Abstract
The exchange factor directly activated by cAMP (Epac) is a newly discovered direct target for cAMP and a guanine-nucleotide exchange factor for the small GTPase Rap. Little is known about the neuronal functions of Epac. Here we show that activation of Epac by specific cAMP analogs or by the pituitary adenylate cyclase-activating polypeptide induces a potent activation of the Ca2+-sensitive big K+ channel, slight membrane hyperpolarization, and increased after-hyperpolarization in cultured cerebellar granule cells. These effects involve activation of Rap and p38 MAPK, which mobilizes intracellular Ca2+ stores. These findings reveal a cAMP Epac-dependent and protein kinase A-independent signaling cascade that controls neuronal excitability.
Keywords: excitability, granule cells, mouse, calcium, PACAP
Cyclic AMP is a second messenger that induces physiological responses ranging from growth and differentiation to hormonal, neuronal, and immunological regulation (1, 2). In the brain, it is involved in memory (3) and cognitive functions (4). The classical and direct targets of cAMP are the protein kinase A (PKA) and nucleotide-gated channels. Another direct target of cAMP has been identified recently and is called exchange factor directly activated by cAMP (Epac) (5, 6). This factor is the product of two genes, Epac1 and Epac2. Epac1 is ubiquitously distributed with predominant expression in the thyroid, kidney, ovary, skeletal muscle, and specific brain regions. Epac2 is predominantly expressed in the brain and adrenal gland (7, 8).
Both Epac1 and Epac2 mediate PKA-independent signal transduction (7, 8). They act as guanine-nucleotide exchange factors (GEFs) for two low molecular weight G proteins, Rap1 and Rap2. The specific Epac activator cAMP analog 8- (4-chlorophenylthio)-2′-O-methyl-cAMP (8-pCPT) distinguishes Epac-mediated from PKA-mediated effects (9). This compound induces secretion of the amyloid peptide precursor protein in cortical primary cultures (10) and insulin in the INS-1 cell line and human pancreatic β-cells (11). Epac-mediated insulin secretion involves ryanodine- and, to a lesser extent, inositol 1,4,5-trisphosphate (IP3)-sensitive Ca2+ channels in a coincident manner with Ca2+ (Ca2+-induced Ca2+ release) (11, 12). Whether the Epac pathway also involves MAPK activation (JNK or ERK) is a matter of intense investigations (5, 6).
It is surprising that in view of the role of cAMP in brain functions (axon growth and regeneration, addiction, synaptic plasticity, and memory), few data are available on the role of Epac in neurons. Epac enhances neurotransmitter release at the glutamatergic synapses of the rat brain calyx of Held (13) and the crayfish neuromuscular junction (14). In dorsal root ganglion neurons, Epac mediates β2-adrenergic receptor/cAMP stimulation of protein kinase Cε (PKCε) and mechanical hyperalgesia (15). Although important, these effects do not shed light on whether and how Epac could affect neuronal ion channels and excitability.
We show here in mouse cultured cerebellar granule cells (CGCs) that Epac can modulate resting membrane potential and after-hyperpolarization (AHP). It also mobilizes Ca2+ from intracellular stores and facilitates big K+ (BK) channel activation. These effects involve p38 MAPK in addition to Rap. These results provide evidence for the existence of cAMP-dependent but PKA-independent regulation of neuronal excitability.
Results
Epac Modulates Membrane Potential.
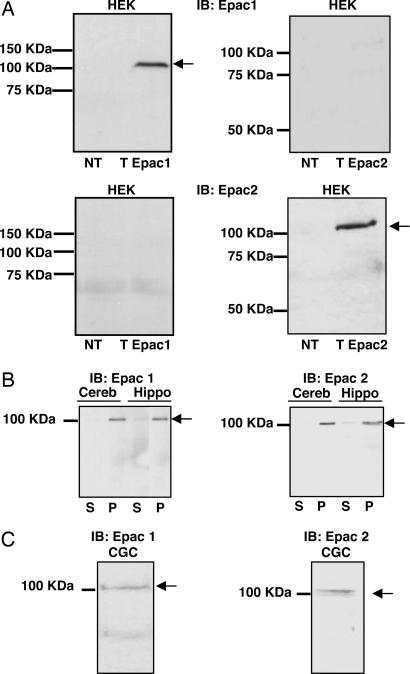
Anti-Epac1 and anti-Epac2 antibodies were characterized in HEK-293 transfected with expression plasmids containing either Epac1 or Epac2 cDNA. Western blot analyses clearly revealed a single immunopositive band corresponding to the apparent molecular weight of either Epac1 or Epac2 protein (Fig. 1A). We used these antibodies to detect Epac1 and Epac2 proteins from adult cerebellar or hippocampal brain extracts (Fig. 1B), as well as CGCs (Fig. 1C). Both Epac1 and Epac2 were expressed in these preparations and associated with particulate fraction.
Fig. 1.
Anti-Epac antibody characterization and endogenous Epac expression in neurons. (A) Western blots were obtained from HEK-293 cells nontransfected (NT) or transfected with Epac1 (T Epac1) or Epac2 (T Epac2) expression plasmids and revealed by using the anti-Epac1 [immunoblot (IB): Epac1] or anti-Epac2 (IB: Epac2) antibodies. (B and C) Western blots obtained from adult mouse cerebellar (B, Cereb), hippocampal (B, Hippo), or CGC extracts (C) by using the anti-Epac1 (Left) or anti-Epac2 (Right) antibodies. For each blot, S and P are soluble and particulate fractions, respectively. A–C are representative of independent experiments.
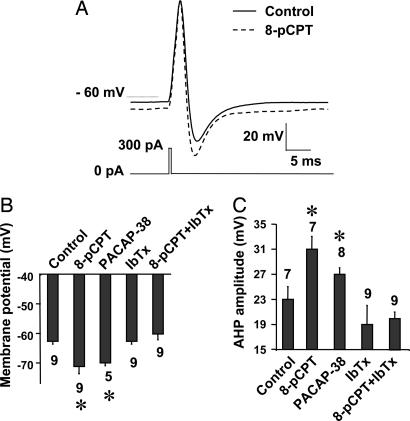
The effect of Epac on resting membrane potential and action potential was then studied in CGCs recorded in whole-cell configuration. The average resting potential was of −63 ± 1 mV (n = 9) and was maintained at this value with current injection. Under these conditions, a brief current pulse evoked an action potential followed by an AHP. One-minute application of the selective membrane-permeant Epac agonist 8-pCPT induced a slight (8-mV) but significant hyperpolarization (Fig. 2 A and B). Additional current was then injected into the neuron to bring the resting membrane potential back to the control value, and the peak amplitudes of the AHP and action potential were measured. 8-pCPT increased amplitude of the AHP (Fig. 2C) without significantly affecting the action potential amplitude, suggesting that Epac could modulate neuronal excitability.
Fig. 2.
Epac and PACAP-38 affect resting membrane potential and AHP by activation of BK channels. (A) Action potential (upper traces) evoked by a brief current pulse (lower trace) in the absence (control) and presence of 8-pCPT (100 μM). These traces were obtained from the same neuron and were representative of 10 different neurons. (B) Resting membrane potential measured in the absence (control) and presence of the indicated drugs [iberiotoxin (IbTx), 0.5 μM; PACAP-38, 10 nM, + U73122, 1 μM]. In this and the following figures, each bar of a histogram represents the mean ± SEM, the number above or below each bar is the number of cells, and the asterisks indicate values significantly different from control. (C) Same as B, but for AHP.
Epac Stimulates BK Channel Opening.
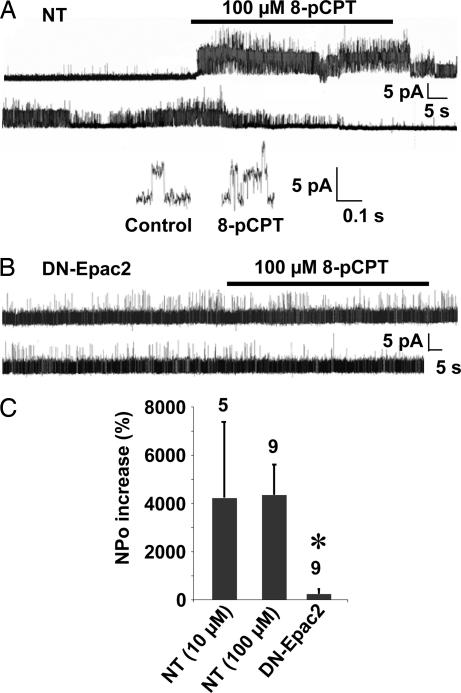
The early phase of AHP depends on BK channel activation (16). Indeed, we found that the BK channel blocker, iberiotoxin (IbTx) (≥20 min), significantly decreased the amplitude of AHP in CGCs (Fig. 2C). More importantly, the toxin abolished the effect of 8-pCPT on AHP (Fig. 2C) and resting potential (Fig. 2B). These results suggest that 8-pCPT activated BK channels in CGCs. Further, BK channels were recorded in cell-attached configuration. The cumulated open probability (NPo) of BK channels increased on bath-applied 8-pCPT (10 and 100 μM; Fig. 3 A and C) without significantly affecting the channel unitary current amplitude (4 ± 0.4 pA in control condition and 4.2 ± 0.6 pA in the presence of 100 μM 8-pCPT, n = 10; Fig. 3A Inset). The onset of the response varied from 2 to 10 sec and lasted for up to several minutes after agonist removal. We verified that 10 and 100 μM 8-pCPT did not activate PKA in CGCs [see Materials and Methods for activity measurements; not shown (9)]. 8-pCPT was significantly less effective in neurons transfected with a dominant-negative form of Epac, DN-Epac2 (Fig. 3 B and C). This finding indicates that Epac, rather than PKA, mediated the effect of 8-pCPT and excludes the possibility of a direct action of the agonist on BK channels.
Fig. 3.
Epac-induced activation of BK channels. (A and B) Cell-attached recordings of BK channels obtained from NT CGCs (A) or CGCs transfected with DN-Epac2 (B). (Inset) Expended trace of the BK channel activity recorded in the absence (control) and presence of 8-pCPT. (C) From left to right, effect on BK channels NPo of 10 μM and 100 μM 8-pCPT in NT and 100 μM 8-pCPT in DN-Epac2-transfected CGCs.
Epac Mobilizes Intracellular Ca2+ Stores.
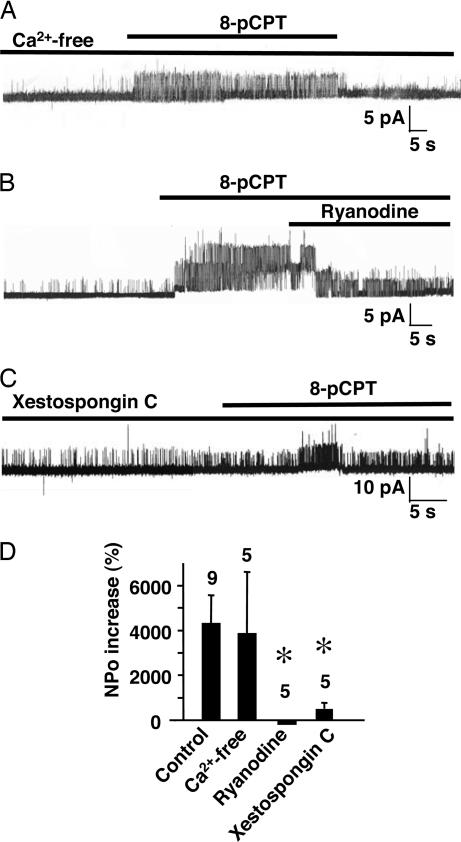
8-pCPT applied in a nominally Ca2+-free extracellular medium significantly increased BK channel NPo, but to a similar extent as in the presence of external Ca2+ (Fig. 4 A and D). However, ryanodine (Fig. 4B) or the IP3 receptor inhibitor xestospongin-C (Fig. 4C) strongly inhibited the 8-pCPT effect (Fig. 4D). These results show that Epac response likely involves mobilization of intracellular Ca2+ stores.
Fig. 4.
Epac mobilization of intracellular Ca2+ activates BK channels. (A) Representative trace of 8-pCPT-induced activation of a BK channel recorded in Ca2+-free external medium. (B) Inhibition of 8-pCPT-induced activation of BK channels by ryanodine (10 μM) in normal external medium. (C) BK channels activated by 8-pCPT after 30-min treatment with xestospongin-C (7.5 μM) in normal external medium. (D) Quantification of the 8-pCPT-induced activation of BK channels in normal, Ca2+-free, and ryanodine- and xestospongin-C-containing external medium.
Rap and p38 MAPK, but Not ERK or JNK, Participate in Epac Activation of BK Channels.
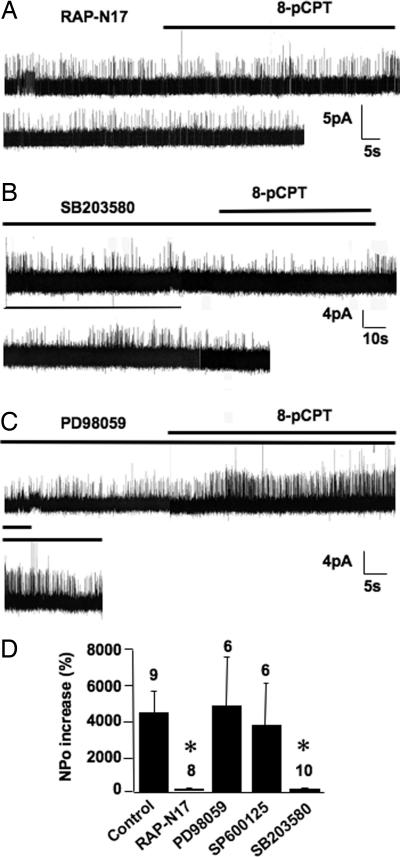
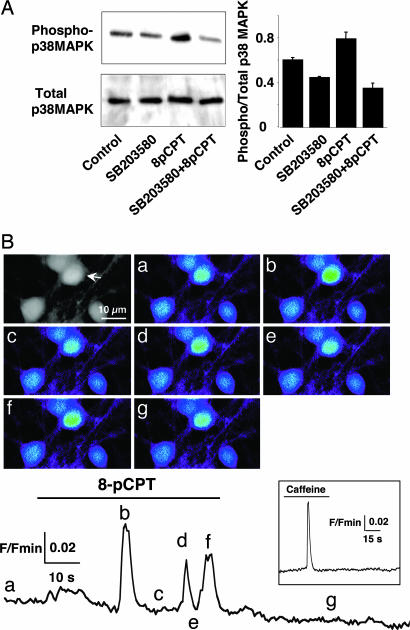
8-pCPT had no significant effect on BK channels recorded in CGCs transfected with the dominant-negative form of Rap, Rap-N17 (Fig. 5 A and D). A pretreatment with the p38 MAPK inhibitor SB203580 (5 min) significantly inhibited the 8-pCPT effect on BK channel NPo (Fig. 5 B and D). Moreover, 10-min stimulation of CGCs with 8-pCPT increased phosphorylation of p38 MAPK (Fig. 6A). These results suggest that Epac mediated opening of BK channels through activation of Rap and p38 MAPK.
Fig. 5.
Rap and p38 MAPK, but not p42/p44 MAPK or c-JunK, are involved in Epac-induced activation of BK channels. (A) BK channel activity recorded in a CGC transfected with Rap-N17. (B and C) BK channel activity recorded in CGCs treated with p38 MAPK (SB203580, 10 μM; B) or p42/p44 MAPK (PD98059, 25 μM; C) inhibitors. (D) Summary of the 8-pCPT effect on BK channel obtained in control neurons, in neurons transfected with Rap-N17, or in neurons treated with PD98059, SP600125, or SB203580 (10 μM).
Fig. 6.
Epac activates p38 MAPK and mobilizes intracellular Ca2+. (A) Western blots (Left) and quantitative histogram (n = 3) (Right) of p38 MAPK phosphorylation obtained from CGCs by using anti-phospho-p38 MAPK (Upper Left) or anti-p38 MAPK antibodies (Lower Left) in the absence of drug (control) or presence of SB203580, 8-pCPT (100 μM; 10 min), or SB203580 + 8-pCPT. (B) (Upper Left) Gray-level image illustrates a field of CGCs loaded with Ca2+-sensitive dye as described in Materials and Methods. 8-pCPT-induced Ca2+ increases in the cell marked with an arrowhead are shown in fluorescence micrographs a–g and plotted as a function of time in Lower. F/Fmin, fluorescence/minimum fluorescence. Color images correspond to an average of three successive confocal images taken at various times indicated by the letters in Lower. (Inset) The Ca2+ variation of this neuron in response to 10 mM caffeine.
The specific antagonists of p42/p44 MAPK and JNK, PD98059 and SP600125, respectively, did not significantly alter activation of BK channels by 8-pCPT (Fig. 5 C and D). This result differs from activation of these kinases by Epac in cell lines (17, 18).
To place the p38 MAPK activation step in the Epac pathway, we directly imaged the 8-pCPT-mediated Ca2+ responses in CGCs (Fig. 6B); 42.8% of the neurons (n = 173) responded to the Epac agonist. This effect was significantly antagonized by SB203580 (25.3% responding neurons, n = 245). The SB203580 treatment did not alter the sensitivity of the neurons to caffeine (96% and 92% neurons responded to caffeine in the absence and presence of SB203580). These results indicate that activation of p38 MAPK occurred upstream to mobilization of intracellular Ca2+ stores.
8-Br-cAMP and Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Mimic the Effect of Epac Agonist.
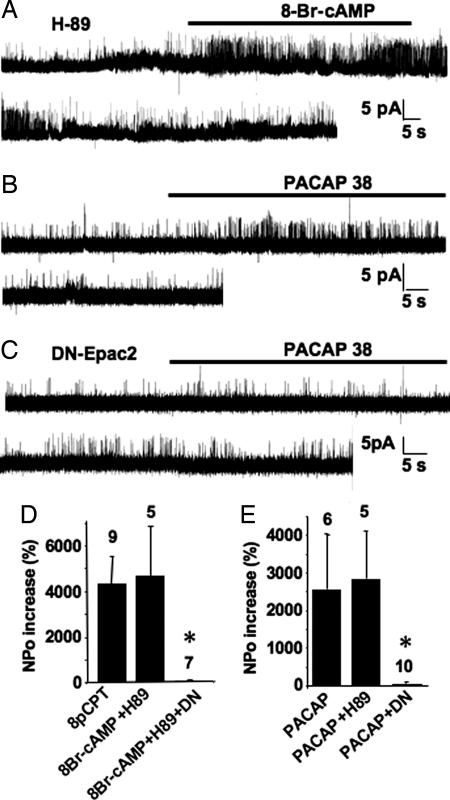
After treatment with the PKA inhibitor H-89, the nonselective cAMP analog 8-Br-cAMP mimicked the effect of 8-pCPT on BK channels in NT (Fig. 7 A and D), but not in DN-Epac2-transfected, CGCs (Fig. 7D). The PACAP receptor activates both adenylyl cyclase and phospholipase C (PLC) (19). We isolated the activation of adenylyl cyclase by using a 30-min pretreatment of CGCs with the PLC inhibitor U73122. Under these conditions, the PACAP receptor agonist PACAP-38 peptide activated BK channels in nontreated (Fig. 7 B and E) and H89-treated (Fig. 7E) neurons. This effect was abolished by transfection of DN-Epac2 (Fig. 7 C and E). These experiments suggest that the PACAP receptor stimulation leads to Epac activation.
Fig. 7.
PKA-independent activation of BK channels induced by cAMP and PACAP-38. (A) Activation of a BK channel by 8-Br-cAMP (100 μM), in the presence of the PKA inhibitor H-89 (10 μM; 5-min pretreatment). (B and C) The PACAP receptor agonist PACAP-38 (10 nM + 1 μM U73122) activated BK channel in nontransfected (B), but not DN-Epac2-transfected CGCs (C). (D) Quantification of BK channel activation induced by 8-pCPT or 8-Br-cAMP in NT and DN-Epac2-transfected CGCs. (E) Quantification of BK channel activation induced by PACAP-38, in control, H89-treated, and DN-Epac2-transfected neurons.
Because PACAP receptor stimulation reproduces Epac activation on BK channels, we examined whether PACAP-38 mimics the effect of 8-pCPT on membrane potential. As expected, PACAP-38 significantly hyperpolarized CGCs (Fig. 2B) and increased amplitude of the AHP (Fig. 2C).
Discussion
We found that both Epac1 and Epac2 are expressed in adult cerebellum and hippocampus, as well as in CGCs. These results are in agreement with previous characterization of tissue localization of Epac mRNA in adult rat brain (8). We show that in CGCs, cAMP can activate Epac1 and Epac2, which in turn stimulates Rap and p38 MAPK, but not p42/p44 MAPK or JNK. This effect results in mobilization of ryanodine- and IP3-sensitive intracellular Ca2+ stores and BK channel opening. It is accompanied by a slight but significant hyperpolarization of the membrane and AHP amplitude increase, which results from activation of BK channels because these effects were blocked by IbTx.
BK channels are opened by both elevated intracellular Ca2+ concentrations and membrane depolarization. In CGCs, the activity of this channel is closely correlated to changes in submembrane intracellular Ca2+ concentration (20). Because Epac can slightly hyperpolarize the membrane, the effect of Epac on BK channel opening may have been underestimated. Nevertheless this effect was probably negligible because no significant change in the amplitude of the cell-attached recorded single-channel current was detected in the presence of the agonist.
Specificity of Epac Signaling in Neurons.
Regulation of BK channels by cAMP is well established. It involves cAMP-dependent activation of PKA and phosphorylation of Ser-869 of the channel α subunit (21, 22). Here we show that cAMP can also regulate BK channel in a PKA-independent manner through activation of Epac by 8-Br-cAMP or PACAP-38 in the presence of the PKA inhibitor, H89. This finding suggests the existence of compartmentalized Epac (PKA excluded) signaling, resulting in elevated local accumulation of cAMP. This result is consistent with low affinity of Epac for cAMP, as compared with PKA (23). It is also in agreement with focalized membrane cAMP signals that have been described in HEK-293 cells expressing cyclic nucleotide-gated channels (24).
The recent availability of the Epac-specific agonist 8-pCPT has been crucial. The specificity of this agonist was further proved here by the complete inhibition of its effect on BK channels following expression of the dominant-negative Epac2. This effect of 8-pCPT was mimicked by 8-Br-cAMP and PACAP receptor stimulation, further supporting a physiological role of Epac in neuronal postsynaptic excitability via BK channel activation, hyperpolarization, and increased AHP. During revision of the present study, a publication appeared showing that PACAP receptors also control the Rit small GTPase signaling via Epac activation in a PKA-independent manner (25).
Complexity of the Epac-Mediated p38 MAPK and Ca2+ Signaling Pathway in Neurons.
Several studies have shown that Epac can mobilize intracellular Ca2+ stores in nonneuronal cells (11, 26, 27). Our results extend this observation to mammalian central neurons. They also suggest that Ca2+ mobilization occurs downstream of p38 MAPK activation. The dual effect of this pathway on ryanodine- and IP3-sensitive Ca2+ stores in CGCs could result from a Ca2+-induced Ca2+ release, probably initiated by activation of ryanodine, rather than IP3 receptors, because ryanodine, but not xestospongin-C, abolished this effect. Alternatively, the 8-pCPT-induced Ca2+ release from IP3-sensitive stores could result from activation of Rap2B and PLCε by Epac as previously described (6, 11, 26). However, we observed that after blockade of PLC with a nonselective inhibitor, the PACAP-38 peptide activates BK channels via Epac in CGCs.
Inhibition of p38 MAPK by SB203580 abolished the 8-pCPT-induced BK channels activation and reduced by ≈50% the number of neurons in which a Ca2+ response to the Epac agonist could be visualized. The most likely explanation for this difference is that in those neurons the Ca2+ rise was not sufficient to activate BK channels, which are known to be sensitive only to high concentrations of intracellular Ca2+. At the present time, the most likely hypothesis is that in CGCs, Gs-coupled receptor stimulation induces cAMP synthesis, which in turn activates Epac. The GEF activity of Epac triggers the Rap-p38 MAPK cascade, which would result in intracellular Ca2+ release from ryanodine- and IP3-sensitive stores. Released Ca2+ then activates BK channels.
Physiological Function of Epac in Neurons.
In neurons, BK channels are localized in the cell body and nerve terminal (28). In the cell body, BK channels have been considered an “emergency brake” contributing to membrane repolarization following action potential, thus decreasing discharge frequency. We found that Epac induced somatic membrane hyperpolarization and increased AHP amplitude, which should limit action potential firing. In calyx of Held presynaptic terminals loaded with 8-pCPT, Epac increased neurotransmitter release, but in a Ca2+-independent manner (29). An Epac-mediated intracellular Ca2+ release and BK channel opening is therefore unlikely at presynaptic sites. Altogether these observations suggest that Epac may have opposite effects on pre- and postsynaptic excitability.
Materials and Methods
Neuronal Preparations.
Experiments were approved by the Ministère de l'Agriculture et de la Forêt (authorization no. 34-103). Primary cultures of CGCs were prepared from 7-day-old Swiss mice (Janvier, Le Genest-St-Isle, France) as described in ref. 30, and experiments were performed after 9–10 days in vitro.
Plasmids and Transfection.
HEK-293 cells were transfected with PMT2 expression plasmids containing Epac1 or Epac2 sequence (31). Cerebellar cultures were transfected with a PRK5 expression plasmid containing DN-Epac2 (32) or a dominant-negative form of Rap (Rap-N17). In neurons, these plasmids were cotransfected with the pEGFP-N1 (Clontech, St-Germain-en-Laye, France) plasmid by using the cationic lipid Transfast (Promega, Charbonnières, France) as described in ref. 33. The GFP fluorescence was reliably used to monitor transfected neurons. We confirmed expression of these plasmids by immunocytochemical analysis and verified that EGFP expression did not modify the Epac response on BK channel. Neurons transfected with EGFP alone were referred to as control neurons.
Immunological and Biochemical Analyses.
Western blotting was performed as previously described (34) with the anti-Epac1 (1:10,000) and anti-Epac2 mouse monoclonal antibodies (1:1,000). An immunopositive band obtained from Epac1- but not Epac2-transfected HEK-293 cells attested to the specificity of the anti-Epac1 antibody (Fig. 1A). Conversely, an immunopositive band was obtained with the anti-Epac2 antibody from Epac2- but not Epac1-transfected HEK-293 cells (Fig. 1A), thus corroborating previous characterization of this antibody (31).
For p38 MAPK activation measurement, CGCs were stimulated with 8-pCPT for 10 min. The CGCs were then incubated in 100 μl of lysis buffer for 20 min at 4°C, and samples were sonicated before centrifugation. Proteins were separated on SDS/10% PAGE gels and transferred to nitrocellulose membrane. The p38 MAPK species were detected by using specific antibodies (1:1,000; Cell Signaling Technology, Beverly, MA) and a horseradish peroxidase-coupled anti-rabbit antibody (1:4,000; New England Biolabs, Ozyme, St-Quentin-Yvelines, France). The PKA activity was measured in CGCs with similar stimulation (EKS-390A kit; Stressgen, Ann Arbor, MI).
Electrophysiology.
Patch-clamp recordings were performed at room temperature. For whole-cell recording, external medium contained 130 mM NaCl, 2 mM CaCl2, 3 mM KCl, 2 mM MgCl2, 10 mM Hepes, 10 mM d-glucose, 0.3 mM tetrodotoxin, pH 7.4 (adjusted with NaOH) and 330 milliosmolal. The pipette medium contained 8.5 mM NaCl, 130 mM KCl, 0.5 mM CaCl2, 2 mM MgCl2, 10 mM Hepes, 10 mM d-glucose, 5 mM EGTA, 4 mM ATP, 0.5 mM GTP, pH 7.2 (adjusted with KOH) and 300 milliosmolal. Cell-attached pipettes were filled with the external medium. Drug solutions were applied with a fast gravity perfusion system. Action potentials were evoked by current pulses (1 msec, 300 pA) at 5-sec intervals, after correction for junctional potential, by using an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Signals were filtered at 1 kHz and acquired at 3 kHz. Data were collected from at least three different dishes prepared from three different cultures.
Analyses were performed with the pClamp 8.0 program (Axon Instruments). Amplitude of action potential and AHP was measured on five successive trials. The threshold for detecting BK channel currents was set at 50% of the open level of the event. Data were used to measure the conductance (200 pS) and NPo of the channels. Drug effects were estimated by calculating the ratio between the maximal change in NPo obtained during the drug application and the average NPo obtained before the drug application. Data were then expressed as mean ± SEM of the indicated number (n) of cells. The mean values were compared by using Wilcoxon's statistical test and considered as significantly different when P ≤ 0.02.
Intracellular Ca2+ Measurements.
Variations in cytosolic Ca2+ concentration was monitored in CGCs loaded with 0.5 μM Oregon Green 488 BAPTA-1/AM and Pluronic acid F127 (Molecular Probes, Eugene, OR; 30 min, 37°C). Real-time Ca2+ signals were imaged (two frames per sec) with a confocal microscope (Odyssey XL, InterVision 1.5.1 software, Noran Instruments, Middleton, WI). Calcium changes were plotted as the F/Fmin ratio. Drugs were pressure-ejected from a micropipette positioned in the vicinity of the cells.
Drugs were from the following sources: Ryanodine, 8-Br-cAMP, SB203580, SP600125, PD98059, Xestospongin-C, and H-89, Sigma (L'Isle D'Abeau, France); 8-pCPT, Biolog (Bremen, Germany); U73122, Calbiochem (Fontenay-Sous-Bois, France); and PACAP-38, Neosystem (Strasbourg, France).
Acknowledgments
We thank Dr. S. Seino (Chiba University, Chiba, Japan) for providing DN-Epac2 plasmid, J. de Gunzburg (Institut Curie, Paris, France) for RAP-N17, and Dr. J. Zhao (Utrecht University, Utrecht, The Netherlands) for anti-Epac1 and anti-Epac2 antibodies. We thank Dr. F. Bertaso, Dr. H. Daniel, and S. Costes for helpful discussions and M.-C. Rousset for technical assistance. This work was supported by Foundation Recherche Médicale, Centre National de la Recherche Scientifique, and Institut National de la Santé et de la Recherche Médical.
Abbreviations
- 8-pCPT
8-(4-chlorophenylthio)-2′-O-methyl-cAMP
- BK
big K+
- CGCs
cultured cerebellar granule cells
- Epac
exchange factor directly activated by cAMP
- IP3
inositol 1,4,5-trisphosphate
- AHP
after-hyperpolarization
- NPo
cumulated open probability
- NT
nontransfected
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PLC
phospholipase C
- PKA
protein kinase A
- GEF
guanine-nucleotide exchange factor.
Footnotes
The authors declare no conflict of interest.
References
- 1.Tasken K, Aandahl M. Physiol Rev. 2004;84:137–167. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- 2.Holz GG. Diabetes. 2004;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang YY, Kandel ER, Varshavsky L, Brandon EP, Qi M, Idzerda RL, McKnight GS, Bourtchouladze R. Cell. 1995;83:1211–1222. doi: 10.1016/0092-8674(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 4.Sur M, Rubenstein JL. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- 5.Seino S, Shibasaki T. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 6.Bos JL. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- 7.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 9.Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- 10.Maillet M, Robert SJ, Cacquevel M, Gastineau M, Vivien D, Bertoglio J, Zugaza JL, Fischmeister R, Lezoualc'h F. Nat Cell Biol. 2003;5:633–639. doi: 10.1038/ncb1007. [DOI] [PubMed] [Google Scholar]
- 11.Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. J Biol Chem. 2003;278:8279–8285. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li WH, Harbeck M, Roe MW, Holz GG. J Physiol. 2005;566:173–188. doi: 10.1113/jphysiol.2005.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaba T, Neher E. Nature. 2003;424:775–778. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- 14.Zhong N, Zucker RS. J Neurosci. 2005;25:208–214. doi: 10.1523/JNEUROSCI.3703-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hucho TB, Dina OA, Levine JD. J Neurosci. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storm JF. J Physiol. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laroche-Joubert N, Marsy S, Michelet S, Imbert-Teboul M, Doucet A. J Biol Chem. 2002;277:18598–18604. doi: 10.1074/jbc.M201868200. [DOI] [PubMed] [Google Scholar]
- 18.Hochbaum D, Tanos T, Ribeiro-Neto F, Altschuler D, Coso OA. J Biol Chem. 2003;278:33738–33746. doi: 10.1074/jbc.M305208200. [DOI] [PubMed] [Google Scholar]
- 19.Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 20.Fagni L, Bossu JL, Bockaert J. Eur J Neurosci. 1991;3:778–789. doi: 10.1111/j.1460-9568.1991.tb01674.x. [DOI] [PubMed] [Google Scholar]
- 21.Nara M, Dhulipala PD, Wang YX, Kotlikoff MI. J Biol Chem. 1998;273:14920–14924. doi: 10.1074/jbc.273.24.14920. [DOI] [PubMed] [Google Scholar]
- 22.Schubert R, Nelson MT. Trends Pharmacol Sci. 2001;22:505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 23.de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. J Biol Chem. 2000;275:20829–20836. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- 24.Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. Proc Natl Acad Sci USA. 2001;98:13049–13054. doi: 10.1073/pnas.221381398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi GX, Rehmann H, Andres DA. Mol Cell Biol. 2006;26:9136–9147. doi: 10.1128/MCB.00332-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, Jakobs KH. Nat Cell Biol. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- 27.Morel E, Marcantoni A, Gastineau M, Birkedal R, Rochais F, Garnier A, Lompre AM, Vandecasteele G, Lezoualc'h F. Circ Res. 2005;97:1296–1304. doi: 10.1161/01.RES.0000194325.31359.86. [DOI] [PubMed] [Google Scholar]
- 28.Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, Storm JF. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaneko M, Takahashi T. J Neurosci. 2004;24:5202–5208. doi: 10.1523/JNEUROSCI.0999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van-Vliet BJ, Sebben M, Dumuis A, Gabrion J, Bockaert J, Pin JP. J Neurochem. 1989;52:1229–1239. doi: 10.1111/j.1471-4159.1989.tb01870.x. [DOI] [PubMed] [Google Scholar]
- 31.Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJT, Collard J, Bos JL. J Biol Chem. 2004;279:35127–35132. doi: 10.1074/jbc.M404917200. [DOI] [PubMed] [Google Scholar]
- 32.Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, et al. Nat Cell Biol. 2000;2:805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- 33.Ango F, Albani-Torregrossa S, Joly C, Robbe D, Michel JM, Pin JP, Bockaert J, Fagni L. Neuropharmacology. 1999;38:793–803. doi: 10.1016/s0028-3908(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 34.Blahos J, Mary S, Perroy J, de Colle C, Brabet I, Bockaert J, Pin JP. J Biol Chem. 1998;273:25765–25769. doi: 10.1074/jbc.273.40.25765. [DOI] [PubMed] [Google Scholar]