Beck, K.-A. Lipper, T. R. Torkelson, E. Löser, and K. K. Beutel. 1986. Chlorinated hydrocarbons, p. 233-398. In W. Gerharts (ed.), Ullmann's encyclopedia of industrial chemistry, vol. A6. VCH, Weinheim, Germany. [Google Scholar]
- 35.Scholz-Muramatsu, H., A. Neumann, M. Meßmer, E. Moore, and G. Diekert. 1995. Isolation and characterization of Dehalospirillum multivorans gen. nov., sp. nov., a tetrachloroethene-utilizing, strictly anaerobic bacterium. Arch. Microbiol. 163:48-56. [Google Scholar]
- 36.Seshadri, R., L. Adrian, D. E. Fouts, J. A. Eisen, A. M. Phillippy, B. A. Methe, N. L. Ward, W. C. Nelson, R. T. Deboy, H. M. Khouri, J. F. Kolonay, R. J. Dodson, S. C. Daugherty, L. M. Brinkac, S. A. Sullivan, R. Madupu, K. E. Nelson, K. H. Kang, M. Impraim, K. Tran, J. M. Robinson, H. A. Forberger, C. M. Fraser, S. H. Zinder, and J. F. Heidelberg. 2005. Genome sequence of the PCE-dechlorinating bacterium Dehalococcoides ethenogenes. Science 307:105-108. [DOI] [PubMed] [Google Scholar]
- 37.Sharma, P. K., and P. L. McCarty. 1996. Isolation and characterization of a facultative bacterium that reductively dehalogenates tetrachloroethene to cis-1,2-dichloroethene. Appl. Environ. Microbiol. 62:761-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sung, Y., K. M. Ritalahti, R. P. Apkarian, and F. E. Löffler. 2006. Quantitative PCR confirms purity of strain GT, a novel trichloroethene-to-ethene-respiring Dehalococcoides isolate. Appl. Environ. Microbiol. 72:1980-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sung, Y., K. M. Ritalahti, J. W. Urbance, S. J. Flynn, J. M. Tiedje, and F. E. Löffler. 2003. Description of Desulfuromonas michiganensis sp. nov., two tetrachloroethene (PCE)-reducing, acetate-oxidizing anaerobic bacteria. Appl. Environ. Microbiol. 69:2964-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanner, R. S., R. S. Wolfe, and L. G. Ljungdahl. 1978. Tetrahydrofolate enzyme levels in Acetobacterium woodii and their implication in the synthesis of acetate from CO2. J. Bacteriol. 134:668-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widdel, F., and N. Pfennig. 1984. Dissimilatory sulfate- or sulfur-reducing bacteria, vol. 1. Williams & Wilkins, Baltimore, MD.
- 42.Wolin, E. A., M. J. Wolin, and R. S. Wolfe. 1963. Formation of methane by bacterial extracts. J. Biol. Chem. 238:2882-2886. [PubMed] [Google Scholar]
- 43.Yang, Y., and P. L. McCarty. 1998. Competition for hydrogen within a chlorinated solvent dehalogenating anaerobic mixed culture. Environ. Sci. Technol. 32:3591-3597. [Google Scholar]
- 44.Zhou, J. M., R. Fries, R. A. Sanford, and J. M. Tiedje. 1995. Phylogenetic analysis of a new group of denitrifiers capable of anaerobic growth on toluene and description of Azoarcus tolulyticus sp. nov. Int. J. Syst. Bacteriol. 45:500-506. [DOI] [PubMed] [Google Scholar]