Abstract
Fetal loss in animals and humans is frequently associated with inflammatory conditions. D6 is a promiscuous chemokine receptor with decoy function, expressed in lymphatic endothelium, that recognizes and targets to degradation most inflammatory CC chemokines. Here, we report that D6 is expressed in placenta on invading extravillous trophoblasts and on the apical side of syncytiotrophoblast cells, at the very interface between maternal blood and fetus. Exposure of D6−/− pregnant mice to LPS or antiphospholipid autoantibodies results in higher levels of inflammatory CC chemokines and increased leukocyte infiltrate in placenta, causing an increased rate of fetal loss, which is prevented by blocking inflammatory chemokines. Thus, the promiscuous decoy receptor for inflammatory CC chemokines D6 plays a nonredundant role in the protection against fetal loss caused by systemic inflammation and antiphospholipid antibodies.
Keywords: trophoblast, leukocyte, placenta
Leukocyte recruitment is a tightly regulated multistep process involving adhesion molecules and locally produced soluble mediators. Among these, chemokines are essential for leukocyte migration and activation. This large family of cytokines has been classified into four subfamilies (CXC, CC, CX3C, and C) according to the relative position of cysteine residues, and in homeostatic (i.e., produced constitutively) and inflammatory (i.e., produced in response to inflammatory or immunological stimuli) categories according to their production (1). Biological activities are mediated by a subfamily of chemoattractant receptors belonging to the large family of seven transmembrane domain G protein-coupled receptors (2, 3). Chemokines also interact with a distinct group of “silent” receptors with structural similarity with conventional receptors but lacking signaling function, which includes the Duffy antigen receptor for chemokines (DARC), CCX-CKR, and D6 (4, 5). D6 binds most inflammatory, but not homeostatic, CC chemokines, internalizes constitutively, and targets the ligand for degradation (6–9). Differently from other chemokine receptors, D6 expression has been reported mainly in nonhematopoietic cells and includes endothelial cells lining afferent lymphatic in certain anatomical sites, such as skin, gut, and lung (10). Evidence from gene-targeted animals indicates that D6 plays a nonredundant role in the control of the local inflammatory response by acting as a decoy and scavenger receptor for inflammatory chemokines in the skin (11, 12). D6 expression has also been detected in placenta (13), but its cellular location and function in this organ have not been previously investigated. Here, we show that trophoblast cells express D6 and use this molecule to scavenge inflammatory CC chemokines. We also provide evidence that D6 is required to prevent excessive placenta leukocyte infiltration in inflammation- and autoantibody-triggered fetal loss animal models, thus protecting the fetus from abortion.
Results and Discussion
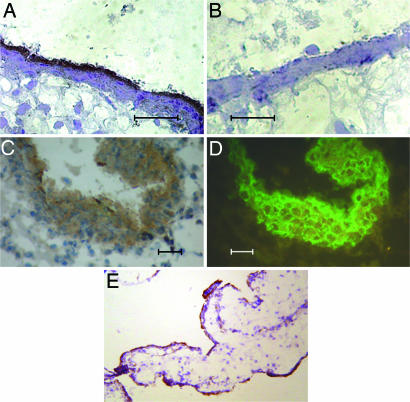
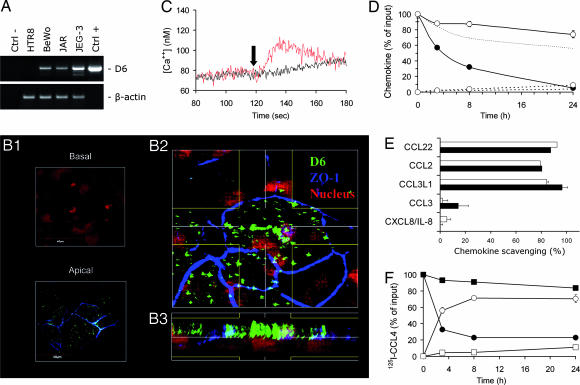
D6 was originally cloned from a placenta expression library (14). We performed Northern blot experiments confirming previous reports on D6 expression in human placenta (13, 14), and we extended this observation to murine placenta (data not shown). Immunohistochemical analysis detected elevated levels of D6 on the syncytiotrophoblast monolayer (Fig. 1A and E) and on cytokeratin 7-positive anchoring and invading trophoblasts (Fig. 1 C and D). Consistent with these findings, the trophoblast-derived choriocarcinoma cell lines JAR, JEG-3, and BeWo also express D6 (Fig. 2A). In particular, confocal microscopy analysis of the apical and basal pole of polarized BeWo cells and XZ projection obtained by three-dimensional reconstruction of sequential XY confocal scanning images clearly demonstrate that D6 is expressed preferentially on the apical side of the polarized trophoblast cells (Fig. 2B).
Fig. 1.
D6 expression in human placenta. First-trimester placenta sections were stained with an anti-D6 mAb (A, C, and E) or an irrelevant antibody (B). Invading extravillous trophoblasts were identified by using an anti-cytokeratin 7 mAb (D). (Scale bars, 20 μm.) (E) Low magnification (×250) of D6 staining on syncytiotrophoblast cells. Results are representative of independent experiments performed on at least three individuals.
Fig. 2.
D6 expression and function in trophoblast cells. (A) RT-PCR analysis of D6 and β-actin expression in choriocarcinoma (BeWo, JAR, and JEG-3) and trophoblast (HTR8) cell lines. Ctrl−, non-retrotranscribed RNA. Ctrl+, hD6/pcDNA6 plasmid. Results obtained in one experiment representative of three performed are shown. (B) Confocal microscopy analysis of polarized BeWo cells stained with an anti-D6 (green) and anti-zonula occludens 1 (blue). Propidium iodide (red) was used for nuclear staining. Fifty consecutive confocal scanning images were obtained in the Z stage (step size, 0.2 μm), and three-dimensional reconstruction was obtained as described in Materials and Methods. (B1) Representative sections of the apical and basolateral regions of the cell monolayer. (B2) Blend projection on the XY axes of the three-dimensional reconstruction. Lines define the area considered in the analysis generating the XZ projection shown in B3. (C) D6/HTR8 and CCR5/HTR8 transfectants (black and red lanes, respectively) were stimulated at indicated time point (arrow) with 300 ng/ml CCL3L1, and intracellular calcium concentrations were recorded over time. Results obtained in one experiment representative of three performed are shown. (D) BeWo cells grown to complete confluence on 0.4-μm pore filters were incubated with 10 ng/ml CXCL8/IL-8 (open symbols) or CCL3L1 (filled symbols) added to the upper chamber. At the indicated time points, the chemokine concentration was measured in the upper (solid line) and lower (dotted line) chambers. The dotted line with no symbols represents CCL3L1 concentrations in the upper chamber in the absence of the cell monolayer. (E) BeWo cells (open columns) and D6/CHO-K1 transfectants (filled columns) were incubated with 10 ng of the indicated chemokines per ml. The chemokine concentration in the supernatant was measured after an 18-h incubation. (F) Kinetics of 125I-CCL4 scavenging. Mock-transfected (squares) and D6-transfected (circles) HTR8 cells were incubated at 37°C with 125I-CCL4 for the indicated time periods. The percentage over total radioactivity input of the trichloroacetic acid (TCA)-soluble (open symbols) and TCA-resistant (filled symbol) fractions recovered in the supernatants are shown. Results in D–F are reported as mean ± SD of triplicate samples of one experiment representative of three performed. SD values are included in the symbol.
Having demonstrated D6 expression in trophoblast cells and corresponding choriocarcinoma cell lines, we investigated its functional role in this cellular context. To this purpose, the trophoblast cell line HTR8, which does not express either D6 (Fig. 2A) or inflammatory CC chemokine receptors CCR1–CCR5 (data not shown), was transfected with either CCR5 or D6. As shown in Fig. 2C, the D6 and CCR5 ligand CCL3L1 was able to induce calcium fluxes in CCR5/HTR8 but not D6/HTR8 transfectants. Similarly, CCL3L1 induced migration of CCR5/HTR8 but not D6/HTR8 transfectants (medium, 10 ± 7 vs. 17 ± 12 cells; 30 ng/ml CCL3L1, 350 ± 14 vs. 10 ± 7 cells, respectively). D6 was shown previously to be unable to facilitate chemokine transfer across the lymphatic endothelial cell monolayer (9). Chemokine transfer through the syncytiotrophoblast monolayer was then evaluated by using the choriocarcinoma BeWo cell line, which expresses some functional properties of the syncytiotrophoblast monolayer (15), as an in vitro model. Under experimental conditions supporting Ig transcytosis (data not shown), no facilitated transfer of CCL3L1 from the upper to the lower compartment (Fig. 2D) nor in the opposite direction (data not shown) was observed. The progressive reduction of CCL3L1 concentration in the upper compartment at a rate significantly higher than that of CXCL8/IL-8, a chemokine not recognized by D6, was suggestive of a D6-dependent chemokine degradation process in trophoblast cells, as demonstrated previously in other cell contexts (6, 9). D6 was therefore tested for its ability to sustain chemokine scavenging in this cellular context. BeWo cells and D6/CHO-K1 transfectants scavenged with a comparable efficacy the D6 ligands CCL2, CCL3L1, and CCL22, whereas the non-D6 ligands CCL3 and CXCL8/IL-8 were not affected (Fig. 2E). Similar results were obtained with the choriocarcinoma cell lines JAR and JEG-3 (data not shown), which also express endogenous D6 (Fig. 2A). The D6-mediated chemokine degradation was demonstrated by using HTR8 cells, which do not express D6. Although mock-transfected cells did not degrade significant amounts of 125I-CCL4, D6/HTR8 transfectants efficiently degraded 125I-CCL4, as demonstrated by the time-dependent decrease of the TCA-precipitable (presumably intact) radioactive fraction, paralleled by the increase of the TCA-soluble (degraded) radioactive fraction (Fig. 2F). A minor (4%) fraction of intracellular radioactivity was also observed in D6 transfectants but not in mock transfectants (data not shown). The specificity of chemokine scavenging and the absence of signaling inflammatory CC chemokine receptors in these cell lines are consistent with D6 accounting for the observed chemokine scavenging and degradation.
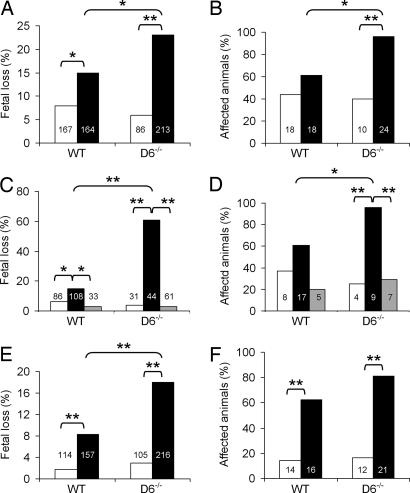
Collectively, these results are consistent with a function for placental D6 as a nonsignaling chemokine scavenger receptor (11, 12), with the possible role of dampening placental inflammation under pathologic conditions. To test this hypothesis, the role of D6 in two animal models of fetal loss associated with inflammatory conditions was investigated. The first model was based on the induction of a systemic inflammatory response in mice by LPS treatment (16), an animal model mimicking a clinical condition frequently associated with abortion and preterm delivery in humans (17). Injection of pregnant D6−/− mice with LPS resulted in a significant increase in fetal loss frequency (Fig. 3A) and in the frequency of mice showing fetal loss (Fig. 3B) compared with LPS-injected WT animals. Both parameters were significantly reduced by a mixture of antibodies blocking inflammatory CC chemokines but not irrelevant antibodies, indicating that inflammatory chemokines play a pathogenic role in this model (Fig. 3 C and D). Treatment with irrelevant antibodies increased LPS embryotoxicity in D6−/− but not in WT mice (Fig. 3 C and D). Although this effect was not investigated in detail in this work, it could be attributed at least in part to the increased production of inflammatory chemokines associated with the simultaneous exposure to LPS and FcγR engagement (18, 19). The complete recovery after treatment with chemokine-blocking antibodies supports this hypothesis (Fig. 3 C and D). The second fetal-loss model was based on the injection of antiphospholipid (aPL) autoantibodies purified from patients affected by the antiphospholipid syndrome (APS) (20, 21). This disorder is characterized by recurrent thrombosis and fetal loss in the presence of pathogenic autoantibodies reacting against phospholipid-binding proteins (mainly β2-glycoprotein I) (21–23). Passive infusion of human aPL was shown to induce fetal loss in pregnant naïve mice by triggering a local inflammatory and necrotic process at the placental level (21, 23). Passive transfer to pregnant mice of IgG fractions from healthy women had no effect on pregnancy, whereas aPL-containing Ig fractions from APS patients induced a significant rate of fetal loss (Fig. 3E) and a significant increase of affected animals (Fig. 3F) in both WT and D6−/− animals. As for the LPS-dependent model, D6−/− animal susceptibility was significantly increased compared with WT animals (Fig. 3E).
Fig. 3.
Role of D6 in LPS- and aPL-induced fetal loss. The percentage of fetal loss (A) and of animals with fetal loss (B) in WT and D6−/− mice injected with saline (open columns) or LPS (filled columns) is shown. The percentage of fetal loss (C) and of animals with fetal loss (D) in WT and D6−/− mice injected with saline (open columns) or LPS after treatment with a mixture of blocking antibodies to chemokines (gray columns) or irrelevant antibodies (filled columns) is also shown. The percentage of fetal loss (E) and of animals with fetal loss (F) in WT and D6−/− mice treated with aPL (filled columns) or IgG from healthy women (open columns) is shown. Numbers inside columns are total numbers of events evaluated. (A, C, and E) Embryonal sacs. (B, D, and F) Injected animals. ∗, P < 0.05; ∗∗, P < 0.01 by Fisher's exact test.
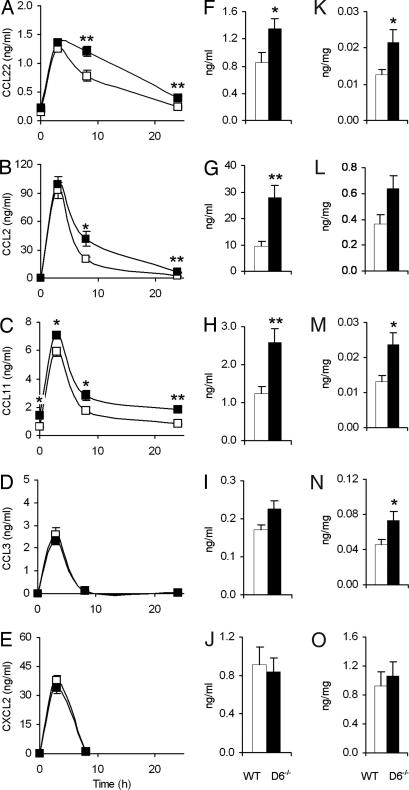
In both experimental conditions, the role of inflammatory chemokines has never been investigated, although the involvement of primary inflammatory cytokines has been previously demonstrated (20, 24). To assess whether the protective function of D6 was indeed related to impaired control of inflammatory chemokines, circulating levels of CC inflammatory chemokines scavenged by D6 were measured after LPS administration. In nonpregnant mice, where kinetic analysis was possible, basal concentrations and peak levels were superimposable in WT and D6−/− mice for CCL22 (Fig. 4A), CCL2 (Fig. 4B), and CCL3 (Fig. 4D), and they were significantly different only for CCL11 (Fig. 4C). On the contrary, at later time points, all inflammatory CC chemokines but CCL3, which disappeared very quickly from circulation, showed significantly higher concentrations in D6−/− mice, similar to what has previously been described in other experimental conditions (11, 12). No difference either in basal conditions or after LPS exposure was observed for CXCL2, which is not recognized by D6 (Fig. 4E). Similar results were obtained when chemokines were measured in sera from LPS-treated pregnant animals (Fig. 4 F–J). To gain insight into the molecular mechanisms responsible for exacerbated LPS-driven fetal loss in D6−/− animals, inflammatory chemokines were investigated in placenta. Compared with WT animals, D6−/− mice displayed higher concentrations of all D6-binding chemokines (Fig. 4 K–N). Levels of CXCL2, which is not recognized by D6, did not differ between WT and D6−/− animals (Fig. 4O). Increased chemokine levels in D6−/− placenta are likely the result of reduced scavenging and not augmented local production because there were no differences in the levels of chemokine mRNA, detected by quantitative PCR analysis, between LPS-injected WT and D6−/− mice (data not shown). These results and the protective effect of chemokine-blocking antibodies highlight a previously unrecognized role of inflammatory chemokines in fetal abortion induced by inflammatory stimuli, and they demonstrate that the absence of the scavenging function of D6 results in a prolonged persistence of chemokines and causes increased susceptibility to inflammation-driven fetal loss.
Fig. 4.
Chemokines in the LPS model of fetal loss. (A–E) Serum chemokine concentrations after LPS treatment in WT and D6−/− male mice. WT (open symbols) and D6−/− (filled symbols) mice were injected i.p. with 1.35 mg/kg LPS. At the indicated time points, circulating chemokine concentrations were measured by ELISA. Data are from seven mice for each time point. (F–J) Serum chemokine concentrations. WT (open columns) and D6−/− (filled columns) mice at day 10 of pregnancy were injected i.p. with 0.4 mg/kg LPS. Circulating chemokine concentrations were measured at 8 h postinjection by ELISA. Data are from nine WT and eight D6−/− mice. (K–O) Chemokine levels in placenta. WT (open columns) and D6−/− (filled columns) mice at day 10 of pregnancy were injected i.p. with 0.4 mg/kg LPS. Chemokine concentrations (expressed as nanograms of chemokine per milligram of total proteins of the lysates) were measured at 8 h postinjection by ELISA. Data are from nine WT and eight D6−/− mice. Results are reported as mean ± SEM. (A, F, and K) CCL22. (B, G, and L) CCL2. (C, H, and M) CCL11. (D, I, and N) CCL3. (E, J, and O) CXCL2.
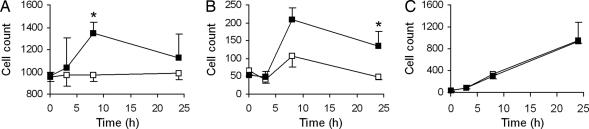
To evaluate the functional implication at placental level of inappropriate control of the chemokine system in the absence of D6, a histological evaluation of infiltrating leukocytes was conducted. Placenta from saline-treated WT and D6−/− animals did not differ for any resident leukocyte population evaluated, including CD68+ macrophages (Fig. 5A), CD3+ T lymphocytes (Fig. 5B), Gr1+ neutrophils (Fig. 5C), and natural killer (NK) cells (data not shown). LPS treatment had no effect on the number of placental NK cells, nor in WT or D6−/− mice (data not shown). On the contrary, LPS induced a significant increase in the number of macrophages (Fig. 5A) and T lymphocytes (Fig. 5B) infiltrating placenta in D6−/− mice but not in WT littermates. LPS also caused a time-dependent increase in the infiltration of neutrophils, but to similar levels in WT and D6−/− mice, consistent with the selective regulation of CC and not CXC chemokines by D6 (Fig. 5C).
Fig. 5.
Placenta-infiltrating leukocytes in the LPS model of fetal loss. Leukocyte infiltration in placenta of WT (open symbols) and D6−/− (filled symbols) mice at day 7 of pregnancy at the indicated time points after i.p. injection of 0.4 mg/kg LPS. The numbers of CD68+ macrophages (A), CD3+ lymphocytes (B), and Gr1+ neutrophils (C) infiltrating the placenta were evaluated on histological sections. Data are from at least three independent animals per group. Results are reported as mean ± SEM. ∗, P < 0.05 by Student's t test.
Decoy receptors are nonsignaling molecules that play a regulatory role in different cytokine and growth factor systems by sequestering agonists and/or components of the signaling receptor complexes (25). Originally formulated for the IL-1 type II receptor (26), the decoy receptor paradigm has now been applied to the IL-1, TNFα, IL-10, IL-4/IL-13, and other receptor families (25). Evidence obtained in vitro suggested that the “silent” chemokine receptor D6 could exert a similar function for inflammatory CC chemokines (9), and subsequent in vivo results demonstrated its role in the control of inflammation in tissues and draining lymph nodes (11, 12). The results reported here demonstrate that D6 is also expressed in placenta by the syncytiotrophoblast, at the very interface with maternal blood, and by invading extravillous trophoblasts.
Chemokines are normally produced by both fetal and maternal components and play a significant role in the extensive leukocyte trafficking observed in placenta, which is required to maintain the balance between protection of the developing embryo/fetus and tolerance of its hemiallogeneic tissues (27). In D6−/− mice we observed normal placenta development and a fertility index comparable with those of WT animals, suggesting that D6 is unlikely to play a major role in homeostatic conditions. On the contrary, results in gene-targeted animals clearly highlight its nonredundant function in the control of placental inflammation of different origin. Interestingly enough, D6 expression on the syncytiotrophoblast monolayer strictly resembles that of the decay-accelerating factor, which has also been proposed as a protective mechanism preventing complement-mediated placenta attack (28). In conclusion, D6 is a unique seven-transmembrane domain chemokine scavenger receptor, strategically located at the fetal–maternal interface to dampen placental inflammation. The chemokine system is a prime target for the development of new therapeutic strategies for diverse disorders (29). The results reported here raise the possibility that strategies blocking inflammatory CC chemokines may protect against unwanted fetal loss in humans.
Materials and Methods
Reagents and Cell Lines.
Recombinant chemokines and ELISA detection kits were purchased from R&D Systems (Minneapolis, MN). LPS (from Escherichia coli 055:B5) and laboratory reagents were purchased from Sigma–Aldrich (St. Louis, MO). The human choriocarcinoma cell lines BeWo, JAR, and JEG-3 (RZPD Consortium, Berlin, Germany) were grown in DMEM/F12 supplemented with 10% FCS. CHO-K1 transfectants were described previously (9). HTR-8/SV40neo trophoblast cells, obtained from explant cultures of human first-trimester placenta immortalized by transfection with the SV40 large-T antigen and expressing phenotypic properties of extravillous placental cytotrophoblasts (30), were grown in RPMI medium 1640 supplemented with 10% FCS and transfected with the hCCR5/pcDNA6 or hD6/pcDNA6 expression plasmids by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) (6). Blasticidin-resistant cells were stained by using mouse anti-human CCR5 mAb (R&D Systems) or rat anti-human D6 mAb (R&D Systems) and sorted by using a FACSVantage flow cytometer (BD Biosciences, San Jose, CA). Tranfectants used in functional tests showed receptor expression levels >90%.
RT-PCR and Quantitative PCR.
Total RNA was extracted from cell pellets or placenta tissues by using TRIzol (Invitrogen) and treated with DNase I (Ambion, Austin, TX) to remove genomic contamination. cDNA was generated by using SuperScript first-strand synthesis systems (Invitrogen). Quantitative PCR was performed by using 2× SYBR green PCR master mix (Applied Biosystems, Foster City, CA) in a 7900HT fast real-time PCR system (Applied Biosystems). Data were normalized for β-actin expression. Primers are listed in supporting information (SI) Table 1.
Immunohistochemistry and Immunofluorescence Analysis.
D6 expression was analyzed in first-trimester human placental tissues obtained after elective termination of pregnancy. Tissue fragments of ≈1 cm3 were embedded in OCT (Sigma), snap-frozen in liquid nitrogen, and kept at −80°C. Immunoperoxidase staining was performed on 6-μm cryostat sections incubated with rat anti-human D6 mAb or an irrelevant isotype control antibody, followed by horseradish peroxidase (HRP)-labeled secondary antibody (Sigma) and enzymatic reaction development by using 3,3′-diaminobenzidine (DAB). Invading extravillous trophoblasts were identified by staining the same section with an anti-human cytokeratin 7 FITC-labeled mouse mAb (Dako Cytomation, Milan, Italy). Leukocyte infiltrate in murine placenta was analyzed at indicated time points after LPS (0.4 mg/kg in 200 μl of saline) injection in mice at day 7 of pregnancy. Placentas were collected, and 8-μm consecutive frozen sections were mounted on Superfrost slides (Bio-Optica, Milan, Italy). After rehydration with PBS, sections were incubated with primary antibodies and subsequently with Envision+ system/HRP-labeled polymer (Dako Cytomation) or biotinylated rabbit anti-rat IgG (Vector Laboratories, Burlingame, CA) followed by ZyMax streptavidin/HRP-conjugated (Zymed Laboratories, San Francisco, CA). Enzymatic reactions were developed by using DAB. Natural killer cells were identified by using a rat anti-mouse LY-49G2 mAb (PharMingen, San Diego, CA), macrophages by using an rat anti-mouse CD68 mAb (HyCult Biotechnology, Uden, The Netherlands), T lymphocytes by using a rabbit anti-human/mouse CD3 polyclonal antibody (Dako Cytomation), and neutrophils were evaluated on the basis of the morphology of cells Gr1+ (rat anti-mouse Gr1 mAb; PharMingen). Positive cells in 10 randomly selected high-power fields for each case were counted by using the WinRec software (Image-Pro Plus, San Diego, CA).
Confocal Microscopy.
BeWo cells were seeded onto 0.4-μm pore size Transwell inserts (Costar, Corning, NY) at 5 × 105 per each 12-mm diameter filter, grown for 4 days at 37°C in 5% CO2, and fixed for 20 min at 4°C with 4% paraformaldehyde in PBS. Inserts were mounted with FluorSave reagent (Calbiochem, San Diego, CA) and stained with a rat anti-human D6 mAb and a purified rabbit polyclonal antibody for zonula occludens 1 (Zymed Laboratories), which defines the transition from the basolateral to the apical side of polarized cells. Appropriate negative controls were performed with irrelevant antibodies. Propidium iodide was used for nuclear staining. As secondary antibodies, Alexa-Fluor goat anti-rabbit 647 and Alexa-Fluor goat anti-rat 488 (Molecular Probes, Eugene, OR) were used. Images were acquired with a FV1000 laser scanning confocal microscope (Olympus, Hamburg, Germany). High-resolution images (1,024 × 1,024 pixels) were acquired sequentially with a ×100 1.4 N.A. Plan-Apochromat oil immersion objective (Olympus), and three-dimensional reconstruction and analysis were performed by using BP-IMARIS-4.2 Imaris colocalization 4.2 software (Bitplane AG, Zurich, Switzerland).
Calcium Flux Assay.
Changes in intracellular calcium concentrations were monitored as described previously (31). Briefly, HTR-8/SV40neo transfectants (107/ml) were incubated with 2 μM Fura 2 acetoxymethyl ester (Calbiochem) and 20% pluronic acid (Molecular Probes) at 37°C for 30 min, washed twice, and resuspended in HBSS (Sigma) containing 1.2 mM CaCl2. Cells (3 × 106 per cuvette) were stimulated at 37°C, and calcium concentrations were recorded in a LS50B spectrophotometer (Perkin–Elmer, Norwalk, CT).
Chemokine Transcytosis Assay.
BeWo cells were seeded onto 0.4-μm pore Transwell clear filters (Costar) and grown to complete confluence for 7 days. Ten nanograms of CXCL8/IL-8 or CCL3L1 per ml was added to each Transwell in the upper chamber, and after the indicated time of incubation at 37°C the chemokine present in the upper and lower chambers was measured by ELISA.
Chemotaxis Assay.
HTR8 transfectants were plated onto 8-μm pore Transwell filters coated with Matrigel (BD Biosciences) with the chemokine. After a 22-h incubation, filters were cut, stained with Diff-Quick (Dade Behring, Duningen, Switzerland), and migrated cells were counted. Results (mean ± SD) are the mean of five counted fields.
Chemokine Scavenging Assays.
Chemokine scavenging was analyzed as described previously (6). Briefly, BeWo cells (2 × 105) were incubated at 37°C in 200 μl of binding buffer (RPMI medium 1640/4 mM Hepes, pH 7.4/1% BSA) supplemented with 20 ng of the indicated chemokines per ml. After 18 h, the chemokine concentration in the supernatant was measured by ELISA. In a series of experiments, chemokine degradation was evaluated by using 125I-CCL4 (GE Healthcare Europe GmbH, Milan, Italy), as described previously (9).
Animals and Animal Models.
The generation and genotyping of D6−/− mice have been described previously (12). D6−/− and WT C57BL/6 mice were bred in a specific pathogen-free/viral antibody-free barrier facility at Charles River Italia (Calco, Italy). Mice (6–8 weeks old) were used in accordance with institutional guidelines in compliance with national (32) and international law and policies (33, 34). All efforts were made to minimize the number of animals used and their suffering. The day of vaginal plug detection was considered as day 0 of pregnancy. In the LPS-dependent fetal-loss model, mice were given an i.p. injection of LPS (0.4 mg/kg in 200 μl of saline) or vehicle on day 7 and killed on day 11. To block inflammatory chemokines, animals were treated with a mixture of goat antibodies to the mouse CC chemokines CCL3L1 (catalog no. AB450NA), CCL4 (catalog no. AB451NA), CCL2 (catalog no. AB479NA) and rat mAb anti-mouse CCL5 (catalog no. MAB478) purchased lyophilized from R&D Systems, resuspended in PBS, and mixed. On days 6–9 of pregnancy, mice were given i.p. injections of 200 μl of the mixture, equivalent to 100 μg of each antibody. On the same days, control mice received i.p. injections equivalent to 400 μg of irrelevant antibodies (normal goat IgG) in 200 μl of PBS. In the aPL-dependent fetal-loss model, mice were infused through the tail vein on day 0 with human serum IgG (10 μg per mouse in 200 μl of saline), purified from antiphospholipid syndrome patients or healthy women as described previously (35, 36), and killed on day 15. Resorbed fetuses were identified by their small size and necrotic or hemorrhagic appearance compared with normal embryos. Results are presented as the percentage of fetal loss, calculated by using the formula R/(R + V) × 100, where R is the number of resorbed fetuses and V is the number of viable fetuses in each experimental group, and as the percentage of females with fetal loss, calculated by using the formula F/(F + N) × 100, where F is the number of pregnant females with any resorption in their litter, and N is the number of pregnant females without, in each group.
Chemokine Levels.
Male mice were given an i.p. injection of 1.35 mg/kg LPS and killed at different time points. Female mice at day 10 of pregnancy were given an i.p. injection of 0.4 mg/kg LPS and killed after 8 h. Chemokine levels in sera and in placenta lysates, prepared as described (11), were measured by ELISA (R&D Systems) according to the manufacturer's instruction.
Statistical Analysis.
Data were analyzed by Student's t test and Fisher's exact test by using Prism4 (GraphPad Software, San Diego, CA). P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Dr. P. K. Lala (Department of Anatomy and Cell Biology, University of Western Ontario, London, ON, Canada) for the HTR8/SVneo cell line; Oriano Radillo for immunohistochemical analysis; and Marco Necci for image management. This work was supported by research grants from the European Community EMBIC (LSHM-CT-2004-512040 to A.M.) and INNOCHEM (LSHB-CT-2005-518167 to A.M. and M.L.), Ministero dell'Istruzione, Università e Ricerca (2005060371 to A.M. and M.L.), and PNR Bio2 (229602-1023/573 to A.M.). This work was conducted with the support of the Fondazione CARIPLO and Italian Association for Cancer Research (1722 to M.L. and 1182 to A.M.).
Abbreviations
- aPL
antiphospholipid autoantibodies
- TCA
trichloroacetic acid.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0607514104/DC1.
References
- 1.Luster AD. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 2.Murphy PM. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 3.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- 4.Locati M, Martinez de la Torre Y, Galliera E, Bonecchi R, Bodduluri H, Vago G, Vecchi A, Mantovani A. Cytokine Growth Factor Rev. 2005;16:679–686. doi: 10.1016/j.cytogfr.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Bonecchi R, Locati M. Nat Rev Immunol. 2006;12:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- 6.Bonecchi R, Locati M, Galliera E, Vulcano M, Sironi M, Fra AM, Gobbi M, Vecchi A, Sozzani S, Haribabu B, et al. J Immunol. 2004;172:4972–4976. doi: 10.4049/jimmunol.172.8.4972. [DOI] [PubMed] [Google Scholar]
- 7.Galliera E, Jala VR, Trent JO, Bonecchi R, Signorelli P, Lefkowitz RJ, Mantovani A, Locati M, Haribabu B. J Biol Chem. 2004;279:25590–25597. doi: 10.1074/jbc.M400363200. [DOI] [PubMed] [Google Scholar]
- 8.Weber M, Blair E, Simpson CV, O'Hara M, Blackburn PE, Rot A, Graham GJ, Nibbs RJ. Mol Biol Cell. 2004;15:2492–2508. doi: 10.1091/mbc.E03-09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fra AM, Locati M, Otero K, Sironi M, Signorelli P, Massardi ML, Gobbi M, Vecchi A, Sozzani S, Mantovani A. J Immunol. 2003;170:2279–2282. doi: 10.4049/jimmunol.170.5.2279. [DOI] [PubMed] [Google Scholar]
- 10.Nibbs RJ, Kriehuber E, Ponath PD, Parent D, Qin S, Campbell JD, Henderson A, Kerjaschki D, Maurer D, Graham GJ, Rot A. Am J Pathol. 2001;158:867–877. doi: 10.1016/s0002-9440(10)64035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez de la Torre Y, Locati M, Buracchi C, Dupor J, Cook DN, Bonecchi R, Nebuloni M, Rukavina D, Vago L, Vecchi A, et al. Eur J Immunol. 2005;35:1342–1346. doi: 10.1002/eji.200526114. [DOI] [PubMed] [Google Scholar]
- 12.Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, McLean P, Alcami A, Lira SA, Wiekowski M, Graham GJ. Nat Immunol. 2005;6:403–411. doi: 10.1038/ni1182. [DOI] [PubMed] [Google Scholar]
- 13.Nibbs RJ, Wylie SM, Yang J, Landau NR, Graham GJ. J Biol Chem. 1997;272:32078–32083. doi: 10.1074/jbc.272.51.32078. [DOI] [PubMed] [Google Scholar]
- 14.Bonini JA, Martin SK, Dralyuk F, Roe MW, Philipson LH, Steiner DF. DNA Cell Biol. 1997;16:1249–1256. doi: 10.1089/dna.1997.16.1249. [DOI] [PubMed] [Google Scholar]
- 15.Ellinger I, Schwab M, Stefanescu A, Hunziker W, Fuchs R. Eur J Immunol. 1999;29:733–744. doi: 10.1002/(SICI)1521-4141(199903)29:03<733::AID-IMMU733>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 16.Sato TA, Keelan JA, Mitchell MD. J Immunol. 2003;170:158–166. doi: 10.4049/jimmunol.170.1.158. [DOI] [PubMed] [Google Scholar]
- 17.Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J. BMJ. 1994;308:295–298. doi: 10.1136/bmj.308.6924.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar V, Ali SR, Konrad S, Zwirner J, Verbeek JS, Schmidt RE, Gessner JE. J Clin Invest. 2006;116:512–520. doi: 10.1172/JCI25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sironi M, Martinez FO, D'Ambrosio D, Gattorno M, Polentarutti N, Locati M, Gregorio A, Iellem A, Cassatella MA, Van Damme J, et al. J Leukocyte Biol. 2006;80:342–349. doi: 10.1189/jlb.1005586. [DOI] [PubMed] [Google Scholar]
- 20.Berman J, Girardi G, Salmon JE. J Immunol. 2005;174:485–490. doi: 10.4049/jimmunol.174.1.485. [DOI] [PubMed] [Google Scholar]
- 21.Rand JH, Wu XX, Andree HA, Lockwood CJ, Guller S, Scher J, Harpel PC. N Engl J Med. 1997;337:154–160. doi: 10.1056/NEJM199707173370303. [DOI] [PubMed] [Google Scholar]
- 22.Levine JS, Branch DW, Rauch J. N Engl J Med. 2002;346:752–763. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- 23.Meroni PL, di Simone N, Testoni C, D'Asta M, Acaia B, Caruso A. Lupus. 2004;13:649–652. doi: 10.1191/0961203304lu2001oa. [DOI] [PubMed] [Google Scholar]
- 24.Erlebacher A, Zhang D, Parlow AF, Glimcher LH. J Clin Invest. 2004;114:39–48. doi: 10.1172/JCI20645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantovani A, Locati M, Vecchi A, Sozzani S, Allavena P. Trends Immunol. 2001;22:328–336. doi: 10.1016/s1471-4906(01)01941-x. [DOI] [PubMed] [Google Scholar]
- 26.Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE, Mantovani A. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- 27.Drake PM, Red-Horse K, Fisher SJ. Rev Endocr Metab Disord. 2002;3:159–165. doi: 10.1023/a:1015463130306. [DOI] [PubMed] [Google Scholar]
- 28.Holmes CH, Simpson KL, Wainwright SD, Tate CG, Houlihan JM, Sawyer IH, Rogers IP, Spring FA, Anstee DJ, Tanner MJ. J Immunol. 1990;144:3099–3105. [PubMed] [Google Scholar]
- 29.Proudfoot AE. Nat Rev Immunol. 2002;2:106–115. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 31.Sozzani S, Zhou D, Locati M, Rieppi M, Proost P, Magazin M, Vita N, van Damme J, Mantovani A. J Immunol. 1994;152:3615–3622. [PubMed] [Google Scholar]
- 32.Presidenza della Repubblica Italiana. Gazzetta Ufficiale. 1992;(supplement 40) D.L. N.116, 18-2-1992. [Google Scholar]
- 33.European Economic Community Council. Official Journal of European Communites. 1987 Directive 86/609, L 358,1. [Google Scholar]
- 34.Institute of Laboratory Animal Resources, Committee on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: Natl Acad Press; 1996. [Google Scholar]
- 35.Blank M, Cohen J, Toder V, Shoenfeld Y. Proc Natl Acad Sci USA. 1991;88:3069–3073. doi: 10.1073/pnas.88.8.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piona A, La Rosa L, Tincani A, Faden D, Magro G, Grasso S, Nicoletti F, Balestrieri G, Meroni PL. Scand J Immunol. 1995;41:427–432. doi: 10.1111/j.1365-3083.1995.tb03588.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.