Abstract
Alzheimer's disease (AD) immunotherapy accomplished by vaccination with β-amyloid (Aβ) peptide has proved efficacious in AD mouse models. However, “active” Aβ vaccination strategies for the treatment of cerebral amyloidosis without concurrent induction of detrimental side effects are lacking. We have developed a transcutaneous (t.c.) Aβ vaccination approach and evaluated efficacy and monitored for deleterious side effects, including meningoencephalitis and microhemorrhage, in WT mice and a transgenic mouse model of AD. We demonstrate that t.c. immunization of WT mice with aggregated Aβ1–42 plus the adjuvant cholera toxin (CT) results in high-titer Aβ antibodies (mainly of the Ig G1 class) and Aβ1–42-specific splenocyte immune responses. Confocal microscopy of the t.c. immunization site revealed Langerhans cells in areas of the skin containing the Aβ1–42 immunogen, suggesting that these unique innate immune cells participate in Aβ1–42 antigen processing. To evaluate the efficacy of t.c. immunization in reducing cerebral amyloidosis, transgenic PSAPP (APPsw, PSEN1dE9) mice were immunized with aggregated Aβ1–42 peptide plus CT. Similar to WT mice, PSAPP mice showed high Aβ antibody titers. Most importantly, t.c. immunization with Aβ1–42 plus CT resulted in significant decreases in cerebral Aβ1–40,42 levels coincident with increased circulating levels of Aβ1–40,42, suggesting brain-to-blood efflux of Aβ. Reduction in cerebral amyloidosis was not associated with deleterious side effects, including brain T cell infiltration or cerebral microhemorrhage. Together, these data suggest that t.c. immunization constitutes an effective and potentially safe treatment strategy for AD.
Keywords: Alzheimer's disease, cytokine, Langerhans cells, vaccine
Alzheimer's disease (AD) is the most common dementing illness and is pathologically characterized by the presence of intracellular neurofibrillary tangles and extracellular senile plaques primarily composed of 40- to 42-aa β-amyloid (Aβ) peptides (1). In a seminal report, Schenk et al. (2) showed that i.p. vaccination of the PDAPP transgenic mouse model of AD with Aβ1–42 plus Freund's adjuvant resulted in dramatic reduction of cerebral amyloidosis. This therapeutic approach is clearly highly efficacious; however, the safety of this strategy has become an important concern. In a recent clinical trial, patients were administered a synthetic Aβ peptide (AN-1792) plus adjuvant, and ≈6% of these patients developed aseptic meningoencephalitis, most likely mediated by brain-infiltrating activated T cells (3, 4). This serious side effect led to suspension of the clinical trial. Furthermore, passive transfer of Aβ antibodies to transgenic AD mice results in cerebral microhemorrhage, a potentially adverse side effect (5, 6). Uncovering of these adverse events has redirected Aβ vaccination strategies toward the goal of developing an approach that is both safe and effective.
Studies examining the brains of Aβ-vaccinated patients developing meningoencephalitis implicate Aβ-reactive T cell subsets as major components of this deleterious response to active Aβ vaccination (7, 8). To subvert possible meningoencephalitis resulting from Aβ vaccination, various strategies have been attempted. Interestingly, recent works suggest that Aβ-derived peptides delivered intranasally (with adjuvant) to mucosal epithelial tissues results in effective clearance of Aβ plaques and improvement of cognitive function in animal models of AD. Moreover, T cell reactivity appeared to be considerably reduced compared with other active immunization strategies. In other studies, differential T cell responses depended on the epitope/fragment of Aβ peptide used for vaccination. Specifically, portions of the Aβ peptide seemed to stimulate different T cell responses, resulting in either proinflammatory T helper (Th) cell type 1 (Th1) responses or antiinflammatory Th cell type 2 (Th2) responses (9, 10). Such findings imply that Aβ vaccination is not only efficacious, but may also prove to be safe and therefore a feasible strategy for AD therapy depending on a number of factors, including route of delivery, adjuvant choice, and Aβ epitope administered.
The skin is a well established effective route for vaccination, including delivery of peptide-based vaccines (11–13). Strong humoral and cellular immune responses have been elicited after transcutaneous (t.c.) vaccination (14), largely owing to the diverse populations of resident antigen-presenting cells (APCs) and other immune cells in the various dermal layers. Subsets of dermal-resident Langerhans cell (LC) precursors, known as migratory CD14+ LC precursors, are important immune regulators that demonstrate “professional” APC capability, including reducing T cell stimulatory function by producing antiinflammatory cytokines (15). Also, skin-resident keratinocytes release the antiinflammatory cytokine IL-10 in response to certain stimuli. Keratinocyte-derived IL-10 serves to buffer harmful proinflammatory immune activation and thereby preserves skin barrier integrity (16).
Taken together, these lines of evidence led us to hypothesize that targeting Aβ immunotherapy to skin tissue might provide an immunotherapeutic approach that is both efficacious and safe. In this study we tested a t.c. Aβ immunization strategy using both WT and the transgenic PSAPP (APPsw, PSEN1dE9) mouse model of AD (17).
Results
t.c. Immunization of Mice with Aβ1–42 Peptide Plus CT Results in High Aβ Antibody Titers.
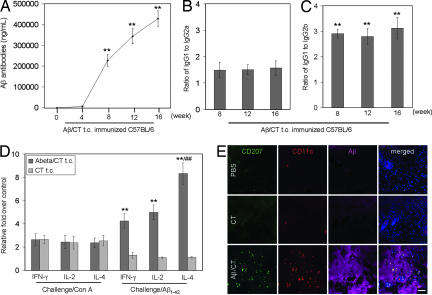
To determine whether t.c. administration of Aβ1–42 to mice could result in Aβ antibody production, 20 nontransgenic C57BL/6 mice at 8 weeks of age were used for this experiment, and 10 mice received aggregated Aβ1–42 peptide with CT (Aβ/CT), whereas the remaining 10 received CT alone. Mice were t.c.-immunized over 16 weeks: weekly for the first 4 weeks and biweekly for the next 12 weeks. Blood samples were taken at weeks 0 (baseline), 4, 8, and 16 (immediately before the death of these mice). Plasma Aβ antibody titers were measured by ELISA. Aβ antibody isotypes were determined by an IgG isotyping assay using an isotype-specific secondary antibody (18). Aβ antibodies were first detected at week 4 in all immunized mice and dramatically increased thereafter (Fig. 1 A; **, P < 0.001). Consistent with our previous studies that used an i.p. route of administration of Aβ plus Fruend's adjuvant (18, 19), Aβ antibodies of the IgG1 isotype were produced at the highest level, whereas IgG2a antibodies directed against Aβ were present in significantly lesser quantity (Fig. 1A; **, P < 0.001). Aβ IgG2b antibody was least detectable (Fig. 1 B and C). t.c. immunization was further evaluated by assaying CT antibody titers in plasma from these mice at weeks 0, 4, 8, and 16 after immunization. A similar pattern of results was observed, albeit CT antibody titers were higher in plasma from mice t.c.-immunized with either Aβ/CT compared with CT alone (data not shown). As an additional control group, we injected WT mice with PBS alone (n = 10) in parallel but were unable to detect Aβ antibody titers in these animals, confirming the specificity of our titer assay (data not shown).
Fig. 1.
Generation of immune responses in WT mice t.c.-immunized with aggregated Aβ1–42 peptide plus CT. (A) Aβ antibody titers were measured by ELISA. Data are presented as mean ± SD (n = 10) of Aβ antibodies (ng/ml plasma). One-way ANOVA followed by post hoc comparison revealed significant differences in anti-Aβ titers when comparing week 4 to weeks 8, 12, or 16 (∗∗, P < 0.001). (B and C) IgG isotypes were determined by an Ig isotyping assay and are represented as ratios (mean ± SD; n = 10) of IgG1 to IgG2a (B) or IgG1 to IgG2b (C). One-way ANOVA followed by post hoc comparison revealed significant differences between the ratio of IgG1 and IgG2a versus IgG1 and IgG2b at each week shown (∗∗, P < 0.001). (D) Splenocytes were individually isolated and cultured from WT mice t.c.-immunized with Aβ1–42/CT, CT alone, or PBS (control). These cells were stimulated with Con A (5 μg/ml) or Aβ1–42 (20 μg/ml) for 48 h. Cultured supernatants were collected from these cells for IFN-γ, IL-2, and IL-4 cytokine analyses by ELISA. Data are presented as relative fold mean ± SD (n = 10) of each cytokine over PBS control. One-way ANOVA followed by post hoc comparison revealed significant differences between groups for levels of each of three cytokines [IFN-γ, IL-2, and IL-4 (∗∗, P < 0.001)] after in vitro Aβ1–42 challenge. As noted, there was also a significant difference in cytokine levels between IL-4 and either IFN-γ or IL-2 after Aβ1–42 challenge (##, P < 0.001). (E) To characterize dermal immune responses to Aβ/CT t.c. immunization, skin tissues were prepared from nontransgenic C57BL/6 mice t.c.-immunized for 18 h with PBS (control, Top), CT alone (Middle), or Aβ/CT (Bottom) as indicated and then analyzed by laser scanning confocal microscopy with the indicated antibodies (antibody 4G8 was used to reveal Aβ). Note the presence of CD207+CD11c+ LCs in Aβ-positive regions in the Aβ/CT t.c.-immunized group. DAPI (blue signal) was used as a nuclear counterstain in merged images shown to the right. (Scale bar: 50 μm.)
Aβ-Specific Immune Responses in Splenocytes from Mice t.c.-Immunized with Aβ Plus CT.
We quantified key cytokines produced by activated T cells (IFN-γ, IL-2, and IL-4) in splenocyte supernatants by ELISA as an indicator of immune responsiveness. Nonspecific mitogenic stimulation of cultured splenocytes with Con A resulted in >2-fold increases in IFN-γ, IL-2, and IL-4 production in cells from mice immunized with Aβ/CT or CT versus PBS-immunized controls (Fig. 1D). No statistically significant difference was noted between Aβ/CT and CT alone groups for each cytokine (P > 0.05). Similar results were observed in cultured splenocytes stimulated with anti-CD3 (data not shown). On the other hand, specific recall stimulation with Aβ1–42 peptide of primary cultured splenocytes from Aβ/CT t.c.-immunized mice resulted in significantly increased production of IFN-γ, IL-2, and IL-4 compared with splenocytes cultured from mice immunized with CT alone (Fig. 1D; **, P < 0.001). Importantly, regarding the antiinflammatory cytokine IL-4, an 8-fold increase in its secretion by splenocytes from Aβ/CT-immunized mice after Aβ1–42 recall stimulation was observed (Fig. 1D; ##, P < 0.001). Taken together with the predominantly IgG1 Aβ-specific humoral response in Aβ/CT t.c.-immunized WT mice, this IL-4 result suggests an antiinflammatory Th2 immune response.
t.c. Immunization with Aβ Plus CT Promotes Recruitment of Dermal LCs.
Skin tissues and frozen sections were prepared from WT mice 18 h after t.c. vaccination with Aβ/CT or CT alone, and they were costained with antibodies against mouse CD207 (Langerin, a pan-LC marker), mouse CD11c [a marker of an LC subset (20)], and/or rabbit anti-human Aβ. Aβ/CT t.c. immunization resulted in LC recruitment into dermal layers compared with CT alone or PBS-immunized controls [Fig. 1E and supporting information (SI) Fig. 5], where dermal LCs were much less frequently observed. Furthermore, these LCs tended to be found in regions of the skin that stained positive for Aβ peptide by 4G8 Aβ antibody (Fig. 1E). These data show the migratory action of LCs in response to Aβ/CT t.c. stimulation and suggest that this effect is important in mediating the initial immune response to Aβ/CT t.c. immunization.
t.c. Immunization of PSAPP Mice with Aβ Plus CT Results in Aβ-Specific Immune Response and Increased Circulating Aβ.
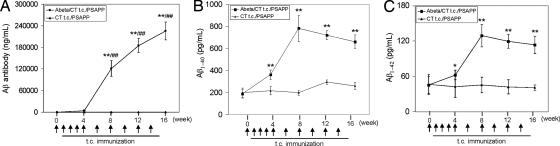
Eighteen transgenic PSAPP mice, which overproduce human Aβ and develop significant amyloid deposits by 8 months of age (17), were immunized at 4 months of age in this study. Half of them (n = 9) received aggregated Aβ1–42 peptide with CT, whereas the remaining half received CT alone. The 16-week procedure that we used was identical to that used above for WT mice. Plasma Aβ antibody titers from these mice were measured by ELISA. Significant increases in Aβ antibody titers were observed in PSAPP mice t.c.-immunized with Aβ/CT (P < 0.001) (Fig. 2A). Similar to WT mice, Aβ antibodies were first detected at week 4 in plasma and dramatically increased thereafter. By contrast, these Aβ antibodies were not detected in plasma from CT-vaccinated control mice (Fig. 2A). Two weeks after the final immunization, primary splenocytes were isolated and cultured from individual mice. Recall stimulation of splenocytes from Aβ/CT t.c.- immunized PSAPP mice with Aβ1–42 peptide resulted in significantly increased production of IFN-γ and IL-2, and particularly IL-4 (data not shown), similar to results from Aβ/CT t.c.-immunized WT mice.
Fig. 2.
Increased systemic Aβ after Aβ1–42/CT t.c. immunization of PSAPP mice. For Aβ analysis, blood samples were individually collected from Aβ/CT or CT alone t.c.-immunized PSAPP mice at the time points indicated. (A) Plasma Aβ antibody titers were measured by ELISA. Data are presented as mean ± SD (n = 9) of Aβ antibodies (pg/ml plasma) (∗∗, P < 0.001) and between time points within the Aβ/CT t.c.-immunized group as indicated (##, P < 0.001). (B and C) Plasma Aβ1–40,42 peptides were measured separately by Aβ ELISA. Data are presented as mean ± SD (n = 9) of Aβ1–40 or Aβ1–42 (pg/ml plasma). ∗, P < 0.05; ∗∗, P < 0.001. Arrows indicate each t.c. immunization with respect to the time of blood sample collection.
In support of peripheral sink hypothesis (21, 22) we quantified Aβ levels in the blood by ELISA and found significantly increased circulating Aβ1–40,42 in PSAPP mice t.c.-immunized with Aβ/CT as early as 4 weeks after immunization (Fig. 2 B and C). Importantly, plasma Aβ1–40,42 levels increased rapidly to the highest values of 781 ± 118 pg/ml and 129 ± 46 pg/ml, respectively, by week 8 (2 weeks after the third booster t.c. immunization). Thereafter, plasma Aβ levels remained relatively constant through to the time of death after 16 weeks of immunization.
PSAPP Mice t.c.-Immunized with Aβ Plus CT Show Reduced Cerebral Amyloidosis in the Absence of T Cell Infiltrates or Cerebral Microhemorrhage.
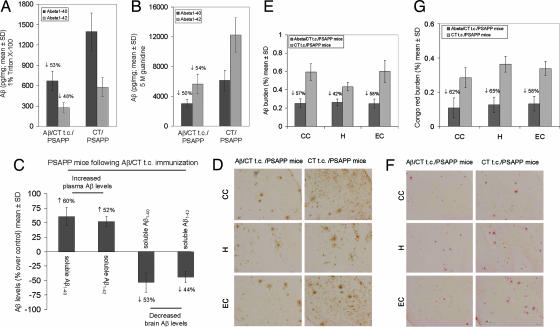
Using a sandwich ELISA-based method, detergent-soluble Aβ1–40,42 levels were reduced by ≈53% and 48%, respectively (P < 0.001) (Fig. 3A). Insoluble Aβ1–40,42 (prepared by acid extraction of detergent-insoluble material in 5 M guanidine) levels were reduced by 50% and 54%, respectively, in Aβ/CT t.c.-immunized PSAPP mice (P < 0.001) (Fig. 3B). We further analyzed Aβ plaques in brains of mice that received t.c. immunization with Aβ/CT or CT alone by 4G8 immnunohistochemistry and Congo red histochemistry (Fig. 3 D and F, respectively). At 10 months of age, Aβ/CT t.c.-immunized PSAPP mice showed 42–58% (Fig. 3E) and 58–65% (Fig. 3G) reductions in 4G8 immunoreactive and Congo red-positive Aβ deposits, respectively, across hippocampal and cortical brain regions examined. Together, these results demonstrate that t.c. immunization with Aβ/CT is effective in reducing cerebral amyloidosis in PSAPP mice.
Fig. 3.
Reduction of cerebral Aβ/β-amyloid pathology in PSAPP mice t.c.-immunized with Aβ1–42/CT. (A and B) Detergent-soluble Aβ1–40,42 peptides (A) and insoluble Aβ1–40,42 prepared from 5 M guanidine extraction (B) were separately measured in brain homogenates by ELISA. Data are presented as mean ± SD (n = 9) of Aβ1–40 or Aβ1–42 (pg/mg protein), and reductions for each group are indicated. (C) A significant inverse correlation (P < 0.001) between plasma and brain-soluble Aβ levels was revealed. Plasma/brain Aβ levels are presented as percentage mean ± SD (n = 9) of soluble circulating/brain Aβ at 16 weeks after t.c. immunization of PSAPP mice with Aβ/CT over CT control mice. (D) Mouse brain coronal paraffin sections were stained with monoclonal anti-human Aβ antibody 4G8. (Left) Aβ1–42/CT t.c. immunized PSAPP mice. (Right) CT t.c.-immunized PSAPP mice. (Top) The cingulate cortex (CC). (Middle) The hippocampus (H). (Bottom) The entorhinal cortex (EC). (E) Percentages (plaque burden, area plaque/total area) of Aβ antibody-immunoreactive Aβ plaques (mean ± SD; n = 9) were calculated by quantitative image analysis, and reductions for each mouse brain area analyzed are indicated. (F) Mouse brain sections from the indicated regions were stained with Congo red. (Left) Aβ1–42/CT t.c.-immunized PSAPP mice. (Right) CT t.c.-immunized PSAPP mice. (G) Percentages of Congo red-stained plaques (mean ± SD; n = 9) were quantified by image analysis, and reductions for each brain region are indicated.
To determine whether this systemic increase in human Aβ1–40,42 was associated with a reduction in cerebral Aβ levels, we performed correlation analysis and noted an inverse correlation between plasma and brain-soluble Aβ (Fig. 3C). In addition, simultaneous analysis of plasma Aβ levels and brain-soluble Aβ levels on a mouse-by-mouse basis provided further evidence of an inverse correlation (SI Fig. 6). These data suggest that circulating Aβ antibodies play an important role in clearance of Aβ from brain to blood via the peripheral sink hypothesis (21, 22). This effect is likely not solely responsible for reduced cerebral amyloidosis, as, interestingly, we detected Aβ antibodies in brain homogenates from Aβ/CT t.c.-immunized PSAPP mice [18.87 ± 6.25 (mean ng/mg of total protein ± SD)], whereas Aβ antibodies were undetectable in PSAPP mice t.c.-immunized with CT alone (data not shown). Given the presence of these Aβ antibodies in the brain, it is possible that additional Aβ clearance mechanisms (i.e., mediated by the Fc receptor on phagocytic microglia) are operating.
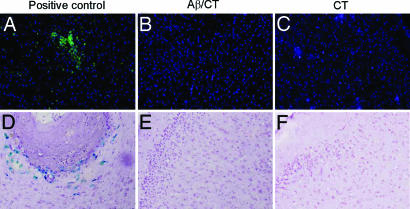
We sought to investigate whether Aβ/CT t.c. immunization might induce T cell infiltration into the brain. We immunostained brain sections from mice immunized with Aβ/CT, CT alone, or, as a positive control, WT mice s.c.-injected with myelin oligodendrocyte protein emulsified in complete Freund's adjuvant (to induce experimental autoimmune encephalomyelitis; brains were isolated 20 days after immunization, when copious amounts of T cells have infiltrated the brain). As shown in Fig. 4A–C, we did not detect CD3+ T cells in brains from mice t.c.-immunized with CT or Aβ/CT; however, this result was not caused by a technical issue as T cells were detected in the positive control tissue (Fig. 4A). Further, we also immunostained brain sections from the above mice with CD4 or CD8 antibodies (which stain different subsets of T cells) and did not detect T cell infiltration in brains of mice t.c.-immunized with CT or Aβ/CT, whereas such cells were detected in our positive control tissue (data not shown).
Fig. 4.
Absence of T cell infiltration or brain microhemorrhage in Aβ/CT t.c.-immunized mice. (A–C) Brain sections were stained for CD3 as an indicator of T cell infiltration. (D–F) Staining for hemosiderin was also performed to identify microhemorrhage in mice immunized with Aβ/CT (B and E) or CT alone (C and F). (A) For CD3 staining, the positive control consisted of CD3-positive brain sections from experimental autoimmune encephalomyelitis mice. (D) For microhemorrhage, experimental sections were compared with sections from mouse brains suffering microhemorrhage. Each panel is representative of staining repeated in triplicate for each brain section for either CD3 or hemosiderin. The brain region shown for each panel is the neocortex. (Magnification: CD3 staining, ×10; microhemorrhage, ×20.)
Further, we carried out microhemorrhage analysis via Perl's Prussian blue stain and did not detect positive staining with this method in either t.c.-immunized group, but we did observe staining in our positive control tissue [in this case, from mice i.p. passively given Aβ antibodies (23)] (Fig. 4 D–F). As an additional indicator of possible blood-brain-barrier breakdown, we analyzed apolipoprotein B (present in blood but not normally in brain) levels in these brain tissues by Western blot and it was undetectable in the t.c.-immunized groups (SI Fig. 7). It is noteworthy that both the Prussian blue stain and apolipoprotein B analyses were negative in t.c.-immunized PSAPP mice, suggesting that detection of Aβ antibodies in the brains of these mice (as mentioned above) was not caused by poor perfusion efficiency or detectable breakdown of the blood-brain-barrier, but rather was likely caused by physiological entry of Aβ antibodies into the brain parenchyma.
Discussion
To translate animal Aβ immunization approaches into successful clinical AD therapies, such strategies should not only be efficacious, but also safe, including avoiding meningoencephalitic reactions to Aβ immunization previously observed in humans (24, 25). Experimental and postmortem evidence suggests that such aseptic meningoencephalitis observed in AD patients after Aβ vaccination resulted from CNS invasion by Aβ-reactive T cells (10, 25). The requirement of Aβ-reactive T cells for cerebral amyloid plaque clearance mediated by active Aβ vaccination strategies still remains unclear (8, 25, 26). A previous study using intranasal delivery of short Aβ-derived peptides lacking T cell reactive epitopes with a specific immune-modulating adjuvant (LT R192G) demonstrated the possibility of potentiating an effective humoral anti-Aβ response while minimizing Aβ reactive T cells (10), suggesting that Aβ-reactive T cells are not necessary for an effective Aβ antibody response. If this proves to be true in humans, such an approach may represent reasonable therapeutic potential; however, it should be noted that anosmia/hyposmia may limit the usefulness of intranasal Aβ immunization. Further evidence that Aβ-reactive T cells are likely not required for Aβ immunotherapy efficacy comes from passive immunization studies, which have shown that humoral responses alone may be sufficient to effectively reduce cerebral amyloid burden, and thereby mitigate neurodegeneration (27, 28).
Here, we investigated the potential of t.c. Aβ immunization for the treatment of AD-like cerebral amyloidosis in transgenic mice. t.c. immunization is an attractive route of delivery, as it is convenient, relatively painless, and minimally invasive. This strategy is also appealing because the epidermal and dermal immune systems provide a unique environment for immune stimulation caused by LC antigen presentation (29–32). Indeed, after Aβ/CT t.c. immunization, we observed cells double-positive for CD207 and CD11c in dermal regions that stained positive for Aβ, showing that these LCs migrate to the t.c. immunization site and likely participate in antigen processing. The skin immune environment has evolved over millennia, owing to constant bombardment of the skin with various antigenic stimuli, resulting in a delicate balance between immunogenic and tolerogenic responses. It is noteworthy that t.c./epicutaneous immunization has been successful in mitigating neurodegenerative disease in both induced and spontaneous forms of experimental autoimmune encephalomyelitis, mouse models of the demyelinating disease multiple sclerosis (33, 34).
To determine the ability of Aβ t.c. immunization to effectively produce Aβ antibodies, we began our investigation in nontransgenic C57BL/6 mice, where we measured the kinetics of Aβ-specific antibody titers. Remarkably, the Aβ antibody response was observed as early as week 4 in all immunized mice and dramatically increased thereafter, remaining elevated through 16 weeks postinitial vaccination. Antibody isotype characterization demonstrated a predominantly IgG1 (IgG1 > IgG2a > IgG2b) response in line with our previous report using an i.p. route of Aβ vaccination plus Freund's adjuvant (19). Production of IgG1 and IgG2b are typically caused by antiinflammatory Th2 cytokine signaling, whereas IgG2a typically results from proinflammatory Th1 signaling (35). The Th2 cytokine profile is likely favorable for inducing antibody production and thus Aβ clearance without the overt proinflammatory (i.e., possibly contributing to autoimmune responses) Th1-type activation that typifies cellular immune responses (19, 36, 37). Accordingly, to circumvent meningoencephalitic reactions, many studies investigating vaccination methods for reducing cerebral amyloidosis in AD have attempted to bias Th cell responses toward Th2 profiles by using various strategies (38–40). The effectiveness and potential safety of these strategies seems promising, but further investigation is needed to confirm whether the link between Th cell responses and meningoencephalitis in AD patients is causative.
Notwithstanding the need for these critical studies, Aβ immunization appears to modulate immune responses based on three major criteria: (i) tissue route of delivery, (ii) antigen epitope used for immunization, and (iii) properties of the coadministered adjuvant. Whether Th2 polarization in this study occurred because of route of delivery, CT adjuvant choice, or the genetic background of the C57BL/6 strain (41, 42) remains to be fully determined in future studies. It has been reported that CT promotes an antiinflammatory Th2 immune response (43), and our data demonstrating IgG1-subtype antibodies produced in the greatest proportion (compared with IgG2a or IgG2b antibodies) supports this notion. Of note, CT antibody titers were observed (data not shown), indicating an immunogenic response to this adjuvant. To confirm specific systemic versus local immune cell activation, we analyzed primary cultures of isolated splenocytes from t.c.-immunized WT mice and found that Aβ/CT t.c. immunization conferred Aβ-specific T cell response as measured by secretion of cytokines IFN-γ, IL-2, and IL-4 upon aggregated Aβ1–42 peptide recall challenge. Importantly, there was a marked increase in IL-4 secretion compared with IFN-γ or IL-2, further suggesting Th2 immune responses after Aβ/CT t.c. immunization. This finding is in agreement with our previous study, where we found Th2-type cytokine responses both in vivo and ex vivo after i.p. Aβ vaccination with Freund's adjuvant (20). Th2-type immune responses are likely preferred to proinflammatory Th1 responses in the Aβ vaccination paradigm, given that proinflammatory Th1 cells likely contributed to the aseptic meningoencephalitis in the human clinical trial of AN-1792 (7, 44). When taken together, these findings show that Aβ/CT t.c. immunization of WT mice produces both Aβ-specific local LC immune response and systemic immune response characterized by high Aβ antibody titers that are sustained throughout the immunization protocol.
To determine the potential therapeutic efficacy of Aβ t.c. immunization, 6-month-old transgenic PSAPP mice [which develop robust amyloid pathology at 8 months of age (17)], were t.c.-immunized against Aβ/CT or CT alone for 16 weeks. Results showed consistently high and sustained Aβ antibody titers throughout the 16-week immunization period only in the Aβ/CT-immunized group. Interestingly, the magnitude of Aβ antibody response in Aβ/CT t.c.-immunized mice was only about half of that in WT mice (compare Figs. 1A and 2A), supporting the notion that transgenic mouse models of AD are hyporesponsive to Aβ vaccination, probably owing to overexpression of the human APP transgene throughout their lives (45). This humoral response correlated with high plasma levels of Aβ1–40,42 peptides, which peaked ≈8 weeks and remained relatively constant to 16 weeks. Immunohistological and histochemical analyses of Aβ-immunoreactive plaques and congophilic plaques, in cortical and hippocampal brain sections showed reductions by ≈50% compared with CT t.c.-immunized PSAPP mice, and a negative correlation existed between brain Aβ and blood Aβ levels after Aβ/CT t.c. immunization. These results show that Aβ/CT t.c. immunization is effective at mitigating cerebral amyloidosis and suggest activation of Aβ brain-to-blood clearance.
For AD immunotherapy approaches to be useful, they must not only be efficacious, but such approaches must also be safe and well tolerated. Importantly, although we did observe peripheral Aβ-specific T cell responses consistent with an antiinflammatory Th2 response (characterized in vivo by IgG1 Aβ antibody production and ex vivo by IL-4 secretion after Aβ recall stimulation of splenocytes) after Aβ/CT t.c. immunization, no signs of aseptic meningoencephalitis and/or cell-mediated immunity were observed in brains as evidenced by lack of CD3-positive T cell infiltrates. However, we did observe evidence of humoral immunity in brain as demonstrated by Aβ antibody titers in brain homogenates, similar to data from previous reports using other modes of Aβ immunotherapy (46, 47), supporting the notion that Aβ antibodies cross the blood-brain-barrier. This observation was not caused by poor perfusion at the time of death, as Perl's stain (which normally detects even trace amounts of iron that could be present because of poor perfusion) results were consistently negative. Finally, other investigators have reported that passive transfer of Aβ antibodies to transgenic AD mice results in cerebral microhemorrhage (5, 6). Importantly, Perl's stain did not show this potentially adverse side effect in mice t.c.-immunized with Aβ/CT. Thus, when taken together, t.c. immunization holds potential as an effective and safe potential treatment strategy for AD.
Materials and Methods
Reagents.
Lyophilized CT, CT antibody, Con A, and mouse CD3 antibody were obtained from Sigma (St Louis, MO). Aβ1–42 peptide was purchased from U.S. Peptides (Rancho Cucamonga, CA). ELISA kits for the detection of IFN-γ, IL-2, and IL-4 were obtained from R & D Systems (Minneapolis, MN). Aβ1–40 and Aβ1–42 ELISA kits were purchased from IBL-American (Minneapolis, MN). Alexa-Fluor-conjugated secondary antibodies (including Alexa-Fluor488, Alexa-Fluor594, and Alexa-Fluor647) were purchased from Invitrogen (Carlsbad, CA).
Animals.
WT C57BL/6 and PSAPP (APPsw, PSEN1dE9) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Brain sections from mice induced with experimental autoimmune encephalomyelitis were provided by T.T. and used as a positive control for CD3 staining. Brain sections for positive microhemorrhage staining from mice i.p. passively given Aβ antibodies were provided by D.M. (23).
t.c. Immunization of Mice.
We first t.c.-immunized WT C57BL/6 mice. These mice (n = 10, five male/five female) were t.c.-immunized with human Aβ1–42 peptide (200 μg per mouse) and CT (10 μg per mouse) or CT alone (10 μg per mouse) in 100 μl of 0.9% saline on a weekly basis for the first month. Thereafter, these mice were continually t.c.-immunized with Aβ1–42 (100 μg per mouse) and CT (5 μg/ml) or CT alone (5 μg per mouse) in 100 μl of 0.9% saline biweekly for the next 12 weeks. t.c. immunization was performed as described (48). To ensure mice immobility for the duration of administration for each immunization, mice were anesthetized. A small lower back section (1–2 cm2) was shaved with an extra precaution not to damage the skin. The skin was then swabbed with acetone to remove surface oils and enhance penetration, allowed to air dry, and then rehydrated by swabbing with 0.9% saline. The shaved edge was coated with a thin petroleum jelly layer to prevent unnecessary leakage of the immunization solution. Last, 100 μl of Aβ1–42 in combination with CT or CT alone in 0.9% saline was placed on the shaved region and allowed to be absorbed for 2 h. At the end, the skin was washed with 0.9% saline and dried, so as to remove any remaining immunization solution. Mice were cleaned thereafter and returned to their cages. We then t.c.-immunized PSAPP mice (n = 9, four male/five female) at 4 months of age by using the same procedure described above.
Splenocyte Cultures.
Cell suspensions of splenocytes from individual mice were prepared and treated as described (19). Lactate dehydrogenase (LDH) release assay was carried out as described (49), and LDH was not detected in any of the wells studied.
Immunofluorescence Staining.
The dorsal skin was removed by careful razor slicing around prelabeled regions (1.5 cm in diameter) where the vaccine was applied. Skin or brain samples were routinely prepared for immunofluorescence staining. The staining was curried out with the following primary antibodies: anti-mouse CD207 (Langerin; 1:250; eBioscience, San Diego, CA), anti-mouse CD11c (1:50; Pierce Biotechnology), and/or anti-human Aβ antibody (clone 4G8; 1:500) or rat anti-mouse CD3 antibodies (1:200) overnight at 4°C, followed by appropriate secondary antibodies conjugated with Alexa-Fluor488, Alexa-Fluor594, and/or Alexa-Fluor647 (1:500) for 45 min. Sections were then washed three times in PBS and mounted with fluorescence mounting media containing DAPI to counterstain cell nuclei, and then viewed under a BX-51 microscope (Olympus, Center City, PA) or visualized in independent channels with a LSM510 META confocal microscope (Zeiss, Thornwood, NY) equipped with a two-photon laser that was used for exciting DAPI.
Aβ Antibody ELISA.
Aβ antibodies in mouse plasma and brain homogenates were measured as described (9). Aβ antibodies were represented as ng per ml of plasma (mean ± SD).
ELISAs for Aβ antibody isotypes were carried out as described (18, 50). The optical density of both ELISAs was immediately determined by a microplate reader at 450 nm. The ratios of IgG1 to IgG2a or IgG1 to IgG2b were calculated for each time point from each mouse individually by using optical density values and then the average ratio for each group (mean ± SD).
Aβ ELISA.
Mouse brains were isolated and prepared as described (51). This fraction represented the detergent-soluble fraction. Aβ1–40,42 species were further subjected to acid extraction of brain homogenates in 5 M guanidine buffer (52), followed by a 1:10 dilution in lysis buffer. Soluble Aβ1–40,42 species were directly detected in plasma, and brain homogenates were prepared with lysis buffer described above at a 1:4 or 1:10 dilution, respectively. Aβ1–40,42 was quantified in these samples with Aβ1–40,42 ELISA kits in accordance with the manufacturer's instructions. Aβ1–40,42 was represented as pg per ml of plasma and pg per mg of total protein (mean ± SD).
Immunohistochemistry and Image Analysis.
Aβ immunostaining and Congo red were performed as described (53). 4G8- and Congo red-positive Aβ deposits were visualized with an Olympus BX-51 microscope. Quantitative image analysis was routinely performed. Data are reported as percentage of immunolabeled area captured (positive pixels) divided by the full area captured (total pixels). See SI Text for more details.
Perl's Prussian Blue Reaction for Ferric Ion-Hemosiderin.
Sections were deparaffinized, hydrated through descending grades of ethanol, washed in distilled water, and incubated for 20 min in a solution containing 20% hydrochloric acid and 10% potassium ferrocyanide. These sections were washed three times for 5 min with H20 and counterstained with hematoxylin solution (Sigma) for 15 s and then mounted (23).
Statistical Analysis.
Means and SDs were calculated according to standard practice (51, 53).
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (J.T.), Alzheimer's Association (J.T.), and Byrd Alzheimer's Center and Research Institute (J.T. and R.D.S.). T.T. is supported by an Alzheimer's Association grant.
Abbreviations
- AD
Alzheimer's disease
- Aβ
β-amyloid
- t.c.
transcutaneous
- CT
cholera toxin
- Th
T helper
- LC
Langerhans cell.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609377104/DC1.
References
- 1.Selkoe DJ. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 3.Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, Millais SB, Donoghue S. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- 4.Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, et al. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 5.Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, Morgan D. J Neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, et al. J Neurosci. 2005;25:629–636. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 8.Ferrer I, Boada Rovira M, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maier M, Seabrook TJ, Lemere CA. Vaccine. 2005;23:5149–5159. doi: 10.1016/j.vaccine.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, Janus C, Lemere CA. J Neurosci. 2006;26:4717–4728. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itoh T, Celis E. J Immunother. 2005;28:430–437. doi: 10.1097/01.cji.0000171289.78495.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beignon AS, Brown F, Eftekhari P, Kramer E, Briand JP, Muller S, Partidos CD. Vet Immunol Immunopathol. 2005;104:273–280. doi: 10.1016/j.vetimm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Dell K, Koesters R, Linnebacher M, Klein C, Gissmann L. Vaccine. 2006;24:2238–2247. doi: 10.1016/j.vaccine.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 14.Giudice EL, Campbell JD. Adv Drug Deliv Rev. 2006;58:68–89. doi: 10.1016/j.addr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Larregina AT, Morelli AE, Spencer LA, Logar AJ, Watkins SC, Thomson AW, Falo LD., Jr Nat Immunol. 2001;2:1151–1158. doi: 10.1038/ni731. [DOI] [PubMed] [Google Scholar]
- 16.Niizeki H, Streilein JW. J Invest Dermatol. 1997;109:25–30. doi: 10.1111/1523-1747.ep12276415. [DOI] [PubMed] [Google Scholar]
- 17.Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, Borchelt DR. Biomol Eng. 2001;17:157–165. doi: 10.1016/s1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- 18.Town T, Tan J, Sansone N, Obregon D, Klein T, Mullan M. Neurosci Lett. 2001;307:101–104. doi: 10.1016/s0304-3940(01)01951-6. [DOI] [PubMed] [Google Scholar]
- 19.Town T, Vendrame M, Patel A, Poetter D, DelleDonne A, Mori T, Smeed R, Crawford F, Klein T, Tan J, Mullan M. J Neuroimmunol. 2002;132:49–59. doi: 10.1016/s0165-5728(02)00307-7. [DOI] [PubMed] [Google Scholar]
- 20.Douillard P, Stoitzner P, Tripp CH, Clair-Moninot V, Ait-Yahia S, McLellan AD, Eggert A, Romani N, Saeland S. J Invest Dermatol. 2005;125:983–994. doi: 10.1111/j.0022-202X.2005.23951.x. [DOI] [PubMed] [Google Scholar]
- 21.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Proc Natl Acad Sci USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuoka Y, Saito M, LaFrancois J, Gaynor K, Olm V, Wang L, Casey E, Lu Y, Shiratori C, Lemere C, Duff K. J Neurosci. 2003;23:29–33. doi: 10.1523/JNEUROSCI.23-01-00029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilcock DM, Alamed J, Gottschall PE, Grimm J, Rosenthal A, Pons J, Ronan V, Symmonds K, Gordon MN, Morgan D. J Neurosci. 2006;26:5340–5346. doi: 10.1523/JNEUROSCI.0695-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janus C. CNS Drugs. 2003;17:457–474. doi: 10.2165/00023210-200317070-00001. [DOI] [PubMed] [Google Scholar]
- 25.Monsonego A, Zota V, Karni A, Krieger JI, Bar-Or A, Bitan G, Budson AE, Sperling R, Selkoe DJ, Weiner HL. J Clin Invest. 2003;112:415–422. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, et al. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 27.Dickstein DL, Biron KE, Ujiie M, Pfeifer CG, Jeffries AR, Jefferies WA. FASEB J. 2006;20:426–433. doi: 10.1096/fj.05-3956com. [DOI] [PubMed] [Google Scholar]
- 28.Ma QL, Lim GP, Harris-White ME, Yang F, Ambegaokar SS, Ubeda OJ, Glabe CG, Teter B, Frautschy SA, Cole GM. J Neurosci Res. 2006;83:374–384. doi: 10.1002/jnr.20734. [DOI] [PubMed] [Google Scholar]
- 29.Schiller M, Metze D, Luger TA, Grabbe S, Gunzer M. Exp Dermatol. 2006;15:331–341. doi: 10.1111/j.0906-6705.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 30.Rozis G, de Silva S, Benlahrech A, Papagatsias T, Harris J, Gotch F, Dickson G, Patterson S. Eur J Immunol. 2005;35:2617–2626. doi: 10.1002/eji.200425939. [DOI] [PubMed] [Google Scholar]
- 31.Renn CN, Sanchez DJ, Ochoa MT, Legaspi AJ, Oh CK, Liu PT, Krutzik SR, Sieling PA, Cheng G, Modlin RL. J Immunol. 2006;177:298–305. doi: 10.4049/jimmunol.177.1.298. [DOI] [PubMed] [Google Scholar]
- 32.Strid J, Callard R, Strobel S. Immunology. 2006;119:27–35. doi: 10.1111/j.1365-2567.2006.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bynoe MS, Evans JT, Viret C, Janeway CA., Jr Immunity. 2003;19:317–328. doi: 10.1016/s1074-7613(03)00239-5. [DOI] [PubMed] [Google Scholar]
- 34.Bynoe MS, Viret C, Flavell RA, Janeway CA., Jr Proc Natl Acad Sci USA. 2005;102:2898–2903. doi: 10.1073/pnas.0409880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbas AK, Murphy KM, Sher A. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 36.Romagnani S. Ann Allergy Asthma Immunol. 2000;85:9–21. doi: 10.1016/S1081-1206(10)62426-X. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz MJ, Chiang S, Muller N, Ackenheil M. Brain Behav Immun. 2001;15:340–370. doi: 10.1006/brbi.2001.0647. [DOI] [PubMed] [Google Scholar]
- 38.Ghochikyan A, Mkrtichyan M, Petrushina I, Movsesyan N, Karapetyan A, Cribbs DH, Agadjanyan MG. Vaccine. 2006;24:2275–2282. doi: 10.1016/j.vaccine.2005.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HD, Cao Y, Kong FK, Van Kampen KR, Lewis TL, Ma Z, Tang DC, Fukuchi K. Vaccine. 2005;23:2977–2986. doi: 10.1016/j.vaccine.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 40.Dasilva KA, Brown ME, Westaway D, McLaurin J. Neurobiol Dis. 2006;23:433–444. doi: 10.1016/j.nbd.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Rosas LE, Keiser T, Barbi J, Satoskar AA, Septer A, Kaczmarek J, Lezama-Davila CM, Satoskar AR. Int Immunol. 2005;17:1347–1357. doi: 10.1093/intimm/dxh313. [DOI] [PubMed] [Google Scholar]
- 42.Fukushima A, Yamaguchi T, Ishida W, Fukata K, Taniguchi T, Liu FT, Ueno H. Exp Eye Res. 2006;82:210–218. doi: 10.1016/j.exer.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Eriksson K, Fredriksson M, Nordstrom I, Holmgren J. Infect Immun. 2003;71:1740–1747. doi: 10.1128/IAI.71.4.1740-1747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Town T, Tan J, Flavell RA, Mullan M. Neuromol Med. 2005;7:255–264. doi: 10.1385/NMM:7:3:255. [DOI] [PubMed] [Google Scholar]
- 45.Monsonego A, Maron R, Zota V, Selkoe DJ, Weiner HL. Proc Natl Acad Sci USA. 2001;98:10273–10278. doi: 10.1073/pnas.191118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 47.Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, Schenk D, Hyman BT. Nat Med. 2001;7:369–372. doi: 10.1038/85525. [DOI] [PubMed] [Google Scholar]
- 48.Skelding KA, Hickey DK, Horvat JC, Bao S, Roberts KG, Finnie JM, Hansbro PM, Beagley KW. Vaccine. 2006;24:355–366. doi: 10.1016/j.vaccine.2005.07.104. [DOI] [PubMed] [Google Scholar]
- 49.Tan J, Town T, Mori T, Wu Y, Saxe M, Crawford F, Mullan M. J Neurosci. 2000;20:7587–7594. doi: 10.1523/JNEUROSCI.20-20-07587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lemere CA, Spooner ET, Leverone JF, Mori C, Clements JD. Neurobiol Aging. 2002;23:991–1000. doi: 10.1016/s0197-4580(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 51.Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K, Zeng J, Morgan D, et al. J Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Gordon M, Tan H, Games D, et al. Proc Natl Acad Sci USA. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan J, Town T, Crawford F, Mori T, DelleDonne A, Crescentini R, Obregon D, Flavell RA, Mullan MJ. Nat Neurosci. 2002;5:1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.