Abstract
Recombinant human cysteine protease inhibitor, stefin A, was expressed in both E. coli and BSC-1 monkey kidney cells utilizing pET and recombinant Vaccinia virus systems, respectively. The expressed protein was purified and analyzed by SDS-PAGE and western blot analysis utilizing a polyclonal antibody against rat cystatin α. In both cases the purified protein appeared as a single band corresponding to the molecular weight of stefin A (~10 kDa). Viability of the expressed stefin A was determined by the inhibition of the plant cysteine protease, papain. Recombinant human stefin A expressed in both E. coli and BSC-1 cells was shown to almost completely inhibit papain. The expression of a fully functional recombinant human stefin A in the bacterial system provides a highly efficient tool for the production of large quantities of the protein. This can be an important tool in kinetic studies as well as in production of antibodies for other analytical studies (immunoblot, immunohistochemical studies, etc.). Expression in the mammalian cells on the other hand, can provide a significant research tool to study the functional roles of stefin A in the mammalian systems such as the regulation of cysteine proteases.
Introduction
Alterations in the balance between cysteine proteases and their endogenous inhibitors have been postulated to contribute to the malignant progression of tumors (for reviews see 1–4). Stefin A is the major cysteine protease inhibitor (CPI) that has been associated most often with malignant progression. More specifically, a decrease in the level of stefin A has been demonstrated in malignant epithelial tumors such as human uterine portico, skin, lung, esophagus, Breast, Prostate and cervix (5–11). More compelling evidence demonstrated continual decrease in the level of stefin A mRNA during the progression of murine skin papillomas to carcinoma (12). Similarly, decreased stefin A transcripts were observed in 4 of 5 breast carcinomas as compared to matched normal breast tissues (13). In addition to decreased amounts of stefin A in malignant tissues, Lah and coworkers (14, 15) have shown that stefin A, isolated from human sarcoma tissue, has an altered inhibitory activity, being less effective than normal stefin A in inhibiting the proteolytic activity of both papain and cathepsin B. Lastly, we have demonstrated in murine tumors that there is an overall decrease of CPI activity accompanied with an enrichment of the activity within the plasma membrane fraction (16). Subsequent work established that the partially purified membrane-associated CPI activity of the highly invasive murine hepatoma, Hepa-cl9, is immunologically related to stefin A (17). Decreased levels, impaired function, and/or membrane enrichment of stefin A may contribute to the imbalance between the CPIs and cysteine proteases, favoring the activity of these enzymes.
The expression of functional recombinant human stefin A in E. coli has been previously reported (18–20). However, the proteins expressed were fusion proteins containing N-terminal extensions or tags. Given the CPIs mode of inhibition, this N-terminal extension may affect the conformational requirements necessary for the formation of the tripartite wedge, unless the external residues are removed. Here we report two methods of expressing functional human recombinant stefin A without N-terminal extension: bacterial and mammalian expression of human recombinant stefin A. The bacterial expression system provided a vehicle for the inexpensive production of large quantities of functional protein, while the mammalian expression system was developed as a tool for probing the molecular mechanisms involved in the malignant progression of epithelial tissues as they relate to the balance of cysteine proteases and their endogenous inhibitors. The studies reported here demonstrate the functional expression of recombinant human stefin A in both bacterial and mammalian cells. This is the first known report of the functional expression of recombinant human stefin A in mammalian cells.
Materials and Methods
Molecular biology reagents
NcoI and BamHI restriction enzymes, Lipofectin, and Opti-MEMI were purchased from GIBCO-BRL (Grand Island, NY); primers for PCR from Operon (Alameda, CA); primers for DNA sequencing from Integrated DNA Technologies, Inc. (Coralville, IA); Sequenase version 2.0 DNA sequencing kit from United States Biochemistry Corp. (Cleveland, OH); [α-35S]-dATP from DuPont NEN (Boston, MA); the E. coli pET expression system, plasmid pET-16b, from Novagen (Madison, WI); the Wizard DNA Clean-up system from Promega (Madison, WI). Plasmid pBR384 was a gift from Dr. Dunne Fong (Rutgers University, Piscataway, NJ).
Protein purification and analysis reagents
The peptide substrate Z-Phe-Arg-NHMec was purchased from Enzyme Systems Products (Livermore, CA); CAPS (3-[cyclohexylamino]-1-propanesulfonic acid), CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propane-sulfonate), DTT (dithiothreitol), EDTA, E-64 (trans-epoxysuccinyl-L-leucylamido (4-guanidino) butane), benzamidine, IPTG (isopropyl-β-D-thiogalactopyranoside), papain, phosphatidylinositol phospholipase C, PMSF (phenylmethyl sulfonyl fluoride), Ponceau S, Tris base and Tween-20 from Sigma (St. Louis, MO); MicroBCA protein assay kits from Pierce Chemical Co. (Rockford, IL); SP Sepharose Fast Flow, Protein A-Superose HR 10/2, from Pharmacia LKB (Piscataway, NJ); Affigel-10, mixed bed resin AG 501-X8 (D), and nitrocellulose membranes from BioRad (Richmond, CA); and precast tricine gels, tricine running buffer, and PVDF membranes from Novex (San Diego, CA). The source of molecular mass markers depended on the technique being used. For SDS-PAGE, BRL LMW markers were purchased from GIBCO-BRL (Grand Island, NY), whereas for immunoblotting, low range biotinylated SDS-PAGE standards from BioRad (Richmond, CA) and rainbow markers from Amersham (Arlington Heights, IL). Polyclonal antibody to-rat epidermis cystatin α was a gift from Dr. Kimie Fukuyuma (University of California at San Francisco, CA). All other chemicals were of reagent grade and were obtained from commercial sources.
Cell Culture
BSC-1 monkey kidney cells were obtained from the American Type Culture Collection (Rockville, MD). Cells were grown and maintained in MEM containing 10% fetal bovine serum.
Cloning of cDNA and Construction of Vaccinia Virus Expression Vector for Recombinant Human Stefin A
The full length coding sequence of human recombinant stefin A was amplified by PCR using a plasmid, pBR384, containing an insert encoding a fusion protein of human stefin A with a 26 amino acid N-terminal extension consisting of 11 amino acids of T7Ø10 protein and 3 residues encoded from BamHI linker sequence plus 12 residues encoded from the 5′-sequence of the stefin A cDNA. A 5′ oligonucleotide [5′-A GCC ACC ATG GAC CTG GAG GC TTA containing an NcoI recognition sequence (underlined)] and a 3′ oligonucleotide [5′-C ACT TTG GAT CCA TGC TGC TA containing a BamHI recognition sequence (underlined)] were designed based upon the published human cDNA sequence (21). The upstream primer changes the isoleucine codon immediately after the ATG initiation codon to a valine codon. The amplified PCR product was subcloned into plasmid vector, pTF7-EMCV-1 (22). The resulting recombinant clones were screened using NcoI/BamHI digestion and their insert sequence verified by dideoxy DNA sequencing (see below) to ensure that the insert was in frame and without any amino acid substitutions.
DNA Sequencing
DNA sequence analysis of the insert in pSA100 was performed using the Sequenase version 2.0 kit following manufacturer’s protocol. Briefly, 5 μg of double stranded DNA template, pSA100, was denatured with alkali reagent (2 M NaOH, 2 mM EDTA), reprecipitated with ethanol, and reconstituted in deionized water. Annealing was performed at 65° C for 2 min using either primer PTF (5′-GAA GGT ACC CCA TTG TAT) or SFA (5′-G GAG ATT GTT GAT AAG). Termination of labeling was accomplished by the addition of the respective cold ddNTPs. Reactions were run on a 6% denaturing DNA electrophoresis gel.
Expression of Recombinant Human Stefin A in Mammalian Cells (vaccinia virus infection/plasmid transfection)
BSC-1 cells (5 x 106/T25 flask) were infected with vTF7-3, a recombinant vaccinia virus containing the bacteriophage T7 RNA polymerase gene (23), at a multiplicity of 30 plaque-forming units (p.f.u.) per cell. The virus was allowed to adsorb for 30 min and the inoculum replaced with 2 ml of Opti-MEM I. The infected BSC-1 cells were then transfected with 30 μg of recombinant plasmid containing stefin A cDNA using Lipofectin according to the directions of the manufacturer. Transfected cells were cultured for 48 hours and then harvested along with the conditioned media.
Cloning and Construction of E. coli Expression Vector for Recombinant Human Stefin A
The human stefin A cDNA insert was isolated and purified from plasmid described above. The insert was cloned into an NcoI/BamHI linearized pET-16b plasmid vector containing the IPTG inducible promoter T7lac. The resulting plasmid containing the 305 base pair cDNA insert encoding human stefin A was designated pETSA. For the purpose of plasmid maintenance and storage the vector was then transferred to the E. coli NovaBlue. Two clones, designated pETSA-A and pETSA-B, were used to transform the E. coli strain BL21 (DE3). Four clones, designated pETSA-A1, pETSA-A2, pETSA-B1, and pETSA-B2, were tested for stefin A production and activity. Vector alone (no insert) and vector containing insert encoding β-galactosidase were used as negative controls for the production of recombinant human stefin A. Bacteria were grown in nutrient broth containing ampicillin. Protein production was induced by the addition of IPTG (1 mM final concentration) and cells were pelleted and lysed according to manufacturer’s protocol. The cell debris (insoluble fraction) was pelleted by centrifugation and both the soluble fraction and the insoluble fraction were analyzed for enrichment of protein corresponding to a Mr of ca 11,000, equivalent to the molecular mass of human stefin A. Finally, immunoblot analyses were performed to confirm the expression of recombinant stefin A on the soluble fractions.
Purification of Recombinant Human Stefin A
Sample preparation
A liquid culture (75 ml) of E. coli strain BL21 (DE3) containing the plasmid pETSA-B1 was grown in nutrient broth containing ampicillin at 37° C. When the bacteria were in log phase growth, expression of recombinant human stefin A was induced by the addition of IPTG (1 mM final concentration) and the incubation was allowed to continue for 3 hr at 37° C. The cells were harvested by centrifugation. The cell pellet was resuspended in 5 ml of lysis buffer (50 mM Tris, 1 mM EDTA, 1 mM DTT, 0.001 mM PMSF, pH 7.5). Lysis was performed by sonication. The soluble fraction was recovered by centrifugation at 10,000 x g. for 10 min at 4ºC. The soluble fraction was placed in a boiling water bath for 10 min. The heat-treated sample was then subjected to papain-affinity chromatography for purification of recombinant stefin A (see below).
Preparation of papain for CPI activity assay and affinity chromatography
Commercially available papain was further purified by the method of Buttle et al. (24) for use as a cysteine protease against which to test inhibitory activity or as a ligand for the preparation of an affinity column.
To ensure that the CPIs are not hydrolyzed by papain during the affinity purification step, the active site of papain was inactivated by the chemical method of carboxymethylation before linkage to the chromatography support resin. A modified method described by Anastasi et al. (25) was used for carboxymethylation and production of the papain affinity column. Briefly, the papain solution was activated by the addition of cysteine (20 mM final concentration). After 10 min incubation at room temperature with constant stirring, iodoacetate was added under a stream of nitrogen gas (200 mM final concentration). The reaction was allowed to proceed at room temperature for 30 min with constant stirring. The solution was dialyzed overnight at 4° C against 100 mM NaH2PO4, 1 mM EDTA, pH 6.0. The carboxymethylated papain (CM-papain) was linked to Affigel-10 according to the manufacturer’s protocol. The resin mixture was then packed in a small column (1.0 cm×20 cm) and equilibrated with loading buffer [50 mM NaH2PO4, 1 mM EDTA, and 0.5 M NaCl, pH 6.5].
Purification of recombinant stefin A
The heat treated recombinant protein sample was applied to the CM-papain affinity column (10 mm diameter, 5 ml bed volume) equilibrated with loading buffer. The column was washed with the same loading buffer at a flow rate of 0.5 ml/min until the A280 approached zero. Nonspecifically bound proteins were eluted from the column using solution B (50 mM NaH2PO4, 1 mM EDTA, 0.1 % CHAPS, and 1 M NaCl, pH 6.5). Specifically bound stefin A was eluted with solution C (10 mM NaH2PO4, 1 mM EDTA, 0.1 % CHAPS, 0.5 M NaCl, pH 11.5). Eluent fractions were assayed for inhibitory activity. Those fractions containing inhibitory activity were combined and neutralized to pH 6.5 with 4 M HCl. Then the fractions were concentrated to a volume of 7 ml using pressure dialysis, YM3 filter.
Protein Assay
Protein concentration was determined using the bicinchoninic acid (micro BCA) kit of Pierce (Rockford, IL) according to the manufacturer’s protocol using bovine serum albumin (BSA) as standard.
Assay of CPI Activity
Inhibitory activity against papain was measured in a stopped assay according to the published protocol of Lah et al. (14). Papain activity was assessed against the substrate Z-Phe-Arg-NHMec. In summary, the appropriate dilution of inhibitor was boiled for 10 min and then pretreated with equal volumes (100 μl each) of 8 mM DTT and 2 nM papain for 10 min at 37° C in acetate buffer (340 mM sodium acetate, 60 mM acetic acid, 4 mM EDTA, pH 6.5). Enzyme reaction was initiated with the addition of 100 μl substrate (final concentration 8.25 mM). The reaction was allowed to proceed for 20 min at 37° C and then stopped by the addition of 400 μl stop solution (100 mM sodium monochloroacetate, 30 mM sodium acetate, 70 mM acetic acid, pH 4.3). The relative increase in fluorescence was measured at an excitation wavelength of 370 nm and emission wavelength of 460 nm. Inhibition constant, Ki, was determined according to the method of Nicklin and Barrett (26)
SDS-PAGE and Immunoblot Analysis
Electrophoresis was performed in either 12% polyacrylamide gels in the buffer system described by Laemmli (27) or 16% tricine polyacrylamide gels using the tricine buffer system (28). Gels were either directly stained for detecting proteins or electrophoretically transferred to nitrocellulose membranes (0.2 μ) for immunoblot analysis. Gels were stained by one of three methods: 1) Coomassie staining, 2) Silver-staining, or 3) double staining. A double staining technique was employed for its ability to detect low levels of proteins on polyacrylamide gels. First the Laemmli (25) Coomassie stain was employed with a de-staining time of 16 hr followed by a seven-step modified silver stain procedure developed by Heukeshoven and Dernick (29). Briefly, the gel was washed for 30 min (protein sensitizing) in 10% glutaraldehyde followed by three 10-minute washes in deionized water. The gel was then washed for 30 min in 0.2 mM DTT (reducing agent) followed by a 30-minute wash in 0.1% silver nitrate and two rapid rinses in deionized water. Color was developed for 5–10 minutes in 3% sodium carbonate containing 0.05% formaldehyde. Background reduction and termination of the color development was performed in 2.5% sodium thiosulfate.
Membranes containing the electrophoretically transferred proteins were developed with an enhanced chemiluminescence western blot detection system using 10% nonfat dry milk and 0.05% Tween-20 as blocking agents, rabbit IgG raised against rat cystatin α or polyclonal antibody to human cystatin C, as primary antibodies, and horseradish peroxidase-conjugated goat anti-rabbit IgG as the secondary antibody according to our published protocols (30).
Amino Acid Sequence Analysis
Purified recombinant human stefin A was run on 16% tricine-SDS-PAGE, electrophoretically transferred to polyvinylidene difluoride (PVDF) membrane (0.2 μm pore size) using 10 mM 3-(cyclohexylamino)-1-propanesulphonic acid, pH 11.0. Staining of the membrane depended on the type of analysis to be performed. For N-terminal sequence analysis, the membrane was stained with Fast Coomassie Blue followed by a methanol/acetic acid/water de-stain. The membrane containing CPI for internal sequence analysis was stained with Ponceau S followed by a methanol/water de-stain. Membranes were sent to Dr. Jan Pohl (Emory University, Atlanta, GA) for microsequence analysis by automated Edman degradation procedure.
Results
Bacterial expression of recombinant stefin A
Human stefin A was expressed in E. coli with the plasmid vector, pETSA, containing human stefin A cDNA under the control of the T7lac promoter. Four clones, pETSA-A1, pETSA-A2, pETSA-B1, and pETSA-B2 were induced for the expression of human recombinant stefin A upon the addition of IPTG. Cell lysates, +/−IPTG, were screened for inhibitory activity (Figure 1). All cell lysates from E. coli transformed with pETSA demonstrated inhibitory activity as compared to controls. The greatest inhibition was observed in the +IPTG cell lysates. Activity in the non-induced cells (−IPTG) represented the level of basal expression of human recombinant stefin A under the control of the T7lac promoter. To confirm the expression of human recombinant stefin A, immunoblot analysis was performed on the cell lysates from the clones using rabbit anti-rat cystatin α IgG. An enrichment of a band corresponding to Mr of ca 10,000 was detected in all +IPTG pETSA cell lysates (Figure 2A) and this band cross-reacted with the antibody indicating that it was indeed recombinant human stefin A (Figure 2B).
Figure 1. Recombinant human stefin A expressed in E. coli inhibited the plant cysteine protease papain.
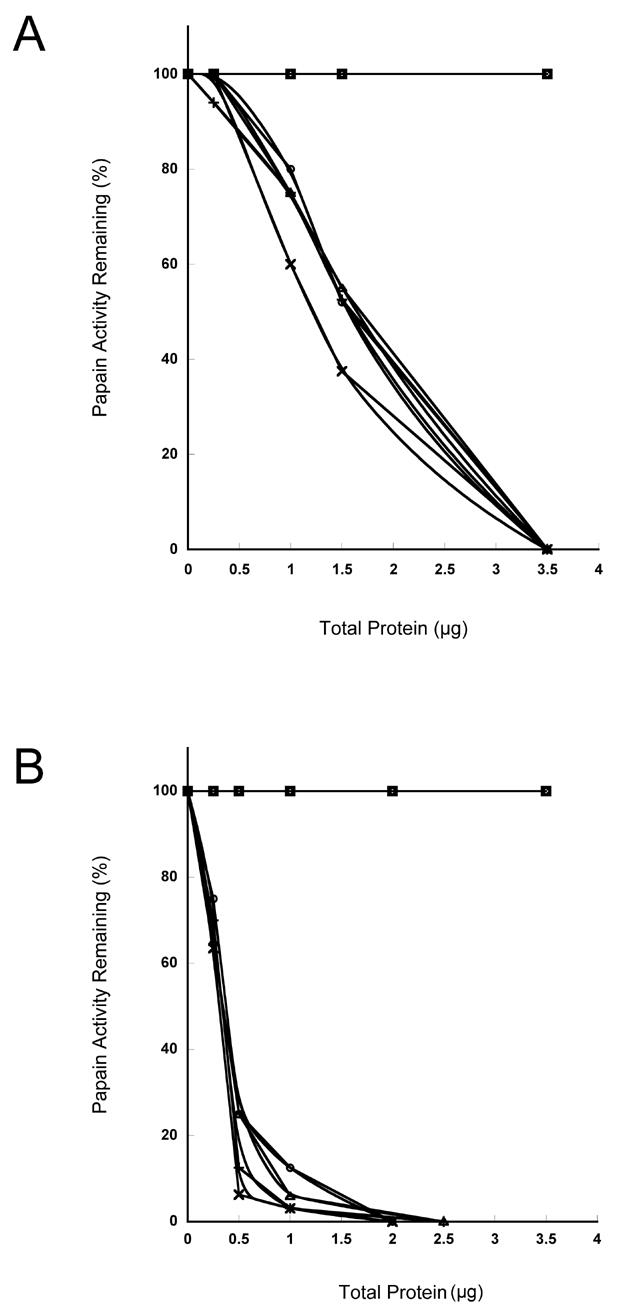
Various dilutions of cell lysates from non-induced (A) and IPTG induced (B) E. coli transformed with vectors containing human stefin cDNA (pETSA), vector alone, or vector containing nucleotide sequence encoding β-gal were assayed for inhibitory activity against papain. The papain activity was measured using the fluorogenic substrate Z-Phe-Arg-NHMec. □, vector alone; ○, PETSA-B2; ◆, vector + β-gal; ×, PETSA-A2; +, PETSA-B1; △, PETSA-A1.
Figure 2. SDS-PAGE and immunoblot analysis of four clones expressing recombinant human stefin.
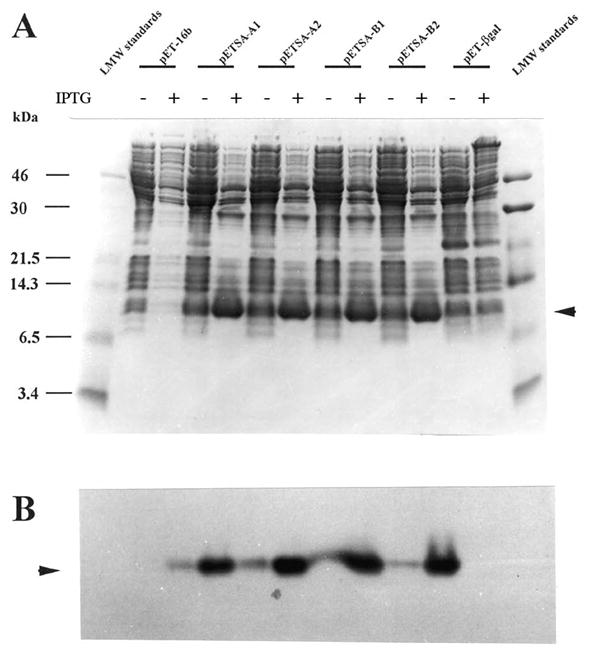
A. Four clones containing the human stefin A cDNA insert were screened for stefin A expression on SDS-PAGE, Coomassie stain (A). Expression was confirmed by immunoblot analysis (B). Arrows indicate increased level of a 10–12 kDa protein in Coomassie stained gel (A). The 10–12 kDa band cross reacted with the polyclonal antibody to rat epidermal cystatin α antibody (B). Plus and minus symbols correspond to lanes containing samples with or without IPTG induction, respectively.
Purification of recombinant human stefin A
In order to obtain milligram quantities of stefin A for further characterization and antibody production, a large culture was grown, induced, and the functional protein purified on a CM-papain affinity column. Taking advantage of the thermostability of stefin A (31), the soluble cell lysate was heat treated (see Materials and Methods) before applying to CM-papain chromatography. Fractions containing inhibitory activity were pooled and the concentration determined to be 22 μg/ml. The purity of the CM-papain pool was analyzed by SDS-PAGE and immunoblot analysis. The band corresponding to ~10,000 Da was the primary protein expressed in the IPTG induced bacterial culture (Figure 3A) and as expected cross-reacted with the polyclonal antibody to rat cystatin α (Figure 3B). We used this antibody because cystatin α is the stefin A equivalent in the rat, sharing 63% sequence identity. Furthermore, we have previously shown that this antibody recognizes human stefin A with high affinity (17). N-terminal amino acid sequence analysis confirmed that the purified protein was recombinant human stefin A (Figure 4).
Figure 3. SDS-PAGE and immunoblot analysis of recombinant human stefin A expressed in E. coli.
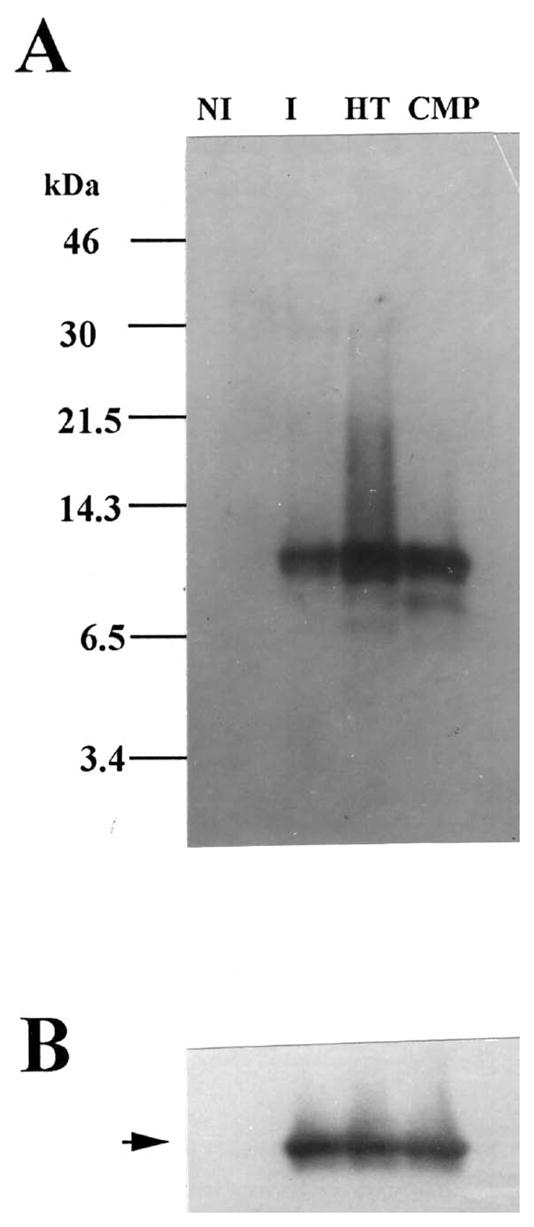
10 μg of protein from the soluble fraction of non-induced cells (NI), induced cells (I), heat treated induced (HT), and the affinity purified (CMP) recombinant stefin A were subjected to SDS-PAGE and immunoblot analysis. Panel A. Silver-stained 16% tricine SDS-PAGE. Panel B. Immunoblot probed with polyclonal antibody to rat epidermis cystatin α.
Figure 4. N-terminal amino acid sequence analysis of recombinant human stefin.

Alignment of N-terminal amino acid sequence of recombinant human stefin A purified from E. coli to those of other cystatins. All amino acid sequencing was performed by Dr. Jan Pohl of the Microchemical Facility, Emory University School of Medicine, Atlanta, GA.
Activity of purified human recombinant stefin A
In order to determine the inhibition constant (Ki), inhibitory activity of the purified recombinant human stefin A from above was measured against purified cysteine protease, papain (Worthington, Inc.), utilizing the stopped assay method (see materials and methods, above). As indicated in Figure 5, recombinant human stefin A expressed in E. coli showed strong inhibitory activity against papain with the Ki of 0.6 nM (Figure 5B).
Figure 5. Determination on the inhibitory rate constant of recombinant human stefin A expressed in E. coli against papain.

Various concentrations of the purified recombinant human stefin A were assayed against the plant cysteine protease papain. Papain activity was measured using the fluorogenic substrate Z-Phe-Arg-NHMec (A) and the inhibitory rate constant calculated (B).
Mammalian expression of recombinant stefin
In addition to expression in bacteria, human stefin A was expressed in mammalian cells. BSC-1 cells were infected with the recombinant vaccinia virus, vTF7-3, and transfected with the plasmid pTF7-EMCV-1 containing stefin A cDNA insert. The cell lysates and the conditioned media of the controls and the cells transfected with stefin A cDNA were assayed for inhibitory activity. The lysates from cells that were over-expressing stefin A showed greater inhibitory activity than the control cell lysates (Figure 6A). However, no detectable difference was seen between the conditioned media of the control cells and those over-expressing stefin A (Figure 6B). These results are consistent with a cytoplasmic localization for stefin A. Immunoblot analysis confirmed the production of recombinant human stefin A in the cell lysate (Figure 7). The recombinant human stefin A migrated with a Mr similar that of stefin A purified from human liver. Stefin A was not detected in the lysates of the control cells. The 32x-concentrated conditioned media from cells transfected with stefin A cDNA as well as the control cells were also analyzed by immunoblot (Figure 7). A band corresponding to stefin A was detected in the condition media from cells transfected with stefin A cDNA but not the condition media from the controls (Figure 7). This indicated that the over expressed stefin A was secreted into the extracellular medium. As stated above, CPI activity measurements assess all CPIs present within a given sample. Therefore, we probed the conditioned media for the presence of another CPI, cystatin C a secreted CPI having a greater inhibitory capability against papain than does stefin A (32). The conditioned media was subjected to immunoblot analysis using rabbit antiserum against human cystatin C. A band migrating with a Mr similar to that of recombinant human cystatin C, ~ 13,000 Da, was detected in all three conditioned media (Figure 8). The presence of cystatin C in the conditioned media provides a plausible explanation for the inhibitory activity results above (see Figure 6).
Figure 6. Recombinant human stefin A expressed in mammalian cells inhibited the plant cysteine protease papain.
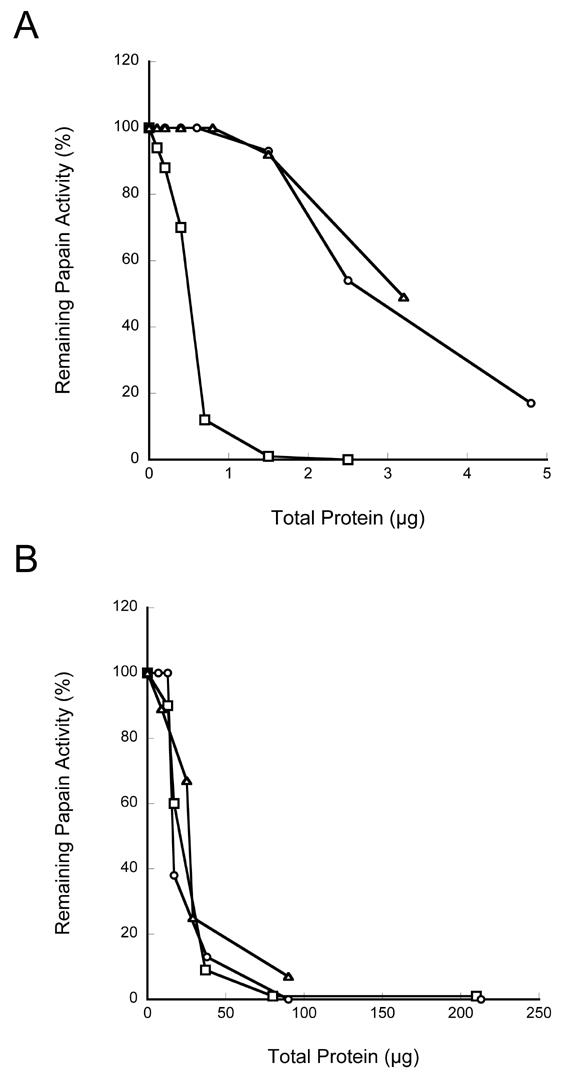
Various levels of cell lysates (A) and conditioned media (B) from BSC-1 cells either non-infected, infected, and infected and transfected with the human stefin A cDNA were assayed for inhibitory activity against papain. The papain activity was measured using the fluorogenic substrate Z-Phe-Arg-NHMec. □, infected/transfected; ○, non-infected; ▵, infected.
Figure 7. Immunoblot analysis of vaccinia virus expression of recombinant human stefin.
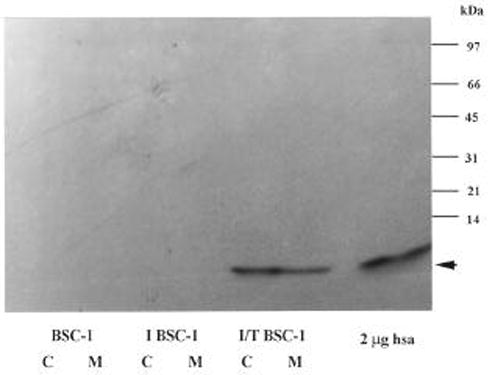
A. Cell lysates (C) and concentrated (32x) conditioned media (M) from wild type BSC-1 cells (BSC-1), infected BSC-1 cells (I BSC-1) and transfected BSC-1 cells (T BSC-1), were subjected to 15% SDS-PAGE, transferred to nitrocellulose membrane, and probed with a polyclonal antibody to rat epidermal cystatin α. hsa, purified human liver stefin A.
Figure 8. Immuno-detection of cystatin C in the conditioned media of BSC-1 cells expressing recombinant human stefin.

A. Conditioned media from wild type BSC-1 cells (C), infected BSC-1 cells (IC), and transfected BSC-1 cells (T) were subjected to 16% tricine SDS-PAGE, transferred to nitrocellulose membrane, and probed with a polyclonal antiserum to human cystatin C. hCC, 0.05 mg human cystatin C.
Discussion
The studies reported here demonstrate the functional expression of recombinant human stefin A in both bacterial and mammalian cells. This is the first known report of the functional expression of recombinant human stefin A in mammalian cells. The expression of functional recombinant human stefin A in E. coli has been previously reported (18–19). However, in most cases the proteins expressed were stefin A fusion protein containing N-terminal extensions. Given the importance of the N-terminus Gly (aa 4) in CPI inhibition, this N-terminal extension may well have affected the conformational requirements necessary for the formation of the tripartite wedge. In addition, this requires extra steps in cleaving the extension and re-purifying the resulting stefin A which could result in loss of optimal yield.
In E. coli expression of recombinant human A was demonstrated through inhibitory activity, immunoblot analysis and N-terminal amino acid analysis. Although two results were obtained by N-terminal amino acid sequence analysis, the second was identical to the first with exception that the initial methionine residue was absent. This was not surprising in that the protein was found in the soluble fraction, and thus would be a substrate for methionine amino peptidase. In addition, there was a single base change at the N-terminus resulting in a single amino acid substitution, isoleucine to valine, at position 2. This was due to the selection of the NcoI restriction site and subsequent upstream primer design that was best suited for the insertion of the stefin A coding sequence into the vectors. These minor modifications however, should not significantly affect the N-terminal structure of the protein. When the method was scaled for a larger volume, the recombinant human stefin A was the major protein present in the soluble fraction of the cell lysate. In addition, kinetic studies against papain revealed that the E. coli expressed stefin A was a highly effective inhibitor (Ki=0.6 nm). Based on the above findings, expression of stefin A in E. coli would appear ideal for the production and purification of large quantities of recombinant human stefin A for the purpose of antibody generation as well as studies on CPI function, kinetic analysis of inhibition of recently cloned cysteine proteases.
In BSC-1 cells, expression of recombinant human stefin A was accomplished through the use of the vaccinia virus expression system. The infect/transfected cell lysate showed a higher degree of inhibition against papain than the control cell lysates. This was confirmed by immunoblot analysis. However, no significant difference in inhibitory activity against papain was detected between the conditioned media from the infect/transfected BSC-1 cells and the conditioned media from control cells. This lack of difference is attributed to the presence of constitutively expressed cystatin C, a secreted cysteine protease inhibitor with lower Ki (5−12M) against papain than that of stefin A (2.7−11M). The production of recombinant human stefin A in mammalian cells provides an important tool for future studies on the roles played by stefin A in biological processes.
Acknowledgments
This study was supported in part by US Public Health Service Grants CA 36481 and CA 56586 to BFS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Catharine C. Calkins, Department of Pharmacology, Wayne State University School of Medicine; Detroit, MI 48201..
Julie Dosescu, Department of Pharmacology, Wayne State University School of Medicine; Detroit, MI 48201..
Nancy A. Day, Department of Pharmacology, Wayne State University School of Medicine; Detroit, MI 48201.
Wei-Ping Ren, Department of Pharmacology, Wayne State University School of Medicine; Detroit, MI 48201..
Rafael Fridman, Department of Pathology and Karmanos Cancer Institute, Wayne State University School of Medicine; Detroit, MI 48201..
Bonnie F. Sloane, Karmanos Cancer Institute, Wayne State University School of Medicine; Detroit, MI 48201.
Kamiar Moin, Karmanos Cancer Institute, Wayne State University School of Medicine; Detroit, MI 48201..
References
- 1.Sloane BF, Moin K, Krepela E, Rozhin J. Cathepsin B and its endogenous inhibitors: the role in tumor malignancy. Cancer Metastasis Rev. 1990;9(4):333–52. doi: 10.1007/BF00049523. [DOI] [PubMed] [Google Scholar]
- 2.Sloane BF. Cathepsin B and cystatins: evidence for a role in cancer progression. Semin Cancer Biol. 1990;1(2):137–52. [PubMed] [Google Scholar]
- 3.Kane SE, Gottesman MM. The role of cathepsin L in malignant transformation. Semin Cancer Biol. 1990;1(2):127–36. [PubMed] [Google Scholar]
- 4.Calkins CC, Sloane BF. Mammalian cysteine protease inhibitors: biochemical properties and possible roles in tumor progression. Biol Chem Hoppe Seyler. 1995;376(2):71–80. [PubMed] [Google Scholar]
- 5.Rinne A, Jarvinen M, Rasanen O, Hopsu-Havu VK. Acid and neutral cysteine proteinase inhibitor in normal uterine portio and in squamo-epithelial metaplasia, dysplasias and infiltrative carcinoma of the uterine portio. Exp Pathol. 1984;26(2):67–70. doi: 10.1016/s0232-1513(84)80071-7. [DOI] [PubMed] [Google Scholar]
- 6.Rinne A, Rasanen O, Jarvinen M, Dammert K, Kallioinen M, Hopsu-Havu VK. Occurrence of acid and neutral cysteine proteinase inhibitors in epidermal malignancies: immunohistochemical study. Acta Histochem. 1984;74(1):75–9. doi: 10.1016/S0065-1281(84)80030-6. [DOI] [PubMed] [Google Scholar]
- 7.Kyllonen AP, Jarvinen M, Hopsu-Havu VK, Dorn A, Rasanen O, Larmi T, Rinne A. Behavior of small molecular cysteine proteinase inhibitors in lung cancers and surrounding tissue. Acta Histochem. 1984;74(1):109–13. [PubMed] [Google Scholar]
- 8.Jarvinen M, Rinne A, Hopsu-Havu VK. Human cystatins in normal and diseased tissues--a review. Acta Histochem. 1987;82(1):5–18. doi: 10.1016/S0065-1281(87)80043-0. [DOI] [PubMed] [Google Scholar]
- 9.Zajc I, Sever N, Bervar A, Lah TT. Expression of cysteine peptidase cathepsin L and its inhibitors stefins A and B in relation to tumorigenicity of breast cancer cell lines. Cancer Lett. 2002;187(1–2):185–90. doi: 10.1016/s0304-3835(02)00452-4. [DOI] [PubMed] [Google Scholar]
- 10.Sinha AA, Quast BJ, Wilson MJ, Fernandes ET, Reddy PK, Ewing SL, Gleason DF. Prediction of pelvic lymph node metastasis by the ratio of cathepsin B to stefin A in patients with prostate carcinoma. Cancer J. 2002;94(12):3141–9. doi: 10.1002/cncr.10604. [DOI] [PubMed] [Google Scholar]
- 11.Eide TJ, Jarvinen M, Hopsu-Havu VK, Maltau J, Rinne A. Immunolocalization of cystatin A in neoplastic, virus and inflammatory lesions of the uterine cervix. Acta Histochem. 1992;93(1):241–8. doi: 10.1016/S0065-1281(11)80215-1. [DOI] [PubMed] [Google Scholar]
- 12.Hawley-Nelson P, Roop DR, Cheng CK, Krieg TM, Yuspa SH. Molecular cloning of mouse epidermal cystatin A and detection of regulated expression in differentiation and tumorigenesis. Mol Carcinog. 1988;1(3):202–11. doi: 10.1002/mc.2940010309. [DOI] [PubMed] [Google Scholar]
- 13.Lah TT, Kokalj-Kunovar M, Drobnic-Kosorok M, Babnik J, Golouh R, Vrhovec I, Turk V. Cystatins and cathepsins in breast carcinoma. Biol Chem Hoppe Seyler. 1992;373(7):595–604. doi: 10.1515/bchm3.1992.373.2.595. [DOI] [PubMed] [Google Scholar]
- 14.Lah TT, Clifford JL, Helmer KM, Day NA, Moin K, Honn KV, Crissman JD, Sloane BF. Inhibitory properties of low molecular mass cysteine proteinase inhibitors from human sarcoma. Biochim Biophys Acta. 1989;993(1):63–73. doi: 10.1016/0304-4165(89)90144-x. [DOI] [PubMed] [Google Scholar]
- 15.Lah TT, Kokalj-Kunovar M, Turk V. Cysteine proteinase inhibitors in human cancerous tissues and fluids. Biol Chem Hoppe Seyler. 1990;371(Suppl):199–203. [PubMed] [Google Scholar]
- 16.Rozhin J, Gomez AP, Ziegler GH, Nelson KK, Chang YS, Fong D, Onoda JM, Honn KV, Sloane BF. Cathepsin B to cysteine proteinase inhibitor balance in metastatic cell subpopulations isolated from murine tumors. Cancer Res. 1990;50(19):6278–84. [PubMed] [Google Scholar]
- 17.Moin K, Emmert LT, Sloane BF. A membrane-associated cysteine protease inhibitor from murine hepatoma. FEBS Lett. 1992;309(3):279–82. doi: 10.1016/0014-5793(92)80789-j. [DOI] [PubMed] [Google Scholar]
- 18.Fong D, Kartasova T, Sloane BF, Chan MM. Bacterial expression of human cysteine proteinase inhibitor stefin A. FEBS Lett. 1989;257(1):55–8. doi: 10.1016/0014-5793(89)81785-5. [DOI] [PubMed] [Google Scholar]
- 19.Estrada S, Nycander M, Hill NJ, Craven CJ, Waltho JP, Björn I. The role of Gly-4 of human cystatin A (stefin A) in the binding of target proteinases. Characterization by kinetic and equiblrium methods of the interactions of cystatin A Gly-4 mutants with papain, cathepsin B and cathepsin L. Biochemistry. 1998;37:7551–7560. doi: 10.1021/bi980026r. [DOI] [PubMed] [Google Scholar]
- 20.Estrada S, Pavlova A, Björk I. The contribution of N-terminal region residues of cystatin A (stefin A) to the affinity and kinetics of inhibition of papain, cathepsin B and cathepsin L. Biochemistry. 1999;38:7339–7345. doi: 10.1021/bi990003s. [DOI] [PubMed] [Google Scholar]
- 21.Kartasova T, Cornelissen BJ, Belt P, van de Putte P. Effects of UV, 4-NQO and TPA on gene expression in cultured human epidermal keratinocytes. Nucleic Acids Res. 1987;15(15):5945–62. doi: 10.1093/nar/15.15.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elroy-Stein O, Fuerst TR, Moss B. Cap-independent translation of mRNA conferred by encephalomyocarditis virus 5′ sequence improves the performance of the vaccinia virus/bacteriophage T7 hybrid expression system. Proc Natl Acad Sci U S A. 1989;86(16):6126–30. doi: 10.1073/pnas.86.16.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuerst TR, Niles EG, Studier FW, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986;83(21):8122–6. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buttle DJ, Kembhavi AA, Sharp SL, Shute RE, Rich DH, Barrett AJ. Affinity purification of the novel cysteine proteinase papaya proteinase IV, and papain from papaya latex. Biochem J. 1989;261(2):469–76. doi: 10.1042/bj2610469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicklin JH, Barrett AJ. Inhibition of cysteine proteinases and dipeptidyl peptidase I by egg-white cystatin. Biochem J. 1984;223:245–253. doi: 10.1042/bj2230245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anastasi A, Brown MA, Kembhavi AA, Nicklin MJ, Sayers CA, Sunter DC, Barrett AJ. Cystatin, a protein inhibitor of cysteine proteinases. Improved purification from egg white, characterization, and detection in chicken serum. Biochem J. 1983;211(1):129–38. doi: 10.1042/bj2110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(259):680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166(2):368–79. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 29.Heukeshoven J, Dernick R. Increased sensitivity for Coomassie staining of sodium dodecyl sulfate-polyacrylamide gels using PhastSystem Development Unit. Electrophoresis. 1988;9(1):60–1. doi: 10.1002/elps.1150090112. [DOI] [PubMed] [Google Scholar]
- 30.Moin K, Day NA, Sameni M, Hasnain S, Hirama T, Sloane BF. Human tumour cathepsin B. Comparison with normal liver cathepsin B. Biochem J. 1992;285 ( Pt 2):427–34. doi: 10.1042/bj2850427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turk V, Bode W. The cystatins: protein inhibitors of cysteine proteinases. FEBS Lett. 1991;22;285(2):213–9. doi: 10.1016/0014-5793(91)80804-c. [DOI] [PubMed] [Google Scholar]
- 32.Barrett AJ. The cystatins: a new class of peptidase inhibitors. TIBS. 1987;12:193–196. [Google Scholar]


