Abstract
Lactic acid bacteria have become a major source of concern for aquaculture in recent decades. In addition to true pathogenic species of worldwide significance, such as Streptococcus iniae and Lactococcus garvieae, several species have been reported to produce occasional fish mortalities in limited geographic areas, and many unidentifiable or ill-defined isolates are regularly isolated from fish or fish products. To clarify the nature and prevalence of different fish-associated bacteria belonging to the lactic acid bacterium group, a collection of 57 isolates of different origins was studied and compared with a set of 22 type strains, using amplified rRNA gene restriction analysis (ARDRA). Twelve distinct clusters were delineated on the basis of ARDRA profiles and were confirmed by sequencing of sodA and 16S rRNA genes. These clusters included the following: Lactococcus raffinolactis, L. garvieae, Lactococcus l., S. iniae, S. dysgalactiae, S. parauberis, S. agalactiae, Carnobacterium spp., the Enterococcus “faecium” group, a heterogeneous Enterococcus-like cluster comprising indiscernible representatives of Vagococcus fluvialis or the recently recognized V. carniphilus, V. salmoninarum, and Aerococcus spp. Interestingly, the L. lactis and L. raffinolactis clusters appeared to include many commensals of fish, so opportunistic infections caused by these species cannot be disregarded. The significance for fish populations and fish food processing of three or four genetic clusters of uncertain or complex definition, namely, Aerococcus and Enterococcus clusters, should be established more accurately.
Several species of lactic acid bacteria account for major infectious diseases in aquaculture. Although clinical cases involving serogroup B streptococci were reported in the United States as early as the beginning of the 1960s (34), they were at first considered a minor source of trouble. A notable exception was Enterococcus seriolicida (20), a junior synonym of Lactococcus garvieae (11), which caused considerable economic losses in Japanese marine-cage aquaculture long before it spread worldwide. In the late 1980s, other species became threatening for the intensive culture of several fish species in various environments and geographic areas. Even though major advances in taxonomic knowledge and characterization procedures have probably favored the identification of new or still unsuspected agents, the concurrent development of international trade, changes in managing procedures, and diversification of domesticated fish species have also been critical in the increasing numbers of lactic acid bacterial infections reported to affect fish (12).
Besides L. garvieae, now firmly established in many European salmonid farms, other illustrative examples are Streptococcus iniae, regularly involved in tilapia and striped bass mortalities in the United States and Israel but widely distributed, in fact, in both fresh and saline waters in all warm areas, and Streptococcus difficilis, a variant form of Streptococcus agalactiae (43). The more opportunistic species Carnobacterium maltaromaticum (formerly called Carnobacterium piscicola) is normally associated with the intestinal microflora of fish. Other representatives with less common occurrence or more limited impact are Streptococcus parauberis in turbot and Vagococcus salmoninarum in salmonids; these may, nonetheless, cause serious damage on a local scale (10, 38).
In France, the involvement of lactic acid bacteria in fish infectious diseases was discovered progressively: C. maltaromaticum was isolated in clinical cases from several fish species (26), and V. salmoninarum was isolated from southwest rainbow trout hatcheries (27). The first cases of L. garvieae infection were experienced concurrently in Brittany and Aquitaine in 1999, more than 5 years after the agent had been identified in Italy and Spain (12). Since chemotherapy appeared to be poorly effective, early detection of new cases, based on rapid and specific diagnostic methods, was the only alternative for preventing further spread of lactococcosis. Unfortunately, identification methods based on phenotypic characterization fall short of basic requirements when applied to bacteria of aquatic origin. Although rapid systems (API 20 Strep, API Rapid ID 32 Strep, and API 50 galleries) may be used, comprehensive tests require several steps and can only provide results after several days or, sometimes, several weeks. Due to the lack of specific documentation and well-defined numerical profiles, result interpretation is especially time-consuming. For these reasons, molecular diagnostic methods based on PCR amplification and/or sequencing of the 16S rRNA gene or other genes of taxonomic interest have been proposed for characterizing most of the newly described agents (1-4, 22, 23, 47). The performance of these techniques, however, has been tested with a limited number of fish-associated bacteria, generally chosen among well-characterized pathogens.
Performing routine examination on increasing numbers of infected fish and tissue samples has also helped researchers to realize that, in fact, recognized pathogens are not the only lactic acid bacteria occurring in fish. A variety of related but different organisms are frequently isolated, rising intriguing questions about their identity, their relationship to pathogens they otherwise closely resemble, like the case with Lactococcus lactis and L. garvieae, and their possible interference in identification tests. To obviate the risk of drawing inaccurate conclusions about closely related and sometimes poorly characterized bacteria, restriction fragment length polymorphism analysis appeared more convenient than PCR amplification based on species-specific primers. Preliminary assays conducted in one of our laboratories had shown that amplified 16S rRNA gene restriction analysis (ARDRA) could be adapted reliably to the specific diagnosis of L. garvieae infection (8). The diversity of the lactic acid bacteria isolated from fish, however, made it difficult to immediately consider extensive use of the method. A cooperative program set up to resolve this issue provided the opportunity to screen and clarify the status of as yet poorly identified species associated with fish. The work was based on the application of ARDRA to a large part (16S-23S) of the rRNA operon; this had already been reported as a reliable technique for identification of lactic acid bacteria (6, 37).
MATERIALS AND METHODS
Isolates.
Fifty-seven field isolates, revived from the Jouy-en-Josas strain collection (JIP) or obtained from different fish disease laboratories, were selected after preliminary tests conducted to establish phenotypic characteristics and/or PCR-restriction fragment length polymorphism profiles according to the method of De Buzon et al. (8). Table 1 summarizes the information available for these strains, together with the results of provisional identification. Twenty-two type strains purchased from different international or national culture collections were also used in various steps of the research (Table 2). All strains were cultured on solid or in liquid brain heart infusion medium enriched with 5% defibrinated horse serum, and aliquots were suspended in 10% glycerol-supplemented medium for freezing preservation at −70°C.
TABLE 1.
Bacteria isolated or collected during this study, with available information about their origins and presumptive identifications, based on phenotypic characteristics in API systems, and the accession numbers of deposited sequencesa
| Isolate | Presumptive identification | Origin | Original area or country | Definitive identification | EMBL library accession no.
|
|
|---|---|---|---|---|---|---|
| 16S rRNA gene | sodA | |||||
| LD40 isolates | ||||||
| C6 | L. lactis/E. faecalis | ? | Poitou-Charentes | |||
| C22 | L. lactis/E. faecalis | ? | Poitou-Charentes | L. lactis | AM490366 | |
| 1609 | E. faecalis | ? | Poitou-Charentes | |||
| 5076 H | S. uberis? | ? | ? | S. dysgalactiae subsp. equisimilis | AM490331 | |
| 5076 NH | L. garvieae? | ? | ? | Aerococcus sp. | AM490332 | |
| 28565-2 | Aerococcus | ? | ? | Aerococcus sp. | AM490327 | |
| 6491-93 | ? | Natural environment | Pyrenees | |||
| 2725-99 | L. garvieae | Oncorhynchus mykiss | ? | |||
| 1798-00 | L. lactis | O. mykiss | Aquitaine | |||
| 2188-00 | E. faecium | O. mykiss | Aquitaine | E. casseliflavus | AM490321 | |
| 3362-01 | A. viridans | O. mykiss | Poitou-Charentes | V. fluvialis/carniphilus | AM490373 | |
| 3363-01 | S. acidominimus | O. mykiss | Poitou-Charentes | |||
| 3364-01 | E. faecalis | O. mykiss | Poitou-Charentes | C. maltaromaticum | Am490329 | |
| 3365-01 | E. faecalis | O. mykiss | Poitou-Charentes | |||
| 3366-01 | S. anginosus | O. mykiss | Poitou-Charentes | L. lactis cremoris | AM490365 | |
| 3525-01 | S. dysgalactiae/S. equisimilis | Acipenser baeri | Aquitaine | S. dysgalactiae subsp. dysgalactiae | AM490330 | |
| 3667-01 | E. faecium/S. bovis | O. mykiss | Brittany | |||
| 2514-02 | L. garvieae | O. mykiss | Aquitaine | |||
| 952-03 | ? | O. mykiss | Aquitaine | |||
| JIP collection | ||||||
| 112-82 (2) | Streptococcus sp. | Paracheirodon innesi | Ile-de-France | |||
| 108-83 (4) | Streptococcus sp. | Channa striata | Thailand | S. iniae | AM490314 | |
| 20-85 (3) | C. piscicola | O. mykiss | Picardy | |||
| CII-5b-88 | Streptococcus sp. | Channa striata | Bangkok | |||
| 31-90 (2) | Streptococcus sp. | Tilapia | Belgium | L. garvieae | AM490328 | |
| 08-92 (1) | S. parauberis? | Tilapia | Lorraine | |||
| 18-93 (2) | V. salmoninarum | O. mykiss | Aquitaine | V. salmoninarum | AM490319 | |
| 18-93 (4) | V. salmoninarum | O. mykiss | Aquitaine | |||
| 10-94 (4) | L. lactis | Pterophyllum scalare | Alsace | |||
| 12-94 (2) | L. piscium? | Betta splendens | MNHN, Paris | |||
| 21-96 (1) | Streptococcus sp. | O. mykiss | Réunion Island | |||
| 21-96 (2) | Streptococcus sp. | O. mykiss | Réunion Island | |||
| 22-96 (2) | L. piscium? | Koi carp | Jura | L. raffinolactis | AM490322 | |
| 23-98 (C11a) | L. lactis | O. mykiss | Aquitaine | |||
| 31-98 (3) | L. lactis? | Tilapia | Brittany | L. lactis subsp. lactis | AM490364 | |
| 25-99 | S. mundtii? | Trout diet | Brittany | E. faecium | AM490323 | |
| 26-99 | Streptococcus sp. | Fish farm water | Brittany | E. faecium | AM490325 | |
| 29-99 | L. garvieae | O. mykiss | Brittany | |||
| 19-00(2) | C. piscicola | P. innesi | Hong Kong | |||
| 20-00 | Leuconostoc? | Carassius carassius | Jura | V. salmoninarum | AM490375 | AM490320 |
| 38-00 (3) | Lactococcus sp. | Symphysodon discus | Ile-de-France | |||
| 10-01 | Leuconostoc? | Koi carp | Jura | L. raffinolactis | AM490367 | AM490311 |
| 14-01 | V. salmoninarum? | C. carassius | Jura | V. fluvialis/carniphilus | AM490371 | |
| Me I.5-02 | C. piscicola | O. mykiss | Brittany | |||
| Other origins | ||||||
| France | ||||||
| 1525A | S. parauberis | Dicentrarchus labrax | SAVU | |||
| 1525E | S. parauberis | D. labrax | SAVU | S. parauberis | AM490317 | |
| 1534E | S. parauberis | D. labrax | SAVU | |||
| Israel | ||||||
| KF 173 | S. iniae | O. mykiss | A. Eldar | |||
| KFP 404 | S. iniae | O. mykiss | A. Eldar | |||
| Spain | ||||||
| 17-02 | Enterococcus sp. | O. mykiss | M. Blanco | E. gallinarum | AM490318 | |
| 18-02 | Enterococcus sp. | O. mykiss | M. Blanco | V. fluvialis/carniphilus | AM490372 | |
| 24-01 | S. mutans? | O. mykiss | Trouw, Spain | |||
| 26-01 | S. mutans? | O. mykiss | Trouw, Spain | Lactococcus sp. | AM490370 | AM490324 |
| 27-01 (2) | Lactococcus sp./Gemella | O. mykiss | Trouw, Spain | V. salmoninarum | AM490374 | AM490326 |
| 28-01 | S. mutans? | O. mykiss | Trouw, Spain | L. raffinolactis | AM490368 | |
| United Kingdom | ||||||
| CF 00021 | L. garvieae | O. mykiss | CEFAS | |||
| CF 01144 | L. garvieae | O. mykiss | CEFAS | |||
| CF 01173 | S. agalactiae | O. mykiss | CEFAS | |||
Abbreviations: CEFAS, Center for Environment, Fisheries and Aquaculture Science, Weymouth, United Kingdom; JIP, Jouy Ichtyopathology collection, INRA, Jouy-en-Josas, France; LV40, Laboratoire Départemental des Landes, Mont-de-Marsan, France; MNHN, Museum National d'Histoire Naturelle, Paris, France; and SAVU, Service d'Assistance Vétérinaire Aquacole d'Urgence, Saint Jean de Vedas, France.
TABLE 2.
Type strains used in the study (accession numbers of the genes sequenced during the study are indicated)
| Species | Collection no.a | Other no. | EMBL accession no.
|
|
|---|---|---|---|---|
| 16S rRNA gene | sodA gene | |||
| Aerococcus viridans | CIP 54.145T | AM490333 | ||
| Carnobacterium maltaromaticum | NCIMB 2264T | AM490310 | ||
| C. inhibens | CIP 106863T | AM490313 | ||
| Enterococcus durans | NCIMB 700596T | |||
| Lactococcus garvieae | CNRZ 1323T | |||
| L. lactis subsp. cremoris | CNRZ 105T | |||
| L. lactis subsp. lactis | CNRZ 142T | |||
| L. lactis subsp. lactis | CNRZ 144T | |||
| L. piscium | CIP 104371T | NCIMB 13196 T | ||
| L. plantarum | CNRZ 1322T | AM490316 | ||
| L. raffinolactis | CNRZ 1214T | AM490369 | AM490315 | |
| Streptococcus acidominimus | CIP 82.4T | |||
| S. agalactiae | CIP 103227T | |||
| S. anginosus | CIP 102921T | |||
| S. bovis | CIP 102302T | |||
| S. dysgalactiae subsp. dysgalactiae | CIP 102914T | |||
| S. dysgalactiae subsp. equisimilis | CIP 105120T | AM490312 | ||
| S. mitis | CIP 103335T | |||
| S. parauberis | NCIMB 702020T | |||
| S. shiloi | ATCC 51499T | = S. iniae | ||
| S. uberis | NCIMB 702038T | |||
| V. salmoninarum | NCIMB 13133T | formerly NCFB 2777 T | ||
ATCC, American Type Culture Collection, Manassas, VA; CIP, Collection de l'Institut Pasteur, Institut Pasteur, Paris, France; CNRZ, Collection CNRZ de Bactéries Lactiques et Propioniques, Unité de Recherches Laitières et de Génétique Appliquée, INRA, Jouy-en-Josas, France; NCFB, National Collection of Food Bacteria; NCIMB, National Collections of Industrial, Food and Marine Bacteria, Aberdeen, Scotland.
DNA extraction.
After verification of the purity and morphological characteristics of the bacteria, 2 ml of a 24- or 48-h broth culture was collected for DNA extraction. DNA extraction was performed using a Wizard genomic DNA purification kit (Promega, Charbonnières-les-Bains, France) according to supplier specifications, with an additional step of lysozyme treatment (1 mg/ml in 40 mM EDTA for 60 min at 37°C) before extraction. DNA quality and quantity were controlled in 1% agarose minigels run in Tris-borate-EDTA buffer (45 mM; pH 7.8 [1 mM EDTA]) at 100 V/cm, stained with ethidium bromide, and observed under UV light. All DNAs were stored at 4°C until further use.
ARDRA.
The technique developed by Gurtler et al. (16) was followed to amplify the extracted DNAs and to prepare the rRNA gene for ARDRA. The primers used were P1 (5′ AGA GTT TGA TCM TGG CTC 3′) and P2 (5′ ACA TCG AGG TGC CAA AC 3′). Amplification was performed in a PTC 100 cycler (MJ Research Inc., Tahoe, NV), using mixes incorporating primers at 20 μM, deoxynucleoside triphosphates at 200 μM, 0.5 μl (2.5 U) of Taqara ExTaq polymerase (Cambrex Bio Science Paris), 5 μl of 10× Taq buffer, and 4 μl of template DNA into distilled water adjusted to a 50-μl final volume. DNA denaturation was performed for 5 min at 94°C, followed by 30 cycles of amplification (30 s at 94°C, 1 min at 57°C, and 6 min at 72°C) and a final elongation step of 7 min at 72°C. The size of the expected 16S-23S fragment, about 5,000 bp, was checked by 1% agarose gel electrophoresis and staining with ethidium bromide. Digestion of the amplified products was tested with several restriction enzymes, including CfoI (Promega), Sau3A (Gibco BRL, Cergy-Pontoise, France), HaeIII, HpaII, MboII, DraI, and SmaI (all from MBI Fermentas, distributed by Coger, Paris, France). It required incubation for 3 h at 37°C of a mixture of enzyme (5 U) and amplified product (4 μl) prepared in the specific buffer supplied by the manufacturer in a final volume of 20 μl. Electrophoretic migration was performed in 1% Seakem GTG agarose (Tebu, Le Perray-en-Yvelines, France) gels in Tris-borate-EDTA buffer at 65 V/cm. The reference marker was Jules (QBiogen), which covers a size range of 100 to 2,000 bp.
Gel analysis.
After preliminary assays aimed at testing seven endonucleases to digest the complete 16S-23S fragment on type strains and comparing the resulting electrophoresis profiles, CfoI and Sau3A were considered to be the most suitable for further analysis of 78 strains, including 21 type strains (Lactococcus raffinolactis type strain DNA was rejected in this step, following quality control) and 57 representative fish isolates (Tables 1 and 2). Numerized pictures of migration gels run under standardized conditions were taken and processed with GelCompar software (Applied-Maths, Sint Martens Latem, Belgium). This made it possible to calibrate, analyze, and combine the results obtained with the different enzymes.
Sequencing.
In order to confirm or determine the identities of dubious species, sequencing was carried out on representative bacterial strains. We chose to use two different genes, the 16S rRNA gene and sodA, depending on the suspected identities of the bacteria and the availability of published information. Amplification and sequencing of the 16S rRNA gene were performed according to the method described by Cibik et al. (7). For the sodA gene, the method of Poyart et al. (30) was applied, modifying only the annealing temperature (50°C instead of 45°C) in order to ensure more stringent conditions. Our sequences, together with those of related species listed in GenBank, were used for comparison and phylogenetic analysis.
RESULTS
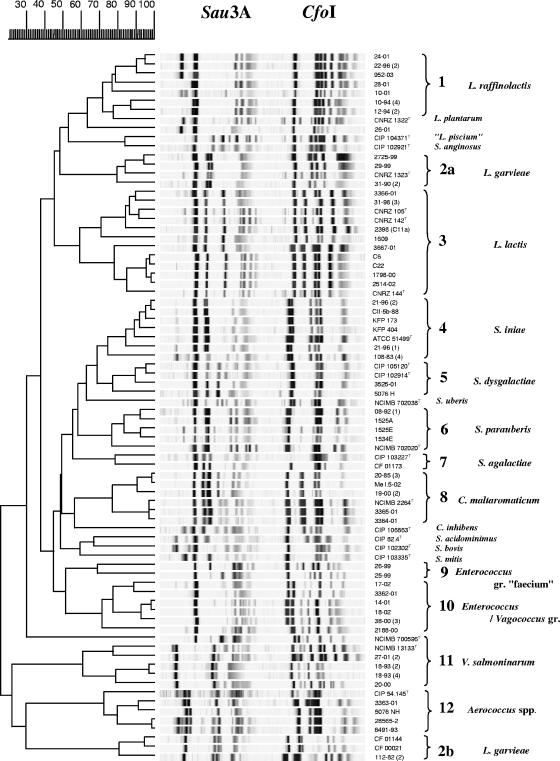
A comparison of the profiles resulting from restriction with Sau3A and CfoI is presented in Fig. 1. Most of the strains clustered in distant but clear-cut groups, some of which could be identified immediately; the others were numbered until additional tests could be performed. Strains that occupied isolated positions in the dendrogram corresponded to unrelated type strains or misidentified non-lactic-acid bacteria and were not considered in the subsequent steps of the study.
FIG. 1.
Comparison of ARDRA profiles resulting from the restriction of 5,000-bp 16S-23S fragments with Sau3A and CfoI, using the Pearson similarity coefficient and the unweighted-pair group method using average linkages program included in GelCompar software (Applied-Maths, Belgium).
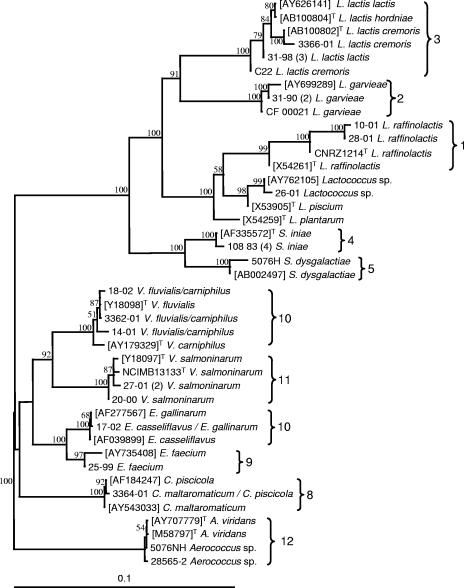
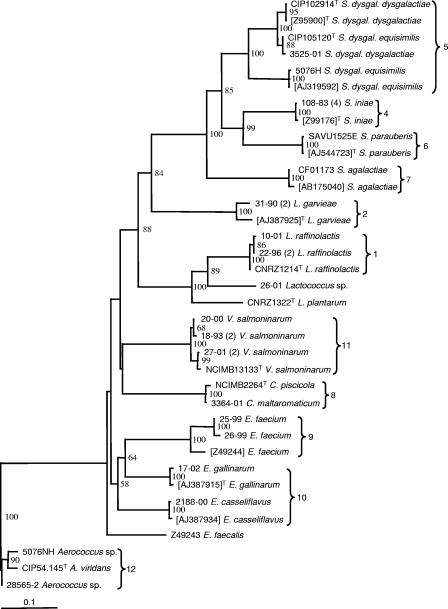
Sequencing was carried out on representative isolates of the different ARDRA clusters to confirm their relationship to representative type strains or to tentatively identify those that could not be related to any type strain. The choice between sequencing of the 16S rRNA and sodA genes was made based on the information available from libraries on reference strains and the recognized limits of 16S rRNA gene sequence discrimination within certain bacterial genera (14, 42). In some cases, both genes were sequenced in order to obtain independent series of data and strengthen the ARDRA results. Figures 2 and 3 show that strong similarity was established between fish isolates and reference strains and that, in every case, the classification of the strains was consistent for all three techniques used in this study.
FIG. 2.
Distance tree resulting from comparison of 16S rRNA gene sequences of selected isolates with reference sequences extracted from the GenBank database. ARDRA groupings are indicated. The tree was constructed using the model of Jukes and Cantor (18) and the neighbor-joining method included in PAUP software (40). Bootstrap values (percentages for 1,000 replicates) of >50% are indicated at the nodes. GenBank accession numbers are shown in square brackets, and “T” indicates type strains. The Aerococcus group was used as an outgroup. Bar, 10% divergence.
FIG. 3.
Distance tree resulting from comparison of sodA gene sequences of selected isolates with reference sequences extracted from the GenBank database. ADRA groupings are indicated. The tree was constructed using the model of Jukes and Cantor (18) and the neighbor-joining method included in PAUP software (40). Bootstrap values (percentages for 1,000 replicates) of >50% are indicated at the nodes. GenBank accession numbers are shown in square brackets, and “T” indicates type strains. The Aerococcus group was used as an outgroup. Bar, 10% divergence.
It eventually became clear that most of our isolates could be assigned to 12 species or groups of interest. An unexpected abundance of isolates (group 1) roughly related to a large branch encompassing Lactococcus plantarum and the type strain supplied as Lactococcus piscium was evidenced by ARDRA. Further sequence comparison showed that all group 1 isolates were closely related to L. raffinolactis (Fig. 2 and 3). While the “type strain” of L. piscium was unexpectedly found to be questionable (Fig. 1), the isolate 26-01 appeared to be roughly related to this species according to published 16S rRNA sequences (Fig. 2). Sequencing results established the unique character of both L. garvieae and L. lactis isolates, especially the former, which split into quite remote subgroups (2a and 2b) in the ARDRA dendrogram. Conversely, the other major fish pathogen, Streptococcus iniae (cluster 4), proved noticeably homogenous in its restriction patterns. Clusters 5 to 7, representing other Streptococcus species, and cluster 8, corresponding to Carnobacterium maltaromaticum, also displayed strong homogeneity and were easily recognized in ARDRA. In the cases of Streptococcus dysgalactiae and S. agalactiae, however, only one or two isolates were available.
The next two clusters were not so easily defined. They included typical enterococci that could not be characterized at the specific level by ARDRA, although some isolates were representative of the “faecium” group (cluster 9). Group 10 strains shared high rRNA gene sequence homology with Enterococcus or Vagococcus spp. Some could be characterized as Enterococcus casseliflavus and Enterococcus gallinarum by sodA gene sequences only (Fig. 3), whereas 16S rRNA analysis eventually revealed several others to be Vagococcus fluvialis or Vagococcus carniphilus, a species recently reported to be in beef meat (39). This was the only case, indeed, in which ARDRA did not enable the assignment of isolates to the correct genus. All group 11 strains were closely related to the V. salmoninarum type strain. All group 12 isolates belonged to the Aerococcus genus, although no further conclusions could be derived regarding the species.
DISCUSSION
The considerable diversity of lactic acid bacteria associated with fish may explain the difficulties encountered when identification procedures are based only on phenotypic characteristics. While Carnobacterium maltaromaticum, Lactococcus lactis, L. garvieae, and Vagococcus salmoninarum were already documented in French fish farm environments, the use of ARDRA made it possible (i) to detect the occurrence of Streptococcus dysgalactiae and S. parauberis in France, (ii) to assign past cases from tropical environments to S. iniae and S. parauberis, and (iii) to establish repeated associations of L. raffinolactis, Aerococcus spp., Enterococcus spp., and Vagococcus spp. with apparently healthy fish. The practical importance of these different clusters for fish health management is worth considering.
All of the isolated streptococci were found to belong to fish pathogenic species and were easily characterized in ARDRA (groups 4 to 7), as demonstrated with Streptococcus iniae. Depending on the diagnosis methods used, confusion is possible with Streptococcus uberis, an agent that may be misidentified as several different organisms (25) but that seems to occur rarely in fish farm environments. A single strain of S. agalactiae, CF 01173, originating from an English trout farm, was recognized in this study. More interesting is the independent isolation of two S. dysgalactiae strains, one of them from diseased sturgeons. The pathogenicity of this species had been reported only once, in Japan (28). The same remark may be made for S. parauberis, whose detrimental impact, until now, seemed to be restricted to turbot (10) in Spanish sea farms. The present isolates were all from French sea bass farms, except for one, Streptococcus sp. strain 08-92 (1), which was isolated from diseased tilapia in 1992.
In most of the groups delineated in Fig. 1 (groups 1, 2, 3, 8, and 11), both pathogenic and nonpathogenic species are represented. Lactococcus raffinolactis has been reported as a contaminant of food products (36), but a debate on possible confusion with the fish pathogen L. piscium has arisen in more recent studies (35). Such confusion cannot be ruled out, as suggested in Fig. 1, where the two species cluster together, and by the presumptive identification of our isolates 12-94 (2) and 22-96 (2) as L. piscium by API systems (Table 1). Whereas additional research easily showed that the ARDRA profile resemblance with Streptococcus anginosus (Fig. 1) was coincidental, it was more difficult to analyze the relationship between L. piscium and L. raffinolactis. Following further 16S rRNA gene sequence checks conducted at the CIP (D. Clermont, personal communication), doubts have arisen with regard to the conformity of CIP 104371T and other type strain cultures maintained in several collections to ATCC HRIA 68, the first culture that was deposited and the most representative of the original description of L. piscium (46). Unlike the isolates described by Sakala et al. (35), the type strain is a fastidious organism and is probably the only one ever to have been isolated from diseased fish (46). Our tentative conclusion is that L. raffinolactis and L. piscium are closely related bacteria regularly isolated from fish and food products, sometimes in the clinical context, although any detrimental effect on health is still far from being demonstrated.
Since close relationships have repeatedly been found in the phylogenetic comparison of L. lactis and L. garvieae, these two species may be considered to represent a large cluster of bacteria frequently associated with the aquatic environment and its inhabitants and characterized by wide variability. This makes clear characterization difficult. L. garvieae represents a threat mainly under intensive farming conditions but may be isolated from almost all fish species (21). In fact, two of our isolates were from ornamental fish. It thus appears to be as ubiquitous as L. lactis, which is not an actual pathogen but may proliferate in weakened or moribund fish, thereby highlighting the importance of differential diagnosis.
Carnobacterium species (group 8) are commonly associated with food processing problems, and some of them (C. maltaromaticum, Carnobacterium divergens, and Carnobacterium inhibens) are normal components of the fish integument and fish intestinal microflora (17, 33). In addition, C. maltaromaticum may act as a common opportunistic pathogen and be likely to produce chronic infection in different species of fish under detrimental conditions (26, 41). The ARDRA approach ascertained the identities of the isolates tested; this is consistent with previous reports which concluded that rRNA molecular techniques were particularly well suited for the specific diagnosis or genotyping of these species (6, 32).
Vagococcus salmoninarum, which is rare but well documented and of serious consequence in salmonid farms where it occurs, is the last pathogen of note among the bacteria identified during this study (27, 38). Until strain 20-00 was isolated from a crucian carp, Carassius carassius, this agent had been reported only from salmonids. The detection of strains (group 10) related to the V. fluvialis/carniphilus cluster (Fig. 2) is too new to form an opinion on their practical significance, but because one of them (14-01) was first misidentified as V. salmoninarum in API systems and as ARDRA did not separate them from enterococci, it is important to be aware of this species interference in current diagnostic procedures.
Up to now, none of the species included in groups 9, 10, and 12 has been demonstrated to threaten fish health. While isolation of Aerococcus sp. strains from freshwater fish on several occasions is an original finding, the occurrence of enterococci in fish or fish environments has been mentioned in rare microflora analyses and surveys (5, 19, 29) and could reasonably have been expected in routine diagnostic results. Enterococcus identification, however, has always been confusing, so among the isolates assigned to these groups, definitive identification was only achieved through ARDRA and sequencing data. In spite of the unexpected position of Enterococcus durans in the dendrogram (apparently due to experimental flaws), strains 25-99 and 26-99, which came from salmonid farm environments, clearly belonged to the “faecium” group recently defined by Poyart et al. (31). While some of the other isolates were consistent with the E. casseliflavus/flavescens (a single species according to Poyart et al. [31]) and E. gallinarum definitions, the identification of others was deferred until 16S rRNA gene sequencing revealed that they were V. fluvialis and V. carniphilus relatives.
It appears that live fish either regularly or transiently host a variety of as yet unsuspected or poorly documented lactic acid bacteria. Since there is little reason to suspect fish pathogenic properties in the new species identified in this study, the main inconvenience for aquaculturists is the risk of misidentification between true pathogens and environmental bacteria. It is therefore important to reconsider the suitability of the techniques employed in routine diagnosis. Although conclusive identification and typing methods have been proposed to improve the diagnosis of lactic acid bacteria in fish, they generally are designed for limited ranges of species of recognized significance (L. garvieae, S. iniae, and Carnobacterium spp.). For more global approaches, ARDRA offers interesting possibilities, provided that the procedure can be simplified and made less cumbersome. Improvement of the already mentioned technique (8) is now in progress, taking into account the issues of the present work.
Another consequence of the presence of such a variety of bacteria is far removed from the aquaculturist's concerns. One cannot help being puzzled by the similarities frequently observed in the lactic acid bacteria isolated from fish and fish products and those that are more generally encountered in the food processing industry (e.g., Lactococcus, Carnobacterium, and Enterococcus) and, sometimes, in human infections (e.g., Streptococcus and Enterococcus). With regard to fish products, the idea that the microbial populations that develop after slaughtering result from terrestrial environments (24) should probably be partly revised, although the responsibility of lactic acid bacteria in food spoilage is generally questioned. Such severe fish pathogens as L. garvieae and S. iniae are sometimes isolated from human or other mammalian clinical cases, and S. iniae has even been suspected of being a zoonotic agent (13, 45). The presence of other pyogenic streptococci and “faecium” group enterococci in fish could give rise to similar conjectures. Indeed, direct contact between fish and consumers does not appear likely to be frequent compared to other epidemiological sources; the relevant question is whether fish and mammal strains are strictly equivalent and share common virulence properties. Rare typing studies conducted with S. iniae (9, 15) and L. garvieae (44) isolates of human and fish origin were able to detect some biochemical and genetic differences, respectively, suggesting that fish isolates are special forms closely adapted to aquatic habitats. Other bacterial species could be investigated in the same way.
To conclude, this research has made it possible to confirm the abundance of lactic acid bacteria in fish and fish farm environments and to assess for the first time the presence or unsuspected frequency of several groups or species. It has added to our knowledge of lactic acid bacteria and to the ways that they can be differentiated using diagnostic procedures and has addressed the refinement of rapid and reliable molecular methods for this purpose. This study paves the way to a better understanding of the species regularly or occasionally encountered in fish health monitoring and to further investigations into their relationships with similar bacteria involved in fish and other animal food processing and in human health.
Acknowledgments
Special thanks are due to the technicians and students who provided precious help or technical expertise throughout this study, namely, Brigitte Kerouault, Elodie Poumerol (INRA), Carinne Bellet, Martine Beyrie (LD40), Thomas Andrieux (Versailles-Saint Quentin University), and Nicolas Bruneau (Centre de Réadaptation Professionnelle, Beauvoir, France). We are indebted to Diane Beaud and Jean-Claude Ogier (INRA, Jouy-en-Josas, France) for their assistance with different stages of ARDRA and to all colleagues who provided original field isolates, including Myriam Algoët (CEFAS, Weymouth, United Kingdom), Maria Blanco-Gutierrez (Facultad Veterinaria, UCM, Madrid, Spain), Avi Eldar (Kimron Veterinary Institute, Bet Dagan, Israel), and Jean-Christophe Raymond (SAVU, France).
This research was supported by a joint Soutien à l'Innovation grant provided by the Office National Interprofessionnel des Produits de la Mer et de l'Aquaculture (OFIMER), with financial assistance from the Comité Interprofessionnel des Produits de l'Aquaculture (CIPA) and the Direction Régionale de l'Agriculture et de la Forêt d'Aquitaine (DRAF).
Footnotes
Published ahead of print on 2 March 2007.
REFERENCES
- 1.Altun, S., Ö. Diler, and A. K. Adiloglu. 2004. Genotyping of Lactococcus garvieae strains from rainbow trout (Oncorhynchus mykiss) by 16S rDNA sequencing. Bull. Eur. Assoc. Fish Pathol. 24:119-124. [Google Scholar]
- 2.Aoki, T., C.-I. Park, H. Yamashita, and I. Horono. 2000. Species-specific polymerase chain reaction primers for Lactococcus garvieae. J. Fish Dis. 23:1-6. [Google Scholar]
- 3.Berridge, B. R., H. Bercovier, and P. F. Frelier. 2001. Streptococcus agalactiae and Streptococcus difficile 16S-23S intergenic rDNA: genetic homogeneity and species-specific PCR. Vet. Microbiol. 78:165-173. [DOI] [PubMed] [Google Scholar]
- 4.Berridge, B. R., J. D. Fuller, J. D. E. Azavedo, D. E. Low, H. Bercovier, and P. F. Frelier. 1998. Development of specific nested oligonucleotide PCR primers for the Streptococcus iniae 16S-23S ribosomal DNA intergenic spacer. J. Clin. Microbiol. 36:2778-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai, Y. M., P. Suyanandana, P. Saman, and Y. Benno. 1999. Classification and characterization of lactic acid bacteria isolated from the intestines of common carp and freshwater prawns. J. Gen. Appl. Microbiol. 45:177-184. [DOI] [PubMed] [Google Scholar]
- 6.Chenoll, E., M. C. Macián, and R. Aznar. 2003. Identification of Carnobacterium, Lactobacillus, Leuconostoc and Pediococcus by rDNA-based techniques. Syst. Appl. Microbiol. 26:546-556. [DOI] [PubMed] [Google Scholar]
- 7.Cibik, R., E. Lepage, and P. Tailliez. 2000. Molecular diversity of Leuconostoc mesenteroides and Leuconostoc citreum isolated from traditional French cheeses as revealed by RAPD fingerprinting, 16S rDNA sequencing and 16S rDNA fragment amplification. Syst. Appl. Microbiol. 23:267-278. [DOI] [PubMed] [Google Scholar]
- 8.De Buzon, S., C. Couture, M. Beyrie, A. Lautraite, and C. Pelletier. 2001. Identification of fish pathogenic gram positive cocci using polymerase chain reaction-restriction fragment length polymorphism analysis, abstr. 1P-053. Abstr. 10th Int. Conf. Eur. Assoc. Fish Pathol.
- 9.Dodson, S. V., J. J. Maurer, and E. B. Shotts. 1999. Biochemical and molecular typing of Streptococcus iniae isolated from fish and human cases. J. Fish Dis. 22:331-336. [Google Scholar]
- 10.Doménech, A., J. F. Fernández-Garayzábal, C. Pascual, J. A. Garcia, M. T. Cutuli, M. A. Moreno, M. D. Collins, and L. Dominguez. 1996. Streptococcosis in cultured turbot, Scophthalmus maximus (L.), associated with Streptococcus parauberis. J. Fish Dis. 19:33-38. [Google Scholar]
- 11.Eldar, A., C. Ghittino, L. Asanta, E. Bozzeta, M. Goria, M. Prearo, and H. Bercovier. 1996. Enterococcus seriolicida is a junior synonym of Lactococcus garvieae, a causative agent of septicemia and meningoencephalitis in fish. Curr. Microbiol. 32:85-88. [DOI] [PubMed] [Google Scholar]
- 12.Eyngor, M., A. Zlotkin, C. Ghittino, M. Prearo, D.-G. Douet, S. Chilmonczyk, and A. Eldar. 2004. Clonality and diversity of the fish pathogen Lactococcus garvieae in Mediterranean countries. Appl. Environ. Microbiol. 70:5132-5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fefer, J. J., R. Ratzan, S. E. Sharp, and E. Saiz. 1998. Lactococcus garvieae endocarditis: report of a case and review of the literature. Diagn. Microbiol. Infect. Dis. 32:127-130. [DOI] [PubMed] [Google Scholar]
- 14.Fox, G. E., J. D. Wisotzkey, and P. Jurtshuk, Jr. 1992. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int. J. Syst. Bacteriol. 42:166-170. [DOI] [PubMed] [Google Scholar]
- 15.Fuller, J. D., D. J. Bast, V. Nizet, D. E. Low, and J. C. S. de Azavedo. 2001. Streptococcus iniae virulence is associated with a distinct genetic profile. Infect. Immun. 69:1994-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurtler, V., V. A. Wilson, and B. C. Mayall. 1991. Classification of medically important clostridia using restriction endonuclease site differences of PCR-amplified 16S rDNA. J. Gen. Microbiol. 137:2673-2679. [DOI] [PubMed] [Google Scholar]
- 17.Jöborn, A., M. Dorsch, J. C. Olsson, A. Westerdahl, and S. Kjelleberg. 1999. Carnobacterium inhibens sp. nov., isolated from the intestine of Atlantic salmon (Salmo salar). Int. J. Syst. Bacteriol. 49:1891-1898. [DOI] [PubMed] [Google Scholar]
- 18.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism, vol. 3. Academic Press, New York, NY. [Google Scholar]
- 19.Kanoe, M., and T. Abe. 1988. Enterococcal isolates from environmental sources. Microbios Lett. 38:15-20. [Google Scholar]
- 20.Kusuda, R., K. Kawai, F. Salati, C. R. Banner, and J. L. Fryer. 1991. Enterococcus seriolicida sp. nov., a fish pathogen. Int. J. Syst. Bacteriol. 41:406-409. [DOI] [PubMed] [Google Scholar]
- 21.Kusuda, R., and F. Salati. 1999. Enterococcus seriolicida and Streptococcus iniae, p. 303-317. In P. T. K. Woo and D. W. Bruno (ed.), Fish diseases and disorders, vol. 3. Viral, bacterial and fungus infections. Cabi International, New York, NY. [Google Scholar]
- 22.Mata, A. I., M. M. Blanco, L. Dominguez, J. F. Fernández-Garayzábal, and A. Gibello. 2004. Development of a PCR assay for Streptococcus iniae based on the lactate oxidase (lctO) gene with potential diagnostic value. Vet. Microbiol. 101:109-116. [DOI] [PubMed] [Google Scholar]
- 23.Mata, A. I., A. Gibello, A. Casamayor, M. M. Blanco, L. Dominguez, and J. F. Fernández-Garayzábal. 2004. Multiple PCR assay for detection of bacterial pathogens associated with warm-water streptococcosis in fish. Appl. Environ. Microbiol. 70:3183-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauguin, S., and G. Novel. 1994. Characterization of lactic acid bacteria isolated from seafood. J. Appl. Bacteriol. 76:616-625. [Google Scholar]
- 25.McDonald, W. L., B. N. Fry, and M. A. Deighton. 2005. Identification of Streptococcus spp. causing bovine mastitis by PCR-RFLP of 16S-23S ribosomal DNA. Vet. Microbiol. 111:241-246. [DOI] [PubMed] [Google Scholar]
- 26.Michel, C., B. Faivre, and B. Kerouault. 1986. Biochemical identification of Lactobacillus piscicola strains from France and Belgium. Dis. Aquat. Organ. 2:27-30. [Google Scholar]
- 27.Michel, C., P. Nougayrède, A. Eldar, E. Sochon, and P. D. Kinkelin. 1997. Vagococcus salmoninarum, a bacterium of pathological significance in rainbow trout Oncorhynchus mykiss farming. Dis. Aquat. Organ. 30:199-208. [Google Scholar]
- 28.Nomoto, R., L. Munasinghe, D. Jin, Y. Shimahara, H. Yasuda, A. Nakamura, N. Misawa, T. Itami, and T. Yoshida. 2004. Lancefield group C Streptococcus dysgalactiae infection responsible for fish mortalities in Japan. J. Fish Dis. 27:679-686. [DOI] [PubMed] [Google Scholar]
- 29.Petersen, A., and A. Dalsgaard. 2003. Species composition and antimicrobial resistance genes of Enterococcus spp., isolated from integrated and traditional fish farms in Thailand. Environ. Microbiol. 5:395-402. [DOI] [PubMed] [Google Scholar]
- 30.Poyart, C., P. Berche, and P. Trieu-Cuot. 1995. Characterization of superoxide dismutase genes from gram-positive bacteria by polymerase chain reaction using degenerate primers. FEMS Microbiol. Lett. 131:41-45. [DOI] [PubMed] [Google Scholar]
- 31.Poyart, C., G. Quesnes, and P. Trieu-Cuot. 2000. Sequencing the gene encoding manganese-dependent superoxide dismutase for rapid species identification of enterococci. J. Clin. Microbiol. 38:415-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rachman, C., P. Kabadjova, R. Valcheva, H. Prevost, and X. Dousset. 2004. Identification of Carnobacterium species by restriction fragment length polymorphism of the 16S-23S rRNA gene intergenic spacer region and species-specific PCR. Appl. Environ. Microbiol. 70:4468-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ringø, E., and F.-J. Gatesoupe. 1998. Lactic acid bacteria in fish: a review. Aquaculture 160:177-203. [Google Scholar]
- 34.Robinson, J. A., and F. P. Meyer. 1966. Streptococcal fish pathogen. J. Bacteriol. 92:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakala, R. M., H. Hayashidani, Y. Kato, C. Kaneuchi, and M. Ogawa. 2002. Isolation and characterization of Lactococcus piscium strains from vacuum-packaged refrigerated beef. J. Appl. Microbiol. 92:173-179. [DOI] [PubMed] [Google Scholar]
- 36.Schillinger, U., and F. K. Lucke. 1986. Lactic acid bacteria on vacuum-packaged meat and influence on shelf life. Fleischwirtschung 66:1515-1520. [Google Scholar]
- 37.Schlegel, L., F. Grimont, P. A. Grimont, and A. Bouvet. 2003. Identification of major streptococcal species by rrn-amplified ribosomal DNA restriction analysis. J. Clin. Microbiol. 41:657-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidtke, L. M., and J. Carson. 1994. Characteristics of Vagococcus salmoninarum isolated from diseased salmonid fish. J. Appl. Bacteriol. 77:229-236. [DOI] [PubMed] [Google Scholar]
- 39.Shewmaker, P. L., A. G. Steigerwalt, R. E. Morey, M.-G. S. Carvalho, J. A. Eliott, K. Joyce, T. J. Barrett, L. M. Teixeria, and R. R. Facklam. 2004. Vagococcus carniphilus sp. nov., isolated from ground beef. Int. J. Syst. Evol. Microbiol. 54:1505-1510. [DOI] [PubMed] [Google Scholar]
- 40.Swofford, D. L. (ed.). 2003. PAUP*. Phylogenetic analysis using parsimony (and other methods), version 4.ob 10. Sinauer Associates, Sunderland, MA.
- 41.Toranzo, A. E., J. L. Romalde, S. Núñez, A. Figueras, and J. L. Barja. 1993. An epizootic in farmed, market-size rainbow trout in Spain caused by a strain of Carnobacterium piscicola of unusual virulence. Dis. Aquat. Organ. 17:87-99. [Google Scholar]
- 42.Ueda, K., T. Seki, T. Kudo, T. Yoshida, and M. Kataoka. 1999. Two distinct mechanisms cause heterogeneity of 16S rRNA. J. Bacteriol. 181:78-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandamme, P., L. A. Devriese, B. Pot, K. Kersters, and P. Melin. 1997. Streptococcus difficile is a nonhemolytic group B, type Ib streptococcus. Int. J. Syst. Bacteriol. 47:81-85. [DOI] [PubMed] [Google Scholar]
- 44.Vela, A. I., J. Vásquez, A. Gibello, M. M. Blanco, M. A. Moreno, P. Liébana, C. Albendea, B. Alcalá, A. Mendez, L. Domínguez, and J. F. Fernández-Garayzábal. 2000. Phenotypic and genetic characterization of Lactococcus garvieae isolated in Spain from lactococcosis outbreaks and comparison with isolates of other countries and sources. J. Clin. Microbiol. 38:3791-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinstein, M. R., M. Litt, D. A. Kertesz, P. Wyper, D. Rose, M. Coulter, A. McGeer, R. Facklam, C. Ostach, B. M. Willey, A. Borczyck, and E. D. Low. 1997. Invasive infections due to a fish pathogen, Streptococcus iniae. N. Engl. J. Med. 337:589-594. [DOI] [PubMed] [Google Scholar]
- 46.Williams, A. M., J. L. Fryer, and M. D. Collins. 1990. Lactococcus piscium sp. nov. a new Lactococcus species from salmonid fish. FEMS Microbiol. Lett. 68:109-114. [DOI] [PubMed] [Google Scholar]
- 47.Zlotkin, A., A. Eldar, C. Ghittino, and H. Bercovier. 1998. Identification of Lactococcus garvieae by PCR. J. Clin. Microbiol. 36:983-985. [DOI] [PMC free article] [PubMed] [Google Scholar]