Abstract
A latent virus-like agent, which we designated zooxanthella filamentous virus 1 (ZFV1), was isolated from Symbiodinium sp. strain CCMP 2465 and characterized. Transmission electron microscopy and analytical flow cytometry revealed the presence of a new group of distinctive filamentous virus-like particles after exposure of the zooxanthellae to UV light. Examination of thin sections of the zooxanthellae revealed the formation and proliferation of filamentous virus-like particles in the UV-induced cells. Assessment of Symbiodinium sp. cultures was used here as a model to show the effects of UV irradiance and induction of potential latent viruses. The unique host-virus system described here provides insight into the role of latent infections in zooxanthellae through environmentally regulated viral induction mechanisms.
The frequency and intensity of coral bleaching have increased in the last two decades, leading to mass mortality of corals and a resulting reduction in the biodiversity of reefs (7, 33). Bleaching has been observed in more than 50 countries and in the three major oceans (41). Many environmental factors have been linked to coral bleaching; these factors include elevated and reduced temperatures (13), exposure to UV radiation (17), and bacterial infections (5, 19). However, the underlying causes of bleaching and the mechanisms involved remain largely unknown.
Reef-building corals associate with a diverse array of eukaryotic and prokaryotic microbes. The coral colony has been modeled as a holobiont comprising multispecies mutualisms consisting of the coral animal, endosymbiotic dinoflagellates (zooxanthellae), bacteria, fungi, protozoans, endolithic algae, and other unknown components (27). Disruption of any of these components may cause physiological changes that result in coral disease or death.
Zooxanthellae (genus Symbiodinium) are now known to comprise numerous clades (2). Zooxanthellae form a symbiotic relationship with many Cnidaria species and are by far the best-understood microbial associates of corals, and they have a clearly beneficial relationship with the coral. They release photosynthetic products to their hosts, providing an important source of organic carbon and nitrogen for host metabolism, growth, and reproduction (12, 24, 40). The symbiosis with zooxanthellae is believed to explain the success of reef-building corals in nutrient-poor tropical seas (15). Disruption of the zooxanthella-host symbiosis (bleaching) can lead to expulsion of zooxanthellae from the host and/or a decrease in the amount of the zooxanthella photosynthetic pigment (15, 18). Zooxanthellae have been shown to undergo necrosis, apoptosis, and lysis during bleaching (4, 31). The expulsion of Symbiodinium cells may help corals adapt to changing environmental conditions by allowing symbiont populations to redistribute themselves (3, 10 ). If the symbiont-host relationship is not recovered, the coral does not survive (33). It has been argued that the random mosaic patterns of bleaching are difficult to attribute to the effect of temperature stress alone as neighboring regions of a colony must be exposed to the same conditions (16). One explanation for the patchy spatial distribution of coral bleaching involves localized infections. Bacterial infection by Vibrio sp. has been shown to be responsible for some types of coral bleaching (5, 28, 37). Given the great abundance of viruses in marine systems, in which concentrations reach >108 particles ml−1 (6, 44), it is likely that viruses are also involved as agents of coral disease (11), and involvement of viruses in the coral bleaching process must be considered. It has been established that the majority of marine viruses infect bacteria, but viruses that infect eukaryotic algae have also been shown to be abundant in the marine environment (8). Virus-like particles (VLPs) have been found in cells of about 50 different algal species representing nearly all major algal classes (26, 38 ), and evidence suggests that viruses play a significant role in the population dynamics and community composition of marine phytoplankton (8, 22, 29, 32). Despite this, there have been relatively few reports on viruses of dinoflagellates (14, 30, 35) and fewer still on the presence of viruses in zooxanthellae. Previous studies in our laboratory have shown that zooxanthellae and corals produce VLPs when they are exposed to stress and elevated temperatures (11, 42, 43), suggesting that zooxanthellae harbor latent viruses. In order to determine whether VLPs are induced by exposure of the zooxanthellae to UV light, we utilized Symbiodinium sp. cultured in vitro to simplify the complexity found in whole organisms containing symbionts.
MATERIALS AND METHODS
Zooxanthella cultures.
During this study, 16 strains of Symbiodinium species isolated from a variety of cnidarian hosts were maintained in culture (nonaxenic). Strains 12, 61, 104,133, 135, 141, 152, 154, 203, 292, 368, 379, 383, and 385 were obtained from a collection at the Marine Biological Association and have been described in a previous study (20). Strains 200X and 200Y were isolated from Acropora formosa. The zooxanthellae were grown in ASP-8A medium with antibiotics (25) and were subcultured monthly. Algal cultures were grown by using a cycle consisting of 16 of light and 8 h of darkness at 26°C. The light intensity used was 40 to 50 mol quanta m−2 s−1. Zooxanthella isolate 292 used in this study has been deposited in the Center for Culture of Marine Phytoplankton (http://ccmp.bigelow.org) as strain CCMP 2465.
Experimental treatments.
Triplicate exponentially growing cultures (50 ml; approximately 105 cells ml−1) were exposed for 2 min in open petri dishes to UV light (254 nm) from a Chromato-Vue transilluminator (model TM-20) which was placed upside down 12 cm above the petri dishes to allow direct radiation. After exposure, the cultures were transferred back into 50-ml flasks and maintained at 26°C with illumination using a cycle consisting of 16 of light and 8 h of darkness. Samples used for enumeration of zooxanthellae and VLPs were collected daily for 1 week. Zooxanthellae were enumerated immediately, and 1-ml aliquots were fixed in 0.5% glutaraldehyde, stored at 4°C for 30 min, snap frozen in liquid nitrogen, and stored at −80°C until they were processed for enumeration of VLPs and bacteria.
Enumeration of zooxanthellae, VLPs, and bacteria by analytical flow cytometry (AFC).
All analyses were performed with a Becton Dickinson FACScan flow cytometer using the Cellquest software. For enumeration of live zooxanthellae, undiluted samples were examined for 2 min using a high flow rate (ca. 50 to 70 μl min−1) with the discriminator set on red fluorescence. For enumeration of VLPs and bacteria, counting was performed using fixed samples diluted 1:10 to 1:1,000 in TE buffer (10 mM Tris, 1 mM EDTA; pH 8.0), filtered with a 50-kDa filter using tangential flow filtration (Vivaflow flip flow), and stained for 10 to 15 min at 80°C with a 10−5 dilution of SYBR green I stain (Molecular Probes). The samples were analyzed for 2 min at a low flow rate (ca. 30 μl min−1) with the discriminator set on green fluorescence (23). VLPs and bacteria were enumerated using plots of side scatter (SSC) versus green fluorescence. All 16 zooxanthella strains were screened and exhibited differential sensitivity to UV induction. Strain CCMP 2465 exhibited the strongest response and was therefore utilized for further assessment.
TEM.
Samples potentially containing VLPs were filtered through 0.45-μm filter units (Whatman) with a BD Plastipak sterile 10-ml syringe to remove debris and large cells. Aliquots were fixed in 0.5% glutaraldehyde, stored at 4°C for 30 min, and snap frozen in liquid nitrogen. “Spot” grids were prepared by placing grids onto 15 to 30 μl of a fixed suspension for 30 min. The excess liquid was removed with filter paper, and each preparation was negatively stained with uranyl acetate (2% [wt/vol] in water). For preparation of thin sections, UV-induced zooxanthellae were pelleted by centrifugation at 14,000 rpm in 1.5-ml Eppendorf tubes for 1 min. The pellets were resuspended, washed, and repelleted twice in filtered seawater (pore size, 0.2 μm). Molten agar (2% Fischer agar in distilled H2O; 0.5 ml) was added to each washed pellet and allowed to set; the excess agar was trimmed from the agar plugs. A 4% osmium tetroxide solution was mixed 1:1 with filtered seawater and added to cover the agar plugs. The preparations were left for 1 h at room temperature, and this was followed by two washes in filtered seawater (pore size, 0.2 μm), dehydration with a graded ethanol series, and infiltration with Spurr's resin in preshaped molds. Dried (48 h, 35°C) blocks were mounted and cut into sections that were 70 to 80 nm thick with a Reichert-Jung Ultracut microtome; the sections were floated onto transmission electron microscopy (TEM) grids, stained with 2% uranyl acetate in 70% ethanol (15 min), washed in double-distilled H2O, stained with Reynolds lead citrate for 15 min, and then rinsed in double-distilled H2O. Prepared grids and thin sections were examined with a JEOL 200 CX TEM (magnification, 80 to 600,000) at 160 kV. Photographs were taken at magnifications between ×5,000 and ×50,000.
Concentration of VLPs.
Zooxanthellae were grown to a density of ca. 105 cells ml−1 in 3 liters of ASP-8A medium and exposed to UV light (254 nm) for 2 min in open petri dishes. VLPs were isolated from the growth media 96 h after exposure to UV. Debris, bacteria, and algal cells were removed by filtration though 0.45-μm-pore size, 47-mm-diameter filters (PALL Corp.). VLPs in the supernatant were concentrated by tangential flow filtration (Vivaflow flip flow) with a 50-kDa cutoff to obtain ca. 50 ml. Further concentration to ca. 3 ml was accomplished by ultracentrifugation with a Beckman L8-M at 100,000 × g for 2 h. VLPs were further purified and concentrated on a CsCl gradient prepared with CsCl in TE buffer at densities of 1.0, 1.2, 1.4, and 1.6 g cm−3, and the pelleted VLPs were added to the 1.0-g cm−3 layer. This preparation was then centrifuged at 100,000 × g for 2 h at 20°C, with the deceleration set at 8. A translucent white band was removed by piercing the ultracentrifuge tube just below the band, which allowed aliquots to be separated. Aliquots were dialyzed (Fisher; diameter, 0.32 mm; 12- to 14-kDa molecular-mass cutoff) overnight against 1 liter of TE buffer at 4°C, and the TE buffer was changed twice in this time. The dialyzed VLP concentrate was stored at 4°C until it was analyzed.
RESULTS AND DISCUSSION
Induction of VLPs by UV irradiation of zooxanthellae.
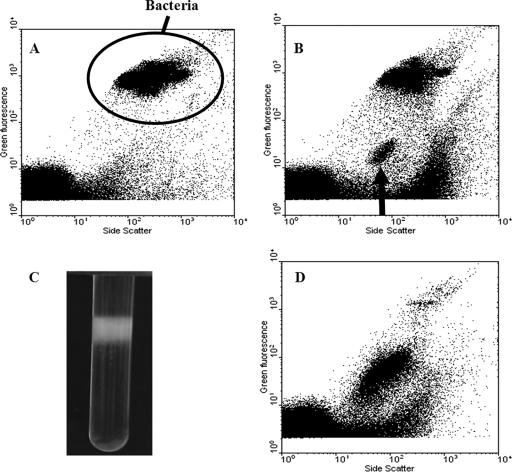
AFC analysis of UV-induced zooxanthella cultures revealed the presence of a separate group of high-SSC, low-green-fluorescence (GFL) particles which were visible ca. 24 h after UV induction. By 96 h, this group was clearly distinguishable (Fig. 1B). It was likely that this group contained VLPs, despite the fact that it had a unique SSC-versus-GFL signal with the SSC higher than that of previously described bacteriophages and viruses (9). The higher SSC suggested that the particles might be filamentous, since SSC is influenced by internal structure and its refractive index (9). Of the zooxanthella isolates screened for UV induction of potential latent viruses, 38% showed proliferation of a group of VLPs similar to that shown in Fig. 1B. VLPs in this high-SSC group were concentrated from a 3-liter culture of zooxanthella strain CCMP 2465. CsCl gradient centrifugation produced a discrete white band at a density of 1.25 g cm−3 (Fig. 1C). AFC analysis of this band revealed that it was dominated by the same high-SSC group of VLPs (Fig. 1D).
FIG. 1.
AFC analysis of VLPs induced following UV treatment of zooxanthella cultures. (A) AFC analysis of control culture of zooxanthellae, showing bacteria (cultures were nonaxenic). (B) AFC analysis of UV-treated culture (after 96 h), showing a new VLP group with high SSC (indicated by arrow). (C) CsCl gradient of the VLPs with a translucent white band at ca. 1.25 g cm−3. (D) AFC analysis of concentrated VLPs taken from the CsCl gradient, showing the presence of the high-SSC VLP group.
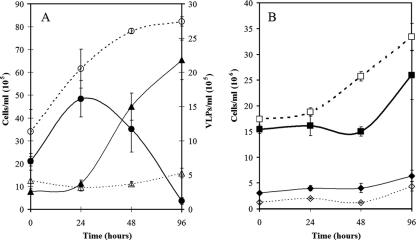
In UV induction experiments, a decline in the zooxanthella concentration was observed starting 24 h after UV induction, which correlated with the appearance and rapid increase in the concentration of the high-SSC VLP group (Fig. 2A). The concentration of the VLPs continued to increase to just over 2 × 106 particles ml−1 at 96 h after UV treatment, at which point the UV-induced zooxanthella culture had lysed.
FIG. 2.
(A) Growth curves showing numbers of cells of zooxanthella strain CCMP 2465 ml−1 in control cultures (○) and UV-treated cultures (•), together with the numbers of the high-SSC VLPs ml−1 in the unirradiated control cultures (▵) and the UV-treated cultures (▴). (B) Concentrations of bacteria in the control cultures (□) and the UV-treated cultures (▪), together with the numbers of bacteriophage particles ml−1 (106) in the control cultures (⋄) and the UV-treated cultures (⧫). The error bars indicate the standard errors for measurements from triplicate cultures.
Since the zooxanthella cultures were nonaxenic, a group with a signal characteristic of bacteria was present in the control (Fig. 1A), as well as in the UV-treated zooxanthella cultures (Fig. 1B). To rule out the possibility that lysogenic bacteria were the source of the high-SSC VLPs, bacterial concentrations were also monitored in the induction experiments. The temporal changes in bacterial concentrations were similar in the control and UV-treated cultures, although there was a slight decrease in the UV-treated cultures between 24 and 48 h, before they again exhibited the same growth rate as the control (Fig. 2B). This initial decrease in bacterial concentrations could have been caused by several factors, such as lysis of UV-sensitive bacteria and/or induction of lysogenic bacteriophage, although there was no apparent increase in the number of bacteriophage particles compared to the control during the induction experiment (Fig. 2B). Bacteriophages usually group in the area above the instrument noise in the bottom left corner of a GFL-versus-SSC plot (Fig. 1A) (9). This group was analyzed, and the number of events remained consistent for the controls and the experimental samples throughout the experiment. While the experimental treatment appeared to have an effect on the numbers of bacteria present in the nonaxenic zooxanthella culture, the clear correlation between the decrease in the size of the zooxanthella population and the concurrent increase in the VLP concentration suggests that bacterial contaminants were not the source of the new VLP group observed by AFC.
Identification of VLPs.
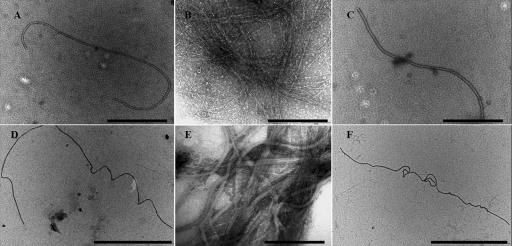
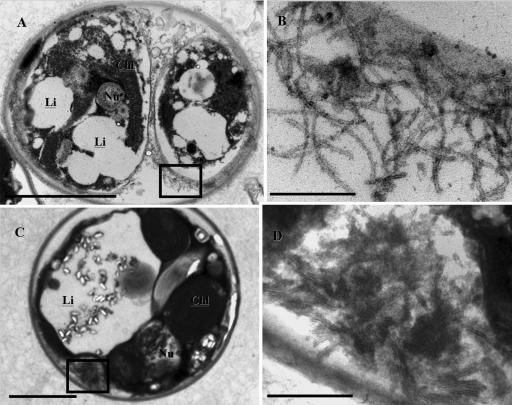
After induction free VLPs in the UV-treated supernatants and concentrates were observed by TEM as long flexible filaments that were 2 to 3 μm long and approximately 30 nm wide (Fig. 3). In VLPs purified by CsCl gradient centrifugation there was an increase in the density of the filamentous VLPs after concentration (3 × 107 VLPs ml−1) (Fig. 3B and E). Thin sections of UV-treated zooxanthellae revealed pockets of filamentous VLPs with similar morphology in the cytoplasm on the periphery of zooxanthella cells (Fig. 4); filamentous VLPs were not seen in controls. The incidence of VLPs observed in the thin sections increased markedly between 39 and 46 h postinduction compared to the incidence in noninduced controls (in which no filaments were observed). The presence of intracellular VLPs provides further evidence that the particles observed were actually infecting the zooxanthellae and not the bacteria present in the cultures. Therefore, we designated the new group of VLPs “zooxanthella filamentous virus 1” (ZFV1).
FIG. 3.
TEM images showing the morphology of the new VLP, ZFV1. The filamentous VLP is ca. 2 to 3 μm long. (A, C, D, and F) Images from spot grids containing unconcentrated lysate. Scale bars = 1 μm. (B and E) Images of VLPs concentrated on a CsCl gradient (shown in Fig. 1C). Scale bars = 200 nm.
FIG. 4.
TEM images showing the presence of filamentous VLPs in thin sections of zooxanthellae prepared 39 h (A and B) and 46 h (C and D) after induction with UV light. Scale bars = 3 μm (A), 200 nm (B), 2 μm (C), and 300 nm (D). The box in panel A indicates the area shown in panel B, and the box in panel C indicates the area shown in panel D. Chl, chloroplast; Nu, nucleus; Li, lipid vacuole.
The majority of algal viruses described to date are members of the Phycodnaviridae, which is a family of large double-stranded DNA viruses that have an icosahedral shape (38, 44), although more recently a number of algal RNA viruses have also been isolated and characterized (21, 34, 36). ZFV1 is morphologically similar to RNA viruses which are known to infect plants, in particular viruses belonging to the family Closteroviridae. Members of this family are filamentous and flexuous, and they are 1,500 to 2,200 nm long (1, 39). However, determination of the taxonomic affiliation of ZFV1 will require further molecular characterization, and this certainly warrants further investigation.
Implications for the mechanism of coral bleaching.
Wilson et al. (43) first suggested that zooxanthellae may harbor a latent infection, after showing that VLPs were present in zooxanthellae of thermally stressed anemones and that isolated VLPs could reinfect zooxanthellae. Further studies have shown that VLPs are present in three species of tropical coral (11). While the hosts of the numerous VLPs remain unknown, the abundance of VLPs and their close association with corals and the symbiotic zooxanthellae is evident. A variety of VLP forms have been observed, and while most of VLPs the are hexagonal, it is notable that a filamentous VLP (up to 3 μm long) whose morphology is similar to that of ZFV1 has been observed in seawater following exposure of the coral Acropora formosa to heat stress (11). Although in our experiments we utilized nonaxenic cultures, our results clearly indicate that zooxanthellae contain a latent virus that is induced by UV treatment, leading to the lysis of the zooxanthellae. Extrapolation from this virus-host interaction and its effects on zooxanthella viability provides a novel link to the impact of latent infection on symbiotic dinoflagellates and the subsequent disruption of reef ecosystems. If stress-induced viral induction in zooxanthellae occurs in the natural reef environment, the presence of this new group of filamentous VLPs is clearly important.
Acknowledgments
We acknowledge help and advice provided by Roy Moate, Peter Bond, and Glen Harper from the Plymouth Electron Microscopy Centre, University of Plymouth.
W.H.W. was supported through the NERC-funded core strategic research program of the Plymouth Marine Laboratory.
Footnotes
Published ahead of print on 9 March 2007.
REFERENCES
- 1.Agranovsky, A. A., D. E. Lesemann, E. Maiss, R. Hull, and J. G. Atabekov. 1995. “Rattlesnake” structure of a filamentous plant RNA virus built of two capsid proteins. Proc. Natl. Acad. Sci. USA 92:2470-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, A. C. 2003. Flexibility and specificity in coral-algal symbiosis: diversity, eco1ogy, and biogeography of Symbiodinium. Annu. Rev. Ecol. Evol. Syst. 34:661-689. [Google Scholar]
- 3.Baker, A. C. 2001. Reef corals bleach to survive change. Nature 411:765-766. [DOI] [PubMed] [Google Scholar]
- 4.Banin, E., Y. Ben-Haim, T. Israely, Y. Loya, and E. Rosenberg. 2000. Effect of the environment on the bacterial bleaching of corals. Water Air Soil Pollut. 123:337-352. [Google Scholar]
- 5.Ben-Haim, Y., M. Zicherman-Keren, and E. Rosenberg. 2003. Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl. Environ. Microbiol. 69:4236-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergh, Ø., K. Y. Børsheim, G. Bratbak, and M. Heldal. 1989. High abundance of viruses found in aquatic environments. Nature 340:467-468. [DOI] [PubMed] [Google Scholar]
- 7.Brown, B. E. 1997. Coral bleaching: causes and consequences. Coral Reefs 16:129-138. [Google Scholar]
- 8.Brussaard, C. 2004. Viral control of phytoplankton populations—a review. J. Eukaryot. Microbiol. 51:125-138. [DOI] [PubMed] [Google Scholar]
- 9.Brussaard, C. P. D. 2004. Optimization of procedures for counting viruses by flow cytometry. Appl. Environ. Microbiol. 70:1506-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buddemeier, R. W., and D. G. Fautin. 1993. Coral bleaching as an adaptive mechanism—a testable hypothesis. BioScience 43:320-326. [Google Scholar]
- 11.Davy, S. K., S. G. Burchett, A. L. Dale, P. Davies, J. E. Davy, C. Muncke, O. Hoegh-Guldberg, and W. H. Wilson. 2006. Viruses: agents of coral disease? Dis. Aquat. Org. 69:101-110. [DOI] [PubMed] [Google Scholar]
- 12.Davy, S. K., I. A. N. Lucas, and J. R. Turner. 1996. Carbon budgets in temperate anthozoan-dinoflagellate symbioses. Mar. Biol. 126:773-783. [Google Scholar]
- 13.Fitt, W. K., B. E. Brown, M. E. Warner, and R. P. Dunne. 2001. Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs 20:51-65. [Google Scholar]
- 14.Franca, S. 1976. On the presence of virus-like particles in the dinoflagellate Gyrodinium resplendens (Hulburt). Protistologica 12:425-430. [Google Scholar]
- 15.Glynn, P. W., N. J. Gassman, C. M. Eakin, J. Cortes, D. B. Smith, and H. M. Guzman. 1991. Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galapagos Islands (Ecuador). I. Pocilloporidae. Mar. Biol. 109:355-368. [Google Scholar]
- 16.Hayes, R. L., and P. G. Bush. 1990. Microscopic observations of recovery in the reef building scleratinian coral, Montastrea annularis, after bleaching on a Cayman reef. Coral Reefs 8:203-209. [Google Scholar]
- 17.Jokiel, P. L., and S. L. Coles. 1990. Response of Hawaiian and other Indo Pacific reef corals to elevated temperatures. Coral Reefs 8:155-162. [Google Scholar]
- 18.Jones, R. J. 1997. Changes in zooxanthellar densities and chlorophyll concentrations in corals during and after a bleaching event. Mar. Ecol. Prog. Ser. 158:51-59. [Google Scholar]
- 19.Kushmaro, A., E. Rosenberg, M. Fine, and Y. Loya. 1997. Bleaching of the coral Oculina patagonica by Vibrio AK-1. Mar. Ecol. Prog. Ser. 147:159-165. [Google Scholar]
- 20.LaJeunesse, T. 2001. Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: in search of a “species” level marker. J. Phycol. 37:866-880. [Google Scholar]
- 21.Lang, A. S., A. I. Culley, and C. A. Suttle. 2004. Genome sequence and characterization of a virus (HaRNAV) related to picoma-like viruses that infects the marine toxic bloom-forming alga Heterosigma akashiwo. Virology 320:206-217. [DOI] [PubMed] [Google Scholar]
- 22.Larsen, A., T. Castberg, R. A. Sandaa, C. P. D. Brussaard, J. Egge, M. Heldal, A. Paulino, R. Thyrhaug, van E. J. Hannen, and G. Bratbak. 2001. Population dynamics and diversity of phytoplankton, bacteria and viruses in a seawater enclosure. Mar. Ecol. Prog. Ser. 221:47-57. [Google Scholar]
- 23.Marie, D., C. P. D. Brussaard, R. Thyrhaug, G. Bratbak, and D. Vaulot. 1999. Enumeration of marine viruses in culture and natural samples by flow cytometry. Appl. Environ. Microbiol. 65:45-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muscatine, L. 1990. The role of symbiotic algae in carbon and energy flux in reef corals, p. 75-87. In Z. Dubinsky (ed.), Ecosystems of the world: coral reefs, vol. 25. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 25.Provasoli, L., J. J. A. McLaughlin, and M. R. Droop. 1957. The development of artificial media for marine algae. Arch. Microbiol. 25:392-428. [DOI] [PubMed] [Google Scholar]
- 26.Reisser, W. 1993. Viruses and virus-like particles of freshwater and marine eucaryotic algae—a review. Arch. Protistenkd. 143:257-265. [Google Scholar]
- 27.Rohwer, F., V. Seguritan, F. Azam, and N. Knowlton. 2002. Diversity and distribution of coral-associated bacteria. Mar. Ecol. Prog. Ser. 243:1-10. [Google Scholar]
- 28.Rosenberg, E., and Y. Ben-Haim. 2002. Microbial diseases of corals and global warming. Environ. Microbiol. 4:318-326. [DOI] [PubMed] [Google Scholar]
- 29.Schroeder, D. C., J. Oke, M. Hall, G. Malin, and W. H. Wilson. 2003. Virus succession observed during an Emiliania huxleyi bloom. Appl. Environ. Microbiol. 69:2484-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sicko-Goad, L., and G. Walker. 1979. Viroplasm and large virus like particles in the dinoflagellate Gymnodinium uberrimum. Protoplasma 99:203-210. [Google Scholar]
- 31.Strychara, K. B., M. Coatesa, P. W. Sammarcob, and T. J. Pivac. 2004. Bleaching as a pathogenic response in scleractinian corals, evidenced by high concentrations of apoptotic and necrotic zooxanthellae. J. Exp. Mar. Biol. Ecol. 304:99-121. [Google Scholar]
- 32.Suttle, C. A. 2000. The ecological, evolutionary, and geochemical consequences of viral infection of cyanobacteria and eukaryotic algae, p. 248-296. In C. J. Hurst (ed.), Viral ecology. Academic Press, New York, NY.
- 33.Szmant, A., and N. J. Gassman. 1990. The effects of prolonged bleaching on the tissue biomass and reproduction of the reef coral Monastrea annularis. Coral Reefs 8:217-224. [Google Scholar]
- 34.Tai, V., J. E. Lawrence, A. S. Lang, A. M. Chan, A. I. Culley, and C. A. Suttle. 2003. Characterization of HaRNAV, a single-stranded RNA virus causing lysis of Heterosigma akashiwo (Raphidophyceae). J. Phycol. 39:343-352. [Google Scholar]
- 35.Tarutani, K., K. Nagasaki, S. Itakura, and M. Yamaguchi. 2001. Isolation of a virus infecting the novel shellfish-killing dinoflagellate Heterocapsa circularisquama. Aquat. Microb. Ecol. 23:103-111. [Google Scholar]
- 36.Tomaru, Y., N. Katanozaka, K. Nishida, Y. Shirai, K. Tarutani, M. Yamaguchi, and K. Nagasaki. 2004. Isolation and characterization of two distinct types of HcRNAV, a single-stranded RNA virus infecting the bivalve-killing microalga Heterocapsa circularisquama. Aquat. Microb. Ecol. 34:207-218. [Google Scholar]
- 37.Toren, A., L. Landau, A. Kushmaro, Y. Loya, and E. Rosenberg. 1998. Effect of temperature on adhesion of Vibrio strain AK-1 to Oculina patagonica and on coral bleaching. Appl. Environ. Microbiol. 64:1379-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Etten, J. L., and R. H. Meints. 1999. Giant viruses infecting algae. Annu. Rev. Microbiol. 53:447-494. [DOI] [PubMed] [Google Scholar]
- 39.van Regenmortel, M. H. V., C. M. Fauquet, D. H. Bishop, E. B. Carsen, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.). 2000. Virus taxonomy: classification and nomenclature of viruses: seventh report of the international committee on taxonomy of viruses. Academic Press Inc., San Diego, CA.
- 40.Wang, J., and A. E. Douglas. 1998. Nitrogen recycling or nitrogen conservation in an alga-invertebrate symbiosis? J. Exp. Biol. 201:2445-2453. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson, C. 1998. Status of coral reefs of the world. Australian Institute of Marine Science, Townsville, Australia.
- 42.Wilson, W. H., A. L. Dale, J. E. Davy, and S. K. Davy. 2005. An enemy within? Observations of virus-like particles in reef corals. Coral Reefs 24:145-148. [Google Scholar]
- 43.Wilson, W. H., I. Francis, K. Ryan, and S. K. Davy. 2001. Temperature induction of viruses in symbiotic dinoflagellates. Aquat. Microb. Ecol. 25:99-102. [Google Scholar]
- 44.Wommack, K. E., and R. R. Colwell. 2000. Virioplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 64:69-114. [DOI] [PMC free article] [PubMed] [Google Scholar]