Abstract
Human progesterone receptors (PR) exist as two functionally distinct isoforms, PR-A and PR-B. The proteins are identical except for an additional 164 residues located at the N terminus of PR-B. To determine the mechanisms responsible for isoform-specific functional differences, we present here a thermodynamic dissection of PR-A–promoter interactions and compare the results to our previous work on PR-B. This analysis has generated a number of results inconsistent with the traditional, biochemically based model of receptor function. Specifically, statistical models invoking preformed PR-A dimers as the active binding species demonstrate that intrinsic binding energetics are over an order of magnitude greater than is apparent. High-affinity binding is opposed, however, by a large energetic penalty. The consequences of this penalty are 2-fold: Successive monomer binding to a palindromic response element is thermodynamically favored over preformed dimer binding, and DNA-induced dimerization of the monomers is largely abolished. Furthermore, PR-A binding to multiple PREs is only weakly cooperative, as judged by a 5-fold increase in overall stability. Comparison of these results to our work on PR-B demonstrates that whereas both isoforms appear to have similar DNA binding affinities, PR-B in fact has a greatly increased intrinsic binding affinity and cooperative binding ability relative to PR-A. These differences thus suggest that residues unique to PR-B allosterically regulate the energetics of cooperative promoter assembly. From a functional perspective, the differences in microscopic affinities predict receptor–promoter occupancies that accurately correlate with the transcriptional activation profiles seen for each isoform.
Keywords: nuclear receptor, protein–DNA interactions, quantitative footprint titrations, statistical mechanics
Progesterone receptors (PR) are members of the nuclear receptor superfamily of ligand activated transcription factors (1). An understanding of PR function is made complicated by the presence of two structurally distinct isoforms: an 83-kDa A-receptor (PR-A) and a 99-kDa B-receptor (PR-B). The two receptors are identical in sequence save for 164 aa located at the N terminus of PR-B. These residues define the B-unique sequence (BUS) (2). Despite their high degree of sequence identity, the isoforms maintain a number of unique biological functions, including differences in transcriptional activity (2, 3), ligand response (4), gene regulation (5), and tissue-specific physiological effects (6, 7). The clinical implications of these differences are seen in work demonstrating that in breast cancer patients, dysregulation of PR-A:PR-B ratios can result in increased frequency of relapse (8). A molecular understanding of PR-A and PR-B functional differences may allow for the development of isoform-specific ligands and thus more targeted treatments of various hormone-dependent diseases.
The residues within BUS have long been assumed to be the primary effector of isoform-specific functional differences, however, the mechanisms by which they do so remain unclear. Shortly after identification of the isoforms, BUS was hypothesized to act as a third transcriptional activation domain, thus explaining the greater transcriptional activity seen for PR-B compared with PR-A (2, 3). However, no evidence of independent activity was observed when the sequence was linked to a heterologous DNA binding domain (DBD). Moreover, biochemical analyses indicated that both isoforms interacted with their palindromic progesterone response elements (PREs) with essentially identical apparent binding affinities (9), suggesting that isoform-specific functional differences could not be accounted for by differences in promoter binding. This interpretation was consistent with studies supporting differential recruitment of coregulators as a mechanism for explaining differences in transcriptional activation (10). Additional work demonstrated that BUS could indeed function as a transcriptional activator, but only in the context of its homologous DBD (2), indicating that regardless of mechanism, specific interactions between BUS and the PR-DBD were necessary for function.
As a step toward understanding the physical principles responsible for higher eukaryotic gene regulation, we are analyzing the thermodynamics of PR isoform interactions with multisite promoters. Our earlier work on the energetics of PR-B self-assembly and cooperative DNA binding found that liganded, preformed solution dimers interact with individual response elements with picomolar, rather than nanomolar binding affinity (11, 12). Moreover, binding to multiple response elements is coupled to highly cooperative assembly reactions. However, high-affinity binding is initiated under conditions in which little to no solution dimers are present, and successive monomer assembly at a palindromic PRE is thermodynamically favored over preformed dimer binding because of an enormous energetic penalty. These results raised the question as to whether the traditional understanding that preformed dimers are truly the predominant binding species is correct (1) and prompted us to ask whether PR-A–promoter interactions follow a similar profile.
We present here a thermodynamic dissection of PR-A interactions with a multisite promoter and compare the results to our previous work on PR-B (12). The analysis makes it clear that whereas PR-B and PR-A appear to have similar binding affinities, the actual energetics of assembly are considerably different, both at the level of the intrinsic binding affinity and the extent of cooperative interactions between palindromic PREs. Thus, the results assign a role for BUS as a modulator of microstate binding energetics, likely via an allosteric mechanism. Importantly, the differences in isoform-specific energetics predict promoter occupancies that closely correlate with the transcriptional activation profiles observed for those promoters in which PR-B is a stronger activator than PR-A. The results may additionally offer a framework for understanding that subset of promoters in which PR-B acts as a similar or weaker transcriptional activator than PR-A.
Results and Discussion
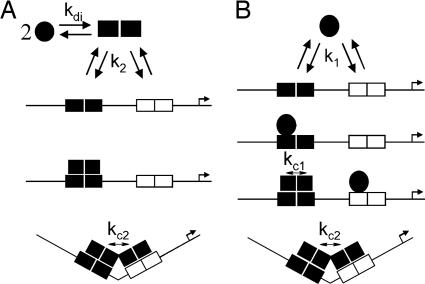
The commonly accepted pathway for describing PR-PRE assembly reactions is that hormone-bound receptors dimerize in solution and then bind at palindromic response elements (1). For promoters containing two tandemly linked PREs (PRE2), dimer binding is coupled to cooperative interactions between each site (12, 13). This series of reactions is presented schematically in Fig. 1A. In light of our previous work demonstrating that PR dimerizes in solution only in the micromolar range (11, 14), and under conditions in which apparent DNA binding affinity is in the nanomolar range (12), we also describe an alternative, thermodynamically equivalent pathway by which PR monomers bind at individual PRE half-sites and cooperatively recruit another monomer to the palindrome (Fig. 1B). Complete saturation at a two-site promoter is coupled to cooperative stabilization as seen in the dimer binding pathway. A subset of the predicted microstates and the microscopic interaction affinities associated with each pathway are also shown in Fig. 1.
Fig. 1.
Schematic of selected assembly states for PR-A–PRE2 interactions. (A) Dimer binding pathway. Circles represent hormone-bound PR-A structure, and squares represent PR-A solution dimers (kdi) or PR-A bound to the PRE2 promoter (k2). (B) Monomer binding pathway. Successive binding at an individual response element (k1) is accompanied by an intrasite cooperative interaction (kc1) represented by a transition from a filled circle to a filled square. Binding at multiple response elements is accompanied by an intersite cooperative interaction (kc2). Arrow refers to the direction of transcriptional start site.
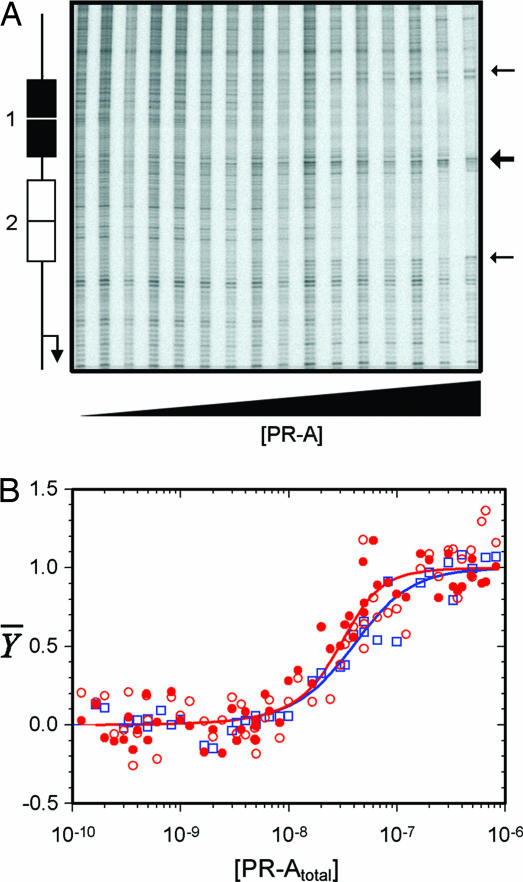
Presented in Fig. 2A is a representative PR-A footprint titration of the PRE2 promoter. It is evident that the receptor binds specifically to each PRE over a broad range of receptor concentrations. Dideoxy sequencing analysis indicates that the nucleotides afforded protection include the entire palindromic PRE and one or two additional flanking nucleotides. As indicated by the arrows, there are three hypersensitive sites that appear upon PR-A binding. The increased nicking seen immediately adjacent to the PREs (small arrows) is observed in titrations using both the PRE2 promoter and PRE1− promoter lacking a functional site 1. These signals originate four to five bases outside the PRE and likely arise because of receptor-mediated DNA bending (15, 16). The more intense hypersensitive signal located equidistant between the two PREs (large arrow) is seen only with the multisite PRE2 promoter; it localizes to two to three base pairs and has been previously interpreted to be due to cooperative receptor interactions between the response elements (12).
Fig. 2.
Quantitative footprint titration of the PRE2 promoter and individual-site binding isotherms obtained for PR-A binding to the PRE2 and PRE1− promoters. (A) PRE2 footprint titration. A schematic of PRE2 promoter structure is shown at the left. Small arrows indicate the appearance of hypersensitive bands seen on both the PRE1− and the PRE2 promoters, and the large arrow indicates the appearance of bands seen only for PRE2. (B) Symbols represent binding at site 1 of the PRE2 promoter (red filled circles), binding at site 2 of the PRE2 promoter (red open circles), and binding at site 2 of the PRE1− promoter (blue open squares). Lines represent best fits to sites 1 and 2 of the PRE2 (red) and site 1 of the PRE1− (blue) data as resolved by a global analysis using the dimer pathway model.
Microstate Energetics of a Preformed Dimer Binding Pathway.
Fig. 2B shows the individual-site binding isotherms generated by PR-A binding to sites 1 and 2 of the PRE2 promoter and to site 2 of the PRE1− promoter. The isotherms were globally fit to a model in which only preformed PR-A dimers are competent to cooperatively bind DNA (see Fig. 1A and Eqs. 2 and 3). As represented by the solid lines, the model well describes the data (SD of 0.062 apparent fractional saturation units). The resolved microscopic interaction energetics are presented in Table 1. As indicated, the intrinsic binding free energy of a liganded PR-A dimer toward an individual palindromic PRE (ΔG°2) was determined to be −11.4 kcal/mol. This value equates to a dissociation constant of 1.0 nM at 4°C, or 35-fold greater than the binding affinity estimated by visual inspection. The discrepancy between the apparent affinity and the true affinity arises because the data are presented in units of total PR-A concentration, rather than in units of the active binding species (i.e., dimer concentration). The intersite cooperativity term (ΔG°c2) was determined to be −0.4 kcal/mol. This weak value equates to only a 2.1-fold increase in overall stability compared with a noncooperative analog and only a 1.4-fold increase on a per PRE basis (17).
Table 1.
Resolved free energy changes and differences for PR-A and PR-B–PRE2 binding interactions
| Interaction free energy | PR-A, kcal·mol−1 | PR-B, kcal·mol−1 | ΔΔG,* kcal·mol−1 |
|---|---|---|---|
| ΔG°2 | −11.4 ± 0.1 | −12.8 ± 0.1 | 1.4 ± 0.1 |
| ΔG°c2† | −0.4 ± 0.2 | −2.5 ± 0.1 | 2.1 ± 0.2 |
| ΔG°1 | −8.4 ± 0.4 | −9.4 ± 0.2 | 1.0 ± 0.4 |
| ΔG°c1 | −1.7 ± 0.9 | −0.9 ± 0.5 | −0.8 ± 1.0 |
| ΔG°c2‡ | −0.9 ± 0.5 | −3.3 ± 0.5 | 2.4 ± 0.7 |
| ΔG°di§ | −7.6 ± 0.6 | −7.2 ± 0.7 | −0.4 ± 0.9 |
Microstate Energetics of a Monomer Binding Pathway.
Analysis of the data by using a monomer binding model generated an SD of the fit of 0.062 apparent fractional saturation units, identical to that of the dimer binding pathway model. Consistent with this, the predicted fractional saturation curves were essentially identical to those seen in Fig. 2B and are thus not shown. As presented in Table 1, the analysis resolved an intrinsic monomer binding affinity (ΔG°1) of −8.4 kcal/mol, which corresponds to a dissociation constant of 238 nM. The intrasite cooperative term (ΔG°c1) was determined to be −1.7± 0.9 kcal/mol, which corresponds to a 22-fold increase in the overall stability of the DNA-induced dimer and a 4.7-fold increase in stability on a per monomer basis (17). The large error value on this term arises from its high correlation with the intrinsic binding affinity parameter (ΔG°1). The resolved intersite cooperativity term (ΔG°c2) was determined to be −0.9 kcal/mol. This free energy change translates into only a 5-fold increase in the overall stability of the PR-A–PRE2 complex relative to a noncooperative analog or a 2.3-fold increase on a per PRE basis (17). This very minor additional stabilization mirrors what was seen in the dimer pathway analysis and demonstrates that regardless of binding model, PR-A cooperative interactions between palindromic PREs are unexpectedly weak (12, 13).
Binding Is Coupled to a Large Energetic Penalty.
Close inspection of the resolved energetics reveals that high-affinity DNA binding, regardless of pathway, is opposed by an enormous energetic penalty. This can be most easily observed by noting that the ability of PR-A monomers to dimerize in solution (−7.6 kcal/mol) is dramatically greater than their ability to dimerize on a response element (−1.7 kcal/mol). In other words, DNA-induced dimerization is accompanied by a +5.9 kcal/mol energetic penalty. Alternatively, it can be seen that successive monomer assembly at a palindrome (−16.8 kcal/mol) is thermodynamically favored over preformed dimer binding (−11.4 kcal/mol) by 5.4 kcal/mol or ≈18,000-fold. This result suggests that energetically costly structural rearrangements in the DNA and/or receptor must accompany complex formation (18). Indeed, a number of studies have demonstrated that steroid receptor–DNA interactions are accompanied by large-scale structural transitions in the N-terminal activation functions and hinge regions (19–23). The observed penalty may also pay for receptor-mediated DNA bending (see Fig. 2A) or DBD-induced compression of the response element minor groove (24). Consistent with this latter set of possibilities, the calculated free energy change for PR-A-induced DNA bending (15, 16) ranges from +3.5 to +4.9 kcal/mol (25), suggesting that bending can account for much, but not all of the penalty to DNA binding. In light of this result, it is probable that proteins such as HMG-1 increase PR binding affinity (9) because of their ability to stabilize a bent DNA conformation, thus reducing the binding penalty for PR and thus increasing the energetics of binding.
Regardless of mechanism, these results make it clear that the penalty greatly constrains the ability of the receptor to dimerize on the palindromic DNA, thus raising the question of whether the DNA-induced dimerization seen for isolated DBDs plays any significant functional role (26). This question may be especially relevant because computational analysis of natural PR-regulated promoters do not reveal an abundance of symmetrically perfect palindromic response elements, but rather appear to be composed of clustered half-sites and/or imperfect palindromic sites (27).
The A-Isoform Binds DNA with Reduced Intrinsic Affinity and Intersite Cooperativity Relative to the B-Isoform.
In addition to the resolved energetics for PR-A–PRE2 interactions, Table 1 also shows the analogous parameters determined for PR-B under identical solution conditions (12), and the arithmetic difference (ΔΔG°) in the respective free energy changes. Statistically significant differences in isoform-specific binding energetics are found in the intrinsic DNA binding affinity (regardless of binding pathway) and the intersite cooperativity (also regardless of pathway). By contrast, the intrasite cooperativity term and the solution dimerization energetics are identical between the isoforms, indicating that BUS plays no role in the penalty to binding. If, as discussed earlier, the penalty pays for DNA bending, this result may suggest that both isoforms are generating similar bend angles as seen for a subset of DNA sequences (15). Consistent with this, the receptor-induced hypersensitive regions seen in the footprinting data do not appear to be influenced by the presence of BUS.
The differences in energetics are not due to differences in protein preparations or activity because we have previously demonstrated that both isoforms are functionally and structurally homogeneous (11, 14). Rather, the results demonstrate that under these conditions, BUS directly modulates a subset of the microscopic binding energetics. Specifically, PR-B dimers bind palindromic response elements with 1.4 kcal/mol greater affinity than PR-A dimers, and PR-B monomers bind with 1.0 kcal/mol greater affinity. These differences translate to a 4- to 6-fold increase in PR-B affinity on a per monomer basis (17). Even more striking is the role of BUS in enhancing cooperative interactions between palindromic response elements: the 2.1 to 2.4 kcal/mol increase in cooperativity translates to a 45- to 78-fold increase in overall stability of the tetrameric complex or an ≈3-fold increase on a per monomer basis (17). Taken together, the residues unique to PR-B account for an ≈2,000- to 6,000-fold increase in PR-B binding stability relative to PR-A on the PRE2 promoter. These results are entirely consistent with recent cellular studies of PR function that hypothesized differential cooperativity as a mechanism for explaining isoform-specific transcriptional activation (27). Finally, the results make it clear that it is unnecessary to invoke differential recruitment of coactivators as an a priori assumption to explain isoform-specific function.
Functional Implications of Differential Isoform-Specific Binding Energetics.
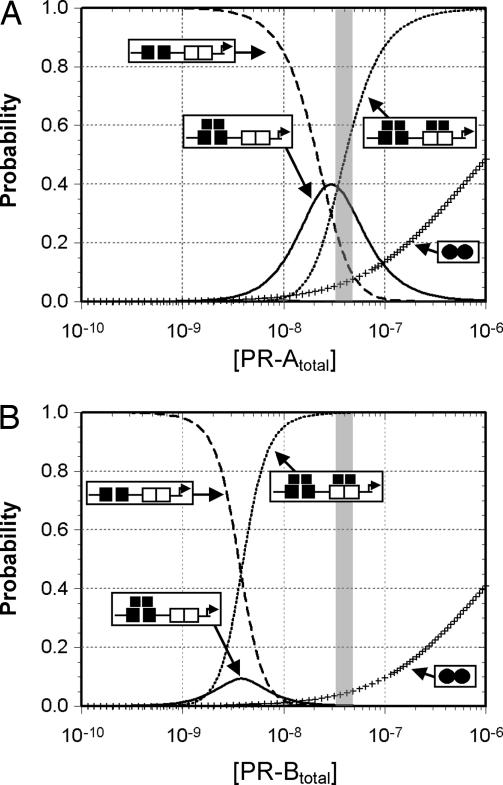
The large difference in isoform-specific binding energetics translates into a greatly reduced PR-A occupancy at the PRE2 promoter relative to PR-B. Shown in Fig. 3 are the calculated probabilities for each receptor–promoter ligation state as a function of total isoform concentration. It is evident that complete saturation of the PRE2 promoter by PR-A occurs at a concentration over an order of magnitude greater compared with PR-B (and under conditions in which little to no solution dimers are present). This difference in promoter occupancies may correlate with the biological activity of each isoform: Transcriptional activation studies of PR isoforms using cell lines containing only one or the other receptor have demonstrated that PR-B is a much stronger transcriptional activator on the PRE2 promoter relative to PR-A (2, 3). Comparing the isoform-specific occupancy of the promoter at the experimentally determined estimate of intracellular receptor concentration (28) (Fig. 3, shaded box) reveals that complete ligation by PR-B (the presumptive transcriptionally active microstate) nears 100% of the population, whereas the PR-A fully ligated state comprises <50%. This difference is due in part to the weaker intrinsic binding energetics of PR-A, but it also originates in the lack of significant PR-A-mediated intersite cooperativity. The impact of weak cooperative stabilization can be seen as the elevated population of PR-A intermediate states relative to those for PR-B.
Fig. 3.
Predicted distribution of each macroscopic PR-A–PRE2 and PR-B–PRE2 ligation state. (A) Distribution of PR-A ligation states as predicted by the dimer-binding pathway energetics (Table 1). (B) Same as A except that PR-B ligation states are displayed. Unligated PRE2 promoter is represented by the dashed line, singly ligated promoter is represented by the solid line, doubly ligated promoter is represented by the dotted line, and the proportion of PR dimers is represented by “+.” A shaded box represents the estimated intracellular PR concentration (28).
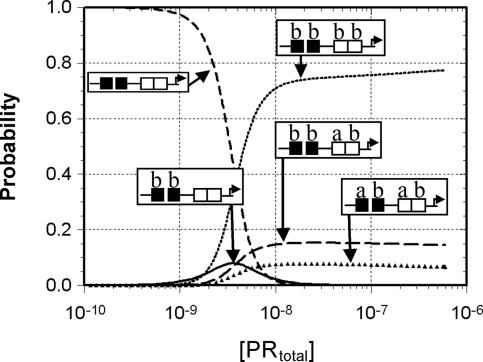
It is important to note that the simulations seen in Fig. 3 were carried out under the assumption that each receptor existed in isolation from the other. However, because the two isoforms exist in human tissues at roughly similar levels (29) and form heterocomplexes on DNA (30), we recalculated the receptor-dependent occupancy of the PRE2 promoter assuming an equimolar ratio of PR-A and PR-B. As seen in Fig. 4, it is evident that the B-isoform almost entirely dominates the assembly reaction because of its more favorable binding energetics. For example, at the estimated physiological concentration of receptor, the PR-B homotetrameric promoter complex accounts for ≈75% of the fully ligated species. By contrast, a homotetrameric PR-A complex never reaches >0.01% of the population. This result raises the question as to what role PR-A could possibly play in mediating PR-regulated transcriptional activity when in the presence of PR-B. Keeping in mind the great importance of cooperative interactions in stabilizing protein–DNA interactions (31, 32), we resimulated the ligation state probabilities assuming that PR-B could not bind cooperatively between the two palindromic response elements (ΔG°c2 = 0). The loss of intersite cooperativity decreases the population of the PR-B homotetrameric promoter complex to only 5% and increases the composite population of PR-A–PR-B heterocomplexes to 95% of the population (data not shown). The question of whether PR-B can in fact generate intersite cooperativity in a promoter-specific fashion is supported by biochemical studies of progesterone receptor and glucocorticoid receptor interactions with the multisite MMTV promoter (33, 34). These studies found no evidence of cooperative interactions between the palindromic PRE and any of the three PRE half-sites.†
Fig. 4.
Predicted distribution of PR–PRE2 ligation states in the presence of equimolar concentrations of both isoforms. Simulations were carried out by using the dimer pathway energetics and assume that (i) the A- and B-isoforms heterodimerize with an average of the two homodimerization affinities, (ii) heterodimers bind a PRE with an average of the PR-A and PR-B homodimer affinity, and (iii) intersite cooperativity occurring within DNA-induced heterotetramers is equal to the average of the intersite cooperativity within a PR-A or PR-B saturated PRE2. Representative ligation states and the ratio of PR-A to PR-B in the PR–PRE2 complex are indicated in the schematics.
It is clear that PR-B has a significant thermodynamic advantage over PR-A in assembling at the PRE2 promoter and that this advantage will only be enhanced at promoters containing additional response elements. Thus, noting that all intensively studied PR-regulated promoters appear to contain multiple PR binding sites (27), one might predict that PR-B should regulate the majority of PR responsive genes. Indeed, microarray analysis revealed that of 94 PR regulated genes, 65 were regulated by PR-B alone, 25 were regulated by both isoforms, and only 4 were regulated solely by PR-A (5). It is thus tempting to speculate that in vivo, promoters exclusively regulated by PR-B preferentially use intersite cooperativity as a means to stabilize the protein–DNA complex. By contrast, jointly regulated promoters may have an architectural layout not conducive toward PR-B-mediated intersite cooperative interactions, thus allowing PR-A to more effectively compete for binding (even if only as part of a heteroprotein complex). How PR-A might exclusively regulate promoters in the presence of both isoforms is somewhat unclear based on our model; for example, preferential intersite cooperative interactions by PR-A are insufficient to compete with PR-B binding because of the latter's greater intrinsic binding affinity. However, our simulations indicate that any decrease in the PR-B:PR-A ratio, as has been observed in a number of breast cancer tumors (35), can easily allow the A-isoform to dominate binding over its traditionally more active counterpart (data not shown). Determination of in vivo PR-A and PR-B occupancy levels at isoform-regulated promoters (using chromatin immunoprecipitation assays, for example) will be needed as a test of these predictions.
What Is the Structural Basis for the Differences in Binding Energetics?
The enhanced binding energetics of PR-B are unlikely to be due to direct BUS–promoter contacts because there is no evidence that the highly acidic sequence (pI ∼ 4.0) interacts with DNA. Furthermore, no additional DNase protection was observed for PR-B relative to PR-A in our footprinting analyses. Likewise, BUS is unlikely to serve as a “cooperativity domain,” per se: It shows no evidence for self-association, even at millimolar protein concentrations (D.L.B. and K. B. Horwitz, unpublished observations). A more likely explanation is that BUS modulates the conformational ensemble of PR-B by enhancing the population of high-affinity binding species, suggesting that BUS acts as a (covalently bound) allosteric effector of receptor function. This interpretation is consistent with limited proteolysis studies demonstrating that BUS destabilizes the structure of N-terminal sequences common to both isoforms (M.T.M. and D.L.B., unpublished work).
In summary, the work presented here represents a rigorous thermodynamic dissection of a full-length nuclear receptor interaction at a multisite promoter and suggests that major aspects of isoform-specific transcriptional activation can be explained at the most fundamental protein–DNA level, i.e., that of the receptor–promoter complex. These results may also have implications for understanding the mechanisms of action for other steroid receptors: The DBDs of steroid receptors are well conserved, although the N-terminal sequences are not. As a consequence, many of the steroid receptors can bind to identical promoter sequences yet somehow differentially regulate transcriptional activity. Our results suggest that a role for steroid receptor N-terminal sequences may be to differentially modulate the extent of cooperative DNA binding energetics, thus regulating the extent of receptor–promoter assembly and transcriptional activation.
Materials and Methods
Purification and Characterization of PR-A.
An expression vector encoding full-length, human PR-A (amino acids 165–933) fused to an N-terminal hexahistidine tag was a gift from Dean Edwards (Baylor College of Medicine, Houston, TX). A detailed description of the PR-A purification process and an analysis of its thermodynamic solution properties was published previously (14). Briefly, analyses of sedimentation velocity and sedimentation equilibrium data demonstrated that self-association could be rigorously described by a monomer–dimer assembly reaction with a dimerization free energy of −7.6 ± 0.6 kcal/mol.
DNA Preparation for DNase I Footprinting.
A vector containing a promoter made up of two tandemly linked PREs (PRE2) was a gift from Kathryn Horwitz (University of Colorado Health Sciences Center). Each PRE corresponds to the palindromic tyrosine aminotransferase promoter sequence, TGTACAGGATGTTCT (36) spaced 25 base pairs. A reduced-valency template (PRE1−) containing a G-to-T point mutation in each half-site of the distal PRE (designated as site 1) was created “in house.” Each template was excised from its respective vector to generate a 1,304-bp promoter fragment and 32P-end-labeled. The proximal PRE of each fragment (site 2) was positioned 100 bp from the 3′ end of the labeled strand.
Individual-Site Binding Experiments.
Experiments were carried out by using quantitative DNase I footprint titrations as originally described by Ackers and coworkers (37, 38) and with modifications described previously (12). Briefly, all reactions were carried out in 20 mM Hepes (pH 8.0)/50 mM NaCl/2.5 mM MgCl2/1 mM CaCl2/1 mM DTT/10−5 M progesterone/100 μg/ml BSA/2 μg/ml salmon sperm DNA at 4°C. Individual-site binding curves were calculated as described by Brenowitz et al. (37), using ImageQuant (GE Healthcare). All studies were carried out by using DNase I concentrations that approximated “single hit” kinetics. Promoter DNA concentrations were well below PR-A intrinsic binding affinity, thus justifying the assumption that PR-Afree ∼ PR-Atotal.
Resolution of Microscopic Interaction Free Energies.
The microscopic interaction energetics were resolved by using a binding model developed for our analysis of PR-B–PRE2 promoter interactions (12). This model makes a number of assumptions that have been extensively analyzed or validated. However, two key issues warrant further discussion. First, resolution of microscopic DNA binding energetics requires that an independently determined solution dimerization constant be used in the fitting analysis (37). As noted above, we previously determined the energetics of PR-A dimerization using analytical ultracentrifugation (14). However, these studies were carried out at 300 mM NaCl rather than the 50 mM NaCl used here. At this lower salt concentration, PR-A becomes insoluble at receptor concentrations >1 μM, thus precluding a comprehensive study comparable to our earlier work. However, a limited analysis of sedimentation data collected at 50 mM NaCl resolved a dimerization free energy of −8.6 ± 0.9 kcal/mol (data not shown). This result is statistically identical to our previously determined value and is consistent with the observation that the weight-average sedimentation coefficient of PR-A is independent of NaCl concentrations ranging from 0.1 M to 1.0 M NaCl (14).
A second concern is based on the assumption that PR-A monomers are competent to bind to PRE half-sites with identical affinity. As noted above, the PREs in the PRE2 and PRE1− promoters are not perfect palindromes. As a consequence, any differences in monomer–half-site binding affinities will be masked by even mild levels of intrasite cooperativity. However, computer simulations indicate that the maximum energetic difference between half-site binding affinities that is still consistent with the experimental data are only 1.3 kcal/mol (data not shown). This result is seen only when the intrasite cooperativity term is fixed at a rarely observed value of −4.5 kcal/mol (corresponding to a 3,000-fold increase in overall stability). The simulations thus suggest that that any difference in monomer–half-site binding energetics is likely to be considerably less than 1.3 kcal/mol and therefore within the error of the data.
The DNase I footprint titration technique resolves the fractional occupancy of binding at each PRE. The statistical thermodynamic expressions that describe the individual-site binding isotherms are constructed by summing the probabilities of each microscopic configuration that contributes to binding at that site. A detailed approach for generating each mathematical formulation was presented previously (31). Briefly, the probability (fS) of any microscopic configuration is defined as (39)
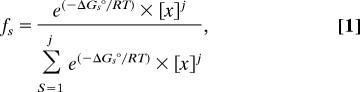 |
where ΔG°s is the free energy of configuration state s relative to the unliganded reference state, x is the PR-A monomer concentration (as calculated from the dimerization constant, kdi), and j is the stoichiometry of PR-A monomer bound to a response element. R is the gas constant, and T is temperature in Kelvin. The relationship between each free energy change and its association constant is defined by means of the standard relationship ΔG°i = −RT ln ki. For example, the fractional saturation (Ȳ) for dimer binding at site 1 of the PRE2 promoter is the sum of probabilities for the isolated dimer binding reaction and the cooperative binding reaction with the adjacently bound dimer. When the equation for PR-A dimer binding to site 1 on the PRE2 promoter is expressed in terms of free PR-A monomer concentration, the fractional saturation is defined as
where kdi and x are as defined previously, k2 is the intrinsic association constant for a preformed dimer binding to a PRE, and kc2 corresponds to the intersite cooperativity term. Because the PREs are identical in sequence, Eq. 2 also describes binding to site 2 of the PRE2 promoter. By using the same approach, the equation describing the fractional saturation of site 2 of the PRE1− promoter is
Equations describing successive monomer binding to the PRE2 and the PRE1− promoters are generated by using a similar approach (12). However, in the latter case the relevant parameters are k1, the intrinsic affinity of monomer binding; kc1, the intrasite cooperativity; and kc2, the intersite cooperativity.
To resolve the interaction parameters describing PR-A–DNA binding, the isotherms from each footprint titration were analyzed simultaneously by using the program Scientist (Micromath). Because protein interactions at DNA binding sites do not afford complete protection from DNase activity, binding data were treated as transition curves fitted to upper (m) and lower (b) endpoints:
Acknowledgments
This work was supported by National Institutes of Health Grant R01-DK061933 (to D.L.B.).
Abbreviations
- DBD
DNA binding domain
- PR
progesterone receptor
- PRE
progesterone response element
- PR-A
A-isoform of PR
- PR-B
B-isoform of PR
- BUS
B-unique sequence
- PRE2
DNA template containing two PREs
- PRE1−
DNA template containing a single PRE.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
Rigorous thermodynamic analysis of the MMTV promoter indicates that neither PR-B nor PR-A can engage in cooperative interactions between the MMTV palindromic site and the half-sites (A.F.H. and D.L.B., unpublished work).
References
- 1.Tsai MJ, O'Malley BW. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 2.Sartorius CA, Melville MY, Hovland AR, Tung L, Takimoto GS, Horwitz KB. Mol Endocrinol. 1994;8:1347–1360. doi: 10.1210/mend.8.10.7854352. [DOI] [PubMed] [Google Scholar]
- 3.Meyer ME, Quirin-Stricker C, Lerouge T, Bocquel MT, Gronemeyer H. J Biol Chem. 1992;267:10882–10887. [PubMed] [Google Scholar]
- 4.Meyer ME, Pornon A, Ji JW, Bocquel MT, Chambon P, Gronemeyer H. EMBO J. 1990;9:3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. J Biol Chem. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 6.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Proc Natl Acad Sci USA. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 8.Hopp TA, Weiss HL, Hilsenbeck SG, Cui Y, Alfred DC, Horwitz KB, Fuqua S. Clin Cancer Res. 2004;15:2751–2760. doi: 10.1158/1078-0432.ccr-03-0141. [DOI] [PubMed] [Google Scholar]
- 9.Onate SA, Prendergast P, Wagner JP, Nissen M, Reeves R, Pettijohn DE, Edwards DP. Mol Cell Biol. 1994;14:3376–3391. doi: 10.1128/mcb.14.5.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. Mol Cell Biol. 2000;20:3102–3115. doi: 10.1128/mcb.20.9.3102-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heneghan AF, Berton N, Miura MT, Bain DL. Biochemistry. 2005;44:9528–9537. doi: 10.1021/bi050609i. [DOI] [PubMed] [Google Scholar]
- 12.Heneghan AF, Connaghan-Jones KD, Miura MT, Bain DL. Biochemistry. 2006;45:3285–3296. doi: 10.1021/bi052046g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai SY, Tsai M-J, O'Malley BW. Cell. 1989;57:443–448. doi: 10.1016/0092-8674(89)90919-7. [DOI] [PubMed] [Google Scholar]
- 14.Connaghan-Jones KD, Heneghan AF, Miura MT, Bain DL. Biochemistry. 2006;45:12090–12099. doi: 10.1021/bi0612317. [DOI] [PubMed] [Google Scholar]
- 15.Petz LN, Nardulli AM, Kim J, Horwitz KB, Freedman LP, Shapiro DJ. J Steroid Biochem Mol Biol. 1997;60:31–41. doi: 10.1016/s0960-0760(96)00171-9. [DOI] [PubMed] [Google Scholar]
- 16.Prendergast P, Pan Z, Edwards DP. Mol Endocrinol. 1996;10:393–407. doi: 10.1210/mend.10.4.8721984. [DOI] [PubMed] [Google Scholar]
- 17.Ackers GK, Shea MA, Smith FR. J Mol Biol. 1983;1:223–242. doi: 10.1016/s0022-2836(83)80234-4. [DOI] [PubMed] [Google Scholar]
- 18.Senear DF, Ross JB, Laue TM. Methods. 1998;16:3–20. doi: 10.1006/meth.1998.0641. [DOI] [PubMed] [Google Scholar]
- 19.Bain DL, Franden MA, McManaman JL, Takimoto GS, Horwitz KB. J Biol Chem. 2000;275:7313–7320. doi: 10.1074/jbc.275.10.7313. [DOI] [PubMed] [Google Scholar]
- 20.Bain DL, Franden MA, McManaman JL, Takimoto GS, Horwitz KB. J Biol Chem. 2001;276:23825–23831. doi: 10.1074/jbc.M102611200. [DOI] [PubMed] [Google Scholar]
- 21.Brodie J, McEwan IJ. J Mol Endocrinol. 2005;34:603–615. doi: 10.1677/jme.1.01723. [DOI] [PubMed] [Google Scholar]
- 22.Kumar R, Thompson EB. Mol Endocrinol. 2003;17:1–10. doi: 10.1210/me.2002-0258. [DOI] [PubMed] [Google Scholar]
- 23.Warnmark A, Treuter E, Wright APH, Gustafsson J-A. Mol Endocrinol. 2003;17:1901–1909. doi: 10.1210/me.2002-0384. [DOI] [PubMed] [Google Scholar]
- 24.Roemer SC, Donham DC, Sherman L, Pon VH, Edwards DP, Churchill MEA. Mol Endocrinol. 2006;20:3042–3052. doi: 10.1210/me.2005-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu-Johnson H-N, Gartenberg MR, Crothers DM. Cell. 1986;47:995–1005. doi: 10.1016/0092-8674(86)90814-7. [DOI] [PubMed] [Google Scholar]
- 26.Tsai SY, Carlstedt-Duke J, Weigel NL, Dahlman K, Gustafsson J-A, Tsai M-J, O'Malley BW. Cell. 1988;55:361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- 27.Tung L, Abdel-Hafiz H, Shen T, Harvell DME, Nitao LK, Richer JK, Sartorius CA, Takimoto GS, Horwitz KB. Mol Endocrinol. 2006;20:2656–2670. doi: 10.1210/me.2006-0105. [DOI] [PubMed] [Google Scholar]
- 28.Theofan G, Notides AC. Endocrinology. 1984;114:1173–1179. doi: 10.1210/endo-114-4-1173. [DOI] [PubMed] [Google Scholar]
- 29.Mote P, Bartow S, Tran N, Clarke C. Breast Cancer Res Treat. 2002;72:163–172. doi: 10.1023/a:1014820500738. [DOI] [PubMed] [Google Scholar]
- 30.Mohamed MK, Tung L, Takimoto GS, Horwitz KB. J Steroid Biochem Mol Biol. 1994;51:241–250. doi: 10.1016/0960-0760(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 31.Ackers GK, Johnson AD, Shea MA. Proc Natl Acad Sci USA. 1982;79:1129–1133. doi: 10.1073/pnas.79.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson AD, Meyer BJ, Ptashne M. Proc Natl Acad Sci USA. 1979;76:5061–5065. doi: 10.1073/pnas.76.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bailly A, Rauch C, Cato ACB, Milgrom E. Mol Cell Endocrinol. 1991;82:313–323. doi: 10.1016/0303-7207(91)90045-t. [DOI] [PubMed] [Google Scholar]
- 34.Perlmann T, Eriksson P, Wrange O. J Biol Chem. 1990;265:17222–17229. [PubMed] [Google Scholar]
- 35.Graham J, Yeates C, Balleine R, Harvey S, Milliken J, Bilous A, Clarke C. Cancer Res. 1995;55:5063–5068. [PubMed] [Google Scholar]
- 36.Jantzen HM, Strahle U, Gloss B, Stewart F, Schmid W, Boshart M, Miksicek R, Schutz G. Cell. 1987;49:29–38. doi: 10.1016/0092-8674(87)90752-5. [DOI] [PubMed] [Google Scholar]
- 37.Brenowitz M, Senear DF, Shea MA, Ackers GK. Methods Enzymol. 1986;130:132–181. doi: 10.1016/0076-6879(86)30011-9. [DOI] [PubMed] [Google Scholar]
- 38.Brenowitz M, Senear DF, Shea MA, Ackers GK. Proc Natl Acad Sci USA. 1986;83:8462–8466. doi: 10.1073/pnas.83.22.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill TL. An Introduction to Statistical Thermodynamics. New York: Dover; 1960. [Google Scholar]