Abstract
Adaptive transcriptional responses to oxygen deprivation (hypoxia) are mediated by the hypoxia-inducible factors (HIFs), heterodimeric transcription factors composed of two basic helix–loop–helix–PAS family proteins. The transcriptional activity of HIF is determined by the hypoxic stabilization of the HIF-α proteins. HIF-1α and HIF-2α exhibit high sequence homology but have different mRNA expression patterns; HIF-1α is expressed ubiquitously whereas HIF-2α expression is more restricted to certain tissues, e.g., the endothelium, lung, brain, and neural crest derivatives. Germ-line deletion of either HIF subunit is embryonic lethal with unique features suggesting important roles for both HIF-α isoforms. Global deletion of Hif-2α results in distinct phenotypes depending on the mouse strain used for the mutation, clearly demonstrating an important role for HIF-2α in mouse development. The function of HIF-2α in adult life, however, remains incompletely understood. In this study, we describe the generation of a conditional murine Hif-2α allele and the effect of its acute postnatal ablation. Under very stringent conditions, we ablate Hif-2α after birth and compare the effect of acute global deletion of Hif-2α and Hif-1α. Our results demonstrate that HIF-2α plays a critical role in adult erythropoiesis, with acute deletion leading to anemia. Furthermore, although HIF-1α was first purified and cloned based on its affinity for the human erythropoietin (EPO) 3′ enhancer hypoxia response element (HRE) and regulates Epo expression during mouse embryogenesis, HIF-2α is the critical α isoform regulating Epo under physiologic and stress conditions in adults.
Keywords: Epo, hypoxia-inducible factor, hypoxia, red blood cell
Hypoxia-inducible factors (HIFs), members of the basic helix–loop–helix (bHLH)-PAS family of transcription factors, are master regulators of oxygen (O2) homeostasis and stimulate genes important for angiogenesis, erythropoiesis and glucose metabolism (1–3). HIFs are heterodimeric factors consisting of α (HIF-1α, -2α, and -3α) and β subunits [HIF-1β or ARNT (arylhydrocarbon-receptor nuclear translocator)]. The activity of HIF is regulated via the labile α subunit, whereas HIF-β is expressed constitutively. At normal O2 levels (≥5% or 36 mmHg) HIF-α is hydroxylated by prolyl hydroxylases (PHDs), allowing recognition by the tumor suppressor protein von Hippel-Lindau (pVHL), and targeting for proteosomal degradation (4–6). Under conditions of low O2 (< 5%), HIF-α is no longer degraded, and translocates into the nucleus where it forms a heterodimeric transcription factor with ARNT. HIF activates the transcription of >150 target genes by binding to HREs in their promoter/enhancer regions (7). The closely related HIF-1α and HIF-2α proteins activate common target genes (e.g., VEGF) but also regulate unique genes, such as glycolytic genes [glucose transporter 1 (Glut-1) and phosphoglycerate kinase (PGK)] or TGF-α and the stem cell factor Oct-4, respectively (8–10, 35). The regulation of different target genes might be due to distinct α subunit expression patterns, as HIF-1α mRNA seems to be expressed ubiquitously whereas HIF-2α mRNA expression is more restricted to endothelial cells, the mesenchyme of the lung, and neural crest derivatives during development (11, 12). Immunohistochemical analysis of HIF-2α in adult rat organs also demonstrated hypoxic stabiliziation of HIF-2α in distinct cell populations in the brain, heart, lung, kidney, liver, pancreas, and intestine (13). HIF activity is essential during embryogenesis as deletion of Arnt, Hif-1α, and Hif-2α in murine models leads to embryonic lethality (14–22). The phenotype of Hif-2α−/− embryos ranges from embryonic lethality between embryonic day (E) 9.5 and E13.5 because of vascular disorganization in the yolk sac and embryo (19), mid-gestational lethality because of bradycardia (17) and perinatal death as a result of defects in lung maturation (18). The variable phenotypes observed are likely due to modifier loci in different mouse strains. These results clearly demonstrate an important role for HIF-2α during embryogenesis and shortly after birth. The role of HIF-2α in the adult, however, has not yet been fully described. Scortegagna et al. (23) obtained a small number of Hif-2α−/− mice that survived until adulthood by generating a unique background in the F1 generation of C57/Black6 and 129 interbred heterozygotes. These mice suffer from multiple organ pathologies, impaired homeostasis of reactive oxygen species (ROS), metabolic abnormalities, mitochondrial distress syndrome, and pancytopenia. However, as a result of strain dependent influences on Hif-2α mutant phenotypes, it is possible that some of the findings described in this study are due to additional loci other than Hif-2α or the impairment of mitochondrial metabolism.
To investigate the adult role of HIF-2α in a controlled fashion, we generated a conditional allele of Hif-2α. By crossing this allele to a ubiquitously expressed, inducible Cre transgene (Ubc-CreERT2) we ablated Hif-2α postnatally, allowing us to investigate the physiologic role of HIF-2α in the adult while excluding possible secondary effects because of the lack of HIF-2α during embryogenesis. This study describes the role of HIF-2α in ensuring proper adult erythropoiesis and the development of anemia when HIF-2α is depleted. Acute deletion of Hif-2α resulted in reduced red blood cell numbers, hemoglobin and hematocrit values. Furthermore, the potential of bone marrow progenitors to form erythroid colonies in vitro was reduced in Hif-2α mutant animals. We also deleted Hif-1α in adults using the inducible Cre system. Surprisingly, we determined that HIF-2α, and not HIF-1α, is the critical HIF complex regulating erythropoietin expression in vivo under both physiologic and stress conditions.
Results
Creation of a Conditional Hif-2α Allele.
We generated a conditional allele of the murine Hif-2α locus by flanking exon 2, encoding the DNA binding bHLH domain with loxP sites [for details, see supporting information (SI) Materials and Methods]. Briefly, the targeting vector shown in Fig. 1A was electroporated into murine ES cells and clones were isolated subsequent to G418/gancyclovir selection. Properly targeted ES cells were confirmed by Southern blot analysis by using a probe within the endogenous locus. A 3-loxP clone was injected into blastocysts. After germ-line transmission, a 3-loxP founder was crossed to an E2A Cre line (24) to obtain 2-loxP mice referred to as Hif-2αfl/fl. These mice appear normal and are unaffected by the introduction of the two loxP sites. In addition, recombined Hif-2α embryos, referred to as Hif-2aΔ/Δ, resemble the germ-line knockout and are embryonic lethal. More precisely, no live born Hif-2aΔ/Δ pups were recovered from Hif-2αfl/Δ inter-crosses; however, all three genotypes were detected at Mendelian ratios at E13.5 (data not shown). As expected Hif-2αfl/Δ mice are viable and healthy.
Fig. 1.
Generation of a conditional Hif-2α allele. (A) Schematic of the targeting vector and recombination steps. (B) PCR genotyping of mice with Cre activated as young pups. Lanes 1 –3 show Hif-2αfl/fl mice without Cre expression. Lane 4* shows a Hif-2αfl/Δ animal without Cre expression. Samples in lanes 5–7 represent DNA from mice which harbor the Cre transgene and show loss of Hif-2α. (C) Southern blot analysis of DNA isolated from the kidney (lanes 1, 4, 7), the liver (lanes 2, 5, 8), and the lung (lanes 3, 6, 9) of Cre negative and positive animals. (D) RT-PCR analysis of cDNA from kidneys and livers of six different animals. Lanes 1 and 7 show floxed mRNA transcripts still containing exon 2 (650 bp). Lanes 2*, 3*, 8*, and 9* express both the floxed and deleted exon 2 mRNA, and a background band. Depletion of Hif-2α results in total loss of exon 2 as displayed in lanes 4–6, and 10–12 (450 bp). (E) β-Galactosidase expression after Cre activation in ROSA26 reporter mice.
Postnatal Deletion of Hif-2α.
As stated above, Hif-2aΔ/Δ pups died during gestation. To investigate the effects of Hif-2α deletion postnatally we used an inducible ubiquitously expressed Cre transgene (Ubc-Cre). This Cre-ERT2 transgene is expressed from the human Ubiquitin C promoter (Y. Ruzankina, C. Pinzon-Guzman, A. Assare, T. Ong, L. Pontano, G. Cotsarelis, V. P. Zediak, M. Velez, A. Bhandoola, and E. J. Brown, personal communication) encoding the Cre protein fused to a modified form of the estrogen receptor that specifically binds tamoxifen with high affinity and is inactive until tamoxifen is administered to the mouse (25). Cre-ERT2 recombinase was activated in adult mice at 6–8 weeks of age by administering tamoxifen through oral gavage. Recombination events occurred with some variability, ranging from 60% to 90% deletion (see Fig. 2D). To obtain reproducible and uniform recombination efficiency and delete Hif-2α earlier in life, we also administered tamoxifen to nursing mothers starting at 3 days postpartum. DNA from tailclippings at weaning (3–4 weeks of age) showed that recombination at this early age is highly efficient and in most cases leads to almost 100% deletion. A representative litter from a Hif-2αfl/fl and Hif-2αfl/Δ/Cre+ cross is presented in Fig. 1B. Tamoxifen was administered to the whole litter. Hif-2αfl/fl and Hif-2αfl/Δ mice lacking the Cre transgene serve as controls for possible background effects resulting from the tamoxifen treatment. Hif-2αfl/fl mice only express the 2-loxP allele (Fig. 1B, lanes 1–3). Hif-2αfl/Δ offspring were obtained from the parental Hif-2αfl/fl by Hif-2αfl/Δ cross when the animal did not inherit the Cre transgene (lane 4*). Hif-2αfl/fl and Hif-2αfl/Δ animals harboring Cre recombined exon 2 so that at weaning only the deleted allele (Hif-2aΔ/Δ) was detectable (lanes 5–7). Southern blot analysis of DNA obtained from various organs demonstrated that recombination occurs in all organs analyzed (brain, heart, kidney, liver, lung) at approximately the same efficiency. Fig. 1C shows the analysis for kidney, liver and lung samples. In addition to the genomic locus, we analyzed mRNA transcripts expressed by the recombined allele. As shown in Fig. 1D, RT-PCR analysis of kidney and liver mRNAs revealed that, no full-length RNA was transcribed in Hif-2aΔ/Δ mice. In contrast, full-length mRNA and mRNA lacking exon 2 were both transcribed in Hif-2αfl/Δ mice, and Hif-2αfl/fl mice expressed only full-length mRNA. We also investigated the recombination efficiency by crossing the Ubc-Cre transgene to a ROSA26 reporter strain (26). Staining for β-galactosidase activity in the brain, kidney and liver (Fig. 1E) confirmed the positive loop-out observed by Southern blot and RT-PCR analysis.
Fig. 2.
Hif-2αΔ/Δ mice are anemic. (A–C) Total red blood cell (RBC) numbers (A), hemoglobin concentration (B) and hematocrit levels (C) are reduced 25%, 32%, and 30%, respectively, in mice with high efficiency of Hif-2α exon 2 deletion. Medium deletion leads to a drop of only 8% (RBCs), 14% (hemoglobin), and 10% (hematocrit levels), and low efficiency of Hif-2α exon 2 deletion does not show a decrease compared with Hif-2αfl/Δ levels. (D) PCR analysis of DNA isolated from earpunches taken 2 weeks after Cre activation. Lanes 1–7 are negative for Cre and therefore either fl/wt (lanes 2, 6, and 7) or fl/Δ (lanes 1 and 3–5). Lanes 8–16 are positive for the Cre transgene and according to PCR have lost 90–100% of Hif-2α expression (lanes 8–10, and 13) or ≈75% of Hif-2α (lanes 12, 14, and 15), or show very weak loop-out, in which case the animal resembles a Hif-2α heterozygous (lanes 11 and 16). (E) Activation of Cre during the first week after birth results in a uniformly high deletion efficiency and leads to a 32% drop in spun hematocrit value. (F) Mice that exhibit Hif-1α deletion fail to develop anemia. (G) Loop-out efficiency for Hif-1α.
Postnatal Ablation of Hif-2α Results in Anemia.
To investigate the role of HIF-2α in adult mice we acutely deleted Hif-2α at 6–8 weeks of age. Genotyping was performed 2 weeks later to confirm that the recombination event had occurred (Fig. 2D). Whole blood cell analysis performed 2 months after Cre activation showed that red blood cell numbers, hematocrit values and hemoglobin levels were decreased in Hif-2aΔ/Δ mice (Fig. 2 A–C). The mean corpuscular volume values were not significantly changed in Hif-2αfl/Δ (48 fl) and Hif-2αΔ/Δ (46 fl) animals. As described in Fig. 2 A–D, the severity of the anemia correlated with the deletion efficiency. The relationship of red blood cell number, hemoglobin, and hematocrit value to the degree of Hif-2α deletion suggests a dosage effect of HIF-2α on red blood cell number and hemoglobin levels. Mice were divided into three groups: (i) high deletion efficiency (n = 5) (90–100% Hif-2α recombination), (ii) medium deletion efficiency (n = 7) (75% recombination), and (iii) low deletion efficiency (n = 3) (50% recombination, resembling a heterozygous animal) and compared with Hif-2αfl/fl and Hif-2αfl/Δ littermates (n = 15) (Fig. 2D). Spun hematocrit analysis performed on blood from mice that had Hif-2α deleted as young pups (Fig. 1B) shows a 32% decrease in mutant hematocrit levels (n = 10) compared with Hif-2αfl/Δ mice (n = 11) (Fig. 2G). For comparison, we also analyzed the hematocrit levels of Hif-1αΔ/Δ mice (27), which, in direct contrast to Hif-2α deletion, does not lead to anemia (Fig. 2H). Blood smears from Hif-2aΔ/Δ mice did not exhibit any abnormalities compared with Hif-2αfl/Δ animals and haptoglobin levels in the serum were not reduced (SI Fig. 6). Reticulocyte numbers, however, were decreased in Hif-2αΔ/Δ animals (data not shown), which is consistent with hypoproliferative anemia and all together excludes red blood cell lysis as an explanation for the anemia observed in Hif-2aΔ/Δ animals.
Deletion of Hif-2α Leads to Decreased Potential of Progenitor Cells to Differentiate into BFU-Es (Burst-Forming Units-Erythroid) and CFU-Es (Colony-Forming Units-Erythroid) in Vitro.
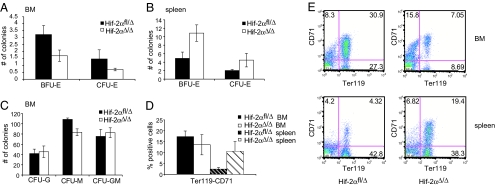
Hif-2aΔ/Δ hematopoietic progenitor cells were tested for their potential to differentiate into committed erythroid cells. Specifically, bone marrow and splenic cells were plated in methylcellulose containing erythropoietin (EPO), which supports erythroid colony growth, but no other cytokines. Progenitor cells from Hif-2aΔ/Δ bone marrow formed ≈50% fewer BFU-E and CFU-E colonies than Hif-2αfl/Δ bone marrow (Fig. 3A). On the contrary, progenitors from mutant spleens formed more BFU-E and CFU-E colonies compared with Hif-2αfl/Δ spleens (Fig. 3B). This progenitor defect is also specific to the erythroid lineage, because CFU-granulocyte (G), CFU-macrophage (M) and CFU-GM numbers were not significantly changed in cultures obtained from mutant bone marrow cells grown in methylcellulose containing stem cell factor, IL-3, IL-6, and EPO (Fig. 3C). In addition, we performed FACS analysis of bone marrow and spleen cells using Ter119 and CD71 (transferrin receptor) as markers for maturing erythroid progenitors. CD71 is expressed on immature erythroid progenitors, which then develop into CD71 and Ter119 double positive cells and finally Ter119 single positive cells, which are mature erythroids. Gating on the CD71+/Ter119+ population revealed fewer precursor cells in the bone marrow and a greater percentage of double positive cells in the spleen of Hif-2aΔ/Δ mice (Fig. 3 D and E), consistent with our result obtained in colony formation assays.
Fig. 3.
Erythroid progenitor cells are decreased in Hif-2αΔ/Δ bone marrow but increased in the spleen. (A) Hif-2αΔ/Δ bone marrow progenitor cells form fewer erythroid colonies in methylcellulose than Hif-2αfl/Δ cells do (n = 4). (B) In contrast, Hif-2αΔ/Δ splenic progenitor cells have a higher potential to form erythroid colonies than cells from Hif-2αfl/Δ spleens (n = 4). (C) There is little difference in the number of nonerythroid colonies formed by Hif-2αfl/Δ and Hif-2αΔ/Δ bone marrow cells (n = 4). (D) The percentage of Ter119high/CD71high erythroid progenitor cells is reduced in Hif-2αΔ/Δ bone marrow, but increased in Hif-2αΔ/Δ spleen (n = 3). (E) Representative FACS blots for Hif-2αfl/Δ and Hif-2αΔ/Δ bone marrow and spleen stained for Ter119 and CD71.
Stress Induced Activation of Erythropoietin Requires Hif-2α.
HIF has been strongly implicated in regulating Epo expression in vivo and in vitro (28–30). We investigated whether Hif-2aΔ/Δ mice were capable of inducing EPO levels in response to stress induced erythropoiesis. Phenylhydrazine (PH), an oxidizing agent that induces severe hemolysis, was injected i.p. for 2 consecutive days to deplete the animals of their red blood cells and induce erythropoiesis. On day 3, hematocrits of all manipulated mice were reduced to 50% of their original value. Hif-2αfl/fl and Hif-2αfl/Δ animals started with a hematocrit of 45–50% which was reduced to 20–25%. Hif-2aΔ/Δ mice exhibited a hematocrit of 28–39% before treatment and 13–20% on day 3. As a result of active erythropoiesis, we also observed an increase in the spleen-to-body weight ratio in Hif-2αfl/Δ animals (0.4–0.7%, n = 6). Hif-2aΔ/Δ spleens were already larger (0.5%, n = 6) and PH treatment did not lead to additional growth of these organs (0.55%, n = 6) (Fig. 4A). Next, EPO levels in the serum were analyzed by ELISA. Whereas there were extremely low levels of circulating EPO in the serum of untreated WT mice, PH treatment leads to a drastic increase in EPO levels, from ≈150 pg/ml in untreated animals to 16,000 pg/ml in those treated with PH (n = 4) (Fig. 4B). Hif-2αfl/Δ mice (n = 5) exhibited significant EPO induction, however not to the extent of WT levels. In contrast, Hif-2aΔ/Δ mice (n = 6) showed a much weaker EPO induction in response to PH treatment, suggesting an important role for HIF-2α in Epo regulation during stress (Fig. 4B). Hif-1α deletion (n = 3), however, does not alter EPO induction (Fig. 4C), suggesting that HIF-2α is the primary HIF-α isoform necessary for stress-related Epo gene regulation in vivo. We also examined circulating VEGF levels by ELISA after PH treatment, but found no genotype specific differences in induction (n = 5) under these stress conditions (SI Fig. 7). To further investigate HIF-2α specific Epo regulation we performed rescue experiments with injections of physiologic levels of exogenous human EPO on Hif-2αfl/Δ and anemic Hif-2aΔ/Δ animals. This treatment lead to an increase in RBCs, hemoglobin and hematocrit after 1 week and elevation to normal levels after 2 weeks. Fig. 4D shows the increase in hematocrit over time.
Fig. 4.
Epo expression after stress-induced erythropoiesis depends on HIF-2α. (A) Spleen-to-body weight ration of PH untreated and treated Hif-2αfl/Δ and Hif-2αΔ/Δ mice. (B) Serum EPO levels from PH treated Hif-2α fl/fl, fl/Δ, and Δ/Δ mice. (C) Hif-1α fl/fl and Δ/Δ mice do not exhibit any defects in EPO stimulation after PH treatment. (D) Hematocrit values increase in Hif-2αfl/Δ and Hif-2αΔ/Δ animals because of EPO treatment and reach normal levels after 2 weeks in Hif-2αΔ/Δ mice.
In Vitro Induction of Epo by HIF-1α and HIF-2α.
To further investigate the role of HIF-1α and HIF-2α in activating Epo transcription we used siRNA to “knockdown” HIF-1α and HIF-2α mRNA and protein expression in Hep3B cells, human hepatocytes expressing both HIF-1α and HIF-2α which produce EPO (8, 29, 31). HIF-1α and HIF-2α mRNA levels were assessed 36 h after siRNA transfection by Q-PCR. Each siRNA is specific for its HIF-target and resulted in ≈80–90% mRNA knockdown (data not shown). Untransfected cells, and cells transfected with control siRNA accumulated HIF-1α and HIF-2α protein when cultured at 1.5% O2 (Fig. 5A, lanes 2 and 4). HIF-1α siRNA specifically depletes HIF-1α protein (lanes 6 and 10), whereas HIF-2α siRNA only depletes HIF-2α (lanes 8 and 10). To investigate EPO induction, cells were cultured at 1.5% O2 for 16 h, and mRNA levels were assessed by using Q-PCR. Hypoxic culture conditions resulted in a 55-fold induction of EPO mRNA transcripts. HIF-2α knockdown severely reduced hypoxic EPO induction by ≈10-fold, whereas HIF-1α knockdown resulted in a moderate decline. This remaining induction might be due to incomplete knockdown of HIF-2α. HIF-1α and HIF-2α knockdown in combination almost completely abolished the induction of EPO transcription, suggesting a cumulative effect of HIF-1α and HIF-2α on EPO transcription (Fig. 5B). We also performed a gain of function experiment transfecting Hep3B cells with increasing concentrations of cDNA encoding a mutated form of HIF-1α and HIF-2α. In this triple mutant (“TM”) isoform the asparagine in the transactivation domain and the two proline residues in the oxygen dependent degradation (ODD) domain have been mutated to alanine, thus rendering it constitutively stable and functional under normoxia. As shown in Fig. 5C, overexpression of exogenous, stabilized HIF-1α in vitro potently induced EPO mRNA expression. Interestingly, HIF-2α over-expression also increased expression of EPO RNA, although not as strongly as HIF-1α. Fig. 5D shows the corresponding levels of over-expressed HIF protein. These data confirm previous results that HIF-1α is a very potent activator of EPO transcription when expressed exogenously (29), but indicates that under physiologic conditions, HIF-2α is the dominant HIF-α isoform regulating EPO expression.
Fig. 5.
In vitro analysis of the ability of HIF-1α and HIF-2α to induce EPO mRNA transcription. (A) siRNA knockdown of HIF-1α and HIF-2α. Western blot for HIF-1α and HIF-2α shows stabilization of the protein under hypoxia (H) and specific loss of the appropriate protein after siRNA transfection. (B) EPO mRNA levels in Hep3B cells. Fold change of mRNA under hypoxia compared with normoxic cells. Nontransfected cells and cells transfected with control siRNA show a 55-fold induction of EPO mRNA under hypoxia. Knocking-down HIF-1α results in a 45-fold increase in EPO mRNA. In contrast, siRNA against HIF-2α resulted in a 5-fold induction and knocking-down both HIFs almost completely ablates EPO mRNA induction under hypoxia. (C) A mutated (“TM”) and therefore stabilized form of HIF-1α and HIF-2α was introduced into Hep3B cells at increasing concentrations, resulting in a strong induction of EPO mRNA for HIF-1α and a less prominent induction for HIF-2α. (D) Western blot displaying the levels of overexpressed protein.
Discussion
Germ-line deletion of Hif-2α demonstrates a crucial function for this transcription factor during embryogenesis associated with a variety of phenotypes (17–19). Because of embryonic lethality, the role of HIF-2α in the adult remains largely unknown. Previous studies suggest a role for HIF-2α in the bone marrow microenvironment during hematopoiesis and that treatment of these pancytopenic mice with exogenous EPO could reverse the hematopoietic phenotype (30, 32).
In an attempt to obtain insight into the postnatal role of HIF-2α through acute deletion, we generated a conditional Hif-2α allele by flanking exon 2 with loxP sites. After crossing the Hif-2αfl/fl allele to an inducible, ubiquitously expressed Cre transgene (Ubc-Cre-ERT2) we acutely ablated Hif-2α in pups shortly after birth, thereby overcoming embryonic lethality and avoiding complex pathologies observed in live-born Hif-2α−/− F1 mice. We activated the Cre transgene as early as 3 days after birth which led to efficient deletion at the genomic locus and inhibited full length Hif-2α mRNA transcription in all organs investigated. This acute loss of HIF-2α resulted in severe anemia which correlated with loop-out efficiency. By deleting Hif-2α shortly after birth we obtained a 32% drop in hematocrits compared with WT animals. Of note, exogenous EPO rescued this phenotype. The anemia was specific to Hif-2α loss because Hif-1α deletion did not alter hematocrits. The potential of Hif-2aΔ/Δ bone marrow and spleen progenitor cells to differentiate into erythroid colonies in vitro displayed a bone marrow progentior defect to form BFU-E and CFU-E colonies, but an increase in colonies formed by splenic cells. These results suggest a role for HIF-2α in adult erythropoiesis in the bone marrow and a potential compensatory function of the spleen in Hif-2aΔ/Δ animals. This possibility is consistent with the observation that Hif-2aΔ/Δ spleens are slightly larger than WT spleens.
Challenging the animals with phenylhydrazine (PH) allowed us to further investigate the role of HIF-2α during erythropoiesis. HIF-2α has a critical function in red blood cell development by activating Epo transcription. Hif-2aΔ/Δ mice displayed a much weaker induction of circulating EPO levels in response to PH than Hif-2αfl/fl animals. As acute loss of Hif-1α after birth does not interfere with Epo induction, these data suggest that HIF-2α is the dominant HIF-α isoform controlling EPO levels in the adult animal. This idea is further supported by the observation that Hif-2α and Epo are expressed in renal interstitial cells, whereas Hif-1α is predominantly expressed in the tubular cells of the kidney (13, 30, 33, 34). In addition to HIF-2α's role in regulating physiologic Epo expression, HIF-2α also regulates Epo in livers lacking pVHL (36). This observation contrasts with the recently described role for HIF-1α to regulate Epo expression in the embryo (28). Our in vivo observation is further supported by in vitro data obtained after siRNA knockdown of either isoform in Hep3B cells. HIF-1α knockdown has only a small effect on EPO transcription compared with the major reduction of EPO mRNA levels observed after HIF-2α inhibition. Over-expression of exogenous, stabilized HIF-1α and HIF-2α confirmed the previously published result for HIF-1α to induce Epo mRNA expression in vitro (29), whereas HIF-2α is strikingly less active in these assays. Interestingly, even though HIF-1α was identified because of its ability to bind to the human EPO 3′ enhancer (31), HIF-2α is the important HIF-α subunit responsible for Epo regulation in vivo.
By generating a conditional allele of murine Hif-2α, we were able to investigate the effect of acutely deleting Hif-2α after birth, and examine the specific physiologic role of HIF-2α during adulthood in a controlled manner. In addition to our findings that HIF-2α regulates adult erythropoiesis we have also shown that it is the important HIF-α subunit regulating EPO in vivo during stress. We did not observe any of the other previously described pathologies (23) in our acute model system, suggesting that these phenotypes may be a result of the mitochondrial defects exhibited by F1 mice. Furthermore, we have created a useful tool for studying the function of HIF-2α in other physiologic and pathologic situations in adulthood.
Materials and Methods
Generation and Analysis of Mice.
Cre-mediated recombination between the loxP sites in the 2-loxP allele produces the 1-loxP allele, which lacks exon 2 and produces a mutant mRNA transcript containing multiple in-frame stop codons downstream of exon 1 sequences. The WT, 2-loxP, and 1-loxP allele can be distinguished by a multiplex PCR [primer (P) 1: 5′-CAGGCAGTATGCCTGGCTAATTCCAGTT-3′; P2: 5′-CTTCTTCCATCATCTGGGATCTGGGACT-3′; P3: 5′-GCTAACACTGTACTGTCTGAAAGAGTAGC-3′]. The WT allele produces a 410-bp fragment (P1 and P2), the 2-loxP allele produces a 444-bp fragment (P1 and P2), and the 1-loxP allele produces a 340-bp fragment (P1 and P3).
Genotyping for Hif-1α, Ubc-Cre-ERT2 and ROSAlacZ has been described (26, 27) (Y. Ruzankina, C. Pinzon-Guzman, A. Assare, T. Ong, L. Pontano, G. Cotsarelis, V. P. Zediak, M. Velez, A. Bhandoola, and E. J. Brown, personal communication).
All procedures involving mice were performed in accordance with National Institutes of Health guidelines for use and care of live animals and were approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC).
Tamoxifen Treatment.
Tamoxifen free base (MP Biomedicals, Solon, OH) was administered at a concentration of 200 μg/g body weight for the adult mice, or 7 mg for the nursing mothers, once a day, 5 times within one week to achieve loop-out in Hif-2αfl/fl, Hif-1αfl/fl, and RosaLacZfl/fl animals.
Phenylhydrazine Treatment.
Hif-2αfl/Δ and Hif-2aΔ/Δ mice were injected i.p. on day 1 and 2 with 30 μg/g phenylhydrazine hydrochloride solution in PBS. Blood (500 μl) was collected on day 3 and mice were killed.
Epo Treatment.
Animals received human recombinant Epo (Epogen; Amgen, Thousand Oaks, CA) 3 times a week at a concentration of 500 units/kg body weight.
EPO and VEGF and Haptoglobin ELISA.
Blood was collected by retroorbital bleed and allowed to clot for 2 h at room temperature or overnight at 4°C. Serum was collected by centrifuging for 20 min at 2000 × g. The ELISA was performed according to the manufacturer's protocol (R & D Systems, Minneapolis, MN; ICL, Newberg, OR).
Knockdown of HIF-α mRNAs Using siRNAs.
Control or siRNAs specific for human HIF-1α or HIF-2α mRNAs were synthesized by Qiagen. Sixty percent confluent Hep3B cells were transfected with siRNAs at a final concentration of 5 nM by using HiPerFect Transfection Reagent according to the manufacturer's protocol. Twenty-four hours posttransfection, cells were cultured at 21% or 1.5% O2 for 12 h and collected to analyze HIF-α RNA, protein, or target genes. The following primers were used to quantify the HIF-1α, HIF-2α, and EPO mRNAs via SYBR-Green based Q-PCR: HIF-1α F, 5′-TTTTACCATGCCCCAGATTCA-3′; HIF-1α R, 5′-AGTGCTTCCATCGGAAGGACT-3′; HIF-2α F, 5′-TACAAGGAGCCCCTGCTTC-3′; HIF-2α R, 5′-TGCTGGATTGGTTCACACATG-3′; EPO F, 5′-TTCGCAGCCTCACCACTCT-3′; and EPO R, 5′-GAGATGGCTTCCTTCTGGGC-3′. The following primers were used as control to normalize RNA levels: β-ACTIN F, 5′-GCCCTGAGGCACTCTTCCA-3′; and β-ACTIN R, 5′-ATGCCACAGGACTCCATGC-3′.
The following protocols can be found in SI Materials and Methods: RNA preparation, blood analysis, CFU assays, flow cytometry, Western blot analysis, β-galactosidase staining, and statistical analysis.
Supplementary Material
Acknowledgments
We thank Anja Runge, Mercy Gohil, Aneesa Sataur, and Yi Pan for technical assistance and members of the M.C.S. laboratory for thoughtful discussions and reading of the manuscript. M.G. is supported by a Ph.D. fellowship from the Boehringer Ingelheim Fonds. This research was supported by National Institutes of Health Grant 66310 and by the Abramson Family Cancer Research Institute. M.C.S. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- HIF
hypoxia-inducible factor
- bHLH
basic helix–loop–helix
- ARNT
arylhydrocarbon-receptor nuclear translocator
- En
embryonic day n
- ROS
reactive oxygen species
- BFU-E
burst-forming unit-erythroid
- CFU-E
colony-forming unit-erythroid
- EPO
erythropoietin
- PH
phenylhydrazine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608382104/DC1.
References
- 1.Schofield CJ, Ratcliffe PJ. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL. Trends Mol Med. 2001;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 3.Giaccia AJ, Simon MC, Johnson R. Genes Dev. 2004;18:2183–2194. doi: 10.1101/gad.1243304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 5.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, et al. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 6.Yu F, White SB, Zhao Q, Lee FS. Proc Natl Acad Sci USA. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semenza GL. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 8.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seagroves TN, Ryan HE, Lu H, Wouters BG, Knapp M, Thibault P, Laderoute K, Johnson RS. Mol Cell Biol. 2001;21:3436–3444. doi: 10.1128/MCB.21.10.3436-3444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain RK, Safabakhsh N, Sckell A, Chen Y, Jiang P, Benjamin L, Yuan F, Keshet E. Proc Natl Acad Sci USA. 1998;95:10820–10825. doi: 10.1073/pnas.95.18.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian H, McKnight SL, Russell DW. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 13.Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, et al. FASEB J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 14.Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- 15.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan HE, Lo J, Johnson RS. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, et al. Nat Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 19.Peng J, Zhang L, Drysdale L, Fong GH. Proc Natl Acad Sci USA. 2000;97:8386–8391. doi: 10.1073/pnas.140087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramirez-Bergeron DL, Runge A, Adelman DM, Gohil M, Simon MC. Dev Cell. 2006;11:81–92. doi: 10.1016/j.devcel.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adelman DM, Maltepe E, Simon MC. Genes Dev. 1999;13:2478–2483. doi: 10.1101/gad.13.19.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, et al. Nat Genet. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 24.Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feil R, Wagner J, Metzger D, Chambon P. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 26.Soriano P. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 27.Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- 28.Yoon D, Pastore YD, Divoky V, Liu E, Mlodnicka AE, Rainey K, Ponka P, Semenza GL, Schumacher A, Prchal JT. J Biol Chem. 2006;281:25703–25711. doi: 10.1074/jbc.M602329200. [DOI] [PubMed] [Google Scholar]
- 29.Wang GL, Semenza GL. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 30.Scortegagna M, Ding K, Zhang Q, Oktay Y, Bennett MJ, Bennett M, Shelton JM, Richardson JA, Moe O, Garcia JA. Blood. 2005;105:3133–3140. doi: 10.1182/blood-2004-05-1695. [DOI] [PubMed] [Google Scholar]
- 31.Wang GL, Jiang B-H, Rue EA, Semenza GL. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA. Blood. 2003;102:1634–1640. doi: 10.1182/blood-2003-02-0448. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberger C, Mandriota S, Jurgensen JS, Wiesener MS, Horstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU. J Am Soc Nephrol. 2002;13:1721–1732. doi: 10.1097/01.asn.0000017223.49823.2a. [DOI] [PubMed] [Google Scholar]
- 34.Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D. FASEB J. 2001;15:2445–2453. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- 35.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. Cancer Cell. 2007 doi: 10.1016/j.ccr.2007.02.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rankin EB, Biju MP, Liu Q, Tha J, Bernhardt WM, Eckardt KU, Wiesener M, Johnson R.S., Simon MC, Keith B, Haase VH. J Clin Invest. 2007 doi: 10.1172/JCI30117. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.