Abstract
The cyanobacterium Microcystis can produce microcystins, a family of toxins that are of major concern in water management. In several lakes, the average microcystin content per cell gradually declines from high levels at the onset of Microcystis blooms to low levels at the height of the bloom. Such seasonal dynamics might result from a succession of toxic to nontoxic strains. To investigate this hypothesis, we ran competition experiments with two toxic and two nontoxic Microcystis strains using light-limited chemostats. The population dynamics of these closely related strains were monitored by means of characteristic changes in light absorbance spectra and by PCR amplification of the rRNA internal transcribed spacer region in combination with denaturing gradient gel electrophoresis, which allowed identification and semiquantification of the competing strains. In all experiments, the toxic strains lost competition for light from nontoxic strains. As a consequence, the total microcystin concentrations in the competition experiments gradually declined. We did not find evidence for allelopathic interactions, as nontoxic strains became dominant even when toxic strains were given a major initial advantage. These findings show that, in our experiments, nontoxic strains of Microcystis were better competitors for light than toxic strains. The generality of this finding deserves further investigation with other Microcystis strains. The competitive replacement of toxic by nontoxic strains offers a plausible explanation for the gradual decrease in average toxicity per cell during the development of dense Microcystis blooms.
Blooms of the cyanobacterium Microcystis can be a major hazard in recreational lakes, drinking water reservoirs, and protected wetland areas (6, 47, 49). Microcystis often forms dense blooms that may cause anoxia when cells die off massively. Moreover, Microcystis can produce the toxin microcystin. This hepatotoxin poses serious health risks for animals and humans (3, 7). Especially in dense scums, the concentration of microcystins may increase dramatically. Microcystin concentrations up to 25,000 μg liter−1 have been reported (10), exceeding the guideline values for recreational waters of 20 μg liter−1 by more than 3 orders of magnitude (5).
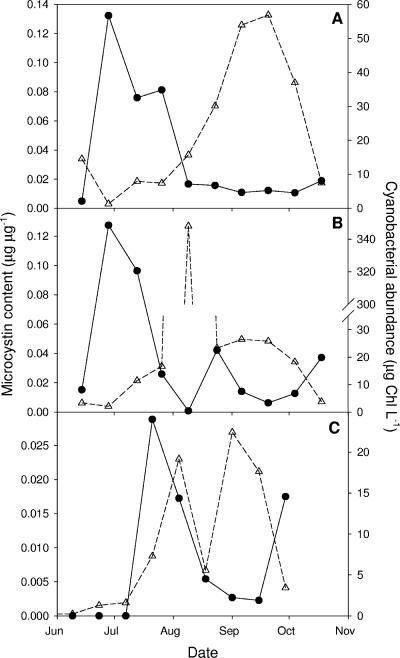
Microcystis populations often consist of mixtures of microcystin-producing and non-microcystin-producing strains (10, 23, 48, 52). Interestingly, several studies show that the average microcystin content expressed per cell is typically high at the onset of Microcystis blooms but much lower at the height of these blooms (22, 51, 53). In other words, with increasing Microcystis biomass, the Microcystis cells become, on average, less toxic. Examples from three Microcystis-dominated Dutch lakes are shown in Fig. 1. This striking seasonal variability in microcystin content of Microcystis blooms exceeds the physiological variability in cellular microcystin content reported for isolated Microcystis strains in laboratory experiments (13, 29, 54). Thus, it seems that the changes in microcystin contents during the development of Microcystis blooms are due to a seasonal succession of toxic and nontoxic strains, in which nontoxic strains prevail at the height of the Microcystis bloom. A seasonal succession of toxic and nontoxic Microcystis geno- or chemotypes has indeed been observed in several lakes (11, 53).
FIG. 1.
Seasonal dynamics of cellular microcystin content (closed circles) and cyanobacterial abundance (open triangles) in three eutrophic Dutch lakes: (A) ′t Joppe, (B) Sloterplas, and (C) De Gouden Ham. All three lakes were dominated by Microcystis. Microcystin contents are expressed per unit of cyanobacterial abundance. Cyanobacterial abundance is expressed as cyanobacterium-bound chlorophyll, which was determined by flow cytometry with lasers specific for phycocyanin and chlorophyll fluorescence. All data are from the summer season of 1999 and were kindly provided by the Dutch Foundation for Applied Water Research (STOWA).
Competition for light is an important selective factor in phytoplankton communities of eutrophic waters (16, 18, 28). Competition models predict that the species (or genotype) with the lowest “critical light intensity” is the best competitor for light, as it can withstand the shading cast by its competitors (15, 50). This model prediction is confirmed by laboratory competition experiments with light-limited phytoplankton (16, 26, 31). Competition for light might play a key role in the seasonal succession of toxic and nontoxic genotypes in Microcystis blooms. The gradual increase in Microcystis biomass during bloom development may cause substantial shading and thereby limit the light available for growth. We therefore hypothesize that the best competitor for light among the Microcystis genotypes present may increase its relative abundance during bloom development, and the toxicity of this strain will then largely determine the overall microcystin content of the Microcystis bloom.
Here, we use competition experiments to investigate competition for light between toxic and nontoxic Microcystis strains. The experiments were carried out in laboratory chemostats specifically designed to study competition for light (16, 17, 31, 41). Toxic and nontoxic strains cannot be distinguished by traditional light microscopic techniques. Therefore, we used two alternative approaches to distinguish the different strains in our competition experiments. In one competition experiment, we used observed differences in pigment composition to monitor the competing strains. In the other competition experiments, we applied recently developed molecular tools based on denaturing gradient gel electrophoresis (DGGE) of the PCR-amplified internal transcribed spacer (ITS) region of the rRNA operon (20, 21) to monitor competition between the Microcystis strains.
MATERIALS AND METHODS
Organisms.
The experiments were performed with two recently isolated Microcystis sp. strains from Lake Volkerak, The Netherlands, and with two Microcystis aeruginosa laboratory strains originating from the NIVA culture collection (Table 1). Volkerak strain V163 and NIVA strain CYA140 both produce microcystin LR. Furthermore, strain V163 produces three other, unidentified, microcystin variants in much lower concentration. Volkerak strain V145 and NIVA strain CYA43 do not produce microcystins. The two NIVA strains and strain V145 contain relatively large amounts of the pigment phycocyanin, which gives these strains a blue-green color. Conversely, strain V163 has a greenish brown appearance as it contains the pigment phycoerythrin and relatively small amounts of phycocyanin. Thus far, phycoerythrin has been found only in one other Microcystis strain (36).
TABLE 1.
Characteristics of the Microcystis strains used in this study
| Code | Origin | Sourcea | Microcystin(s) produced | Presence of phycoerythrin | Competition expt no. |
|---|---|---|---|---|---|
| V145 | Lake Volkerak, The Netherlands | UvA | None | − | 1 |
| V163 | Lake Volkerak, The Netherlands | UvA | LR and 3 unidentified variants | + | 1 |
| CYA43 | United States | NIVA | None | − | 2 and 3 |
| CYA140 | Bendig's Pond, Bruno, Canada | NIVA | LR | − | 2 and 3 |
UvA, algal culture collection of the University of Amsterdam, Amsterdam, The Netherlands; NIVA, algal culture collection of the Norwegian Institute for Water Research, Oslo, Norway.
Light-limited chemostats.
Experiments were conducted in light-limited chemostats (16, 17), using flat culture vessels with a working volume of 1.85 liters and an optical path length (“mixing depth”) of 5 cm. The dilution rate was fixed at 0.011 h−1 by a continuous inflow of nutrient-saturated mineral medium (46). The chemostats were bubbled with a constant inflow of filtered and moistened air to ensure homogeneous mixing and to provide sufficient amounts of inorganic carbon. Temperature was kept constant at 20 ± 1°C by means of a stainless steel cooling element placed within the culture vessel. The specific design of the chemostats allowed an accurate definition of the light conditions (17). The light source consisted of four white fluorescent tubes (Philips PL-L/24W/840/4P) directed towards the front surface of the culture vessel. Incident light intensity (Iin) and the light intensity penetrating through the vessel (Iout) were measured with a LI-COR SA 190 quantum sensor at 10 evenly spread points on the front surface and back surface of the culture vessel, respectively. Iin was set at 25 ± 1 μmol photons m−2 s−1 for all experiments. Iout was measured every 3 to 4 days.
Experiments.
The Microcystis strains were grown in monoculture experiments and competition experiments. The monoculture experiments were performed for four reasons: (i) to ensure that the strains could all survive in monoculture under the imposed experimental conditions, (ii) to determine the microcystin content of the toxic strains under the imposed experimental conditions, (iii) to assess changes in pigment concentration during the experiments, and (iv) to determine the critical light intensities of the strains. The critical light intensity (I*out) of each strain was measured as the light intensity penetrating through the monoculture once the monoculture had reached a steady state (15, 16, 31). We ran three competition experiments. In competition experiment 1, the toxic strain V163 and nontoxic strain V145 were inoculated at low initial population densities at a cell ratio of 1:1. Likewise, in competition experiment 2, the toxic strain CYA140 and nontoxic strain CYA43 were inoculated at a cell ratio of 1:1. In competition experiment 3, the toxic strain CYA140 and nontoxic strain CYA43 were inoculated at a cell ratio of 9:1 to give the toxic strain an initial advantage.
Sampling.
Cultures were not completely axenic. However, frequent examination by phase-contrast microscopy indicated that heterotrophic bacteria amounted to less than 1% of the total biovolume. Furthermore, we used our DGGE analysis (see below) to check for contamination with other cyanobacterial species: the primers used for PCR and DGGE were cyanobacterium specific, and contamination by other cyanobacteria would have been detected as an additional band in the DGGE profiles. Contamination by other cyanobacteria was not detected.
Samples were taken from day 1 (inoculation) until the cultures had maintained a steady state (constant population density and constant Iout) for at least 1 week. During the entire experimental period, samples were taken once every 4 days from the monocultures and once every 2 days from the competition experiments. Samples were divided into subsamples for analysis of cell counts (Casycounter, type Casy 1 TTC; Schärfe System, Germany), light absorption spectra, DGGE profiles, and intracellular and extracellular microcystin concentrations.
Microcystin analysis.
For intracellular microcystin analysis, 10 ml of culture suspension was filtered in triplicate using GF/C filters (pore size, ∼1.2 μm; 25-mm diameter; Whatman, Maidstone, United Kingdom). The filters were lyophilized, and subsequently 1.5 ml of 75% (vol/vol) aqueous methanol was added for extraction of microcystins according to Fastner et al. (9), with an extra step for grinding the filters in a Mini Beadbeater (Biospec products, Bartlesville, OK) with 0.5-mm silica beads (45). Dried extracts were stored at −20°C and dissolved in 50% MeOH for analysis of microcystin content using high-performance liquid chromatography with photodiode array detection (KONTRON Instruments, Watford, United Kingdom). Extracts were separated on a LiChrospher 100 RP-18 (5 μm) LichorChart 250-4 cartridge system (Merck, Darmstadt, Germany), using a gradient of 30 to 70% (vol/vol) aqueous acetonitrile (with 0.05% [vol/vol] trifluoroacetic acid) at a flow rate of 1 ml min−1. Microcystins were identified using their typical UV spectra (24). Total microcystin concentrations were quantified as the sum of all microcystin peaks using a microcystin LR gravimetrical standard provided by the Laboratory of Microbiology of the University of Dundee.
Extracellular microcystins were obtained from the 10 ml of filtered culture suspension mentioned above. The filtrate was lyophilized and subsequently resuspended in 150 μl Milli-Q water. Prior to analysis the samples were vortexed, boiled in a water bath for 1 h (27), and centrifuged for 3 min at 18,300 × g. Extracellular microcystin concentrations were below the detection limit of the high-performance liquid chromatograph (2.5 ng microcystin). Therefore, they were determined using an enzyme-linked immunosorbent assay. The enzyme-linked immunosorbent assay was performed according to the protocol of the microcystin plate kit (EnviroLogix, Inc.; catalog no. EP 022).
Light absorbance spectra.
Because strain V145 has a higher content of the pigment phycocyanin than strain V163, we could deduce the population dynamics of the two strains in competition experiment 1 from the relative concentration of phycocyanin. For this purpose, 2 ml of culture suspension was pressurized at 10 × 105 Pa to collapse the gas vesicles of the cells. Next, the culture suspension was transferred to a quartz cuvette (10-mm width) and its light absorbance spectrum was scanned from 350 to 700 nm with a bandwidth of 0.4 nm using an Aminco DW-2000 double-beam spectrophotometer. Mineral medium without Microcystis was used for baseline measurements. After baseline correction, the relative concentration of phycocyanin in the culture was estimated by expressing light absorption by phycocyanin (at 627 nm) as a percentage of the light absorption by the first chlorophyll peak (at 438 nm).
DGGE profiling.
Strain CYA140 and strain CYA43 have a very similar pigment composition. Previous work, however, has shown that different Microcystis strains can be differentiated at high resolution using DGGE analysis of the ITS region (20, 21). Therefore, we prepared a range of different mixtures of the two strains, to assess whether the relative abundances of the two strains could be quantified using the relative band intensities of strain-specific bands in DGGE profiles. Since this worked out very well, we decided to monitor the population dynamics of Microcystis strains CYA140 and CYA43 in competition experiments 2 and 3 using their relative band intensities in DGGE profiles of the samples. After sampling, 2 ml of the culture suspension was transferred to Eppendorf tubes and put under pressure (10 × 105 Pa) to collapse the gas vesicles of the cells. Subsequently, the Eppendorf tubes were centrifuged at 18,300 × g and the supernatants were removed. The Eppendorf tubes were stored at −20°C until further processing. We used a xanthogenate-based protocol for DNA isolation (43). We applied DGGE analysis to sections of the ITS between the 16S and 23S rRNA genes. The PCR amplification protocol and the ITSa primer set used for the ITS region were based on Janse et al. (20). PCR products were separated on a 1.5-mm-thick, vertical DGGE gel containing 8% (wt/vol) polyacrylamide (37.5:1 acrylamide/bisacrylamide ratio) and a linear gradient of the denaturants urea and formamide. After staining of the gel in water containing 0.5 μg ml−1 ethidium bromide, an image of the gel was recorded with a charge-coupled device camera system (Imago; B&L Systems, The Netherlands). DGGE gel pictures were analyzed using the Phoretics-1D package (Nonlinear Dynamics, United Kingdom). Lanes were created manually with a fixed width. Subsequent lanes represented subsequent sampling days. Peaks smaller than 1% of the maximum peak were discarded. Relative densities of Microcystis bands were calculated by dividing the peak intensity of the band concerned by the sum of the peak intensities from all Microcystis bands in that lane. Here, peak intensity is defined as the sum of all pixel values within the band boundaries. The DGGE profiles were run in duplicate to check the consistency of the results.
RESULTS
Monoculture experiments.
All strains were able to grow well in monoculture. Figure 2 shows examples of monoculture experiments of strain V145 and strain CYA140. Cell densities increased to steady-state values of about 6 million cells ml−1 for strain V145 and 23 million cells ml−1 for strain CYA140. This difference in steady-state cell densities can be attributed to a difference in cell size, since strain V145 had an average cell diameter of 5.2 μm, while strain CYA140 had an average cell diameter of only 3.9 μm. Hence, in terms of biovolume, the steady states of the two strains were quite similar. With increasing cell density, Iout decreased. The I*outs of strain V145 and strain CYA140 were both around 1.3 μmol photons m−2 s−1 (Fig. 2). Strain CYA43 reached a similar I*out of about 1.3 μmol photons m−2 s−1, whereas the I*out of the toxic strain V163 was higher, 4.6 μmol photons m−2 s−1. The steady-state microcystin contents in the monoculture experiments were around 24 fg cell−1 in strain V163 (standard deviation [SD] = 11; n = 4) and around 40 fg cell−1 in strain CYA140 (SD = 6, n = 11).
FIG. 2.
Time course of cell number (open triangles) and Iout (closed circles) in monoculture experiments with (A) the nontoxic strain V145 and (B) the toxic strain CYA140. A steady state was reached in about 20 to 30 days.
Toxic strain V163 versus nontoxic strain V145. (i) Approach.
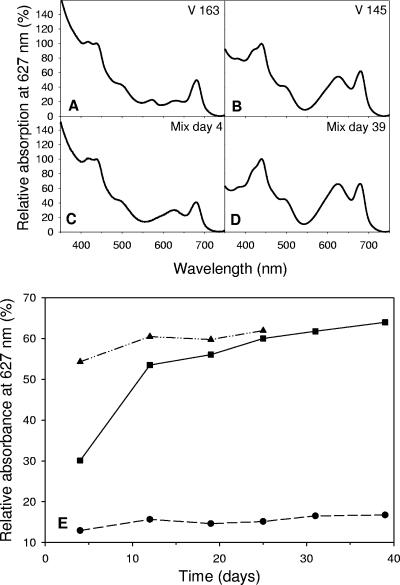
Strains V163 and V145 used in competition experiment 1 differed in their pigment compositions. Toxic strain V163 contains accessory pigments absorbing light between 350 and 400 nm, probably playing a role in UV protection, the red pigment phycoerythrin (peak absorbance at 570 nm), and a relatively low content of the blue-green pigment phycocyanin (peak absorbance at 627 nm) (Fig. 3A). Nontoxic strain V145 lacks phycoerythrin but has a much higher content of the pigment phycocyanin (peak absorbance at 627 nm) (Fig. 3B).
FIG. 3.
Light absorption spectra, normalized on the first chlorophyll peak at 438 nm, of the monoculture experiments of (A) toxic strain V163 and (B) nontoxic strain V145 and of the competition experiment between these two strains on (C) day 4 and (D) day 39. (E) Changes in the relative absorption at 627 nm, the characteristic wavelength for phycocyanin, show the displacement of the toxic strain V163 by the nontoxic strain V145 during the competition experiment (solid squares connected by solid line). Changes in the relative absorption at 627 nm during the monoculture experiments of strain V145 (solid triangles connected by a dashed-and-dotted line) and strain V163 (circles connected by dashed line) are also indicated.
(ii) Population dynamics.
The differences in pigment composition between the two strains were used to monitor the competition experiment. The absorbance spectrum of the mixture in the competition experiment shifted from a spectrum quite similar to that of toxic strain V163 at day 4 (Fig. 3C) towards a spectrum similar to that of nontoxic strain V145 at the end of the competition experiment (Fig. 3D). In fact, the changes in the light absorbance spectra of the competition experiment indicated that the toxic strain V163 was competitively replaced by the nontoxic strain V145 within about 2 weeks (Fig. 3E).
(iii) Microcystin concentration and Microcystis biomass.
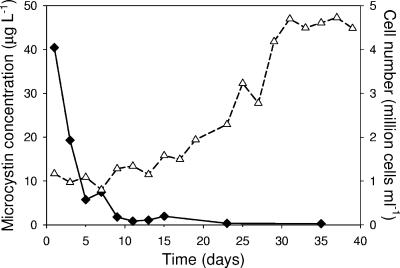
The increasing dominance of the nontoxic strain V145 was confirmed by changes in microcystin concentration. While the total Microcystis population increased more than fourfold, the total microcystin concentration in the competition experiment decreased to nearly zero in 10 days (Fig. 4).
FIG. 4.
Time course of the total microcystin concentration (solid diamonds) and cell number (open triangles) in competition experiment 1 between the nontoxic strain V145 and the toxic strain V163. Data represent the mean of three replicate measurements.
Toxic strain CYA140 versus nontoxic strain CYA43. (i) Approach.
In the next two competition experiments, we used two strains (nontoxic strain CYA43 and toxic strain CYA140) with similar pigment compositions. We therefore used DGGE analysis of the ITS region to distinguish the two strains. DGGE profiles from the monocultures of strains CYA43 and CYA140 each yielded a single band when amplified with rRNA ITSa primers. The positions of the bands on the gels differed clearly, thus allowing identification of the two strains in the competition experiments. To test whether the DGGE profiles also allowed quantification of the relative abundances of the two strains, we prepared mixtures of the two strains in a range of different relative abundances. This yielded corresponding ratios of the band intensities in rRNA-ITS DGGE profiles (Fig. 5A). Furthermore, all DGGE profiles were run in duplicate, and the duplicates always showed very similar results. Therefore, we conclude that the relative band intensities indeed enable semiquantitative monitoring of competition between the two strains (Fig. 5B).
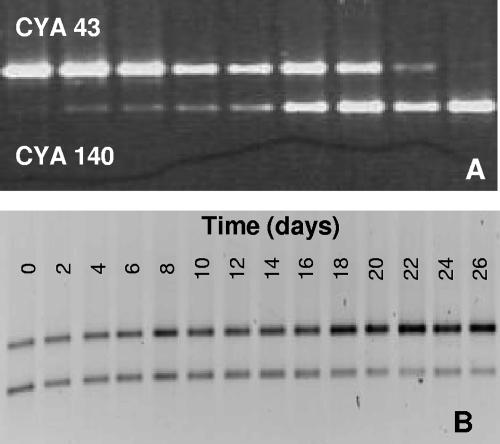
FIG. 5.
DGGE gels loaded with PCR products after amplification with ITSa primers of the rRNA ITS region for different mixtures of strain CYA43 and strain CYA140. (A) DGGE gel of strains CYA43 and CYA140 mixed in the following ratios (from left to right): 99:1, 5.67:1, 2.45:1, 1.33:1, 0.89:1, 0.59:1, 0.32:1, 0.14:1, and 0.01:1. (B) DGGE gel of competition experiment 2, in which the toxic strain CYA140 is gradually displaced by the nontoxic strain CYA43.
(ii) Population dynamics.
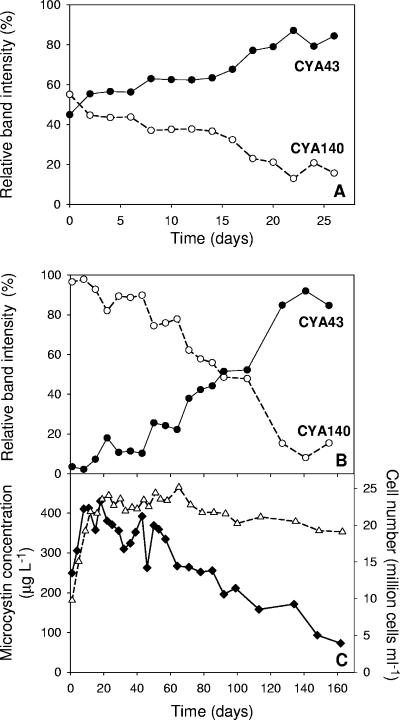
We carried out two experiments with different initial ratios of toxic versus nontoxic cells. Experiment 2 was started with equal amounts of toxic and nontoxic cells (1:1), while experiment 3 was started with many more toxic than nontoxic cells (9:1) to give the toxic strain an initial advantage. Analysis of the relative band intensities in the two competition experiments revealed that the ratio between strains CYA140 and CYA43 changed towards dominance of the nontoxic strain CYA43 in both experiments (Fig. 6A and B). In experiment 2, the relative band intensity of toxic strain CYA140 was reduced to less than 20% within 25 days (Fig. 6A). In experiment 3, which started with a high initial density of toxic cells, the toxic strain also gradually declined, but it took much longer (around 120 days) before the toxic strain was reduced to less than 20% of the total Microcystis population (Fig. 6B).
FIG. 6.
Time courses of competition between the toxic strain CYA140 (open circles) and the nontoxic strain CYA43 (solid circles), deduced from the relative band intensities on the DGGE gels. (A) At the start of competition experiment 2, the competing strains CYA43 and CYA140 were inoculated in a 1:1 ratio. (B) At the start of competition experiment 3, the competing strains CYA43 and CYA140 were inoculated in a 1:9 ratio to give the toxic strain CYA140 an initial advantage. (C) Time course of the total microcystin concentration (solid diamonds) and total cell density (open triangles) in the latter competition experiment shown in panel B. Data for total microcystin concentration and total cell density represent the mean of three replicate measurements. Data for the relative band intensities of the two strains are based on duplicate DGGE profiles.
(iii) Microcystin concentration and Microcystis biomass.
The total microcystin concentration increased during the first 15 days of competition experiment 3, in parallel with the increase in the total Microcystis population (Fig. 6C). However, once the experiment reached its highest cell densities (approximately 25 million cells ml−1), the total microcystin concentration started to decline. After about 140 days, the total microcystin concentration was reduced to less than 20% of its highest value, reflecting the competitive replacement of the toxic strain CYA140 by the nontoxic strain CYA43.
DISCUSSION
In this study, we investigated competition for light between different strains of the Microcystis genus. Traditionally, phytoplankton competition studies make use of microscopy and/or flow cytometry to monitor the population dynamics of competing species (12, 16, 31, 40, 41, 44). However, the Microcystis strains in our competition experiments could not be distinguished microscopically. Therefore, in our experiment 1, we made use of the observation that the two strains differ in their pigment compositions (Fig. 3), which allowed monitoring of the relative abundances of the two strains during the competition experiment. In experiments 2 and 3, we applied a recently developed molecular approach that can distinguish different strains by DGGE profiles of the ITS region of the rRNA operon (20, 21). This molecular technique allowed recognition of the different strains, and we showed that in our experiments the relative band intensities in the DGGE profile also enabled semiquantitative estimates of the abundances of these strains (Fig. 5). The population dynamics deduced from the DGGE analysis were confirmed by independent measurements of changes in the total microcystin concentration in the competition experiments (Fig. 6).
Competition for light.
Earlier laboratory studies revealed subtle differences in the light-dependent growth responses of various Microcystis strains (2, 13). Nevertheless, our results show that these subtle differences among Microcystis strains are sufficient to cause competitive replacement (Fig. 3 and 6). Competition theory predicts that, in well-mixed waters, the phytoplankton species with the lowest critical light intensity will be the best competitor for light (15, 50). This prediction is confirmed by a series of competition experiments (16, 26, 31). In our study, the critical light intensity of toxic strain V163 was higher than the critical light intensity of nontoxic strain V145. As predicted by the theory, the toxic strain V163 was indeed competitively displaced by the nontoxic strain V145 (Fig. 3). Competitive displacement took less than 2 weeks. The critical light intensities of strains CYA140 and CYA43 were very similar. Therefore, competition theory predicts that these two strains should be more or less equal competitors for light. However, the nontoxic strain CYA43 became dominant in the competition experiments. Even in competition experiment 3, where toxic strain CYA140 was given a strong initial advantage, the nontoxic strain CYA43 became dominant in the end. Competitive displacement of CYA140 by CYA43 took much longer, however, than competitive displacement of V163 by V145 (compare the time scales of Fig. 3 and Fig. 6), which is consistent with the prediction that the difference in competitive abilities between CYA140 and CYA43 must have been small.
Our competition experiments suggest that nontoxic strains are better competitors for light than toxic strains. In each of the competition experiments, toxic strains were competitively excluded by nontoxic strains. One might expect a tradeoff between the costs and benefits of toxin production (33). Strains that invest their resources in microcystin production and the microcystin synthetase complex may have fewer resources available to invest in other cellular functions. Although the physiological costs of microcystin production have not yet been fully elucidated, this might indeed imply that toxic strains are usually poorer competitors than nontoxic strains. However, the numbers of toxic and nontoxic Microcystis strains that we investigated are relatively small, and we explored only one set of environmental conditions. Further research with more strains competing under a wide range of different environmental conditions will be needed to shed more light on the generality of this finding.
Allelopathic interactions?
Several studies have suggested that microcystins and other toxic peptides produced by cyanobacteria may have allelopathic effects on other phytoplankton and plants (32, 35-38; however, see reference 25). In particular, microcystins can function as inhibitors of photosynthetic activity (14, 39, 42). Mathematical theory predicts that, at least in well-mixed chemostats, the winner of allelopathic interactions between toxin-producing and toxin-sensitive strains will depend on the initial abundances of these strains (4, 8, 19). That is, allelopathic interactions will be effective only if toxin-producing strains are sufficiently abundant and produce enough toxin to suppress toxin-sensitive strains. Our experiments were run with very high cell densities (up to 25 million cells ml−1) typical of dense cyanobacterial blooms. Moreover, in one of our competition experiments, the toxic strain was given a much higher initial abundance than the nontoxic strain. Yet, in the end, in all competition experiments the nontoxic strain became dominant. Furthermore, the outcome of the competition experiment with strains V145 and V163 followed the prediction on the basis of their growth in monocultures: i.e., the strain with the lowest critical light intensity, V145, won the competition. Any effect of microcystins or other allelopathic substances would have counteracted this result. Hence, our findings do not support the suggestion that microcystins play an ecologically important role as allelopathic compounds in Microcystis population dynamics.
One explanation for the absence of allelopathic effects might be that the non-microcystin-producing strains used in our study were resistant to microcystins. An alternative explanation might be that the extracellular microcystin concentrations in our experiments never exceeded 20 μg liter−1, which is only 5% of the cell-bound microcystin in the experiments. Despite high Microcystis densities, the extracellular microcystin concentrations in our experiments may still have been too low to have a significant negative effect on the growth of non-microcystin-producing Microcystis strains. Similarly, Babica et al. (1) recently concluded that ecologically relevant microcystin concentrations, as commonly found in Microcystis blooms, are generally too low to have allelopathic effects on other photoautotrophic organisms.
Seasonal dynamics of toxic and nontoxic strains.
Controlled laboratory experiments provide very simple environments in comparison to the full complexity of real aquatic ecosystems. For instance, in our laboratory experiments we found competitive exclusion, resulting in the dominance of a single strain. Under field conditions, however, coexistence of several Microcystis genotypes is often found (23, 30, 34, 55). It might be that ecologically relevant aspects not investigated in our experiments, like zooplankton grazing or nutrient limitation, promote the coexistence of multiple strains in natural waters. Furthermore, differences in pigment composition may enable strains containing phycoerythrin, like strain V163, to use another part of the light spectrum, which may prolong their coexistence with strains containing phycocyanin (41).
Yet, despite the simplified environments in laboratory experiments, there are striking similarities between the population dynamics in our competition experiments and the seasonal dynamics of toxic and nontoxic Microcystis strains in natural waters. In eutrophic lakes, the increase in total Microcystis biomass during the growing season is often accompanied by a decrease of the average microcystin concentration per cell (Fig. 1) (see also references 22 and 51). We hypothesize that this seasonal pattern reflects a competitive replacement from toxic to nontoxic Microcystis strains within Microcystis blooms. The population dynamics in our competition experiments indicate that the strain composition within Microcystis populations determines the overall microcystin concentration. Moreover, the toxic strains were weaker competitors for light than the nontoxic strains, resulting in gradually declining microcystin concentrations during the competition experiments (Fig. 4 and 6). Hence, our laboratory experiments demonstrate that, in principle, competition for light can drive a seasonal succession from toxic to nontoxic strains in dense Microcystis blooms.
Acknowledgments
We thank G. Zwart for critical reading of the manuscript.
The research of W.E.A.K. and I.J. was funded by the Technology Foundation (STW; project ACH 4874). L.T., S.H., J.H., and P.M.V. were supported by the Earth and Life Sciences Foundation (ALW), which is subsidized by The Netherlands Organization for Scientific Research (NWO). L.T. and P.M.V. were additionally supported by a European Union grant within the program PEPCY.
Footnotes
Published ahead of print on 2 March 2007.
REFERENCES
- 1.Babica, P., L. Bláha, and B. Maršálek. 2006. Exploring the natural role of microcystins: a review of effects on photoautotrophic organisms. J. Phycol. 42:9-20. [Google Scholar]
- 2.Böttcher, G., I. Chorus, S. Ewald, T. Hintze, and N. Walz. 2001. Light-limited growth and microcystin content of Planktothrix agardii and Microcystis aeruginosa in turbidostats, p. 115-132. In I. Chorus (ed.), Cyanotoxins—occurrence, causes, consequences. Springer, Berlin, Germany.
- 3.Carmichael, W. W. 2001. Health effects of toxin-producing cyanobacteria: “the CyanoHABs.” Hum. Ecol. Risk Assess. 7:1393-1407. [Google Scholar]
- 4.Chao, L., and B. R. Levin. 1981. Structured habitats and the evolution of anticompetitor toxins in bacteria. Proc. Natl. Acad. Sci. USA 78:6324-6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chorus, I. 2005. Current approaches to cyanotoxin risk assessment, risk management and regulations in different countries. Federal Environmental Agency, Berlin, Germany.
- 6.Chorus, I., and J. Bartram. 1999. Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. WHO, E & F Spon, London, United Kingdom.
- 7.Codd, G. A., L. F. Morrison, and J. S. Metcalf. 2005. Cyanobacterial toxins: risk management for health protection. Toxicol. Appl. Pharmacol. 203:264-272. [DOI] [PubMed] [Google Scholar]
- 8.Durrett, R., and S. A. Levin. 1997. Allelopathy in spatially distributed populations. J. Theor. Biol. 185:165-171. [DOI] [PubMed] [Google Scholar]
- 9.Fastner, J., I. Flieger, and U. Neumann. 1998. Optimised extraction of microcystins from field samples: a comparison of different solvents and procedures. Water Res. 32:3177-3181. [Google Scholar]
- 10.Fastner, J., U. Neumann, B. Wirsing, J. Weckesser, C. Wiedner, B. Nixdorf, and I. Chorus. 1999. Microcystins (hepatotoxic heptapeptides) in German fresh water bodies. Environ. Toxicol. 14:13-22. [Google Scholar]
- 11.Fastner, J., M. Erhard, and H. von Döhren. 2001. Determination of oligopeptide diversity within a natural population of Microcystis spp. (cyanobacteria) by typing single colonies by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 67:5069-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grover, J. P. 1991. Dynamics of competition among microalgae in variable environments: experimental tests of alternative models. Oikos 62:231-243. [Google Scholar]
- 13.Hesse, K., and J. G. Kohl. 2001. Effects of light and nutrient supply on growth and microcystin content of different strains of Microcystis aeruginosa, p. 104-114. In I. Chorus (ed.), Cyanotoxins—occurrence, causes, consequences. Springer, Berlin, Germany.
- 14.Hu, Z. Q., Y. D. Liu, and D. H. Li. 2004. Physiological and biochemical analyses of microcystin-RR toxicity to the cyanobacterium Synechococcus elongates. Environ. Toxicol. 19:571-577. [DOI] [PubMed] [Google Scholar]
- 15.Huisman, J., and F. J. Weissing. 1994. Light-limited growth and competition for light in well-mixed aquatic environments: an elementary model. Ecology 75:507-520. [Google Scholar]
- 16.Huisman, J., R. R. Jonker, C. Zonneveld, and F. J. Weissing. 1999. Competition for light between phytoplankton species: experimental tests of mechanistic theory. Ecology 80:211-222. [Google Scholar]
- 17.Huisman, J., H. C. P. Matthijs, P. M. Visser, H. Balke, C. A. M. Sigon, J. Passarge, F. J. Weissing, and L. R. Mur. 2002. Principles of the light-limited chemostat: theory and ecological applications. Antonie Leeuwenhoek 81:117-133. [DOI] [PubMed] [Google Scholar]
- 18.Huisman, J., J. Sharples, J. Stroom, P. M. Visser, W. E. A. Kardinaal, J. M. H. Verspagen, and B. Sommeijer. 2004. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 85:2960-2970. [Google Scholar]
- 19.Hulot, F. D., and J. Huisman. 2004. Allelopathic interactions between phytoplankton species: the roles of heterotrophic bacteria and mixing intensity. Limnol. Oceanogr. 49:1424-1434. [Google Scholar]
- 20.Janse, I., M. Meima, W. E. A. Kardinaal, and G. Zwart. 2003. High-resolution differentiation of cyanobacteria by using rRNA-internal transcribed spacer denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 69:6634-6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janse, I., W. E. A. Kardinaal, M. Meima, J. Fastner, P. M. Visser, and G. Zwart. 2004. Toxic and nontoxic Microcystis colonies in natural populations can be differentiated on the basis of rRNA gene internal transcribed spacer diversity. Appl. Environ. Microbiol. 70:3979-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kardinaal, W. E. A., and P. M. Visser. 2005. Dynamics of cyanobacterial toxins: sources of variability in microcystin concentrations, p. 41-63. In J. Huisman, H. C. P. Matthijs, and P. M. Visser (ed.), Harmful cyanobacteria. Springer, Dordrecht, The Netherlands.
- 23.Kurmayer, R., E. Dittmann, J. Fastner, and I. Chorus. 2002. Diversity of microcystin genes within a population of the toxic cyanobacterium Microcystis spp. in Lake Wannsee (Berlin, Germany). Microb. Ecol. 43:107-118. [DOI] [PubMed] [Google Scholar]
- 24.Lawton, L. A., C. Edwards, and G. A. Codd. 1994. Extraction and high-performance liquid-chromatographic method for the determination of microcystins in raw and treated waters. Analyst 119:1525-1530. [DOI] [PubMed] [Google Scholar]
- 25.LeBlanc, S., F. R. Pick, and R. Aranda-Rodriguez. 2005. Allelopathic effects of the toxic cyanobacterium Microcystis aeruginosa on duckweed, Lemna gibba L. Environ. Toxicol. 20:67-73. [DOI] [PubMed] [Google Scholar]
- 26.Litchman, E. 2003. Competition and coexistence of phytoplankton under fluctuating light: experiments with two cyanobacteria. Aquat. Microb. Ecol. 31:241-248. [Google Scholar]
- 27.Metcalf, J. S., and G. A. Codd. 2000. Microwave oven and boiling waterbath extraction of hepatotoxins from cyanobacterial cells. FEMS Microbiol. Lett. 184:241-246. [DOI] [PubMed] [Google Scholar]
- 28.Mur, L. R., and H. Schreurs. 1995. Light as a selective factor in the distribution of phytoplankton species. Water Sci. Technol. 32:25-34. [Google Scholar]
- 29.Oh, H.-M., S. J. Lee, M.-H. Jang, and B.-D. Yoon. 2000. Microcystin production by Microcystis aeruginosa in a phosphorus-limited chemostat. Appl. Environ. Microbiol. 66:176-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouellette, A. J. A., S. M. Handy, and S. W. Wilhelm. 2006. Toxic Microcystis is widespread in Lake Erie: PCR detection of toxin genes and molecular characterization of associated cyanobacterial communities. Microb. Ecol. 51:154-165. [DOI] [PubMed] [Google Scholar]
- 31.Passarge, J., S. Hol, M. Escher, and J. Huisman. 2006. Competition for nutrients and light: stable coexistence, alternative stable states, or competitive exclusion? Ecol. Monogr. 76:57-72. [Google Scholar]
- 32.Pflugmacher, S. 2002. Possible allelopathic effects of cyanotoxins, with reference to microcystin-LR, in aquatic ecosystems. Environ. Toxicol. 17:407-413. [DOI] [PubMed] [Google Scholar]
- 33.Riley, M. A., and D. M. Gordon. 1999. The ecological role of bacteriocins in bacterial competition. Trends Microbiol. 7:129-133. [DOI] [PubMed] [Google Scholar]
- 34.Sanchis, D., C. Padilla, F. F. Del Campo, A. Quesada, and S. Sanz-Alferez. 2005. Phylogenetic and morphological analyses of Microcystis strains (Cyanophyta/Cyanobacteria) from a Spanish water reservoir. Nova Hedwigia 81:431-448. [Google Scholar]
- 35.Schagerl, M., I. Unterrieder, and D. G. Angeler. 2002. Allelopathy among cyanoprokaryota and other algae originating from Lake Neusiedlersee (Austria). Int. Rev. Hydrobiol. 87:365-374. [Google Scholar]
- 36.Schatz, D., Y. Keren, O. Hadas, S. Carmeli, A. Sukenik, and A. Kaplan. 2005. Ecological implications of the emergence of non-toxic subcultures from toxic Microcystis strains. Environ. Microbiol. 7:798-805. [DOI] [PubMed] [Google Scholar]
- 37.Sedmak, B., and G. Kosi. 1998. The role of microcystins in heavy cyanobacterial bloom formation. J. Plankton Res. 20:691-708. [Google Scholar]
- 38.Sedmak, B., and T. Elersek. 2005. Microcystins induce morphological and physiological changes in selected representative phytoplanktons. Microb. Ecol. 50:298-305. [DOI] [PubMed] [Google Scholar]
- 39.Smith, G. D., and N. T. Doan. 1999. Cyanobacterial metabolites with bioactivity against photosynthesis in cyanobacteria, algae, and higher plants, J. Appl. Phycol. 11:337-344. [Google Scholar]
- 40.Sommer, U. 1986. Nitrate- and silicate competition among Antarctic phytoplankton. Mar. Biol. 91:345-351. [Google Scholar]
- 41.Stomp, M., J. Huisman, F. de Jongh, A. J. Veraart, D. Gerla, M. Rijkeboer, B. W. Ibelings, U. I. A. Wollenzien, and L. J. Stal. 2004. Adaptive divergence in pigment composition promotes phytoplankton biodiversity. Nature 432:104-107. [DOI] [PubMed] [Google Scholar]
- 42.Sukenik, A., R. Eshkol, A. Livne, O. Hadas, M. Rom, D. Tchernov, A. Vardi, and A. Kaplan. 2002. Inhibition of growth and photosynthesis of the dinoflagellate Peridinium gatunense by Microcystis sp. (cyanobacteria): a novel allelopathic mechanism. Limnol. Oceanogr. 47:1656-1663. [Google Scholar]
- 43.Tillett, D., and B. A. Neilan. 2000. Xanthogenate nucleic acid isolation from cultured and environmental cyanobacteria. J. Phycol. 36:251-258. [Google Scholar]
- 44.Tilman, D. 1977. Resource competition between planktonic algae: an experimental and theoretical approach. Ecology 58:338-348. [Google Scholar]
- 45.Tonk, L., P. M. Visser, G. Christiansen, E. Dittmann, E. O. F. M. Snelder, C. Wiedner, L. R. Mur, and J. Huisman. 2005. The microcystin composition of the cyanobacterium Planktothrix agardhii changes toward a more toxic variant with increasing light intensity. Appl. Environ. Microbiol. 71:5177-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Liere, L., and L. R. Mur. 1978. Light-limited cultures of the blue-green alga Oscillatoria agardhii. Mitt. Int. Ver. Theor. Angew. Limnol. 21:158-167. [Google Scholar]
- 47.Verspagen, J. M. H., J. Passarge, K. D. Jöhnk, P. M. Visser, L. Peperzak, P. Boers, H. J. Laanbroek, and J. Huisman. 2006. Water management strategies against toxic Microcystis blooms in the Dutch delta. Ecol. Appl. 16:313-327. [DOI] [PubMed] [Google Scholar]
- 48.Via-Ordorika, L., J. Fastner, M. Hisbergues, E. Dittmann, M. Erhard, J. Komarèk, R. Kurmayer, and I. Chorus. 2004. Distribution of microcystin-producing and non-microcystin-producing Microcystis in European freshwater bodies: detection of microcystins and mcy genes in single colonies. Syst. Appl. Microbiol. 27:592-602. [DOI] [PubMed] [Google Scholar]
- 49.Visser, P. M., B. W. Ibelings, L. R. Mur, and A. E. Walsby. 2005. The ecophysiology of the harmful cyanobacterium Microcystis: features explaining its success and measures for its control, p. 109-142. In J. Huisman, H.C.P. Matthijs, and P.M. Visser (ed.), Harmful cyanobacteria. Springer, Dordrecht, The Netherlands.
- 50.Weissing, F. J., and J. Huisman. 1994. Growth and competition in a light gradient. J. Theor. Biol. 168:323-336. [Google Scholar]
- 51.Welker, M., H. von Döhren, H. Tauscher, C. E. W. Steinberg, and M. Erhard. 2003. Toxic Microcystis in shallow Lake Müggelsee (Germany): dynamics, distribution, diversity. Arch. Hydrobiol. 157:227-248. [Google Scholar]
- 52.Welker, M., M. Brunke, K. Preussel, I. Lippert, and H. von Döhren. 2004. Diversity and distribution of Microcystis (Cyanobacteria) oligopeptide chemotypes from natural communities studied by single-colony mass spectrometry. Microbiology 150:1785-1796. [DOI] [PubMed] [Google Scholar]
- 53.Welker, M., L. Šejnohová, D. Némethová, H. von Döhren, J. Jarkoský, and B. Marŝálek. 2007. Seasonal shifts in chemotype composition of Microcystis sp. communities in the pelagial and the sediment of a shallow reservoir. Limnol. Oceanogr. 52:609-619. [Google Scholar]
- 54.Wiedner, C., P. M. Visser, J. Fastner, J. S. Metcalf, G. A. Codd, and L. R. Mur. 2003. Effects of light on the microcystin content of Microcystis strain PCC 7806. Appl. Environ. Microbiol. 69:1475-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson, A. E., O. Sarnelle, B. A. Neilan, T. P. Salmon, M. M. Gehringer, and M. E. Hay. 2005. Genetic variation of the bloom-forming cyanobacterium Microcystis aeruginosa within and among lakes: implications for harmful algal blooms. Appl. Environ. Microbiol. 71:6126-6133. [DOI] [PMC free article] [PubMed] [Google Scholar]