Abstract
We used bovine intestinal organ culture to study infection by enterohemorrhagic Escherichia coli serogroups O157, O26, and O111. We show colonization and attaching and effacing lesion formation on explants derived from the ileum, colon, and rectum. Intimin and Tir were detected at the sites of adherent bacteria; Tir was essential for colonization.
Enterohemorrhagic Escherichia coli (EHEC) strains are defined by their ability to produce one or more Shiga toxins and form attaching and effacing (A/E) lesions on intestinal epithelia (25). Ruminants are an important reservoir of EHEC (13), and human infections are frequently associated with contact with ruminant feces (21). The EHEC serogroup associated predominantly with human infections in the United States and Europe is O157, but other serogroups, including O26, O103, and O111, are also frequently implicated (4).
The genes required for the formation of A/E lesions by EHEC O157:H7 are carried on two separate pathogenicity islands: the locus of enterocyte effacement (LEE) (24) and prophage CP-933U/Sp14 (6, 14). The LEE encodes transcriptional regulators, the adhesin intimin (18), a type III secretion system (responsible for effector protein translocation), chaperones, translocators (EspA, EspD, and EspB), and six effector proteins, including Tir (translocated intimin receptor) (20), the only LEE effector so far implicated in A/E lesion formation. Prophage CP-933U/Sp14 carries the genes encoding the effector protein TccP/EspFU (14).
In calves, clinical signs of natural EHEC infections range from subclinical to dysentery depending on the serogroup involved. Natural and experimental infection of normal cattle with EHEC O157:H7 results in efficient colonization of the intestinal tract in the absence of clinical signs (5, 9, 41). In contrast, EHEC serogroups O5, O26, and O118 are commonly associated with diarrhea in calves, which imposes a significant economic burden on livestock producers (7, 29, 40). Extensive adherence and A/E lesion formation occur with EHEC serogroups O5, O26, and O111 in the bovine large intestine (29, 36, 37), while sparsely distributed microcolonies of EHEC O157:H7 have been reported at the same bovine intestinal site in age-matched calves (37). In older cattle, EHEC O157:H7 has been reported to exhibit tropism for lymphoid follicle-dense mucosa in the terminal rectum (26) and to form A/E lesions at this site (27).
Although cattle represent a major reservoir of EHEC, the mechanisms underlying the intestinal carriage and virulence of EHEC are poorly understood. Worldwide, very few laboratories have the capacity to study EHEC-intestine interactions in vivo owing to the cost and infrastructure required to accommodate large animals at high containment. As such, intestinal in vitro organ culture (IVOC) provides a powerful system for studying the interaction of bacteria with the mucosal surfaces of their respective hosts. Indeed, the interaction of EHEC with human intestinal explants is now the gold standard for the assessment of host-pathogen interaction (12, 14, 30). A bovine IVOC (bIVOC) model using terminal ileum, colon, and rectum segments from adult cattle has previously been described (3) and refined (2) for EHEC O157:H7. However, although optimal conditions have been successfully described in these papers, a lack of consistency, reproducibility, and convincing results is noted in subsequent studies (8, 22, 31). Moreover, bIVOC has yet to be utilized to study the colonization and A/E lesion formation by non-O157:H7 EHEC. The aim of this study was to adapt the bIVOC model described by Baehler and Moxley (2, 3) to investigate A/E lesion formation by wild-type and mutant EHEC O157 strains and non-O157 EHEC on different intestinal sites by using light and electron microscopy. Moreover, we sought to ascertain the role and the expression of the bacterial virulence determinants intimin and Tir during this interaction.
Interaction of EHEC O157, O26, and O111 with bIVOC.
Intestinal tissue tropism of EHEC O157, O26, and O111 was investigated using explants of the terminal ileum and colon and lymphoid follicle-dense mucosa of the terminal rectum by using the previously described conditions (2). All of the strains used in this study (Table 1) were grown overnight in tryptic soy broth and then transferred into fresh, sterile tryptic soy broth and grown to early log phase for 2.5 to 3 h prior to infection. When appropriate, nalidixic acid was used at a final concentration of 20 μg ml−1. Seven Friesian bull calves of 6 to 9 weeks of age were used in seven different experiments. Fresh segments from the terminal ileum and terminal colon and from the last centimeter of the terminal rectum were excised, rinsed in cold, sterile phosphate-buffered saline, and immersed in cold complete RPMI 1640 medium (15) without d-mannose for 30 min. Twelve-millimeter2 explants were cut and placed on biopsy foam pads (Curtin Matheson Scientific, Inc., TX) in multidish four-well Nunclon Delta surface tissue culture plates (Invitrogen), with up to four explants being placed on each pad. Complete RPMI 1640 medium was added to each well, and each explant was inoculated once with 25 μl of the appropriate log-phase broth culture, corresponding to approximately 107 bacteria per explant.
TABLE 1.
Strains used in this study
| Strain | Description | Reference(s) or source |
|---|---|---|
| 85-170 Nalr | EHEC O157:H7, spontaneous stx1 and stx2 mutant, eaeγ, nalidixic acid resistant | 35 |
| EDL933 | EHEC O157:H7, stx1/stx2 positive, eaeγ | ATCC |
| TUV 93-0 | EDL933, EHEC O157:H7, stx, eaeγ | ATCC |
| TUV 93-0Δtir | Δtir in TUV 93-0 | 19 |
| 193 Nalr | EHEC O26:H−, stx1 positive, eaeβ | 23, 37 |
| E45035N | EHEC O111:H−, stx1 positive, isolated from the feces of a patient with hemolytic-uremic syndrome | 28 |
| 331S89 Nalr | EHEC O26:H11, stx1 positive, eaeβ, isolate from a newborn calf with diarrhea | 17 |
| EC920201 | EHEC O111:H−, stx1 positive | 33 |
As in all recent studies of bIVOC, explants were maintained in a 5%-CO2-in-95%-O2 atmosphere (8, 22, 31), which is optimal for infection of human IVOC (12, 14, 30); we used these conditions in our initial incubations with EHEC O157:H7. However, although tissue preservation was excellent, we observed inefficient adhesion with no A/E lesion formation (data not shown).
The fact that an oxygen-enriched environment impaired the ability of EHEC O157:H7 to induce A/E lesions supports the hypothesis that high oxygen levels negatively regulate the expression levels of some proteins involved in A/E lesion formation (3, 10). Indeed, Ando et al. recently reported that under anaerobic growth conditions, the maturation of a functional type III secretion system apparatus is accelerated in EHEC O157:H7 (1). In contrast, maintaining the explants at 37°C on a seesaw rocker (18 cycles min−1) in a humidified atmosphere of 5% CO2 in air for a total of 8 h, as was originally reported by Baehler and Moxley (2, 3), resulted in good tissue preservation, efficient adhesion, and A/E lesion formation. Thus, all subsequent incubations were performed using these growth conditions. Because Stx-positive strains were used, all incubations were performed in a category III laboratory. To prevent bacterial overgrowth and acidic pH, hourly changes with sterile fresh complete RPMI 1640 medium were carried out during culture, starting 2 h after the initial inoculation of the explants. Uninfected explants were also cultured in each experiment in order to confirm the absence of endogenous infection and that no external contamination occurred during the experimental process.
Hematoxylin- and eosin-stained sections revealed that the architecture of uninfected tissue was well preserved following an 8-h incubation compared to biopsy samples collected at the outset (Fig. 1). Following the staining of O antigen on formalin-fixed, paraffin-embedded sections (14, 16), EHEC O157, O26, and O111 were found to be intimately associated with the epithelium of the terminal ileum, colon, and rectum (Table 2 and Fig. 2A and B). Chi-square 2-by-2 statistical analysis of the number of explants with intimately adherent bacteria revealed that EHEC O157 binds better than does EHEC O111 to explants derived from the terminal ileum (P ≤ 0.05). No significant difference was found between Shiga toxin (Stx)-negative (TUV 93-0) (Table 2) and Stx1/Stx2-positive (EDL933) EHEC O157 with respect to the number of explants with intimately adherent bacteria (P ≤ 1) (Table 2). Interestingly, a recent report from Robinson et al. showed that the Stx2 of EHEC O157:H7 promotes intestinal colonization in the mouse model, presumably by increasing the surface expression of the host cell-encoded intimin receptor nucleolin (32). The mechanism by which Stx induces surface expression of nucleolin on the bovine gut cell is not known.
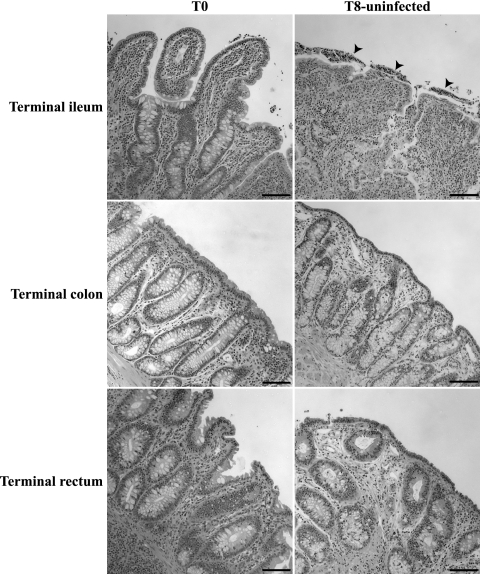
FIG. 1.
Preservation of the tissue architecture after 8-h (T8) incubation of uninfected bovine explants. While shorter villi and some desquamated cells in the lumen (arrowheads) were observed in the terminal ileum, excellent preservation of the crypt epithelium was observed with explants of the terminal colon and the terminal rectum under the same culture conditions, as in uncultured, uninfected explants (T0). Hematoxylin and eosin staining; bar = 100 μm.
TABLE 2.
Adherence of EHEC O157:H7, O26, and O111:H− strains to bovine explants ex vivo
| Serogroup for strain | No. of explants with intimately adherent bacteria/ total no. (% positive explants)
|
||
|---|---|---|---|
| Terminal ileum | Terminal colon | Terminal rectum | |
| O157:H7 | |||
| 85-170 Nalr | 18/19 (95) | 11/15 (73) | 17/20 (85) |
| TUV 93-0 | 11/12 (92) | 11/14 (79) | 16/18 (89) |
| TUV 93-0Δtir | 0/7 (0) | 0/8 (0) | 0/8 (0) |
| EDL933 | 5/5 (100) | 4/6 (67) | 6/6 (100) |
| O26 | |||
| 193 Nalr | 6/6 (100) | 5/7 (71) | 11/11 (100) |
| 331S89 Nalr | 3/4 (75) | 0/4 (0) | 2/4 (50) |
| O111:H− | |||
| E45035N | 4/7 (57) | 3/7 (43) | 7/10 (70) |
| EC920201 | 3/4 (75) | 4/4 (100) | 2/4 (50) |
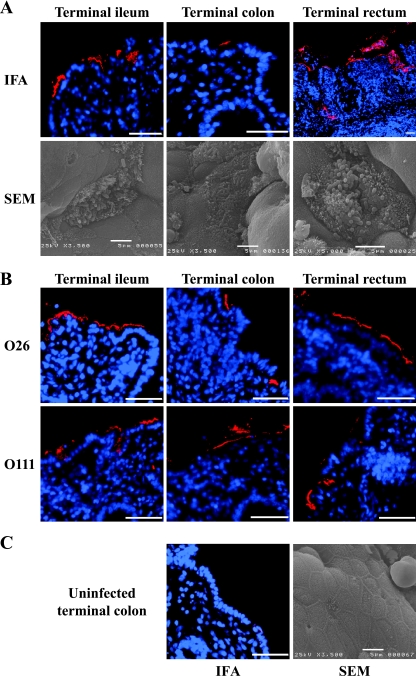
FIG. 2.
Interaction of EHEC O157 strain TUV 93-0 (A), EHEC O26 (193 Nalr), and O111 (E45035N and EC920201) (B) with bovine intestinal mucosa. (A) Foci of intimately adherent O157-positive bacteria were observed following the immunostaining of a formalin-fixed, paraffin-embedded section (immunofluorescence assay [IFA]), and typical A/E lesions, accompanied by localized effacement of the brush border, microvillous elongation, were observed by SEM. Similar findings were observed by using EHEC of O26 and O111 serogroups (B). (C) No bacteria of the O157, O26, or O111 serogroup were observed on uninfected explants by either IFA or SEM. Representative micrographs are shown for each reporter technique. Hoechst 33342 (blue, false color), nuclei and bacteria; tetramethyl rhodamine isothiocyanate (TRITC) (red, false color), O157-positive bacteria. Bar = 50 μm (IFA) or 5 μm (SEM).
Applying the statistical analysis to EHEC O26 revealed significantly more explants from the terminal ileum and rectum with intimately adherent bacteria than explants from the terminal colon (for ileum versus colon, P was ≤0.05; for colon versus rectum, P was ≤0.025). Our findings are of significant interest and will need to be more thoroughly investigated.
Infection with EHEC O157 resulted in a consistent pattern of colonization, characterized by scattered foci of intimately adherent bacteria in the terminal ileum and colon, whereas foci covering a larger surface of the epithelium were frequently observed in the terminal rectum (Fig. 2A). These results are in agreement with observations made in vivo following deliberate oral challenges for EHEC O157 (26, 37). Some EHEC O157-positive bacteria were also found deep in the crypts in the terminal rectum (Fig. 2A). In contrast, EHEC O111 and O26 showed extensive foci of intimately adherent bacteria in the terminal ileum and rectum, although the foci of intimately adherent bacteria appeared to cover less epithelial surface in the terminal rectum than those of EHEC O157 (Fig. 2B). Loosely associated bacteria were also frequently observed in explants derived from the terminal colon. No foci of intimately adherent bacteria were found on explants derived from the terminal colon infected with O26 strain 331S89 (Table 2). This finding shows variation in tissue tropism between strains of the same serogroup. The presence of typical A/E lesions (characterized by intimate adherence, localized effacement of the brush border, and microvillus elongation) was confirmed by scanning electron microscopy (SEM) and transmission electron microscopy (Fig. 3) for all the strains assessed (except for O26 strain 331S89) in all the intestinal sites examined, although the elongation of microvilli was less obvious in the terminal colon (Fig. 2A).
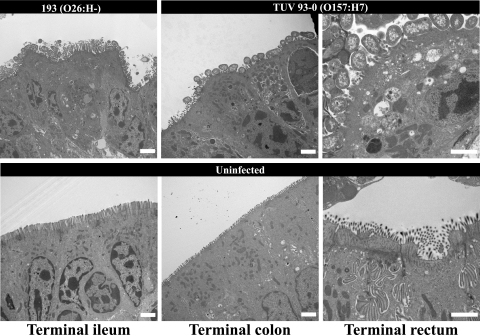
FIG. 3.
Representative transmission electron microscopy micrographs showing EHEC O26:H− (193 Nalr) and O157:H7 (TUV 93-0) inducing typical A/E lesions in the terminal ileum, colon, and rectum. A/E lesions were characterized by intimately adherent bacteria, effacement of the brush border, and F-actin accumulation underneath adherent bacteria. No A/E lesions were observed on uninfected sections. Bar = 2 μm.
The results we obtained by using the bIVOC model are consistent with the previous observation that EHEC O157 can adhere and induce A/E lesions at intestinal sites other than the terminal rectum, which include explants derived from the terminal ileum ex vivo (31) and in a ligated ileal loop model in the presence of the neuroendocrine hormone norepinephrine (38, 39). We previously reported that in age-matched neonatal calves EHEC O26:H− strain 193 Nalr formed extensive microcolonies in spiral colon, whereas EHEC O157:H7 strain EDL933 formed only sparse microcolonies, despite the calves shedding comparable numbers of bacteria (37). EHEC O111:H− strain E45035N similarly formed dense microcolonies in the colon of neonatal calves (36). Though the overall numbers of explants infected with EHEC strains of serogroups O26 and O111 may not reflect this trend, the histological appearance of extensive microcolonies as apposed to diffusely attached EHEC O157 supports these findings and may reflect interactions between bacteria that facilitate colony spreading in the non-O157 strains. Further, the explants cultured in the present experiments derived from 6-week-old animals and the tissue tropism of the O26 and O111 strains were studied only in 4-day-old calves. Importantly, no bacteria of the endogenous gut flora were seen, even in association with uninfected control tissue taken at time zero (T0). This suggests that the gut microflora is not closely associated with the mucosa. As such, the relationship between EHEC and the gut microflora is not addressed in this study. However, A/E lesions were readily seen in our study after the 8-h incubation and it is known from signature-tagged mutagenesis (11, 37) and targeted mutagenesis that these are pivotal for the colonization of calves and adult cattle.
Our data also confirm the development of extensive microcolonies on terminal rectum mucosa by E. coli O157 (26); however, it is unclear whether this reflects an increased number of initial adherence events or spread over the mucosal surface due to enhanced growth, interbacterial interactions, or other factors. We report for the first time that EHEC strains of serogroups O26 and O111 can adhere to the terminal rectum mucosa. This colonization pattern, if proven in experimentally inoculated animals, may support the value of intervention strategies aimed at clearing EHEC of various serotypes by direct application of antimicrobial agents to the terminal rectum mucosa.
Localization of intimin and Tir at the site of intimately adherent EHEC O157:H7.
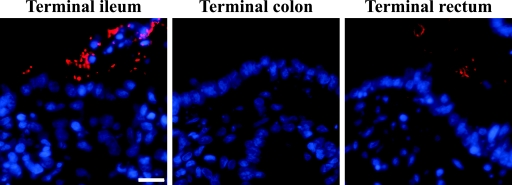
The role of Tir in intimate adherence of EHEC O157:H7 strain TUV 93-0 was first investigated using a Δtir derivative. Only a few O157-positive bacteria were observed close to the ileal epithelium following the immunostaining of a formalin-fixed, paraffin-embedded section, whereas virtually no O157-positive bacteria were found in the terminal colon and rectum (Fig. 4). A consistent phenotype was observed for samples processed for SEM (data not shown). The ability of the Δtir derivative to induce A/E lesions was restored when it was trans complemented with its isogenic tir gene (data not shown). This shows that tir is required for the development of A/E lesions by EHEC O157:H7, as observed in vivo in calves (38).
FIG. 4.
Interaction of a Δtir derivative of EHEC O157:H7 strain TUV 93-0 with the bovine intestinal mucosa. Only a few O157-positive bacteria were observed close to the ileal epithelium following the immunostaining of a formalin-fixed, paraffin-embedded section, whereas no O157-positive bacteria were found in the terminal colon and terminal rectum. Hoechst 33342 (blue, false color), nuclei and bacteria; tetramethyl rhodamine isothiocyanate (TRITC) (red, false color), O157-positive bacteria. Bar = 10 μm.
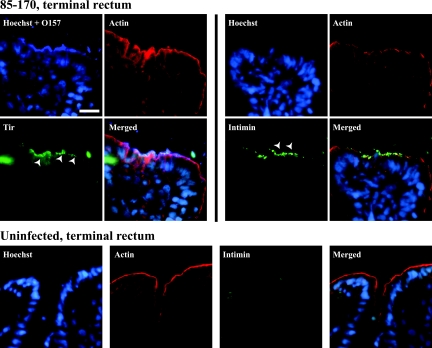
The expression of the intimin and Tir by intimately adherent bacteria was also further investigated by using cryosections. Because of the category III laboratory restrictions, we did not process any frozen samples from Stx-positive EHEC O26- and O111-infected explants. Immunostaining with rabbit anti-Tir EHEC localized Tir to the apical surface of enterocytes at almost every site of intimately adherent bacteria in each of the intestinal sites (terminal ileum, colon, and rectum) (Fig. 5), and its presence was always associated with O157-positive bacteria. For its part, intimin was detected on both luminal and intimately adherent bacteria in most of the investigated sites (Fig. 5). The absence of A/E lesions using a TUV 93-0Δtir derivative, combined with the expression of intimin and Tir in foci of intimately adherent bacteria, is in agreement with the findings of Vlisidou et al., showing that the intimin-Tir interaction is a relevant mechanism in the colonization and development of A/E lesions in calves (38). In our study, in most cases, a rim of F-actin, which was found to be continuous and regularly distributed at the luminal surfaces of the enterocyte layers of uninfected explants after 8 h (Fig. 5, bottom panels), appeared to be disrupted, at least locally, in the presence of intimately adherent bacteria (Fig. 5). Neither Tir nor intimin was detected on uninfected cryosections (Fig. 5, bottom panels). Our data also support a recent observation in neonatal piglets and calves, showing that immunostained Tir is observed at the site of bacterial adherence following deliberate oral challenge with the EHEC strain 86-24 (34).
FIG. 5.
Detection of intimin γ and Tir at the site of interaction of EHEC O157:H7 strain 85-170 Nalr with the bovine intestinal mucosa. Tir was found at the apical surface of infected enterocytes (arrowheads in the “Tir” panel), whereas intimin γ was detected on both luminal and intimately adherent bacteria (arrowheads in the “Intimin” panel of the “85-170, terminal rectum” section). An F-actin rim, which was found to be continuous and regularly distributed at the apical surface of the enterocytes on uninfected explants (“Actin” panel of the “Uninfected, terminal rectum” section), appeared to be disrupted, at least locally, in the presence of intimately adherent bacteria. Similar results were obtained with EHEC O157:H7 strain TUV 93-0. Neither Tir nor intimin was detected on uninfected cryosections. Representative micrographs from the terminal rectum are shown, and similar findings were observed in the terminal ileum and colon. Hoechst 33342 (blue, false color), nuclei and bacteria; Cy5 (intense blue, false color), O157-positive bacteria; Cy2 (green, false color), intimin γ or Tir; phalloidin-tetramethyl rhodamine isothiocyanate (TRITC) (red, false color), F-actin. Bar, 10 μm.
In conclusion, this study shows that O157 and non-O157 EHEC strains can colonize and induce A/E lesions on bovine intestinal explants from various sites, including the terminal rectum. It also strengthens the fact that intimin-Tir interaction is the major mechanism of colonization of the bovine gut. Considering that cattle are the major source of environmental and food contamination by EHEC, reducing bacterial carriage will significantly reduce the risk to humans. To this end, we have to increase our understanding of bacterium-host cell interactions. Considering the difficulties of studying the association of EHEC with the bovine gut mucosa in vivo, the bovine IVOC model provides a practical way forward to study bacterial pathogenesis, host specificity, and the host response.
Acknowledgments
We thank Carlton L. Gyles and Jacques Mainil for kindly providing us with strains EC920201 and 331S89, respectively, Stephanie Schüller for helpful scientific discussions, and Magalie Lemoine for helpful discussions regarding bovine IVOC.
This work was supported by the Medical Research Council (MRC) and the Department for the Environment, Food and Rural Affairs (project OZ0707). Francis Girard is supported by a postdoctoral fellowship from the Natural Sciences and Engineering Research Council (NSERC) of Canada and by a Canada-United Kingdom Millennium Research Award, awarded by NSERC and the Royal Society of London, United Kingdom.
Footnotes
Published ahead of print on 9 March 2007.
REFERENCES
- 1.Ando, H., H. Abe, N. Sugimoto, and T. Tobe. 2007. Maturation of functional type III secretion machinery by activation of anaerobic respiration in enterohaemorrhagic Escherichia coli. Microbiology 153:464-473. [DOI] [PubMed] [Google Scholar]
- 2.Baehler, A. A., and R. A. Moxley. 2002. Effect of culture conditions on Escherichia coli O157:H7-mediated attaching-effacing lesions in a bovine large intestinal mucosal explant model. FEMS Microbiol. Lett. 212:107-110. [DOI] [PubMed] [Google Scholar]
- 3.Baehler, A. A., and R. A. Moxley. 2000. Escherichia coli O157:H7 induces attaching-effacing lesions in large intestinal mucosal explants from adult cattle. FEMS Microbiol. Lett. 185:239-242. [DOI] [PubMed] [Google Scholar]
- 4.Bettelheim, K. A. 2000. Role of non-O157 VTEC. Symp. Ser. Soc. Appl. Microbiol. 29:38S-50S. [DOI] [PubMed] [Google Scholar]
- 5.Brown, C. A., B. G. Harmon, T. Zhao, and M. P. Doyle. 1997. Experimental Escherichia coli O157:H7 carriage in calves. Appl. Environ. Microbiol. 63:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campellone, K. G., D. Robbins, and J. M. Leong. 2004. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev. Cell 7:217-228. [DOI] [PubMed] [Google Scholar]
- 7.Chanter, N., G. A. Hall, A. P. Bland, A. J. Hayle, and K. R. Parsons. 1986. Dysentery in calves caused by an atypical strain of Escherichia coli (S102-9). Vet. Microbiol. 12:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cookson, A. L., and M. J. Woodward. 2003. The role of intimin in the adherence of enterohaemorrhagic Escherichia coli (EHEC) O157:H7 to HEp-2 tissue culture cells and to bovine gut explant tissues. Int. J. Med. Microbiol. 292:547-553. [DOI] [PubMed] [Google Scholar]
- 9.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeVinney, R., M. Stein, D. Reinscheid, A. Abe, S. Ruschkowski, and B. B. Finlay. 1999. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect. Immun. 67:2389-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dziva, F., P. M. van Diemen, M. P. Stevens, A. J. Smith, and T. S. Wallis. 2004. Identification of Escherichia coli O157:H7 genes influencing colonization of the bovine gastrointestinal tract using signature-tagged mutagenesis. Microbiology 150:3631-3645. [DOI] [PubMed] [Google Scholar]
- 12.Fitzhenry, R. J., D. J. Pickard, E. L. Hartland, S. Reece, G. Dougan, A. D. Phillips, and G. Frankel. 2002. Intimin type influences the site of human intestinal mucosal colonisation by enterohaemorrhagic Escherichia coli O157:H7. Gut 50:180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gansheroff, L. J., and A. D. O'Brien. 2000. Escherichia coli O157:H7 in beef cattle presented for slaughter in the U.S.: higher prevalence rates than previously estimated. Proc. Natl. Acad. Sci. USA 97:2959-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garmendia, J., A. D. Phillips, M. F. Carlier, Y. Chong, S. Schuller, O. Marches, S. Dahan, E. Oswald, R. K. Shaw, S. Knutton, and G. Frankel. 2004. TccP is an enterohaemorrhagic Escherichia coli O157:H7 type III effector protein that couples Tir to the actin-cytoskeleton. Cell. Microbiol. 6:1167-1183. [DOI] [PubMed] [Google Scholar]
- 15.Girard, F., I. Batisson, G. M. Frankel, J. Harel, and J. M. Fairbrother. 2005. Interaction of enteropathogenic and Shiga toxin-producing Escherichia coli and porcine intestinal mucosa: role of intimin and Tir in adherence. Infect. Immun. 73:6005-6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girard, F., I. P. Oswald, I. Taranu, P. Helie, G. D. Appleyard, J. Harel, and J. M. Fairbrother. 2005. Host immune status influences the development of attaching and effacing lesions in weaned pigs. Infect. Immun. 73:5514-5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goffaux, F., J. Mainil, V. Pirson, G. Charlier, P. Pohl, E. Jacquemin, and B. China. 1997. Bovine attaching and effacing Escherichia coli possess a pathogenesis island related to the LEE of the human enteropathogenic Escherichia coli strain E2348/69. FEMS Microbiol. Lett. 154:415-421. [DOI] [PubMed] [Google Scholar]
- 18.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenny, B. 2001. The enterohaemorrhagic Escherichia coli (serotype O157:H7) Tir molecule is not functionally interchangeable for its enteropathogenic E. coli (serotype O127:H6) homologue. Cell. Microbiol. 3:499-510. [DOI] [PubMed] [Google Scholar]
- 20.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic Escherichia coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 21.Locking, M. E., S. J. O'Brien, W. J. Reilly, E. M. Wright, D. M. Campbell, J. E. Coia, L. M. Browning, and C. N. Ramsay. 2001. Risk factors for sporadic cases of Escherichia coli O157 infection: the importance of contact with animal excreta. Epidemiol. Infect. 127:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Low, A. S., F. Dziva, A. G. Torres, J. L. Martinez, T. Rosser, S. Naylor, K. Spears, N. Holden, A. Mahajan, J. Findlay, J. Sales, D. G. E. Smith, J. C. Low, M. P. Stevens, and D. L. Gally. 2006. Cloning, expression, and characterization of fimbrial operon F9 from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 74:2233-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mainil, J. G., C. J. Duchesnes, S. C. Whipp, L. R. Marques, A. D. O'Brien, T. A. Casey, and H. W. Moon. 1987. Shiga-like toxin production and attaching effacing activity of Escherichia coli associated with calf diarrhea. Am. J. Vet. Res. 48:743-748. [PubMed] [Google Scholar]
- 24.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naylor, S. W., A. J. Roe, P. Nart, K. Spears, D. G. Smith, J. C. Low, and D. L. Gally. 2005. Escherichia coli O157:H7 forms attaching and effacing lesions at the terminal rectum of cattle and colonization requires the LEE4 operon. Microbiology 151:2773-2781. [DOI] [PubMed] [Google Scholar]
- 28.Nicholls, L., T. H. Grant, and R. M. Robins-Browne. 2000. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol. Microbiol. 35:275-288. [DOI] [PubMed] [Google Scholar]
- 29.Pearson, G. R., K. J. Bazeley, J. R. Jones, R. F. Gunning, M. J. Green, A. Cookson, and M. J. Woodward. 1999. Attaching and effacing lesions in the large intestine of an eight-month-old heifer associated with Escherichia coli O26 infection in a group of animals with dysentery. Vet. Rec. 145:370-373. [DOI] [PubMed] [Google Scholar]
- 30.Phillips, A. D., and G. Frankel. 2000. Intimin-mediated tissue specificity in enteropathogenic Escherichia coli interaction with human intestinal organ cultures. J. Infect. Dis. 181:1496-1500. [DOI] [PubMed] [Google Scholar]
- 31.Phillips, A. D., S. Navabpour, S. Hicks, G. Dougan, T. Wallis, and G. Frankel. 2000. Enterohaemorrhagic Escherichia coli O157:H7 target Peyer's patches in humans and cause attaching/effacing lesions in both human and bovine intestine. Gut 47:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson, C. M., J. F. Sinclair, M. J. Smith, and A. D. O'Brien. 2006. Shiga toxin of enterohemorrhagic Escherichia coli type O157:H7 promotes intestinal colonization. Proc. Natl. Acad. Sci. USA 103:9667-9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandhu, K. S., and C. L. Gyles. 2002. Pathogenic Shiga toxin-producing Escherichia coli in the intestine of calves. Can. J. Vet. Res. 66:65-72. [PMC free article] [PubMed] [Google Scholar]
- 34.Sinclair, J. F., E. A. Dean-Nystrom, and A. D. O'Brien. 2006. The established intimin receptor Tir and the putative eucaryotic intimin receptors nucleolin and β-1 integrin localize at or near the site of enterohemorrhagic Escherichia coli O157:H7 adherence to enterocytes in vivo. Infect. Immun. 74:1255-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevens, M. P., A. J. Roe, I. Vlisidou, P. M. van Diemen, R. M. La Ragione, A. Best, M. J. Woodward, D. L. Gally, and T. S. Wallis. 2004. Mutation of toxB and a truncated version of the efa-1 gene in Escherichia coli O157:H7 influences the expression and secretion of locus of enterocyte effacement-encoded proteins but not intestinal colonization in calves or sheep. Infect. Immun. 72:5402-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens, M. P., P. M. van Diemen, G. Frankel, A. D. Phillips, and T. S. Wallis. 2002. Efa1 influences colonization of the bovine intestine by Shiga toxin-producing Escherichia coli serotypes O5 and O111. Infect. Immun. 70:5158-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Diemen, P. M., F. Dziva, M. P. Stevens, and T. S. Wallis. 2005. Identification of enterohemorrhagic Escherichia coli O26:H− genes required for intestinal colonization in calves. Infect. Immun. 73:1735-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlisidou, I., F. Dziva, R. M. La Ragione, A. Best, J. Garmendia, P. Hawes, P. Monaghan, S. A. Cawthraw, G. Frankel, M. J. Woodward, and M. P. Stevens. 2006. Role of intimin-Tir interactions and the Tir-cytoskeleton coupling protein in the colonization of calves and lambs by Escherichia coli O157:H7. Infect. Immun. 74:758-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vlisidou, I., M. Lyte, P. M. van Diemen, P. Hawes, P. Monaghan, T. S. Wallis, and M. P. Stevens. 2004. The neuroendocrine stress hormone norepinephrine augments Escherichia coli O157:H7-induced enteritis and adherence in a bovine ligated ileal loop model of infection. Infect. Immun. 72:5446-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wieler, L. H., E. Vieler, C. Erpenstein, T. Schlapp, H. Steinruck, R. Bauerfeind, A. Byomi, and G. Baljer. 1996. Shiga toxin-producing Escherichia coli strains from bovines: association of adhesion with carriage of eae and other genes. J. Clin. Microbiol. 34:2980-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wray, C., I. M. McLaren, L. P. Randall, and G. R. Pearson. 2000. Natural and experimental infection of normal cattle with Escherichia coli O157. Vet. Rec. 147:65-68. [DOI] [PubMed] [Google Scholar]