Abstract
Vertebrates display diverse patterns of neuromuscular innervation, but little is known about how such diversity is generated. In mammals, neuromuscular junctions form predominantly at equatorial locations, giving rise to a focal innervation pattern along a central endplate band. In addition, vertebrate striated muscles exhibit two nonfocal neuromuscular patterns, myoseptal and distributed innervation. Although agrin-MuSK-rapsyn signaling is essential for the focal innervation pattern, it is unknown whether the same genetic program also controls synaptogenesis at nonfocal innervation sites. Here we show that one of three transcripts generated by the zebrafish unplugged locus, unplugged FL, encodes the zebrafish MuSK ortholog. We demonstrate that UnpFL/MuSK is critical for the assembly of focal synapses in zebrafish and that it cooperates with dystroglycan in the formation of nonfocal myoseptal and distributed synapses. Our results provide the first genetic evidence that neuromuscular synapse formation can occur in the absence of MuSK and that the combinatorial function of UnpFL/MuSK and dystroglycan generates diverse patterns of vertebrate neuromuscular innervation.
Keywords: muscle-specific kinase, neuromuscular junction, synaptogenesis
The formation of synapses requires coordinated localization of pre- and postsynaptic elements at prospective sites of innervation. In the developing mammalian muscle, motor axons grow together through the central region of the muscle and then extend individual branches that terminate onto myofibers at an equatorial position (1). Acetylcholine receptors (AChRs) aggregate in apposition to the presynaptic nerve terminals, and the result is a focal band of neuromuscular junctions (NMJs). Focal innervation patterns are most prevalent and best studied in mammals. However, patterns of nonfocal innervation, in which synapses form at multiple sites distributed along the length or at the myoseptal ends of muscle fibers, are widespread in vertebrates such as avians, amphibians, and teleost fish and are also present in mammals, e.g., in extraocular and laryngeal muscle (2–6). The diversity of innervation patterns present in vertebrates raises the question of whether these different types of NMJs develop in similar ways and under the same molecular controls.
Genetic and molecular studies in mice have established that the agrin-MuSK-rapsyn pathway controls neuromuscular synapse formation at focal sites of innervation, although recent studies suggest a more complex view in which the assigned roles of some of these core molecular players have been revised (reviewed in refs. 7 and 8). It remains unchallenged that postsynaptic differentiation is directed by the muscle-specific receptor tyrosine kinase, MuSK (9). Before receiving signals from the nerve, MuSK patterns the presumptive postsynaptic region by localizing AChRs and other postsynaptic proteins to the central endplate zone (10, 11). Upon release of nerve-derived agrin, MuSK recruits rapsyn, an obligate intracellular effector, which clusters AChRs beneath the nerve (12–16). Interestingly, agrin also binds one component of the DGC complex, α-dystroglycan (α-DG), with high affinity, and this interaction has been implicated in agrin-mediated AChR cluster maturation (17–19).
Most genetic studies on the role of MuSK have focused on selected groups of rodent muscle, mainly the diaphragm, which display focal innervation patterns (9, 20). MuSK homologs have been identified in several nonmammalian vertebrates in which nonfocal innervation is more prevalent (21–24), but there are no genetic studies addressing whether the MuSK pathway is involved in the formation of nonfocal patterns of innervation. Here we examine the role of MuSK signaling in the formation of NMJs in the zebrafish because they exhibit focal and nonfocal innervation patterns. We have previously reported that the zebrafish unplugged gene encodes two MuSK homolog isoforms, unplugged Full-Length (UnpFL) and Splice Variant 1 (UnpSV1), and that unplugged exerts its role in motor axon guidance through the SV1 isoform (Fig. 1A) (25). In contrast to the mouse MuSK knockout (9), unplugged-null mutants develop neuromuscular junctions and are viable (25). Here we show that the UnpFL isoform is the MuSK ortholog, and we provide the first genetic evidence that vertebrate NMJs can form in the absence of UnpFL/MuSK. Finally, we show that dystroglycans are critical in the formation of synapses at nonfocal sites of innervation. Based on our results, we propose a model by which combinatorial functions of UnpFL and dystroglycans generate diverse patterns of neuromuscular innervation.
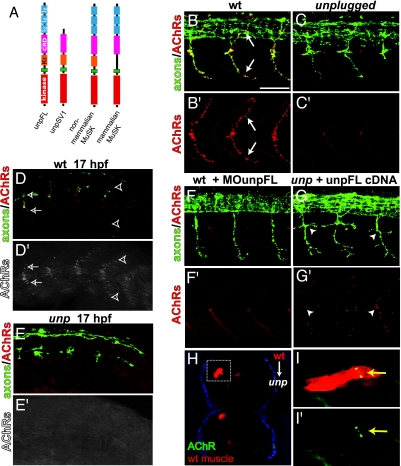
Fig. 1.
UnpFL is essential for focal innervation. (A) Domain organization of UnpFL and UnpSV1, nonmammalian MuSK, and mammalian MuSK. (B and B′) In wild-type embryos (lateral view), en passant equatorial AChR clusters (red, α-BTX) are apposed to primary motor axons (green, znp-1/SV2, arrows). (C and C′) In unplugged mutants, equatorial AChR clusters are absent. (D and D′) Before the arrival of growth cones (caudal segments, right), AChRs are prepatterned in a central band (open arrowheads). As growth cones approach (rostral somites, left), they colocalize with discrete AChR clusters (open arrows). (E and E′) In unplugged mutants, AChR prepatterning is absent. (F and F′) Injection of MO against UnpFL abolishes AChR clusters. (G and G′) Mosaic expression of UnpFL in unplugged muscle restores AChR clustering (arrowhead). (H) Cross-section of chimeric unplugged embryo with wild-type muscle clones (red) and AChR clusters (yellow, dashed box). (I and I′) Lateral view of same clones. (Scale bar: 50 μm.)
Results and Discussion
unplugged Is Essential for Focal Equatorial Synapses and AChR Prepatterning.
The axial muscle of zebrafish exhibits three patterns of neuromuscular innervation: (i) focal innervation along the center of the myotomes, (ii) distributed innervation, and (iii) myoseptal innervation. We previously showed that unplugged-null mutants exhibit AChR clusters at distributed and myoseptal synaptic sites but did not examine AChRs at focal sites (25). Because the unplugged gene displays a high degree of sequence similarity to mammalian MuSKs, we wondered whether unplugged plays a role in the development of focal synapses, analogous to those absent in mice lacking MuSK. In wild-type embryos at 26 h postfertilization (hpf), dense AChR clusters form along the length of the dorsal and ventral primary motor axons (Fig. 1B). These synapses have an en passant morphology and are restricted to the center of muscle fibers, giving rise to a focal innervation pattern (26). unplugged mutant embryos lack these en passant synapses (Fig. 1C) (100% hemisegments affected), demonstrating that unplugged is essential for the formation of synapses in focal innervation.
In 17-hpf wild-type embryos, the appearance of early equatorial synapses is preceded by a diffuse band of small aneural AChR clusters located along the presumptive axonal path in the dorsal and ventral myotomes (Fig. 1D) (27). These AChR clusters are morphologically similar to those observed in prepatterned mammalian endplate bands, where MuSK initiates clustering of AChRs before signals from the nerve growth cone (10, 11). As motor growth cones extend into the myotome, AChR clusters become restricted along the axon while aneural clusters disperse (27, 28). In unplugged mutant embryos, AChR prepatterning is completely absent (Fig. 1E). Thus, similar to mammalian MuSK, unplugged is essential for both the formation of focal NMJs and initiation of the AChR prepattern.
UnpFL Is the Zebrafish MuSK Ortholog.
The unplugged locus generates three splice variants, of which the UnpFL encodes a protein isoform most similar to mammalian MuSK (25) (Fig. 1A). To determine whether the UnpFL variant is critical for AChR clustering, we reduced UnpFL expression by using antisense oligonucleotide morpholinos (MOs) previously shown to block expression of functional UnpFL protein (25). Analysis of MO-injected embryos at 26 hpf revealed that knockdown of UnpFL resulted in either a strong reduction or a complete absence of AChR clusters (Fig. 1F) [reduction in 45.2% and absence in 54.8% of hemisegments (n = 520); see Materials and Methods for details]. Furthermore, expression of myc-tagged UnpFL cDNA in myotomal cells in unplugged mutant embryos was sufficient to rescue AChR clustering (Fig. 1G) (n = 292 UnpFL-myc-positive hemisegments displaying AChR clusters in 62 unplugged embryos examined). These AChR clusters were consistently apposed to motor axons, even along aberrant axonal projections. In contrast, injection of UnpSV1 cDNA into unplugged mutants rarely resulted in AChR clusters, and those that formed were of abnormal morphology and present only on few fibers (n = 11 UnpSV1-GFP-positive hemisegments displayed AChR clusters in 62 unplugged embryos). Finally, analyses of chimeric embryos, in which labeled wild-type cells were transplanted into unplugged host embryos, revealed that only wild-type-derived medial myofibers (i.e., those apposed to motor axons) formed AChR clusters (n = 114 wild-type medial muscle clones with AChR clusters in 50 chimeric embryos) (Fig. 1 H and I). Thus, like mammalian MuSK (23), unplugged functions cell-autonomously in muscle to initiate synapse formation. Taken together, these data demonstrate that UnpFL is a zebrafish MuSK ortholog and induces neuromuscular synapse formation.
The unplugged gene plays distinct roles in motor axon guidance and neuromuscular synapse formation, mediated by two receptor tyrosine kinase isoforms, UnpSV1 and UnpFL, respectively. The two isoforms differ in their ectodomain modules and expression profiles. UnpSV1 is expressed only transiently and acts cell-nonautonomously in adaxial cells, which initially border the future axonal path (25, 29, 30). Furthermore, the UnpSV1 ectodomain lacks the Ig domains required for agrin responsiveness (31) and signals independent of rapsyn to provide guidance information for advancing growth cones in a contact-independent manner (25, 30). In contrast, UnpFL is expressed slightly later (25) and throughout the myotome (unpublished data), where it acts cell-autonomously to direct the formation of focal synapses with motor axons. After synaptogenesis, UnpFL mRNA and protein expression are maintained, and both are concentrated at mature neuromuscular synapses (25). Unlike UnpSV1, the UnpFL ectodomain contains the Ig domains required for agrin responsiveness, and functions through twitch once/rapsyn as agrin morphants and twitch once mutants both lack focal NMJs (32, 33). Thus, UnpFL-mediated AChR clustering likely employs the agrin-MuSK-rapsyn signaling pathway. Together, these data suggest that UnpSV1 and UnpFL receptors have distinct roles in motor axon guidance and synapse formation, respectively, by being activated by different signals and engaging different effector pathways.
Distributed and Myoseptal Innervation Patterns Develop in the Absence of unplugged/MuSK.
After the formation of en passant focal synaptic contacts along the center of medial muscle fibers, motor axons subsequently make two types of nonfocal synaptic contacts. As motor axons advance along the vertical myosepta, robust AChR clusters assemble beneath. In addition, motor axons give rise to lateral branches that form synapses distributed along myofibers throughout the myotome (27, 34, 35). Thus, by 120 hpf, axial muscle exhibits two distinguishable patterns of nonfocal innervation: distributed myoneural (Fig. 2A, arrowhead) and myoseptal (Fig. 2A, arrow) innervation, resulting in polyneuronally and multiterminally innervated muscle fibers (36).
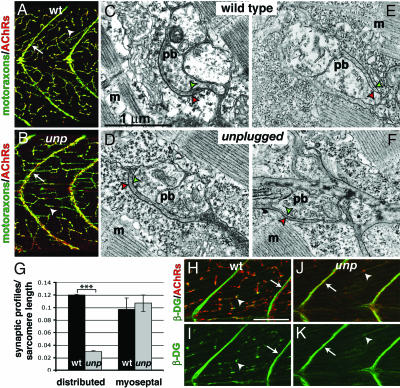
Fig. 2.
Neuromuscular synapse formation in the absence of unplugged/MuSK. (A and B) Wild-type and unplugged larvae stained for motor axons and nerve terminals (green, SV2) and AChRs (red, α-BTX). Nerve terminals and AChR clusters colocalize along muscle fibers for a distributed innervation pattern (arrowhead) and along vertical myosepta for myoseptal innervation (arrow) in wild type (A) and unplugged-null mutants (B). (Scale bar: 50 μm.) (C–F) Electron micrographs of NMJ in distributed (C and D) and myoseptal (E and F) innervation in unplugged mutants and wild type. NMJs consist of a motor nerve presynaptic bouton (pb) with synaptic vesicles clustered around active zone densities and pre- and postsynaptic membranes (green and red arrowheads, respectively) lining the synaptic cleft. (G) Presynaptic bouton profiles were scored based on the presence of characteristic synaptic vesicles and a presynaptic membrane density apposed to a postsynaptic density. Frequency of presynaptic bouton profiles in distributed innervation in unplugged compared with wild type is significantly reduced (P < 0.001, Student's t test); no significant difference was observed in the frequency of myoseptal nerve bouton profiles (P = 0.9077). (H–K) Lateral view of dorsal somitic muscle stained for β-DG (green) and AChRs (red). (H and I) In wild type, β-DG localization is prominent along myosepta (arrow) and along muscle fibers (arrowhead), which colocalize with AChR receptors (H). In unplugged mutants, β-DG immunoreactivity is robust along the myosepta, in apposition to myoseptal AChR clusters (arrow, K). Along muscle fibers, β-DG present in smaller aggregates, coextensive with the reduction of AChR clusters (arrowhead in J). (Scale bar: 50 μm.)
Although unplugged mutants lack focal synapses, at 120 hpf they developed distributed and myoseptal AChR clusters (Fig. 2B) (25). Electron microscopy sections of 120-hpf unplugged mutant muscle revealed that distributed myoneural (Fig. 2D) and myoseptal (Fig. 2F) synapses exhibit characteristic NMJ morphology and were indistinguishable from wild type (Fig. 2 C and E), but that presynaptic profiles along the length of unplugged muscle fibers were reduced by 75% when compared with wild type (Fig. 2G) [P < 0.001, Student's t test; from three wild-type (n = 65 sections) and three unpluggedbr307 (n = 71 sections) 120-hpf larvae]. In contrast, the frequency of presynaptic bouton profiles along the myosepta in unplugged muscle was equivalent to wild-type muscle (Fig. 2G) [P = 0.91, Student's t test; from three wild-type (n = 47 sections) and three unpluggedbr307 (n = 50 sections) 120-hpf larvae]. Thus, the requirement for UnpFL in neuromuscular synapse formation differs according to the pattern of innervation. Although UnpFL/MuSK is required for AChR clustering at focal synaptic sites, it plays a limited role in the formation of distributed synapses and is not essential for myoseptal innervation.
Dystroglycans Colocalize with AChRs at Distributed and Myoseptal Synapses.
The presence of NMJs in unplugged-null mutants suggests the existence of additional MuSK homologs or a MuSK-independent pathway. Exhaustive searches from zebrafish genomic sequence and PCR-based screens failed to identify additional MuSK homologs (data not shown); therefore, we considered alternative pathways that direct AChR clustering. Dystroglycans, which form heterodimers, are strong candidates because α-DG binds agrin with high affinity, and the transmembrane β-DG has been shown to bind rapsyn (18, 19, 37–39). Because of the early lethality of dystroglycan-deficient mice, the contribution of dystroglycan in relation to MuSK signaling and its potential role in early steps of NMJ development are poorly understood (40, 41).
In 28-hpf wild-type larvae, β-dystroglycan (β-DG) is undetectable at focal innervation sites, consistent with previous MO knockdown results showing that dystroglycan is dispensable for the formation of AChR clusters in focal innervation (40). At 96 hpf β-DG is strongly localized along myosepta in apposition to AChR clusters (Fig. 2 H and I, arrows). β-DG was also present along the length of muscle fibers, forming aggregates that colocalized with AChR clusters (Fig. 2 H and I, arrowheads). In unplugged mutant larvae, β-DG robustly colocalized with myoseptal AChRs (Fig. 2 J and K, arrows). In contrast, β-DG aggregates along unplugged muscle fibers were reduced in size and in number (Fig. 2 J and K, arrowheads), reflecting the reduced number of AChR clusters in distributed innervation (Fig. 2G). Furthermore, the presence of AChR clusters associated with β-DG shows that unplugged/MuSK is not required to recruit dystroglycan to AChR clusters.
To determine whether dystroglycans play a role in the formation of distributed NMJs, we reduced α-DG and β-DG protein levels in the developing embryo by injecting an antisense dystroglycan MO (dag1-MO) used previously by Parsons et al. (40). Because maximal knockdown of dystroglycan (dag1) resulted in severe muscular dystrophy (40), we first determined a dose that caused an almost complete reduction of β-DG protein levels without overtly affecting muscle fiber morphology and integrity [supporting information (SI) Fig. 5 A–I]. Reduction of dystroglycan protein levels in dag1 morphant larvae persisted at least until 96 hpf (SI Fig. 5J), enabling us to examine the role of dystroglycans in NMJ formation.
Absence of UnpFL/MuSK Reveals a Critical Role for Dystroglycans in the Formation of Distributed and Myoseptal Synapses.
To examine the role of dystroglycans, we injected dag1-MO into wild-type and unplugged larvae. To quantify the relative effects of dystroglycan reduction on AChR aggregation, we measured the area, number, and synaptic localization of AChR clusters present at distributed synaptic sites (three selected fields per larvae; n = 8 larvae). In 96-hpf wild-type dag1-MO-injected larvae, AChR clusters were present along muscle fibers and the myosepta (Fig. 3A, A′, B, and B′), and the number of clusters (Fig. 3F) and the percent localization of AChR clusters to presynaptic SV2-positive sites (Fig. 3G) were similar to those in uninjected wild-type larvae (see SI Table 1) (cluster number/area, P = 0.992; percentage of colocalization, P = 0.562; Mann–Whitney test). Only the AChR cluster area in dag1 morphants showed a slight reduction in cluster area distribution compared with uninjected wild type (Fig. 3E, Kolmogorov–Smirnov test). Thus, in the presence of UnpFL/MuSK, removal of dystroglycans does not impair AChR cluster formation at distributed and at myoseptal synaptic sites.
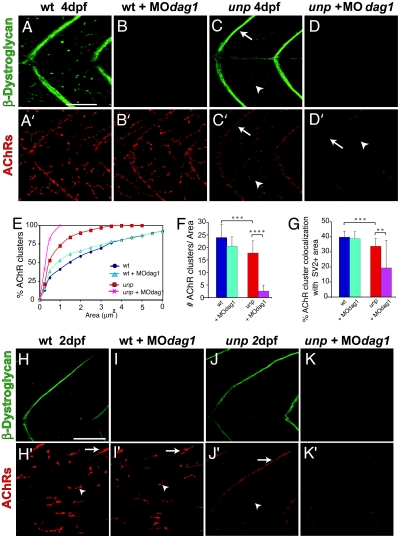
Fig. 3.
In the absence of unplugged/MuSK, dystroglycan directs AChR clustering. (A–D and A′–D′) Projection of three confocal sections stained for β-DG (green, A–D) and AChRs (red, A′–D′). (E) Cumulative histogram of AChR cluster area distribution. Significant difference in AChR area distribution was observed between (i) wild type and unplugged, (ii) wild type and dag1-MO-injected wild type, and (iii) unplugged and MOdag1-injected unplugged (Kolmogorov–Smirnov test). (F) Quantification of dystroglycan knockdown on AChR cluster number per field (3,075 μm2). (G) Quantification of the percentage of AChR clusters colocalizing with SV2-positive presynaptic areas. Asterisks indicate significant differences (∗∗, P < 0.01; ∗∗∗, P < 0.001; ∗∗∗∗, P < 0.0001; Mann–Whitney or Kruskal–Wallis ANOVA). (H–K and H′–K′) Confocal section of 48-hpf embryos stained for β-DG (H–K) and AChRs (H′–K′). In unplugged mutants, small AChR clusters emerge along myosepta and muscle fibers (J′). In dag1-MO-injected unplugged mutants, β-DG protein is strongly reduced (K), and AChRs clusters are absent (K′). (Scale bar: 50 μm.)
In contrast, removal of dystroglycans in unplugged mutants interfered with AChR clustering. Compared with the distributed AChR clusters present along uninjected unplugged muscle fibers (Fig. 3C′), the number and size of these AChR aggregates in dag1-MO-injected unplugged larvae were significantly reduced or almost absent (Fig. 3D′). Similarly, AChR localization was also markedly reduced along the myosepta when compared with uninjected unplugged larvae (Fig. 3D′, arrow). Quantification of distributed AChR clusters revealed that dystroglycan knockdown in unplugged mutants resulted in a significant reduction in AChR area (Fig. 3E, Kolmogorov–Smirnov test) and a 6-fold reduction in the number of distributed clusters (Fig. 3F) compared with uninjected unplugged mutant larvae (Fig. 3G) (P < 0.0001, Mann–Whitney test) (see SI Table 1). Thus, removal of dystroglycans in unplugged mutants further reduced or even eliminated AChR clustering at distributed synaptic sites. Among the remaining AChR clusters in dag1-MO-injected unplugged larvae, few colocalized with SV2-positive terminals compared with uninjected unplugged (P < 0.01, Mann–Whitney test). Thus, at nonfocal synaptic sites dystroglycans direct an alternative AChR clustering pathway, which is revealed only in the absence of UnpFL/MuSK signaling.
To distinguish whether the reduction of AChR clusters results from impaired AChR cluster maintenance or from a defect in cluster formation, we examined distributed AChR clusters as they first form. In both uninjected and dag1-MO injected wild-type embryos at 48 hpf, neural AChR clusters are present along the vertical myosepta and distributed along muscle fibers (Fig. 3 H′ and I′). In unplugged mutants, the first small AChR clusters emerge at distributed and myoseptal synaptic sites, consistent with the initiation of distributed and myoseptal innervation (Fig. 3J′). In dag1-MO-injected unplugged mutants, AChRs were only diffusely localized along the myosepta and along muscle fibers but no AChR clusters were detectable, strongly suggesting that dystroglycan is critical in their aggregation (Fig. 3K′) (n = 40 embryos; four individual experiments). We cannot exclude the possibility that dystroglycan acts to stabilize nascent AChR clusters, but the absence of AChR clusters at the earliest stages of synaptogenesis strongly argues that dystroglycan is sufficient for synapse formation in nonfocal innervation. Together, these genetic analyses demonstrate that dystroglycans do not require the function of unplugged/MuSK to induce postsynaptic differentiation. Although UnpFL/MuSK is required for the assembly of focal synapses, UnpFL/MuSK signaling cooperates with dystroglycans to direct synapse formation in distributed and myoseptal innervation, resulting in the generation of diverse patterns of neuromuscular innervation.
Finally, we asked whether dystroglycans can compensate for the absence of unplugged in the formation of focal synapses. Because β-DG expression is undetectable at focal innervation sites, we used a muscle-specific α-actin promoter (42, 43) to force expression of Myc-tagged full-length dystroglycan (dag1, giving rise to α-DG and β-DG after posttranslational cleavage). We first ensured that this construct produces functional dystroglycan protein, as injection of this plasmid lacking the MO target sequence into dag1-MO-injected unplugged embryos resulted in β-DG expression. Moreover, at 96 hpf, AChR clustering and colocalization with dystroglycan at distributed and myoseptal innervation sites was restored, demonstrating that the construct produces functional dystroglycan (Fig. 4A–F). In contrast, analysis of 28-hpf embryos injected with the same construct revealed that in unplugged muscle fibers dystroglycan fails to restore AChR clusters at focal synapses (Fig. 4 G and H). Furthermore, forced expression of dystroglycan in wild-type muscle fibers did not lead to colocalization of dystroglycan and AChR clusters at focal sites of innervation (Fig. 4 I and J). Thus, focal synapse assembly requires only unplugged/MuSK signaling, completely independent of dystroglycan. Second, unplugged/MuSK is not sufficient to recruit dystroglycan to AChR clusters, but additional, yet unknown, mechanisms exist to control the selective localization of dystroglycan to distributed and myoseptal synapses.
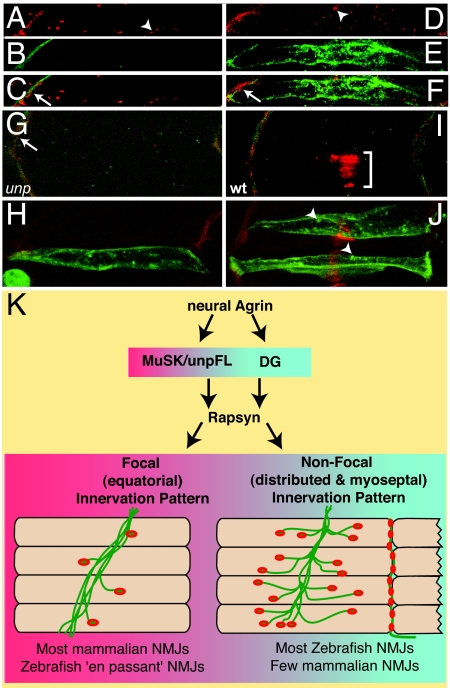
Fig. 4.
At focal synapses dystroglycan fails to induce AChR clustering. (A–C) Endogenous β-DG (green) and AChRs clusters (red) in wild type. Forced expression of dystroglycan-myc in dag1-MO-injected unplugged mutant larvae restores β-DG localization and clustering of AChRs at distributed (arrowhead) and myoseptal (arrow) sites (D–F). (G–J) High-magnification confocal images of 28-hpf axial muscle pioneers stained for β-DG (green) and AChRs (red). unplugged mutants retain myoseptal AChR localization (arrow) but lack focal AChRs (G), even when dystroglycan is overexpressed (H). Wild-type muscle pioneers display large AChR clusters (I, bracket) lacking dystroglycan. Forced dystroglycan expression in muscle pioneers results in aggregates (J, arrowheads), which fail to colocalize with AChR clusters. (K) Proposed model: upon release of neural agrin, unplugged/MuSK recruits rapsyn, leading to focal innervation. At sites of distributed and myoseptal innervation, unplugged/MuSK and dystroglycan, upon activation by neural agrin, cooperate to recruit rapsyn and direct AChR clustering.
The presence of morphologically intact neuromuscular synapses in unplugged-null mutants is the first genetic evidence that fully functional vertebrate neuromuscular synapses can form in the complete absence of MuSK. Recent studies have challenged some of the widely held paradigms on the molecular mechanisms underlying neuromuscular synaptogenesis (reviewed in ref. 8). For example, cell type-specific Neuregulin-1 or ErbB receptor-deficient mice and other double mutant combinations have redefined the roles of Neuregulin ErbB signaling and the role of presynaptic agrin during this process (44–46). Our results that functional neuromuscular synapses can form in the absence of unplugged/MuSK challenge the conventional view that vertebrate NMJ formation requires MuSK, at least at nonfocal sites of innervation, and suggest the existence of other genetic pathways that direct neuromuscular synapse formation.
The development of NMJs at nonfocal synaptic sites in the complete absence of unplugged/MuSK signaling reveals a previously unappreciated role for dystroglycans in synapse formation. Genetic studies in mice have shown that dystroglycans are required for later aspects of AChR cluster maturation rather than formation, and that dystroglycans are not sufficient to compensate for the absence of MuSK (9, 41, 47, 48). Here, we demonstrate that dystroglycans are sufficient to direct neuromuscular synapse formation at distributed and myoseptal sites of innervation (Fig. 4K). Although we cannot exclude the possibility that this alternative pathway is only engaged when challenged by the absence of MuSK signaling, we propose that, for synapse assembly at nonfocal innervation sites, this pathway occurs concurrently or even cooperates with MuSK signaling. Interestingly, some ligands and downstream effectors may be shared between MuSK and dystroglycan. For example, agrin, which enhances MuSK signaling, could potentially initiate or promote dystroglycan-mediated AChR clustering. α-DG is known to bind agrin and laminins, and these interactions have been functionally implicated in AChR clustering in vitro (18, 37, 49, 50). Similarly, the MuSK effector rapsyn is required for AChR clustering in all patterns of innervation (32). Thus, rapsyn likely participates in dystroglycan-directed AChR clustering by way of its direct interactions with β-DG and AChRs (12, 38, 51). Although elucidation of the precise signaling pathway remains, our results provide clear genetic evidence that dystroglycans play a critical role in the formation of vertebrate neuromuscular synapses.
What is the biological significance of dystroglycan's ability to direct synapse formation at distributed and myoseptal synaptic sites? Patterns of distributed and/or myoseptal innervation are widespread in fish and amphibian species using near synchronous and unilateral activation of body wall muscles to generate rapid, undulating movements, as well as in birds (2–4). Patterns of distributed innervation are also present in some mammalian muscles, most notably in laryngeal as well as in extraocular muscle, one of the fastest mammalian muscles (5, 6). We propose that dystroglycans control an evolutionary more primordial postsynaptic differentiation program to provide massive (distributed) and fast muscle activation. The requirement for MuSK signaling may have evolved with progressive positioning of innervation sites to the center of the muscle, to ultimately provide focal and monosynaptic input to individual muscle fibers, providing mammals with superior control over individual muscles fibers. In mammals, where focal innervation predominates, MuSK signaling superimposed the primordial “dystroglycan program,” resulting in the equatorial pattern of focal neuromuscular synapses (Fig. 4K).
Our studies demonstrate that UnpFL/MuSK is critical for synapse development at focal innervation sites but only plays a limited role at nonfocal synaptic sites. This distinction raises the interesting possibility that unplugged/MuSK signaling not only serves postsynaptic differentiation, but that it may pattern muscle fibers to confine structural and signaling components to a central region. Polarization of muscle cells in such a manner may be a prerequisite for synapse formation; by ensuring the focal enrichment of postsynaptic components, synapses can be rapidly assembled at a defined innervation site (10, 11, 52). In addition to patterning the presumptive innervation site, unplugged/MuSK signaling may also pattern other components along the central muscle cell surface, for example, by modifying the extracellular matrix to guide growth cones along their intended axonal path (25). Interestingly, components of the planar cell polarity pathway with known roles in regulating the organization and dynamics of the cytoskeleton, such as APC, Dishevelled, and small GTPases Rac and Rho, have been identified as downstream effectors of MuSK and agrin-mediated AChR clustering (53–56). Although the roles of these factors in vertebrate NMJ development have yet to be examined in vivo, they are involved in multiple morphogenetic processes and neuronal connectivity (57–60). The presence of a frizzled-like cysteine-rich domain in MuSKs implicates Wnt signaling in MuSK-induced postsynaptic differentiation, and future studies may reveal a Wnt-related MuSK pathway.
Materials and Methods
Zebrafish Strains and Breeding.
Wild-type and unplugged mutant strains were maintained in a Tu or TL genetic background and raised as described (61). For all experiments we used unpluggedtbr307- and unpluggedtbb72-null alleles (25).
Antisense MO Oligonucleotide Injections.
Approximately 5–6 ng of unplugged FL MO (25) and/or α-DG and β-DG MO (40) was injected into one-cell embryos. Dag-1 morphants exhibiting trunk defects and muscular dysmorphogenesis (40) were discarded.
Chimeric Embryos.
Chimeric embryos were generated by performing cell transplantations of rhodamine-labeled wild-type donor cells into unplugged mutant hosts as previously described in ref. 29.
Electron Microscopy.
At 4–5 days after fertilization, larvae were fixed and processed as described previously (26). Synaptic profiles were quantified by the presence of a presynaptic bouton morphology, characterized by clustered synaptic vesicles and pre- and postsynaptic membrane densities.
Additional Details.
Supplementary Material
Acknowledgments
We thank laboratory members for helpful discussions and advice on the manuscript. This work was supported by grants from the National Science Foundation and the National Institutes of Health (to M.G.).
Abbreviations
- AChR
acetylcholine receptor
- NMJ
neuromuscular junction
- hpf
hours postfertilization
- MO
morpholino
- α-DG
α-dystroglycan
- β-DG
β-dystroglycan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610822104/DC1.
References
- 1.Burden SJ. J Neurobiol. 2002;53:501–511. doi: 10.1002/neu.10137. [DOI] [PubMed] [Google Scholar]
- 2.Coers C. Int Rev Cytol. 1967;22:239–267. doi: 10.1016/s0074-7696(08)61837-9. [DOI] [PubMed] [Google Scholar]
- 3.Silver A. J Physiol. 1963;169:386–393. doi: 10.1113/jphysiol.1963.sp007263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bone Q. Int Rev Neurobiol. 1964;33:99–147. doi: 10.1016/s0074-7742(08)60768-0. [DOI] [PubMed] [Google Scholar]
- 5.Feindel W, Hinshaw JR, Weddell G. J Anat. 1952;86:35–48. [PMC free article] [PubMed] [Google Scholar]
- 6.Khanna S, Richmonds CR, Kaminski HJ, Porter JD. Invest Ophthalmol Vis Sci. 2003;44:1918–1926. doi: 10.1167/iovs.02-0890. [DOI] [PubMed] [Google Scholar]
- 7.Hesser BA, Henschel O, Witzemann V. Mol Cell Neurosci. 2006;31:470–480. doi: 10.1016/j.mcn.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Kummer TT, Misgeld T, Sanes JR. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 9.DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, et al. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- 10.Yang X, Arber S, William C, Li L, Tanabe Y, Jessell TM, Birchmeier C, Burden SJ. Neuron. 2001;30:399–410. doi: 10.1016/s0896-6273(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 11.Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- 12.Apel ED, Glass DJ, Moscoso LM, Yancopoulos GD, Sanes JR. Neuron. 1997;18:623–635. doi: 10.1016/s0896-6273(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 13.Gautam M, Noakes PG, Mudd J, Nichol M, Chu GC, Sanes JR, Merlie JP. Nature. 1995;377:232–236. doi: 10.1038/377232a0. [DOI] [PubMed] [Google Scholar]
- 14.Froehner SC, Luetje CW, Scotland PB, Patrick J. Neuron. 1990;5:403–410. doi: 10.1016/0896-6273(90)90079-u. [DOI] [PubMed] [Google Scholar]
- 15.Phillips WD, Noakes PG, Roberds SL, Campbell KP, Merlie JP. J Cell Biol. 1993;123:729–740. doi: 10.1083/jcb.123.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noakes PG, Phillips WD, Hanley TA, Sanes JR, Merlie JP. Dev Biol. 1993;155:275–280. doi: 10.1006/dbio.1993.1025. [DOI] [PubMed] [Google Scholar]
- 17.Hopf C, Hoch W. J Biol Chem. 1996;271:5231–5236. doi: 10.1074/jbc.271.9.5231. [DOI] [PubMed] [Google Scholar]
- 18.Sugiyama J, Bowen DC, Hall ZW. Neuron. 1994;13:103–115. doi: 10.1016/0896-6273(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson C, Montanaro F, Lindenbaum M, Carbonetto S, Ferns M. J Neurosci. 1998;18:6340–6348. doi: 10.1523/JNEUROSCI.18-16-06340.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gautam M, DeChiara TM, Glass DJ, Yancopoulos GD, Sanes JR. Brain Res Dev Brain Res. 1999;114:171–178. doi: 10.1016/s0165-3806(99)00013-9. [DOI] [PubMed] [Google Scholar]
- 21.Jennings CG, Dyer SM, Burden SJ. Proc Natl Acad Sci USA. 1993;90:2895–2899. doi: 10.1073/pnas.90.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ip FC, Glass DG, Gies DR, Cheung J, Lai KO, Fu AK, Yancopoulos GD, Ip NY. Mol Cell Neurosci. 2000;16:661–673. doi: 10.1006/mcne.2000.0892. [DOI] [PubMed] [Google Scholar]
- 23.Valenzuela DM, Stitt TN, DiStefano PS, Rojas E, Mattsson K, Compton DL, Nunez L, Park JS, Stark JL, Gies DR, et al. Neuron. 1995;15:573–584. doi: 10.1016/0896-6273(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 24.Fu AK, Smith FD, Zhou H, Chu AH, Tsim KW, Peng BH, Ip NY. Eur J Neurosci. 1999;11:373–382. doi: 10.1046/j.1460-9568.1999.00443.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Lefebvre JL, Zhao S, Granato M. Nat Neurosci. 2004;7:1303–1309. doi: 10.1038/nn1350. [DOI] [PubMed] [Google Scholar]
- 26.Lefebvre JL, Ono F, Puglielli C, Seidner G, Franzini-Armstrong C, Brehm P, Granato M. Development (Cambridge, UK) 2004;131:2605–2618. doi: 10.1242/dev.01123. [DOI] [PubMed] [Google Scholar]
- 27.Panzer JA, Gibbs SM, Dosch R, Wagner D, Mullins MC, Granato M, Balice-Gordon RJ. Dev Biol. 2005;285:340–357. doi: 10.1016/j.ydbio.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 28.Flanagan-Steet H, Fox MA, Meyer D, Sanes JR. Development (Cambridge, UK) 2005;132:4471–4481. doi: 10.1242/dev.02044. [DOI] [PubMed] [Google Scholar]
- 29.Zeller J, Granato M. Development (Cambridge, UK) 1999;126:3461–3472. doi: 10.1242/dev.126.15.3461. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Granato M. Development (Cambridge, UK) 2000;127:2099–2111. doi: 10.1242/dev.127.10.2099. [DOI] [PubMed] [Google Scholar]
- 31.Zhou H, Glass DJ, Yancopoulos GD, Sanes JR. J Cell Biol. 1999;146:1133–1146. doi: 10.1083/jcb.146.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ono F, Shcherbatko A, Higashijima S, Mandel G, Brehm P. J Neurosci. 2002;22:6491–6498. doi: 10.1523/JNEUROSCI.22-15-06491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim MJ, Liu IH, Song Y, Lee JA, Halfter W, Balice-Gordon RJ, Linney E, Cole GJ. Glycobiology. 2007;17:231–247. doi: 10.1093/glycob/cwl069. [DOI] [PubMed] [Google Scholar]
- 34.Myers PZ, Eisen JS, Westerfield M. J Neurosci. 1986;6:2278–2289. doi: 10.1523/JNEUROSCI.06-08-02278.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Downes GB, Granato M. Dev Biol. 2004;270:232–245. doi: 10.1016/j.ydbio.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 36.Westerfield M, McMurray JV, Eisen JS. J Neurosci. 1986;6:2267–2277. doi: 10.1523/JNEUROSCI.06-08-02267.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campanelli JT, Roberds SL, Campbell KP, Scheller RH. Cell. 1994;77:663–674. doi: 10.1016/0092-8674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 38.Fuhrer C, Gautam M, Sugiyama JE, Hall ZW. J Neurosci. 1999;19:6405–6416. doi: 10.1523/JNEUROSCI.19-15-06405.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahl J, Campanelli JT. J Neurosci. 2003;23:392–402. doi: 10.1523/JNEUROSCI.23-02-00392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parsons MJ, Campos I, Hirst EM, Stemple DL. Development (Cambridge, UK) 2002;129:3505–3512. doi: 10.1242/dev.129.14.3505. [DOI] [PubMed] [Google Scholar]
- 41.Cote PD, Moukhles H, Lindenbaum M, Carbonetto S. Nat Genet. 1999;23:338–342. doi: 10.1038/15519. [DOI] [PubMed] [Google Scholar]
- 42.Ono F, Higashijima S, Shcherbatko A, Fetcho JR, Brehm P. J Neurosci. 2001;21:5439–5448. doi: 10.1523/JNEUROSCI.21-15-05439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higashijima S, Okamoto H, Ueno N, Hotta Y, Eguchi G. Dev Biol. 1997;192:289–299. doi: 10.1006/dbio.1997.8779. [DOI] [PubMed] [Google Scholar]
- 44.Escher P, Lacazette E, Courtet M, Blindenbacher A, Landmann L, Bezakova G, Lloyd KC, Mueller U, Brenner HR. Science. 2005;308:1920–1923. doi: 10.1126/science.1108258. [DOI] [PubMed] [Google Scholar]
- 45.Misgeld T, Kummer TT, Lichtman JW, Sanes JR. Proc Natl Acad Sci USA. 2005;102:11088–11093. doi: 10.1073/pnas.0504806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaworski A, Burden SJ. J Neurosci. 2006;26:655–661. doi: 10.1523/JNEUROSCI.4506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobson C, Cote PD, Rossi SG, Rotundo RL, Carbonetto S. J Cell Biol. 2001;152:435–450. doi: 10.1083/jcb.152.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grady RM, Zhou H, Cunningham JM, Henry MD, Campbell KP, Sanes JR. Neuron. 2000;25:279–293. doi: 10.1016/s0896-6273(00)80894-6. [DOI] [PubMed] [Google Scholar]
- 49.Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Cell. 1994;77:675–686. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 50.Burgess RW, Skarnes WC, Sanes JR. J Cell Biol. 2000;151:41–52. doi: 10.1083/jcb.151.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cartaud A, Coutant S, Petrucci TC, Cartaud J. J Biol Chem. 1998;273:11321–11326. doi: 10.1074/jbc.273.18.11321. [DOI] [PubMed] [Google Scholar]
- 52.Arber S, Burden SJ, Harris AJ. Curr Opin Neurobiol. 2002;12:100–103. doi: 10.1016/s0959-4388(02)00296-9. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Jing Z, Zhang L, Zhou G, Braun J, Yao Y, Wang ZZ. Nat Neurosci. 2003;6:1017–1018. doi: 10.1038/nn1128. [DOI] [PubMed] [Google Scholar]
- 54.Luo ZG, Wang Q, Zhou JZ, Wang J, Luo Z, Liu M, He X, Wynshaw-Boris A, Xiong WC, Lu B, Mei L. Neuron. 2002;35:489–505. doi: 10.1016/s0896-6273(02)00783-3. [DOI] [PubMed] [Google Scholar]
- 55.Weston C, Yee B, Hod E, Prives J. J Cell Biol. 2000;150:205–212. doi: 10.1083/jcb.150.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weston C, Gordon C, Teressa G, Hod E, Ren XD, Prives J. J Biol Chem. 2003;278:6450–6455. doi: 10.1074/jbc.M210249200. [DOI] [PubMed] [Google Scholar]
- 57.Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- 58.Hall AC, Lucas FR, Salinas PC. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 59.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Nat Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 60.Ciani L, Salinas PC. Nat Rev Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 61.Mullins MC, Hammerschmidt M, Haffter P, Nüsslein-Volhard C. Curr Biol. 1994;4:189–202. doi: 10.1016/s0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.